Abstract
This study revisits the issue of the spectral ripple resolution abilities of cochlear implant (CI) users. The spectral ripple resolution of recently implanted CI recipients (implanted during the last 10 years) were compared to those of CI recipients implanted 15 to 20 years ago, as well as those of normal-hearing and hearing-impaired listeners from previously published data from Henry, Turner, and Behrens [J. Acoust. Soc. Am. 118, 1111–1121 (2005)]. More recently, implanted CI recipients showed significantly better spectral ripple resolution. There is no significant difference in spectral ripple resolution for these recently implanted subjects compared to hearing-impaired (acoustic) listeners. The more recently implanted CI users had significantly better pre-operative speech perception than previously reported CI users. These better pre-operative speech perception scores in CI users from the current study may be related to better performance on the spectral ripple discrimination task; however, other possible factors such as improvements in internal and external devices cannot be excluded.
I. INTRODUCTION
In 1984, the first multichannel cochlear implant (CI) was approved by the U.S. Food and Drug Administration (FDA). Since then, improvements in design, speech processing strategies, surgical methods, and professionals' collaborated efforts have led to the CI becoming a commonplace rehabilitation strategy to restore hearing and communication to many people with severe to profound hearing impairment. Today, most CI recipients reach high-performance levels for understanding open-set speech in quiet without visual cues and can talk on the phone. These outcomes, in turn, have extended the criteria for CI candidacy in regards to the degree of hearing loss and performance with best-aided conditions. The first CI approved by the FDA in 1984 was exclusively for use in profoundly deaf adults ages 18 and older with hearing loss greater than 100 dB. By 2000, the implantable age was extended to babies as young as 12 months of age. Current CI candidacy guidelines also include adults with more residual hearing with 50%–60% accuracy in open-set sentence recognition scores. At present, more than 320 000 individuals worldwide, including adults and children, have received the CI (National Institute on Deafness and Other Communication Disorders, 2015). The population of CI users now includes many patients with more pre-operative residual hearing and shorter periods of deafness than in earlier years (Sampaio et al., 2011). Both of these factors have been shown to be strong predictors of post-implant speech recognition abilities with a CI (e.g., Rubinstein et al., 1999; Gomaa et al., 2003; Leung et al., 2005).
Limitations for even the best CI users are apparent in challenging listening conditions, including music appreciation and understanding speech in background noise (e.g., Qin and Oxenham, 2003; Stickney et al., 2004; Gfeller et al., 2002; Gfeller et al., 2007). These limitations are inevitable because of the reduced spectral resolution restricted by the device design that uses only a few electrode channels. Friesen et al. (2001) suggested that 6–8 effective channels of stimulation were all that was possible with CIs, while normal-hearing (NH) listeners with CI simulations were capable of using more than 16 channels of stimulation. In the case of these long-electrode CI users, most implanted in the 1990s, these 6–8 “effective” or “usable” channels are spread along the entire (approximately 24 mm) length of the electrode array. Their general conclusion of this study was that there might be a limitation on usable channels in CI patients. This is most likely due to channel interactions, which are located some distance from the site of nerve stimulation or due to limited nerve ending survival. A greater number of effective channels are critical for accurate speech recognition, particularly in complex backgrounds (e.g., Nelson et al., 2003; Qin and Oxenham, 2003). This potential limitation has important clinical implications.
Clinical experience from other clinics, as well as the authors' own institution, shows that today's cochlear implant patients perform at levels surpassing previous patients for many speech recognition tasks (e.g., Wilson and Dorman, 2008; Zeng et al., 2008; Shannon, 2010). The data for long-electrode patients in the Henry et al. (2005) study were collected more than 15 years ago at the author's present laboratory. One possible explanation is related to the fact that the populations of patients implanted today can differ significantly from those implanted with long-electrodes in the 1990s. Today's patients might have better pre-operative hearing and shorter durations of deafness. Both of these factors might be associated with a higher survival of neural elements in the cochlea and/or centrally than in previous implant patients, who typically had much less (or no) pre-operative hearing and often had longer durations of deafness than the patients today. Thus, perhaps a more current population of long-electrode cochlear implant patients, who would have better pre-operative speech scores, etc., might produce better spectral resolution than the data provided in Henry et al. (2005). That is the basic rationale underlying the current study.
The primary purpose of this study is to measure spectral ripple resolution in CI recipients who were implanted more recently, and to compare these results to a previous study from a University of Iowa laboratory conducted more than 10 years ago (Henry et al., 2005). Spectral ripple discrimination ability data have been collected from CI users in the same laboratory for over 15 years using the same measure, the spectral ripple discrimination test. It was developed using a method based on the “ripple phase reversal test” used in Supin et al. (1994, 1997, 1999) studies. The spectral ripple discrimination test involves discriminating two rippled noise stimuli in which the frequency positions of the peaks and valleys are interchanged. While the spectral ripple depth was held constant, the spectral ripple spacing was varied. Wider spectral ripple spacing is thought to make it easier to detect a reversal in peak and valley positions than narrower spectral ripple spacing. The highest discriminable spectral ripple density in ripples per octave is defined as the threshold for spectral peak resolution. Higher spectral ripple discrimination thresholds indicate better spectral discrimination performance.
This test has been used to investigate differences in spectral ripple discrimination thresholds among various listeners with NH, hearing impaired listening acoustically (HI), and CI listeners (Henry and Turner, 2003; Henry et al., 2005). Using logarithmically spread ripples, Henry et al. (2005) showed that spectral ripple resolution varied widely across listening groups; NH listeners obtained the highest spectral ripple discrimination thresholds with an average of 4.84 ripples/octave, followed by HI listeners with an average of 1.77 ripples/octave. The CI users had the poorest spectral ripple discrimination thresholds with an average of 0.62 ripples/octave. Most importantly, they found a strong relationship between the spectral ripple discrimination thresholds and both vowel (r2 = 0.64, p < 0.0001) and consonant (r2 = 0.66, p < 0.0001) recognition scores across the listening groups. Predicting outcomes using this test has potential as a quick and easy test to assist in optimizing speech processor programming. Also of importance in the Henry et al. (2005) study was the finding that essentially all the CI users performed more poorly than the sensorineural HI patients who listened with acoustic hearing and whose hearing loss ranged from mild to profound. This might not be the case today.
In subsequent studies from other laboratories, this and similar measures of testing spectral peak resolution have become a popular test to measure spectral peak resolution across various listeners, particularly in CI users, despite the fact that the details of the spectral ripple stimuli used were slightly different (e.g., Won et al., 2007; Won et al., 2010; Won et al., 2011a; Won et al., 2011b; Anderson et al., 2011). Other researchers used spectral modulation thresholds (i.e., minimum depth of modulation required for discrimination), rather than spectral ripple discrimination thresholds, to measure spectral ripple resolution (e.g., Litvak et al., 2007; Saoji et al., 2009; Zhang et al., 2013). In general, these previous studies demonstrated that spectral ripple resolution could provide predictive information about speech recognition abilities in quiet and noise and in music perception. Researchers suggest that these tasks can provide a good measure of peripheral spectral resolution of the auditory system for CI users (e.g., Won et al., 2011b; Jones et al., 2013).
