Abstract
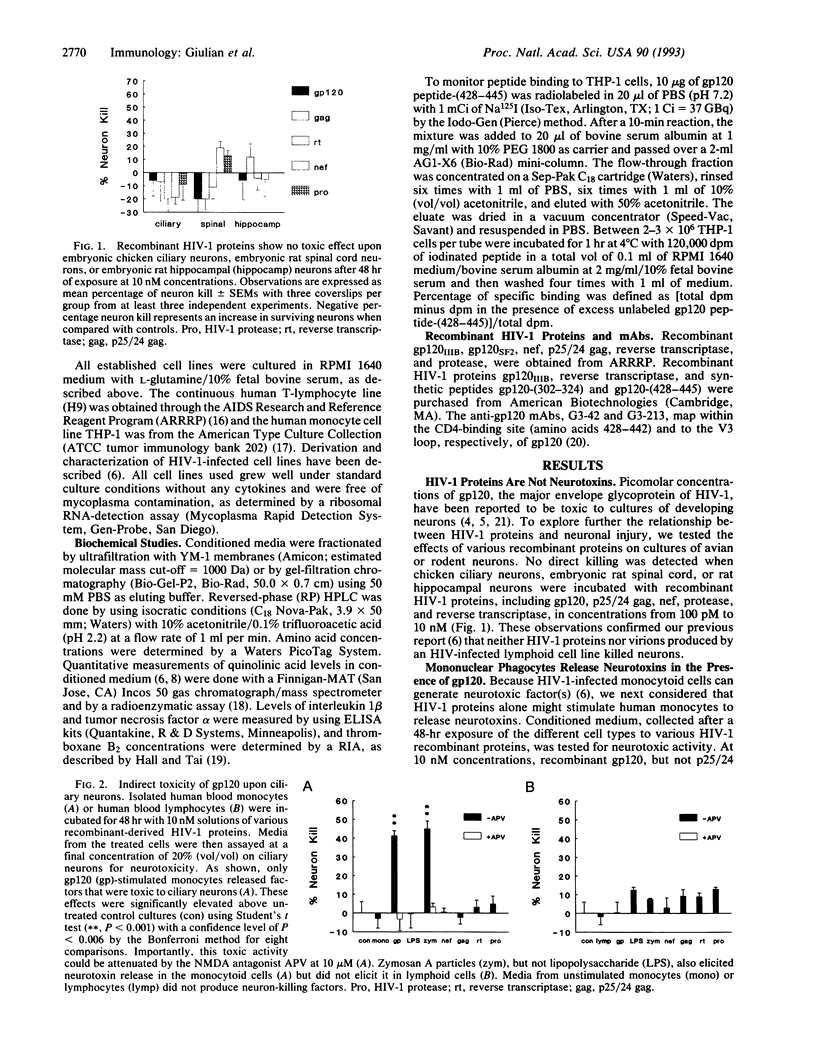
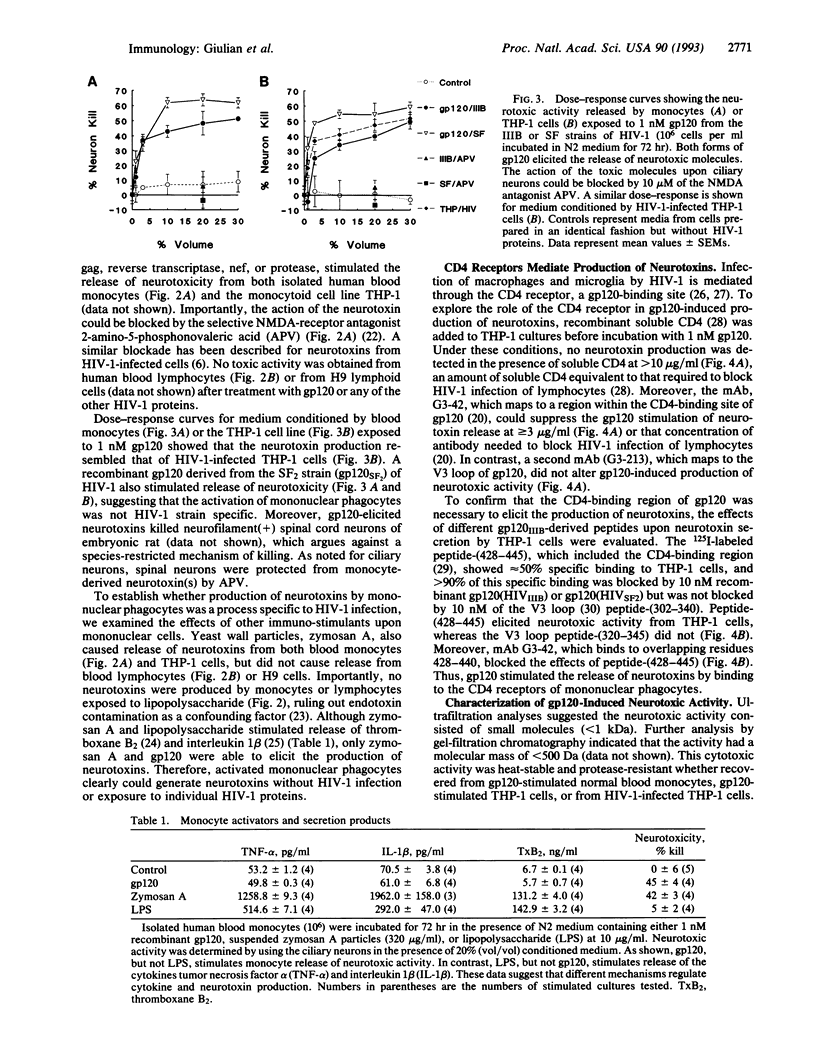
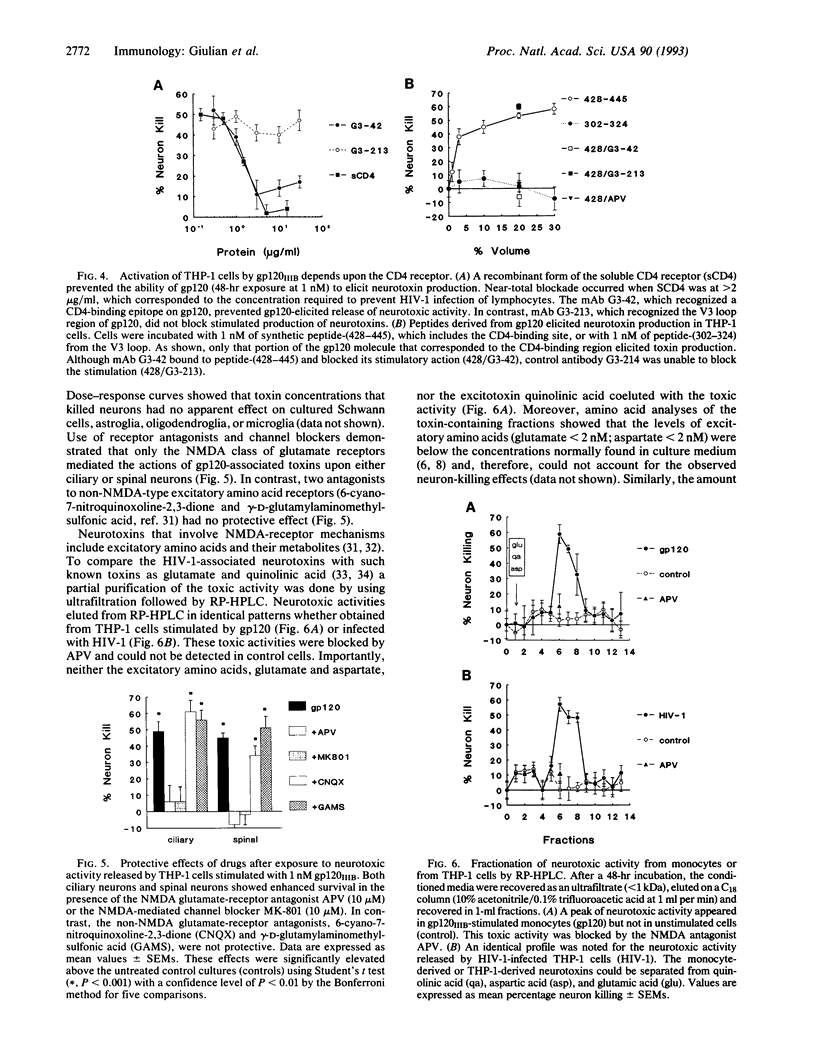
Mononuclear phagocytes infected with human immunodeficiency virus 1 (HIV-1) produce soluble factors that kill neurons in culture. To define the molecular events that lead to neuron killing, HIV-1 proteins were tested for the ability to trigger release of neurotoxins from human monocytes and lymphocytes. None of the recombinant-derived HIV-1 proteins examined (reverse transcriptase, protease, gag, nef, or gp120) were directly neurotoxic at concentrations from 100 pM to 10 nM. The envelope glycoprotein gp120 did, however, stimulate both isolated human blood monocytes and the monocytoid line THP-1 (but not lymphocytes or the lymphoid cell line H9) to discharge neurotoxic factors. These toxins consisted of heat-stable, protease-resistant molecules (< 500 Da) that copurified with neurotoxins from HIV-1-infected THP-1 cells and were blocked by antagonists to N-methyl-D-aspartate receptors. Release of neurotoxins through gp120 stimulation involved monocytoid CD4 receptors because toxin production could be inhibited either by a monoclonal antibody to the CD4-binding region of gp120 or by soluble CD4 receptors. Alternatively, production of neuron-killing factors could be induced with a peptide from the CD4-binding region of gp120. These data show that the HIV-1 envelope glycoprotein alone can stimulate neurotoxin release by binding to CD4 receptors of mononuclear phagocytes. Such neurotoxic factors may, in turn, contribute to the central nervous system dysfunction associated with HIV-1 by acting on neurons through N-methyl-D-aspartate receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Humes J. L. Physiological and pharmacological regulation of prostaglandin and leukotriene production by macrophages. J Leukoc Biol. 1984 Jan;35(1):1–10. doi: 10.1002/jlb.35.1.1. [DOI] [PubMed] [Google Scholar]
