Abstract
Angiogenesis is the formation of new blood vessels from pre-existing vessels and normally occurs during the process of inflammatory reactions, wound healing, tissue repair, and restoration of blood flow after injury or insult. Stimulation of angiogenesis is a promising and an important step in the treatment of peripheral artery disease. Reactive oxygen species have been shown to be involved in stimulation of this process. For this reason, we have developed and validated a non-equilibrium atmospheric temperature and pressure short-pulsed dielectric barrier discharge plasma system, which can non-destructively generate reactive oxygen species and other active species at the surface of the tissue being treated. We show that this plasma treatment stimulates the production of vascular endothelial growth factor, matrix metalloproteinase-9, and CXCL 1 that in turn induces angiogenesis in mouse aortic rings in vitro. This effect may be mediated by the direct effect of plasma generated reactive oxygen species on tissue.
I. INTRODUCTION
Peripheral Artery Disease (PAD) is characterized by a decrease in blood flow to the extremities (ischemia) and is associated with pain and often loss of limb function or even the limb itself. A major goal of therapy is to stimulate vasculogenesis in ischemic limbs, either through the growth of new vessels or through collateral angiogenesis from existing vessels.1 Angiogenesis, the growth of new blood vessels, normally occurs during the process of inflammatory reactions, wound healing, tissue repair, and restoration of blood flow after injury or insult. Physiologically, angiogenesis is required to meet the increased nutritional and oxygen needs during embryogenesis and growth, but is a rare phenomenon in healthy adult tissues.2
Formation of new capillaries is a multi-step process. It begins with the degradation of endothelial basement membrane and the local extracellular matrix to make space for the growing vessels. This is followed by induction of migration and proliferation of endothelial cells. Finally, factors that inhibit angiogenesis are produced to regulate the vascular network and allow for maturation of capillaries.3 Hence, there is a balance between growth promoting and growth inhibiting processes resulting in a tight control over angiogenesis.
Any disturbance in the equilibrium results in either too much or too little capillary growth and is recognized as an essential component of many serious diseases such as cancers, cardiovascular disease, strokes, diabetic ulcers, and macular degeneration.4
Various growth factors essential for angiogenesis have been identified. Vascular Endothelial Growth Factor (VEGF-A) is one of the most important regulators of angiogenesis.4,5 Its production by macrophages and stromal cells is stimulated by Reactive Oxygen Species (ROS).6,7 Macrophages are also a potent source of Matrix metalloproteinase-9 (MMP-9), also known as Collagenase, which breaks down the basal lamina in blood vessels and extracellular matrix to make room for new vessels.8 Another important molecule, CXCL 1, is a chemokine secreted by both macrophages and endothelial cells. It is important for migration and proliferation of endothelial cells.9,10 Angiogenesis is a complex process and multiple other factors play a role, for example, Interleukin 8, Interleukin 19, Fibroblast Growth Factor, Platelet Derived Endothelial Growth Factor, etc.11–13 The key cells involved in the process of angiogenesis include endothelial cells, stromal cells, and macrophages.13 Their activation, juxtacrine communication, and coordination are a tightly regulated process. A number of factors required for angiogenesis are produced by endothelial cells and macrophages, mononuclear phagocytic cells that are ubiquitously present.14,15
Available pro-angiogenic strategies for PAD include growth factor/cytokine therapy, gene therapy, and stem cell therapy. While promising in the laboratory, growth factor based therapies have only been partially successful in clinical trials.11 Current research approaches are focused on modulation of growth factor delivery to achieve sustained, temporal release by employing newly engineered biomaterials.16 Stem cell based therapies face other limitations and their clinical efficacy is not yet well proven.17 Genetic engineering approaches are being used to optimize the survival, homing, and growth factor production by stem cells.18 Combination therapies are also being tried. Based on limitations of current, systemic therapies, what is needed is a non-invasive, localized therapy to induce angiogenesis in the ischemic limb. Dielectric barrier discharge (DBD) plasma is a promising, non-invasive approach for the treatment of PAD.