In recent years, as the positive outcomes of CIs have become more evident, the candidacy criteria for cochlear implantation have been relaxed, and hearing-impaired people with more residual hearing are receiving CIs. Also, changes in the internal and external devices could affect CI listeners' performances on spectral ripple discrimination thresholds. It is therefore important to now revisit spectral resolution abilities in more recent CI recipients. This study investigates spectral ripple resolution ability and speech perception abilities of CI recipients implanted over the last 10 years, and compare these results with those of Henry et al. (2005), which included CI recipients who were implanted 15 to 20 years ago. The focus here is on comparison with the Henry et al. (2005) data since the same stimuli, equipment and testing procedures are still available at the authors' present laboratory, therefore enabling direct comparison with previous spectral ripple resolution results for NH, HI, and CI listeners. In Henry et al. (2005), the CI patients performed, on average, only at about one-third the spectral ripple spacing of the HI subjects. The first question is: is this result still true of patients implanted later than those who participated in Henry et al. (2005)? Related to this question, is it still true that cochlear implant patients essentially always perform more poorly on the spectral discrimination task than hearing-impaired patients listening with acoustic hearing? An additional question is, does the strong correlation between spectral ripple discrimination thresholds and speech perception still hold with the new CI data?
II. METHODS
A. Participants
In the Henry et al. (2005) study, 12 NH young adults, 32 HI adults, and 23 CI subjects participated. They were all native English speakers. All NH subjects had audiometric thresholds better than 15 dB hearing level. All 32 HI listeners had sensorineural hearing loss ranging from mild to profound. The mean and dispersion of their audiometric thresholds for each frequency are summarized in Table I.
TABLE I.
Audiometric thresholds and standard deviation across frequencies for the hearing impaired subjects in the Henry et al. (2005) study.
| 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz | |
|---|---|---|---|---|---|---|
| Mean | 32 | 32 | 43 | 56 | 63 | 68 |
| SD | 16 | 16 | 21 | 18 | 17 | 17 |
| Range | 10–60 | 10–70 | 15–100 | 25–100 | 15-no response | 15-no response |
CI listeners in the Henry et al. (2005) study received their CIs between 1996 and 2002 at the University of Iowa Hospitals and Clinics. Nineteen subjects used the CI24M and 4 used the CI24R internal device. All tests were done using the laboratory SPrint body worn speech processor (most of these patients used a Sprint processor for everyday use as well), using each subject's everyday MAP; the speech processing strategies used in their MAP included spectral peak (SPEAK), continuous interleaved sampling (CIS), and advanced combination encoder (ACE). All of them had at least 6 months of experience with their implant prior to testing. The details of each subject are shown in Table II, reprinted from Henry et al. (2005).
TABLE II.
Individual subject details for cochlear implant participants in the Henry et al. (2005) study. Prog. = progressive; ACE used eight maxima unless otherwise noted.
| Subject | Age (years) | Duration of profound deafness (years) | CI experience (years) | Etiology | Implant type | Processing Strategy, Number of maxima | Pulse rate (pps/ch), Number of channels | CNC word score (% correct) | Average dynamic range (dB) |
|---|---|---|---|---|---|---|---|---|---|
| CI1 | 74 | 12 | 6 | Infection | CI24M | ACE | 720, 22 | 86 | 8.6 |
| CI2 | 47 | 13 | 3 | Congenital, prog. | CI24M | ACE | 900, 22 | 39 | 12.5 |
| CI3 | 64 | 4 | 5 | Unknown | CI24M | ACE | 720, 18 | 56 | 9.7 |
| CI5 | 73 | 8 | 4 | Congenital, prog. | CI24M | ACE | 900, 20 | 64 | 7.6 |
| CI6 | 73 | 1 | 2 | Meniere's disease | CI24M | SPEAK | 250, 18 | 54 | 4.9 |
| CI7 | 44 | 0.5 | 3 | Autoimmune disease | CI24M | ACE | 1200, 22 | 72 | 15.5 |
| CI10 | 75 | 25 | 5 | Congenital, prog. | CI24M | ACE | 720, 20 | 68 | 7.7 |
| CI11 | 77 | 40 | 3 | Unknown | CI24M | CIS | 900, 6 | 4 | 6.9 |
| CI13 | 49 | 2 | 2 | Unknown | CI24M | CIS | 2400, 6 | 74 | 12.0 |
| CI14 | 55 | 5 | 4 | Unknown | CI24M | ACE,10 | 1200, 22 | 18 | 4.0 |
| CI15 | 81 | 3 | 4 | Unknown | CI24M | SPEAK | 250, 19 | 54 | 4.9 |
| CI16 | 37 | 2 | 3 | Unknown | CI24R | ACE, 12 | 720, 22 | 42 | 8.8 |
| CI18 | 57 | 36 | 6 | Unknown | CI24M | ACE,12 | 1200, 20 | 50 | 9.0 |
| CI19 | 79 | 0.5 | 5 | Viral infection | CI24M | SPEAK | 250, 20 | 66 | 2.5 |
| CI20 | 47 | 7 | 4 | Unknown | CI24M | ACE | 900, 20 | 22 | 16.5 |
| CI22 | 63 | 0.3 | 3 | Infection | CI24M | ACE | 720, 22 | 82 | 10.9 |
| CI23 | 75 | 8 | 3 | Unknown | CI24M | SPEAK | 250, 20 | 42 | 4.9 |
| CI24 | 85 | 11 | 2 | Unknown | CI24R | SPEAK | 250, 18 | 24 | 5.8 |
| CI25 | 76 | 10 | 4 | Unknown, prog. | CI24M | ACE | 900, 22 | 68 | 11.6 |
| CI26 | 62 | 1 | 3 | Meniere's disease | CI24M | ACE | 720, 22 | 54 | 9.5 |
| CI27 | 47 | 28 | 0.5 | Infection | CI24R | ACE | 900, 22 | 64 | 19.2 |
| CI28 | 41 | 3 | 2 | Hereditary | CI24R | ACE | 900, 22 | 84 | 9.5 |
| CI29 | 49 | 8 | 3 | Unknown, prog. | CI24M | CIS | 900, 6 | 58 | 7.7 |
In the current study, a total of 28 CI subjects participated with a total of 32 implanted ears were tested. Twelve of these were bilateral CI users; both CI ears were tested in four subjects when time allowed. All subjects were native English speakers and received their implants between 2003 and 2013 at the University of Iowa Hospitals and Clinics. Twenty-two participants had internal devices, CI24R(CS), CI24RE(CA), CI422, or CI512 manufactured by Cochlear Ltd. (Sydney, Australia) and six participants had CII or HiRes90K manufactured by Advanced Bionics Corp. (Valencia, CA, USA). The internal device and speech processing strategies used in their MAPs included ACE and ACE (RE) (Cochlear Ltd), HiRes (HiResolution) Optima, HiRes -P (paired) or HiRes-S (sequence) with or without Fidelity 120 feature (Advanced Bionics). They had a minimum of 6 months of CI experience prior to testing. Individual subject details for the current study are shown in Table III, including subject ID, age, duration of severe-profound deafness, duration of CI experience, etiology, implant type, speech processor type, and years implanted.