- Brenneman D. E., Westbrook G. L., Fitzgerald S. P., Ennist D. L., Elkins K. L., Ruff M. R., Pert C. B. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Okuno E., Brougher D. S., Schwarcz R. A radioenzymatic assay for quinolinic acid. Anal Biochem. 1986 Oct;158(1):98–103. doi: 10.1016/0003-2697(86)90595-6. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D. Reactive glia as rivals in regulating neuronal survival. Glia. 1993 Jan;7(1):102–110. doi: 10.1002/glia.440070116. [DOI] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Corpuz M. Brain glia release factors with opposing actions upon neuronal survival. J Neurosci. 1993 Jan;13(1):29–37. doi: 10.1523/JNEUROSCI.13-01-00029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Tai H. H. Purification of thromboxane synthetase and evidence of two distinct mechanisms for the formation of 12-L-hydroxy-5,8,10-heptadecatrienoic acid by porcine lung microsomes. Biochim Biophys Acta. 1981 Sep 24;665(3):498–503. doi: 10.1016/0005-2760(81)90263-0. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Rubinow D., Lane C., Markey S. P. Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann Neurol. 1989 Aug;26(2):275–277. doi: 10.1002/ana.410260215. [DOI] [PubMed] [Google Scholar]
- Honoré T. Excitatory amino acid receptor subtypes and specific antagonists. Med Res Rev. 1989 Jan-Mar;9(1):1–23. doi: 10.1002/med.2610090102. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Jordan C. A., Watkins B. A., Kufta C., Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991 Feb;65(2):736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P. K., Offermann J. T., Lipton S. A. Neuronal injury due to HIV-1 envelope protein is blocked by anti-gp120 antibodies but not by anti-CD4 antibodies. Neurology. 