We have previously shown that DBD plasma can stimulate the migration of cultured macrophages in an in vitro wound healing scratch assay.19 Short-pulsed non-equilibrium atmospheric pressure plasmas have been shown to produce high concentrations of reactive oxygen and reactive nitrogen species, charges, and ultraviolet radiation.20,21 Specifically, the authors have developed a Floating Electrode-DBD (FE-DBD) system that allows safe application of Non-Thermal Plasma (NTP) to cells and living tissues without damage.22,23 FE-DBD systems generate plasma in direct contact with tissue by applying fast, high voltage pulses between a quartz dielectric-covered copper electrode and the biological target. Active species known for their biochemical activity, generated by NTP, include H2O2, OH, N2+, O2−, O2(1Δg), NO, ONOO•, and others.24
We hypothesize that since non-thermal plasma can stimulate macrophage function,19 cells important for VEGF-A, MMP-9, and CXCL 1 secretion, and since ROS are important signaling molecules for angiogenesis via VEGF-A secretion, DBD treatment of aortic rings will induce angiogenesis. In this manuscript, we use mouse aortic rings to determine if non-thermal DBD plasma treatment can elicit angiogenesis. This assay is a commonly used, physiologically relevant model to identify angiogenic factors in the absence of ischemia and inflammation.
II. MATERIALS AND METHODS
A. Aortic ring assay
The aortic ring assay was performed as described by Jain et al.12 Aorta from C57B6 mice (8–10 weeks old) were removed from arch to renal arteries under sterile conditions and placed in sterile phosphate buffered saline (PBS) containing penicillin and streptomycin (pen-strep). Perivascular fat was removed and the aorta was cut into 3 mm rings. Matrigel (BD 356231) was thawed overnight at 4 °C and kept on ice throughout the procedure to prevent polymerization. Fifty microliter of matrigel was added to each well of a 24 well plate. To identify the safe therapeutic dose range, each aortic ring was plasma treated at a different energy (0.3 J corresponding to 50 Hz, 4.6 J corresponding to 830 Hz, and 5.6 J corresponding to 1000 Hz) and then placed in matrigel in the prepared plate. The plate was incubated for 30 min at 37 °C. 100 μl of matrigel was added on top to cover each ring, and the plate was incubated for another 15 min at 37 °C. Then 700 μl of MCDB media (Corning 15–100-CV) containing pen-strep, L-glutamine, and 10% Fetal Bovine Serum (FBS) by volume was slowly added to each well. Four hundred ng/ml VEGF-A was added to appropriate wells. Aortic rings were examined daily, and digital images were taken at day 7 for quantitative analysis of the area of vessel outgrowth by the SPOT Advanced imaging program (Media Cybernetics, Sterling Heights, MI). Microvessel outgrowth was calculated using the Image J analysis program by circling the extent of microvessel outgrowth at 7 days, and subtracting the area of the aortic ring. All animal procedures were approved by the Institutional Animal Care and Use Committee of Temple University. At the end of the experiment, RNA was extracted from representative aortic rings and analyzed by Reverse Transcription Polymerase Chain Reaction (qRT-PCR). The following experimental groups were included in the study:
-
(a)
Negative control: Aortic rings with no treatment
-
(b)
Plasma experimental group: Aortic rings with msDBD plasma treatment
-
(c)
Positive control: Aortic rings with VEGF treatment
-
(d)
Synergy experimental group: Aortic rings with VEGF and msDBD plasma treatment
B. Microsecond pulsed DBD treatment
Treatment of the aortic rings was performed on glass slides with microsecond pulsed DBD (msDBD) plasma (Fig. 1). Plasma was produced by applying a voltage pulse of approximately 20 kV between the high voltage electrode and a grounded metal plate underneath the slide.24,25 Aortic rings were at floating potential. Treatment time and gap distance were fixed at 10 s and 2 mm, respectively, and the frequency was internally controlled. Three frequencies, 50, 830, and 1000 Hz, were used for low, medium, and high plasma treatment. The energy per pulse of a single msDBD plasma discharge was measured and calculated using the methods stated in our previous work.26 This was found to be 0.56 mJ/pulse, and the total energy delivered to the cells (dose) during treatment for the three frequencies are 0.3 mJ (50 Hz), 4.6 mJ (830 Hz), and 5.6 mJ (1000 Hz). Tissue was immediately transferred to a 24-well plate well containing matrigel.