TABLE III.
Individual subject details for the current study. Prog. = progressive; ACE used eight maxima.
| Subject | Age (years) | Duration of profound deafness (years) | CI experience (yrs) | Etiology | Implant type | Speech processor | Processing Strategy, Number of maxima | Pulse rate (pps/ch), Number of channels |
|---|---|---|---|---|---|---|---|---|
| CI37 | 41 | 14 | 9 | Unknown, Meningitis | HiRes 90 K | Harmony | HiRes-S w/F120 | 3712, 14 |
| CI51 | 76 | 7 | 8 | Unknown | CI24RE(CA) | Freedom | ACE | 900, 22 |
| CI54 | 69 | 7 | 4 | Unknown, prog. | CI512 | CP810 | ACE | 900, 19 |
| CI56 | 63 | 9 | 10 | Unknown | CI24RE(CA) | Freedom | ACE(RE) | 1800, 22 |
| CI61 | 76 | 0.6 | 7 | Head trauma | CI24RE(CA) | CP810 | ACE | 900, 22 |
| CI62 | 57 | 41 | 6 | Unknown | CI24RE(CA) | CP810 | ACE | 900, 22 |
| CI70 | 58 | 5 | 6 | Unknown | CI24RE(CA) | CP810 | ACE | 900, 21 |
| CI74L | 63 | 2 | 6 | Hereditary-other | CI24RE(CA) | Freedom | ACE | 900, 21 |
| CI74R | 63 | 2 | 6 | Hereditary-other | CI24RE(CA) | Freedom | ACE | 900, 22 |
| CI77 | 67 | 2 | 8 | Unknown | HiRes90K | Harmony | HiRes-S w/F120 | 3093,16 |
| CI81 | 50 | 28 | 2 | Unknown | CI24RE(CA) | Freedom | ACE | 1200, 22 |
| CI89 | 72 | 6 | 4 | Unknown, prog. | CI512 | CP810 | ACE | 900, 22 |
| CI90 | 60 | 3 | 3 | Unknown | CI512 | CP810 | ACE | 900, 22 |
| CI91 | 58 | 17 | 8 | Otosclerosis | HiRes 90 K | Harmony | HiRes-S w/F120 | 1326, 16 |
| CI95 | 89 | 7 | 7 | Unknown, prog. | CI24RE(CA) | CP810 | ACE | 900, 22 |
| CI97L | 64 | 12 | 4 | Unknown | CI512 | CP810 | ACE | 900, 22 |
| CI97R | 64 | 16 | 4 | Unknown | CI512 | CP810 | ACE | 900, 22 |
| CI99 | 61 | 38 | 11 | Unknown | CI24R(CS) | CP810 | ACE | 900, 22 |
| SE4 | 65 | 2 | 6 | Autoimmune disease | CI24RE(CA) | CP810 | ACE | 1200, 20 |
| CI101 | 57 | 0 | 2 | Unknown | CI24RE(CA) | CP810 | ACE | 900, 19 |
| CI103 | 20 | 10 | 2 | Unknown | HiRes 90 K | Neptune | HiRes-P w/F120 | 2062, 16 |
| CI102 | 79 | 2 | 0.5 | Unknown | CI422 | CP910 | ACE | 900, 19 |
| CI105 | 60 | 0.5 | 0.5 | Meniere's disease | CI422 | CP920 | ACE | 900, 20 |
| CI109 | 25 | 11 | 11 | Unknown | CI24R(CS) | Freedom | ACE | 900, 19 |
| CI111 | 73 | 56 | 2 | Unknown | CI422 | CP810 | ACE | 500, 16 |
| CI112 | 70 | 0.9 | 1 | Unknown | CI24RE(CA) | CP910 | ACE | 900, 22 |
| CI114 | 73 | 0.1 | 4 | Autoimmune disease | HiRes 90 K | Harmony | HiRes-S w/F120 | 3712, 16 |
| CI115 | 59 | 44 | 11 | Unknown, measles | CII | Harmony | HiRes Optima-S | 2062, 16 |
| CI116L | 55 | 17 | 3 | Unknown | CI512 | CP810 | ACE | 900, 22 |
| CI116R | 55 | 25 | 5 | Unknown | CI24RE(CA) | CP810 | ACE | 900, 20 |
| CI118L | 54 | 11 | 2 | Unknown, prog. | CI512 | CP810 | ACE | 900,22 |
| CI118R | 54 | 9 | 5 | Unknown, prog. | CI24RE(CA) | CP810 | ACE | 900, 22 |
B. Procedures and tests
All experiments were conducted in a sound booth. All stimuli were presented at an average level of 65 dB sound pressure level (SPL) via a loudspeaker located approximately 1 m from a subject. A touch screen was used for a subject to choose a response for all experiments. The non-tested ear was plugged and muffed. Bilateral users were tested with one CI at a time. In the current study, subjects used their own speech processors using their everyday MAP. All procedures, instruments, and stimuli were identical between the two studies, with the exception of some of the speech tests.
1. Spectral ripple discrimination thresholds
Spectral ripple noise stimuli of 100 to 5000 Hz bandwidth with peak-to-valley ratios of approximately 30 dB were synthesized on an Apple Mac Pro computer by algebraically summing 200 pure-tone frequency components with amplitudes determined by a sinusoidal envelope with ripples spaced on a logarithmic frequency scale. The starting phases of the individual frequency components were randomized for each stimulus to avoid fine structure pitch cues that may be perceptible to listeners. The frequency of the spectral envelope of the stimulus complex was varied in 14 steps from 0.125 to 11.314 ripples/octave. The spectral envelope phase of the stimulus complex was set to zero at the low-frequency edge of the complex for the standard (reference) stimulus. The inverted (test) stimulus had a reversed phase. The stimuli were of 500 ms duration, had 150 ms rise/fall time, and were shaped with a filter that approximated the long-term speech spectrum (Byrne et al., 1994). The overall levels of the rippled noise sound files were then approximately equalized. To limit the use of loudness cues, the presentation level of each stimulus roved randomly in 1-dB steps within an 8 dB range. Subjects were instructed to choose the interval, which was different from the other two in terms of pitch or quality, rather than loudness in a 3-interval forced choice task. Feedback was provided after each trial. The test started at an easy-to-distinguish ripple frequency of 0.176 ripples/octave, and the spectral ripple frequency was varied using a two-down and one-up procedure based on the subject's response; this led to 70.7% accuracy (Levitt, 1971). Each run stopped after 12 reversals. The spectral ripple discrimination threshold for each run was calculated as the mean of the ripple frequencies for the last 8 of 12 reversals (Henry and Turner, 2003). The final discrimination threshold for the spectral ripple resolution was reported using the average thresholds from three test runs. At least one practice run was completed before testing.