1990 Nov;40(11):1757–1761. doi: 10.1212/wnl.40.11.1757. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Scadden D. T., Amirault C., Woon A., Vannier E., Dinarello C. A., Groopman J. E. Human immunodeficiency virus does not induce interleukin-1, interleukin-6, or tumor necrosis factor in mononuclear cells. J Virol. 1990 Jun;64(6):2901–2906. doi: 10.1128/jvi.64.6.2901-2906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Piani D., Frei K., Do K. Q., Cuénod M., Fontana A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991 Dec 9;133(2):159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J., Davies S. W. Excitatory receptors and their role in excitotoxicity. Biochem Soc Trans. 1987 Apr;15(2):218–219. doi: 10.1042/bst0150218. [DOI] [PubMed] [Google Scholar]
- Sabatier J. M., Vives E., Mabrouk K., Benjouad A., Rochat H., Duval A., Hue B., Bahraoui E. Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1. J Virol. 1991 Feb;65(2):961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Stone T. W., Connick J. H. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985 Jul;15(3):597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- Sun N. C., Ho D. D., Sun C. R., Liou R. S., Gordon W., Fung M. S., Li X. L., Ting R. C., Lee T. H., Chang N. T. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry Clotilde, Chamak Brigitte, Mallat Michel. Cytotoxic Effect of Brain Macrophages on Developing Neurons. Eur J Neurosci. 1991 Oct;3(11):1155–1164. doi: 10.1111/j.1460-9568.1991.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Masliah E., Morey M., Lemere C., DeTeresa R., Grafe M., Hansen L., Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991 Jun;29(6):651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]