FIG. 1.
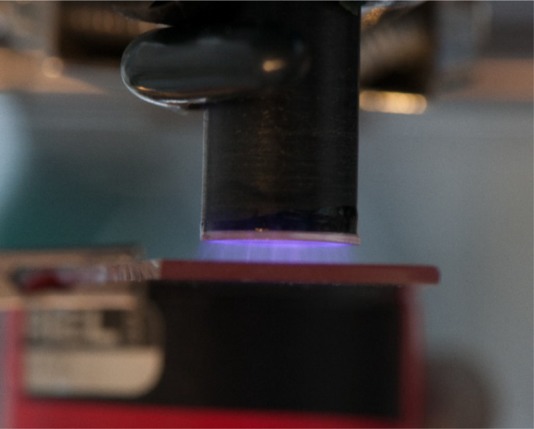
Photograph of the microsecond-pulsed dielectric barrier discharge generated on top of a glass slide.
C. RT-PCR
Total RNA was isolated from control and stimulated aortic rings. One microgram of total RNA was reverse transcribed by standard methods as described.12 MMP-9 and GAPDH mRNA were targeted using primer pairs from Integrated DNA Technologies, (Coralville, IA), SYBR green was used for detection and amplified using an Eppendorf MCEP RealPlex 4X thermocycler.
The following primer pairs were used:
Mouse VEGF-A: Forward: GGCAGCTTGAGTTAAACGAAC; Reverse: TGGTGACATGGTTAATCGGTC
Mouse CXCL1: Forward: AGAACATCCAGAGCTTGAAGG; Reverse: CAATTTTCTGAACCAAGGGAGC
Mouse MMP-9: Forward: GATCCCCAGAGCGTCATTC; Reverse: CCACCTTGTTCACCTCATTTTG
Mouse GAPDH: Forward: GCAAGGACACTGAGCAAGAG; Reverse: GGGTCTGGGATGGAAATTGT
D. Statistical analysis
Results are expressed as mean ± SEM. Differences between groups were evaluated with the use of ANOVA, with the Newman-Keuls method applied to evaluate differences between individual mean values and by paired t tests where appropriate, respectively. Differences were considered significant at a level of P < 0.05.
III. RESULTS AND DISCUSSION
A. Plasma treatment stimulates angiogenesis in aortic rings
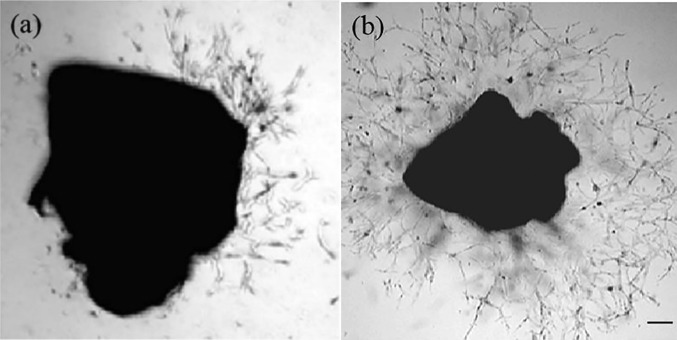
A microsecond pulsed atmospheric pressure DBD device was used for in vitro treatment of dissected aortic rings. The voltage was set to 20 kV, and treatment time and distance were set at 10 s and 2 mm, respectively. Aortic rings were treated at 50 Hz, 830 Hz, and 1000 Hz and immediately placed in matrigel. They were observed daily for the outgrowth of new blood vessels. On day 7, pictures were taken and analyzed with ImageJ. Growth factors present in matrigel resulted in some capillary growth in non-plasma treated aortic rings (Figure 2(a)). This was used as our negative control. VEGF supplemented samples served as our positive control (Figure 2(b)).