2. Consonant recognition
The consonant recognition test was given to subjects in a closed-set using 16 consonants presented in an /a/-consonant-/a/ syllable context (Turner et al., 1995). Four speakers including two males and two females produced one token at a time at random. Each consonant in an /aCa/ syllable was randomly repeated by the four speakers, i.e., a total of 64 tokens per run. This procedure was repeated three times for a total of 192 test items. An average of the three runs was calculated for the final score shown in percentage of correct answers. One practice run was completed for all subjects before testing. This was the same consonant test employed in Henry et al. (2005).
3. Speech recognition thresholds in two-talker speech maskers
Instead of the vowel recognition test used in Henry et al. (2005), the speech recognition test in noise (Turner et al., 2004) was included in this current study. The speech recognition threshold (SRT) in two-talker speech maskers was measured using a closed set of 12 spondees in two-speaker background masking material; both spondees and two-talker speech maskers were presented from the same loud speaker. One female speaker spoke 1 of 12 spondees in a random order at an average of 65 dB SPL. The two-speaker, one female and one male, speech maskers were varied to obtain the signal-to-noise ratio (SNR) where the subject could identify the spondee with 50% accuracy. The SNR was decreased or increased by 2 dB based on the subject's response. A single run stopped the procedure after 14 reversals. The SNR values for the last ten reversals were averaged for the threshold for each run. Participants completed at least four runs, and the last three runs were used to calculate the final SRT score. Previously, Henry et al. (2005) obtained SRT scores at the time of testing for both spectral ripple discrimination thresholds and consonant recognition; however, these were not reported at the time. They are included here.
4. Subject characteristics
Subject characteristics include: individual pre-operative audiograms, duration of deafness, and duration of CI use. These characteristics of subjects for Henry et al. (2005) were compared to those of subjects in current studies. Pre-operative speech perception scores in Henry et al. (2005) were compared to the current study's CI patients using Consonant-Nucleus-Consonant (CNC) (Lehiste and Peterson, 1959; Peterson and Lehiste, 1962) and the Hearing in Noise Test (HINT) (Nilsson et al., 1994) scores.
III. RESULTS
Two subjects (CI89 and CI102) in the current study were excluded from data analysis. While CI89 was able to perform the spectral ripple discrimination task, he showed difficulty following the instruction for speech perception testing; especially associating the button labels with consonant sounds. CI102 had very good post-operative residual hearing from 125 to 1500 Hz. This patient's unusually good results could be the result of listening with acoustic hearing. For these reasons, results from these subjects were not included in the analysis.
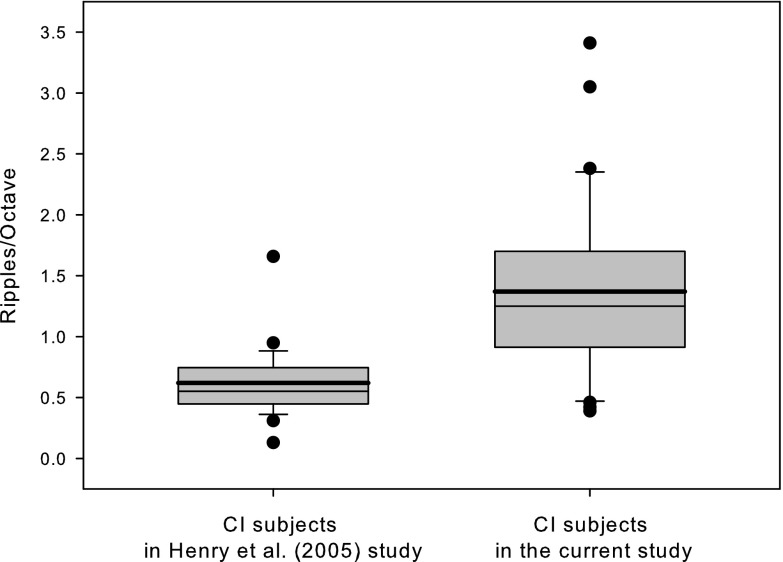
Figure 1, shown as a box-and-whisker plot, compares the current subjects' spectral ripple discrimination thresholds with the Henry et al. (2005) subject's spectral ripple discrimination thresholds. While the spectral ripple discrimination thresholds obtained from CI subjects in Henry et al. (2005) ranged from 0.13 to 1.66 ripples/octave [mean = 0.62, median = 0.55, standard deviation (SD) = 0.29], the spectral ripple discrimination thresholds obtained from participants in the current study ranged from 0.39 to 3.41 (mean = 1.37, median = 1.25, SD = 0.72). A t-test shows that the mean spectral ripple discrimination thresholds obtained from subjects in the current study were significantly better than those obtained from the subjects from Henry et al. (2005) (t = −5.22, df = 40, p < 0.0001). The equality of variances test (Folded F) indicates unequal variances (F = 6.26, p < 0.0001), so degrees of freedom were adjusted from 51 to 40.
FIG. 1.
Comparison of spectral ripple thresholds between cochlear implant subjects in the Henry et al. (2005) and the current studies. This box-and-whisker plot shows results from Henry et al. (2005) in the left panel and results from the current study in the right panel. The boxes extend from the 25th to 75th percentiles and the whiskers show data ranged from the 10th to 90th percentiles. Within the box, the thin solid line shows the median and the thick solid line show the mean spectral ripple thresholds of each group. Individual dots are outliers; scores are outside of 10th and 90th percentiles.
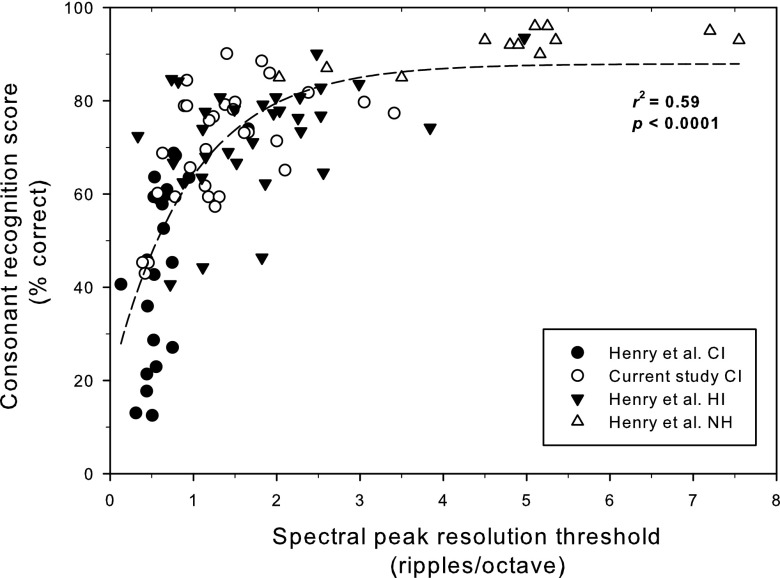
Figure 2 shows the relationship between spectral ripple discrimination thresholds and consonant recognition scores across normal hearing listeners (NH), hearing impaired (HI) listeners, and CI users. The current study's CI subjects are marked with open circles, and the CI subjects from Henry et al. (2005) are marked with closed circles. The data from NH subjects and HI subjects are also taken from the Henry et al. (2005) study.
FIG. 2.
The relationship between spectral ripple discrimination thresholds and consonant recognition across normal hearing listeners (NH), hearing impaired (HI) listeners, and cochlear implant (CI) users. Closed circles indicate CI subjects' data from Henry et al. (2005). Open circles indicate CI subjects' data from the current study. Closed triangles indicate HI data and open triangles indicate NH data, both from Henry et al. (2005).
Interestingly, CI subjects' in the current study showed considerable overlap with HI subjects whose average spectral ripple discrimination thresholds were 1.77 ripples/octave (SD = 0.96). When spectral ripple discrimination thresholds were compared between these two groups, they were not significantly different (t = 1.87, df = 60, p = 0.0662). The consonant recognition scores were not significantly different between the HI subjects from Henry et al. (2005) and the current CI subjects (t = 0.70, df = 60, p = 0.4892); HI subjects obtained, on average, 73% correct (SD = 12.19) and CI subjects in the current study obtained 70% correct (SD = 12.67). However, the CI subjects in the current study obtained significantly higher scores than the CI subjects in the Henry et al. (2005) study, who obtained an average of 45% correct (SD = 19.42).
Henry et al. (2005) showed a significant positive relationship between spectral ripple resolution and consonant recognition (r2 = 0.66, p < 0.0001), using nonlinear regression analysis based on the fitted function (P = ae−S/b + c), where P is the percent correct score, S is the spectral ripple discrimination threshold, and a, b, c are fitting parameters (a = −72.24, b = 1.33, and c = 94.76).
Using the regression function given by Henry et al. (2005), the predicted consonant recognition scores were computed for the current CI subjects based on their spectral ripple discrimination thresholds. When the prediction was compared to the actual consonant recognition scores of the current CI subjects, they were significantly correlated (r = 0.65, p < 0.0001).
Including the more recently implanted CI users tested in this study with the Henry et al. (2005) data, Fig. 2 shows that the positive correlation between spectral ripple discrimination thresholds and consonant recognition still holds for this expanded data set (r2 = 0.59, p < 0.0001) on the best fitted function [P = a(1 − e (−sb)) + c, where a = 68.82, b = 1.05, and c = 19.09]. While the current CI subjects have better spectral resolution and consonant recognition abilities than the Henry et al. (2005) CI subjects, the strong positive relationship between spectral ripple discrimination thresholds and consonant recognition scores still holds.
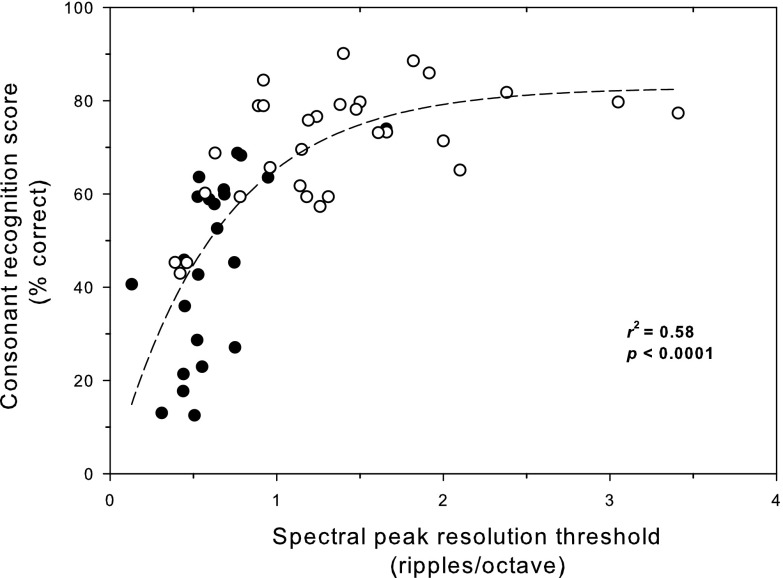
This relationship was also explored in CI users alone in Fig. 3. Here the difference between the Henry et al. (2005) CI subjects and the current CI subjects is clearly seen. A nonlinear regression analysis shows a significant correlation between spectral ripple discrimination thresholds and consonant recognition (r2 = 0.58, p < 0.0001) on the same form of fitted function used in Fig. 2 [P = a(1 − e (−sb)) + c, where a = 83.26, b = 1.56, and c = −0.42].
FIG. 3.
The relationship between spectral ripple discrimination thresholds and consonant recognition in CI users alone. Closed circles indicate CI subjects' data from Henry et al. (2005). Open circles indicate CI subjects' data from the current study.
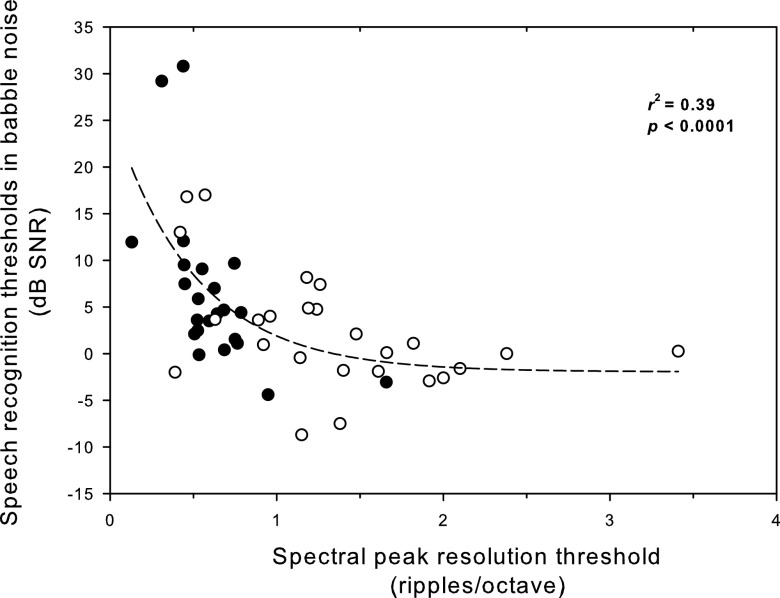
In this study, the SRT in two-talker speech maskers from the current study's CI subjects were also compared to those of CI subjects in the Henry et al. (2005). The SRT in two-talker speech maskers ranged from −4.4 to 30.8 dB SNR in the CI subjects from Henry et al. (2005) (mean = 6.65, median = 4.4, SD = 8.54), and ranged from −8.7 to 17 dB SNR in the current study's CI subjects (mean = 2.33, median = 0.95, SD = 6.40). A t-test shows that the mean SRT obtained from subjects in the current study were significantly better than those obtained from the subjects from Henry et al. (t = 2.00, df = 46, p = 0.0519). In Fig. 4 nonlinear regression analyses show significant correlations between spectral ripple discrimination thresholds and speech recognition thresholds in two-talker speech maskers (r2 = 0.39, p < 0.0001), using the fitted function (P = ae (−sb)+ c, where a = 28.33, b = 2.00, and c = −1.95).