FIG. 2.
Microvessel outgrowth in mouse aortic rings. Angiogenesis occurs when endothelial cells sprout from pre-existing vessels to form new structures. For these experiments, mouse thoracic aorta was sectioned, placed onto growth factor reduced matrigel, and incubated in media containing normal saline or VEGF as a positive control. Sprouting from rings was analyzed daily, and on the seventh day photographed and outgrowth area quantitated by image analysis. In (a), a representative image of an aortic ring without plasma treatment is shown. Aortic ring cultured in VEGF-A supplemented media is shown in (b). Scale bar: 200 μm.
The results of exposure of aortic rings to different plasma doses are summarized in Table I. Capillary outgrowth was observed in tissue samples following 50 Hz treatment of plasma with and without VEGF-A. However, at higher dose treatments, no growth was observed. Previous studies looking at survivability of mammalian cells following the plasma treatment show a dose-dependent relationship between treatment and cell viability.25 There are several mechanisms proposed for this loss of viability, of which the most plausible is the dose-dependent generation of ROS, which leads to formation of single-stranded DNA breaks. DNA damage induces a cascade of cellular responses including activation of multiple cellular kinases, leading to arrest of DNA replication and repair and induction of apoptosis. Investigation into the precise mechanisms for this loss of viability is beyond the scope of the present manuscript. Since in the present study, rings treated with higher plasma doses were incapable of capillary outgrowth, even in the presence of VEGFA, we conclude that the tissue was sufficiently damaged by the plasma treatment to preclude DNA replication and endothelial cell sprouting, which is consistent with a loss of viability.
TABLE I.
msDBD treatment dose optimization. Mouse thoracic aorta was sectioned, placed onto growth factor reduced matrigel, and incubated in media containing normal saline, VEGF-A as a positive control or exposed to treatment with different doses of msDBD plasma. Below, the different plasma regimes are represented as the treatment frequency. A combination of both plasma and VEGF treatment was also studied. Sprouting from rings was analyzed daily, and on the seventh day photographed and outgrowth area quantitated by image analysis. Capillary outgrowth was seen at 50 Hz. At higher doses, no growth was observed.
| Microvessel formation | |
|---|---|
| Treatment | Growth |
| Untreated | + |
| VEGF | ++ |
| 50 Hz | ++++ |
| 50 Hz + VEGF-A | ++++ |
| 830 Hz | No growth |
| 830 Hz + VEGF-A | No growth |
| 1000 Hz | No growth |
| 1000 Hz + VEGF-A | No growth |
Consequently, while in this study viability was not determined, we hypothesize that the higher doses are damaging the cells, or at the very least are not angiogenic. The fact that 50 Hz-treated aortic rings could sprout microvessels indicates that this dose does not reduce viability.
Quantification of the images showed that at non-damaging regimes of msDBD, the plasma treatment was more effective in inducing angiogenesis than VEGF-A, a documented promoter of angiogenesis.5 The results are presented in Figure 3. There was an approximately two-fold increase in the area covered by the newly growing capillary vessels. In addition, VEGF-A did not appear to further enhance the effect of DBD plasma.
FIG. 3.