FIG. 4.
The relationship between spectral ripple discrimination thresholds and speech recognition thresholds in two-talker speech maskers in CI users. Closed circles indicate CI subjects' data from Henry et al. (2005), and open circles indicate CI subjects' data from the current study.
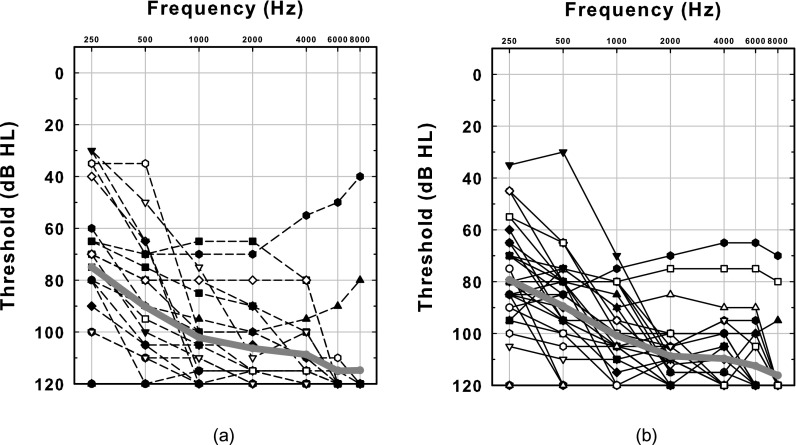
There was no significant difference in the pre-operative audiograms between the Henry et al. (2005) subjects and the current study subjects [Figs. 5(a) and 5(b)]. The duration of deafness was also not significantly different between the two groups (t = −0.89, df = 51, p = 0.3756): the Henry et al. (2005) CI subjects had on average 9 year duration of deafness (SD = 11.45), while the current study's CI subjects had 13 years on average (SD = 14.65). However, the duration of CI use prior to the study in CI patients between the Henry et al. (2005) (mean = 3.46, SD = 1.36) and the current studies (mean = 5.45, SD = 3.09) was significantly different (t = −3.16, df = 42, p = 0.0029). The equality of variances test (Folded F) indicates unequal variances (F = 5.18, p = 0.0002), so degrees of freedom were adjusted from 51 to 42.
FIG. 5.
Audiograms obtained preoperatively for all CI subjects in the two studies. (a) The audiograms of the subjects who participated in the Henry et al. (2005) study. (b) The audiograms of the current study. The thick grey line shows the mean threshold for each frequency for all subjects in each group.
The primary difference between the two groups of CI subjects was found in the pre-operative speech scores. All subjects in Henry et al. (2005) and the majority of subjects in the current study had speech perception scores in both CNC and HINT tests. The current study's CI participants had significantly higher pre-operative CNC (t = −2.19, df = 35, p = 0.0356) and HINT scores (t = −2.65, df = 45, p = 0.0111) than the CI participants from Henry et al. When pre-op speech performance was statistically controlled, the difference between groups was still significant (F(1,31) = 19.32, p < 0.0001).
IV. DISCUSSION
The primary purpose of this study was to test if the spectral ripple resolution values of contemporary CI listeners in the current study are similar to findings in the study by Henry et al. (2005). As hypothesized, the new group of long-electrode patients had better spectral resolution than the group of subjects who participated in the Henry et al. (2005) study (Fig. 1). In fact, many of the CI patients in the current study achieved better than 1 ripple/octave, scores comparable to HI listeners in Henry et al. (2005).
This finding raises a question: What is the source of the difference between the two studies? One possible explanation could be increased peripheral cochlear nerve fibers. The improved performance in the more current CI users might be associated with better pre-operative patient characteristics (such as shorter duration of deafness, better speech perception etc.). This “increased number” of responding nerve fibers could allow for more accurate spectral resolution, regardless of current spread and electrode interaction. Pre-operative patient characteristics might indicate the degree of neural survival (e.g., Peterson et al., 2010). Pre-operative pure-tone audiograms, as well as the duration of deafness, were also compared between the two groups. Based on findings from previous studies (e.g., Rubinstein et al., 1999; Gomaa et al., 2003; Leung et al., 2005), it was predicted that the CI subjects in this study might have better pre-operative residual hearing and shorter deafness duration than the CI subjects in Henry et al. (2005). However, there were no significant differences in either of these two factors between the two studies. The primary difference between the two groups of CI subjects was found in their pre-operative speech perception scores. However, the fact that the difference between groups still exists after controlling pre-op speech performance indicates that the pre-op speech scores is just one factor, rather than explaining the difference of spectral ripple discrimination scores between the two groups.
Additionally, the duration of CI use prior to the study in CI patients between the Henry et al. (2005) and the current study was significantly different. Although previous studies show a plateau in performance within the first year of CI use for post-lingual recipients (e.g., Holden et al., 2013) and the longer duration of CI use positively correlates to higher speech perception scores for pre-lingual recipients (e.g., Sarant et al., 2001; Davidson et al., 2011), in our laboratory, it was observed that some CI listeners' performances improved over time. Recently, the spectral ripple discrimination thresholds were retested in several subjects (CI2, CI7, CI13, and CI27) from the Henry et al. (2005) study, whose duration of implant use at the time of the Henry et al. (2005) was less than 5 years (mean = 2 years, SD = 1.02). At the time of retesting, they had more than 10 years of CI use (mean = 12.5 years, SD = 2.06). Three of the four had improved over time; two of them did considerably better. For example, CI7 showed improvement of spectral ripple discrimination scores from 0.79 to 1.27 ripples/octave and CI 27 improved from 0.75 to 1.60 ripples/octave.
One might think that the use of a newer speech processor in the more recently implanted listeners might lead to better spectral ripple discrimination resolution abilities than the Henry et al. (2005) CI subjects. To explore this, the spectral ripple discrimination thresholds for two subjects (CI20 and CI26) who were available for further testing were obtained, using both a SPRINT speech processor as used in the Henry et al. (2005) and their current CP810 speech processors. The difference between spectral ripple discrimination scores obtained using the SPRINT and their current CP810 were 0.09 ripples/octave for CI20 and 0.07 ripples/octave for CI26. For this limited sample of subjects, the speech processor itself did not seem to affect the spectral ripple discrimination results.
One might think that the effect of internal implant type might lead to better spectral ripple resolution abilities. In fact, the Henry et al. (2005) study had mainly CI24M implants, straight arrays while many in the current study used CI24RE(CA) implants, peri-modiolar arrays. While earlier research has not shown much of a difference in speech perception between peri-modiolar and standard electrode arrays, some psychophysics studies have indicated more localized neural excitation with contour arrays (e.g., Cohen et al., 2006), and more recent research has indicated that peri-modiolar electrode arrays may enhance speech perception (e.g., Holden et al., 2013). It may be that those with the contour peri-modiolar electrode arrays have better spectral ripple resolution. In the present study there was no significant difference between spectral ripple discrimination thresholds with CI24RE(CA) vs CI422/CI512 within the current group of subjects at σ = 0.05 level. However, at the present time, this must also be only a tentative conclusion.
The second question of this study asks if the strong positive correlation between ripple discrimination thresholds and speech perception still holds in this expanded data set that includes more recently implanted patients. The strong relationship between spectral ripple resolution and consonant recognition still holds. A moderate correlation also exists between spectral ripple resolution and speech recognition in noise. This supports the findings from the study by Won et al. (2007) using two-talker babble background noise (29 CI subjects, r2 = −0.30, p = 0.002). However, Anderson et al. (2011) did not find such a correlation between spectral ripple discrimination and speech perception in noise for either word recognition in a sentence (12 CI subjects, r2 = 0.07, p = 0.43) or vowel recognition in the presence of noise (14 CI subjects, r2 = 0.22, p = 0.09).
The finding that spectral ripple resolution in CI patients is better than previously reported has clinical significance. Today's CI patients have the potential to achieve better consonant recognition in quiet and speech recognition in noise than previously assumed. The finding that spectral resolution in some of our CI patients is better than that obtained by HI patients also lends support to current more expansive candidacy criteria for CIs in that today's cochlear implant patients do not necessarily perform more poorly on spectral resolution tasks and speech recognition than some moderate to severely hearing impaired acoustic hearing patients.
ACKNOWLEDGMENTS
The authors gratefully appreciate the subjects who generously gave their time to participate in this study. We acknowledge the adult CI team at the University of Iowa Hospitals and Clinics for scheduling and collecting baseline speech perception data from our subjects. This study was supported by the National Institute on Deafness and Other Communication Disorders grants, R01DC000377 and P50 DC000242.
References
- 1. Anderson, E. S. , Nelson, D. A. , Kreft, H. , Nelson, P. B. , and Oxenham, A. J. (2011). “ Comparing spatial tuning curves, spectral ripple resolution, and speech perception in cochlear implant users,” J. Acoust. Soc. Am. 130, 364–375. 10.1121/1.3589255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Byrne, D. , Dillon, H. , Tran, K. , Arlinger, S. , Wilbraham, K. , Cox, R. , Hagerman, B. , Hetu, R. , Kei, J. , Lui, C. , Kiessling, J. , Nasser Kotby, M. , Nasser, N. H. A. , El Kholy, W. A. H. , Nakanishi, Y. , Oyer, H. , Powell, R. , Stephens, D. , Meredith, R. , Sirimanna, T. , Tavartkiladze, G. , Frolenkov, G. , Westerman, S. , and Ludvigsen, C. (1994). “ An international comparison of long-term average speech spectra,” J. Acoust. Soc. Am. 96, 2108–2120. 10.1121/1.410152 [DOI] [Google Scholar]
- 3. Cohen, L. T. , Saunders, E. , Knight, M. R. , and Cowan, R. S. (2006). “ Psychophysical measures in patients fitted with Contour and straight Nucleus electrode arrays,” Hear. Res. 212, 160–175. 10.1016/j.heares.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 4. Davidson, L. S. , Geers, A. E. , Blamey, P. J. , Tobey, E. , and Brenner, C. (2011). “ Factors contributing to speech perception scores in long-term pediatric CI users,” Ear Hear. 32, 19S–26S. 10.1097/AUD.0b013e3181ffdb8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friesen, L. M. , Shannon, R. V. , Baskent, D. , and Wang, X. (2001). “ Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants,” J. Acoust. Soc. Am. 110, 1150–1163. 10.1121/1.1381538 [DOI] [PubMed] [Google Scholar]
- 7. Gfeller, K. , Turner, C. , Oleson, J. , Zhang, X. , Gantz, B. , Froman, R. , and Olszewski, C. (2007). “ Accuracy of cochlear implant recipients on pitch perception, melody recognition, and speech reception in noise,” Ear Hear 28, 412–423. 10.1097/AUD.0b013e3180479318 [DOI] [PubMed] [Google Scholar]
- 8. Gfeller, K. , Turner, C. W. , Woodworth, G. , Mehr, M. , Fearn, R. , Knutson, J. F. , Witt, S. , and Stordahl, J. (2002). “ Recognition of familiar melodies by adult cochlear implant recipients and normal-hearing adults,” Cochlear Implants Int. 3, 29–53. 10.1179/cim.2002.3.1.29 [DOI] [PubMed] [Google Scholar]
- 9. Gomaa, N. A. , Rubinstein, J. T. , Lowder, M. W. , Tyler, R. S. , and Gantz, B. J. (2003). “ Residual speech perception and cochlear implant performance in postlingually deafened adults,” Ear Hear. 24, 539–544. 10.1097/01.AUD.0000100208.26628.2D [DOI] [PubMed] [Google Scholar]
- 10. Henry, B. A. , and Turner, C. W. (2003). “ The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners,” J. Acoust. Soc. Am. 113, 2861–2873. 10.1121/1.1561900 [DOI] [PubMed] [Google Scholar]
- 11. Henry, B. A. , Turner, C. W. , and Behrens, A. (2005). “ Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners,” J. Acoust. Soc. Am. 118, 1111–1121. 10.1121/1.1944567 [DOI] [PubMed] [Google Scholar]
- 12. Holden, L. K. , Finley, C. C. , Firszt, J. B. , Holden, T. A. , Brenner, C. , Potts, L. G. , Gotter, B. D. , Vnderhoof, S. S. , Mispagel, K. , Heydebrand, G. , and Skinner, M. W. (2013). “ Factors affecting open-set word recognition in adults with cochlear implants,” Ear Hear. 34, 342–360. 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones, G. L. , Won, J. H. , Drennan, W. R. , and Rubinstein, J. T. (2013). “ Relationship between channel interaction and spectral-ripple discrimination in cochlear implant users,” J. Acoust. Soc. Am. 133, 425–433. 10.1121/1.4768881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lehiste, I. , and Peterson, G. E. (1959). “ Linguistic considerations in the study of speech intelligibility,” J. Acoust. Soc. Am. 31, 280–286. 10.1121/1.1907713 [DOI] [Google Scholar]
- 15. Leung, J. , Wang, N. Y. , Yeagle, J. D. , Chinnici, J. , Bowditch, S. , Francis, H. W. , and Niparko, J. K. (2005). “ Predictive models for cochlear implantation in elderly candidates,” Arch. Otolaryngol. Head Neck Surg. 131, 1049–1054. 10.1001/archotol.131.12.1049 [DOI] [PubMed] [Google Scholar]
- 41. Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- 16. Litvak, L. M. , Spahr, A. J. , Saoji, A. A. , and Fridman, G. Y. (2007). “ Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners,” J. Acoust. Soc. Am. 122, 982–991. 10.1121/1.2749413 [DOI] [PubMed] [Google Scholar]
- 17.National Institute on Deafness and Other Communication Disorders (2015). “ Cochlear implants,” http://www.nidcd.nih.gov/health/hearing/pages/coch.aspx (Last viewed September 25, 2015).