msDBD treatment increases microvessel outgrowth in mouse aortic rings. Mouse thoracic aorta was sectioned, treated with plasma and immediately placed onto growth factor reduced matrigel, and incubated in media containing normal saline or VEGF-A. Sprouting from rings was analyzed daily, and on the seventh day photographed and outgrowth area quantitated by image analysis. The results of image quantification are summarized in (a). Representative image of aortic rings seven days after plasma treatment at 50 Hz is shown in (b). Aortic ring that was plasma treated at 50 Hz and incubated for seven days in the presence of VEGF-A is shown in (c). Scale bar: 200 mm.
B. Plasma treatment induces expression of key angiogenic genes in aortic rings
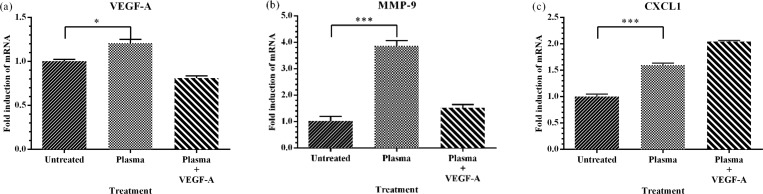
The fact that plasma-treated rings could sprout microvessels in the absence of VEGF-A suggests that 50 Hz treatment alone could initiate a series of cellular events leading to angiogenesis. Angiogenesis is regulated by coordinated expression of key genes. To define a potential mechanism by which DBD plasma may be inducing angiogenesis in the aortic rings, we examined the expression of three key genes involved in angiogenesis, VEGF-A, MMP-9, and CXCL1. Total RNA was extracted and quantitative Reverse-Transcription-PCR was performed using appropriate primers as listed in Sec. II C. Expression was normalized to GAPDH, a housekeeping gene. Our results show that plasma significantly increased expression of all three genes as compared to the untreated controls (Fig. 4). Curiously, addition of VEGF-A to incubation medium also significantly decreased the expression of VEGF-A below basal levels from plasma treated aortic rings. This may indicate a feedback mechanism for regulating VEGF-A levels. The maximum effect of DBD plasma was seen on MMP-9, where a four-fold increase was observed. Once again, in samples supplemented with VEGF-A, the increase was not as robust. Expression of the chemokine CXCL1 significantly increased by 50% following plasma treatment and doubled when plasma treatment was used in combination with VEGF-A. Expression of VEGF-A is particularly informative in this experimental system. VEGF-A plays a central role in neovascularization and is considered to be the main, dominant inducer of angiogenic gene expression.4 VEGF-A is essential in adult wound healing, tumor angiogenesis, and vascular sprouting. The differentiation of endothelial cells is dependent upon the expression of VEGF-A; and the lack of VEGF-A results in impaired neo-vascularization. The chemokine (C-X-C motif) ligand 1 (CXCL1) is a small cytokine belonging to the CXC chemokine family. Originally identified for its leukocyte chemotactic properties, CXCL1 is secreted by endothelial cells and macrophages, and has been shown to regulate endothelial cell motility, proliferation, viability, and angiogenesis.9,10 MMP-9, in particular, plays an important role in angiogenesis as it is a key regulator of growth plate angiogenesis. MMP-9 is also required for the recruitment of endothelial stem cells, a critical component of angiogenesis.27 Expression of both CXCL1 and MMP-9 is inducible by VEGF-A, suggesting that plasma treatment induction of VEGF-A in aortic rings may be the primary mechanism for the observed angiogenic effects. Future experiments are necessary to determine if plasma treatment induction of VEGF-A is the only mechanism whereby plasma treatment induces angiogenesis.
FIG. 4.
msDBD treatment induces expression of key angiogenic genes. Mouse aortic rings were treated with plasma, in the presence or absence of VEGF-A, and incubated as described. At 7 days, gene induction of VEGF-A, MMP-9, and CXCL1, known regulatory genes for angiogenesis was quantitated by quantitative reverse transcription PCR. Data are shown as mean ± SEM. Asterisk indicates a significant difference compared with untreated rings. *p < 0.05, ***p < 0.001.