- 18. Nelson, P. B. , Jin S.-H., Carney, A. E. , and Nelson D. A. (2003). “ Understanding speech in modulated interference: Cochlear implant users and normal-hearing listeners,” J. Acoust. Soc. Am. 113, 961–968. 10.1121/1.1531983 [DOI] [PubMed] [Google Scholar]
- 19. Nilsson, M. , Soli, S. D. , and Sullivan, J. (1994). “ Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise,” J. Acoust. Soc. Am. 95, 1085–1099. 10.1121/1.408469 [DOI] [PubMed] [Google Scholar]
- 20. Peterson, G. E. , and Lehiste, I. (1962). “ Revised CNC lists for auditory tests,” J. Speech Lang. Hear. Disord. 27, 62–70. 10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- 21. Peterson, N. R. , Pisoni, D. B. , and Miyamoto, R. T. (2010). “ Cochlear implants and spoken language processing abilities: Review and assessment of the literature,” Restor. Neurol. Neuros. 28, 237–250. 10.3233/RNN-2010-0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qin, M. K. , and Oxenham A. J. (2003). “ Effects of simulated cochlear-implant processing on speech reception in fluctuating maskers,” J. Acoust. Soc. Am. 114, 446–454. 10.1121/1.1579009 [DOI] [PubMed] [Google Scholar]
- 23. Rubinstein, J. T. , Parkinson, W. S. , Tyler, R. S. , and Gantz, B. J. (1999). “ Residual speech recognition and cochlear implant performance: Effects of implantation criteria,” Am. J. Otol. 20, 445–452. [PubMed] [Google Scholar]
- 24. Sampaio, A. L. , Araujo, M. F. , and Oliveira, C. A. (2011). “ New criteria of indication and selection of patients to cochlear implant,” Int. J. Otolaryngol. 2011, 573968. 10.1155/2011/573968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saoji, A. A. , Litvak, L. , Spahr, A. J. , and Eddins, D. A. (2009). “ Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners,” J. Acoust. Soc. Am. 126, 955–958. 10.1121/1.3179670 [DOI] [PubMed] [Google Scholar]
- 26. Sarant, J. Z. , Blamey, P. J. , Dowell, R. C. , Clark, G. M. , and Gibson, W. P. R. (2001). “ Variation in speech perception scores among children with cochlear implants,” Ear Hear. 22, 18–28. 10.1097/00003446-200102000-00003 [DOI] [PubMed] [Google Scholar]
- 27. Shannon, R. V. (2010). “ Restoration of hearing by electrical stimulation of the human cochlea, brainstem, and midbrain,” in Biomedical Engineering: Principles of the Bionic Man, edited by Hung G. K. ( World Scientific Publishing Co., Singapore: ), pp. 205–232. [Google Scholar]
- 28. Stickney, G. S. , Zeng, F. G. , Litovsky, R. V. , and Assmann, P. F. (2004). “ Cochlear implant speech recognition with speech maskers,” J. Acoust. Soc. Am. 116, 1081–1091. 10.1121/1.1772399 [DOI] [PubMed] [Google Scholar]
- 29. Supin, A. , Popov, V. V. , Milekhina, O. N. , and Tarakanov, M. B. (1994). “ Frequency resolving power measured by rippled noise,” Hear. Res. 78, 31–40. 10.1016/0378-5955(94)90041-8 [DOI] [PubMed] [Google Scholar]
- 30. Supin, A. Ya. , Popov, V. V. , Milekhina, O. N. , and Tarakanov, M. B. (1997). “ Frequency-temporal resolution of hearing measured by rippled noise,” Hear. Res. 108, 17–27. 10.1016/S0378-5955(97)00035-X [DOI] [PubMed] [Google Scholar]
- 31. Supin, A. , Popov, V. V. , Milekhina, O. N. , and Tarakanov, M. B. (1999). “ Ripple depth and density resolution of rippled noise,” J. Acoust. Soc. Am. 106, 2800–2804. 10.1121/1.428105 [DOI] [PubMed] [Google Scholar]
- 32. Turner, C. W. , Gantz, B. J. , Vidal, C. , Behrens, A. , and Henry, B. A. (2004). “ Speech recognition in noise for cochlear implant listeners: Benefits of residual acoustic hearing,” J. Acoust. Soc. Am. 115, 1729–1735. 10.1121/1.1687425 [DOI] [PubMed] [Google Scholar]
- 33. Turner, C. W. , Souza, P. E. , and Forget, L. N. (1995). “ Use of temporal envelope cues in speech recognition by normal and hearing-impaired listeners,” J. Acoust. Soc. Am. 97, 2568–2576. 10.1121/1.411911 [DOI] [PubMed] [Google Scholar]
- 34. Wilson, B. S. , and Dorman, M. F. (2008). “ Cochlear implants: Current designs and future possibilities,” J. Rehabil. Res. Dev. 45, 695–730. 10.1682/JRRD.2007.10.0173 [DOI] [PubMed] [Google Scholar]
- 35. Won, J. H. , Clinard, C. G. , Kwon, S. , Dasika, V. K. , Nie, K. , Drennan, W. R. , Tremblay, K. L. , and Rubinstein, J. T. (2011a). “ Relationship between behavioral and physiological spectral-ripple discrimination,” J. Assoc. Res. Otolaryngol. 12, 375–393. 10.1007/s10162-011-0257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Won, J. H. , Drennan, W. R. , Kang, R. S. , and Rubinstein, J. T. (2010). “ Psychoacoustic abilities associated with music perception in cochlear implant users,” Ear Hear. 31, 796–805. 10.1097/AUD.0b013e3181e8b7bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Won, J. H. , Drennan, W. R. , and Rubinstein, J. T. (2007). “ Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users,” J. Assoc. Res. Otolaryngol. 8, 384–392. 10.1007/s10162-007-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Won, J. H. , Jones, G. L. , Drennan, W. R. , Jameyson, E. M. , and Rubinstein, J. T. (2011b). “ Evidence of across-channel processing for spectral-ripple discrimination in cochlear implant listeners,” J. Acoust. Soc. Am. 130, 2088–2097. 10.1121/1.3624820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeng, F.-G. , Rebscher, S. , Harrison, W. , Sun, X. , and Feng, H. (2008). “ Cochlear implants: System design, integration, and evaluation,” IEEE Rev. Biomed. Eng. 1, 115–142. 10.1109/RBME.2008.2008250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang, T. , Spahr, A. J. , Dorman, M. F. , and Saoji, A. (2013). “ The relationship between auditory function of non-implanted ears and bimodal benefit,” Ear Hear. 34, 133–141. 10.1097/AUD.0b013e31826709af [DOI] [PMC free article] [PubMed] [Google Scholar]