IV. CONCLUSIONS
Microsecond pulsed dielectric barrier discharges stimulate the production of VEGF-A, MMP-9, and CXCL 1 that in turn induce angiogenesis in mouse aortic rings in vitro. This effect may be mediated by the direct effect of plasma generated reactive oxygen species on macrophages. This is the first report that correlates plasma dose and proangiogenic factors for the promotion of neovascularization from aortic rings. Further exploration of plasma-tissue interactions is required to optimize treatment regimes as a potential non-invasive therapeutic approach for PAD.
ACKNOWLEDGMENTS
This work was supported by Grant Nos. HL115575 and HL117724 from the National Heart Lung and Blood Institute of the National Institutes of Health to MVA.
References
- 1. Collinson D. and Donnelly R., “ Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation?,” Eur. J. Vasc. Endovasc. Surg. 28(1), 9–23 (2004). 10.1016/j.ejvs.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 2. Ferrara N., “ Role of vascular endothelial growth factor in regulation of physiological angiogenesis,” Am. J. Physiol. 280(6), C1358–C1366 (2001). [DOI] [PubMed] [Google Scholar]
- 3. Demir R., Kayisli U., Cayli S., and Huppertz B., “ Sequential steps during vasculogenesis and angiogenesis in the very early human placenta,” Placenta 27(6), 535–539 (2006). 10.1016/j.placenta.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 4. Carmeliet P., “ Mechanisms of angiogenesis and arteriogenesis,” Nat. Med. 6(4), 389–396 (2000). 10.1038/74651 [DOI] [PubMed] [Google Scholar]
- 5. Hoeben A., Landuyt B., Highley M. S., Wildiers H., Van Oosterom A. T., and De Bruijn E. A., “ Vascular endothelial growth factor and angiogenesis,” Pharmacol. Rev. 56(4), 549–580 (2004). 10.1124/pr.56.4.3 [DOI] [PubMed] [Google Scholar]
- 6. Ushio-Fukai M. and Alexander R. W., “ Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD (P) H oxidase,” Mol. Cell. Biochem. 264(1–2), 85–97 (2004). 10.1023/B:MCBI.0000044378.09409.b5 [DOI] [PubMed] [Google Scholar]
- 7. Fay J., Varoga D., Wruck C. J., Kurz B., Goldring M. B., and Pufe T., “ Reactive oxygen species induce expression of vascular endothelial growth factor in chondrocytes and human articular cartilage explants,” Arthritis Res. Ther. 8(6), R189 (2006). 10.1186/ar2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vu T. H., Shipley J. M., Bergers G., Berger J. E., Helms J. A., Hanahan D., Shapiro S. D., Senior R. M., and Werb Z., “ MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes,” Cell 93(3), 411–422 (1998). 10.1016/S0092-8674(00)81169-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhawan P. and Richmond A., “ Role of CXCL1 in tumorigenesis of melanoma,” J. Leukocyte Biol. 72(1), 9–18 (2002). [PMC free article] [PubMed] [Google Scholar]
- 10. Zaja-Milatovic S. and Richmond A., “ CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing,” Histol. Histopathol. 23(11), 1399 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deveza L., Choi J., and Yang F., “ Therapeutic angiogenesis for treating cardiovascular diseases,” Theranostics 2(8), 801 (2012). 10.7150/thno.4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain S., Gabunia K., Kelemen S. E., Panetti T. S., and Autieri M. V., “ The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells,” Arterioscler., Thromb., Vasc. Biol. 31(1), 167–175 (2011). 10.1161/ATVBAHA.110.214916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sunderkötter C., Steinbrink K., Goebeler M., Bhardwaj R., and Sorg C., “ Macrophages and angiogenesis,” J. Leukocyte Biol. 55(3), 410–422 (1994). [DOI] [PubMed] [Google Scholar]
- 14. Lavin Y., Winter D., Blecher-Gonen R., David E., Keren-Shaul H., Merad M., Jung S., and Amit I., “ Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment,” Cell 159(6), 1312–1326 (2014). 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosselin D., Link V. M., Romanoski C. E., Fonseca G. J., Eichenfield D. Z., Spann N. J., Stender J. D., Chun H. B., Garner H., and Geissmann F., “ Environment drives selection and function of enhancers controlling tissue-specific macrophage identities,” Cell 160(1), 351–352 (2015). 10.1016/j.cell.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeon O., Kang S.-W., Lim H.-W., Chung J. H., and Kim B.-S., “ Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly (L-lactide-co-glycolide) nanospheres and fibrin gel,” Biomaterials 27(8), 1598–1607 (2006). 10.1016/j.biomaterials.2005.08.030 [DOI] [PubMed] [Google Scholar]
- 17. Fadini G. P., Agostini C., and Avogaro A., “ Autologous stem cell therapy for peripheral arterial disease: meta-analysis and systematic review of the literature,” Atherosclerosis 209(1), 10–17 (2010). 10.1016/j.atherosclerosis.2009.08.033 [DOI] [PubMed] [Google Scholar]
- 18. Iwaguro H., Yamaguchi J.-i., Kalka C., Murasawa S., Masuda H., Hayashi S.-i., Silver M., Li T., Isner J. M., and Asahara T., “ Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration,” Circulation 105(6), 732–738 (2002). 10.1161/hc0602.103673 [DOI] [PubMed] [Google Scholar]
- 19. Miller V., Lin A., Fridman G., Dobrynin D., and Fridman A., “ Plasma stimulation of migration of macrophages,” Plasma Processes Polym. 11(12), 1193–1197 (2014). 10.1002/ppap.201400168 [DOI] [Google Scholar]
- 20. Fridman A., Plasma Chemistry ( Cambridge University Press, 2008). [Google Scholar]
- 21. Fridman A., Chirokov A., and Gutsol A., “ Non-thermal atmospheric pressure discharges,” J. Phys. D: Appl. Phys. 38(2), R1 (2005). 10.1088/0022-3727/38/2/R01 [DOI] [Google Scholar]
- 22. Fridman G., Friedman G., Gutsol A., Shekhter A. B., Vasilets V. N., and Fridman A., “ Applied plasma medicine,” Plasma Processes Polym. 5(6), 503–533 (2008). 10.1002/ppap.200700154 [DOI] [Google Scholar]
- 23. Fridman G., Peddinghaus M., Balasubramanian M., Ayan H., Fridman A., Gutsol A., and Brooks A., “ Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air,” Plasma Chem. Plasma Process. 26(4), 425–442 (2006). 10.1007/s11090-006-9024-4 [DOI] [Google Scholar]
- 24. Dobrynin D., Fridman G., Friedman G., and Fridman A., “ Physical and biological mechanisms of direct plasma interaction with living tissue,” New J. Phys. 11(11), 115020 (2009). 10.1088/1367-2630/11/11/115020 [DOI] [Google Scholar]
- 25. Kalghatgi S., Kelly C. M., Cerchar E., Torabi B., Alekseev O., Fridman A., Friedman G., and Azizkhan-Clifford J., “ Effects of non-thermal plasma on mammalian cells,” PloS One 6(1), e16270 (2011). 10.1371/journal.pone.0016270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin A., Chernets N., Han J., Alicea Y., Dobrynin D., Fridman G., Freeman T. A., Fridman A., and Miller V., “ Non-equilibrium dielectric barrier discharge treatment of mesenchymal stem cells: Charges and reactive oxygen species play the major role in cell death,” Plasma Processes Polym. (published online). [DOI] [PMC free article] [PubMed]
- 27. Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N. R., Crystal R. G., Besmer P., Lyden D., and Moore M. A., “ Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand,” Cell 109(5), 625–637 (2002). 10.1016/S0092-8674(02)00754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]