Abstract
Recent studies implicating the fallopian tube as the site of putative precursors of ovarian serous carcinoma and the hypothesis that injury, inflammation and repair of the ovarian surface epithelium at the time of ovulation may be contributing factors to ovarian carcinogenesis, prompted us to undertake a comprehensive analysis of the immune cells in the normal fallopian tube. Accordingly, the aim of this study was to provide a base line for future studies exploring the relationship of inflammation to the early events of ovarian carcinogenesis by characterizing the immune cell repertoire in thirteen normal human fallopian tube, combining digital microscopy of immunostained slides and flow cytometry of fresh single cell suspensions, with a panel of markers that identify the most important adaptive and innate immune cells. We found that CD45+ leucocytes are regularly observed in the fallopian tube and are mainly composed of CD163+ macrophages, CD11c+ dendritic cells and CD8+ T-cells. In addition, there are minor populations of CD56+ NK cells, CD4+ T-cells, CD20+ B-cells, TCRγδ+ T-cells, and, among dendritic cells, CD207(Langerin)+ Langerhans cells. The cellular mapping that we performed indicates that the local immune system in the human fallopian tube is composed of a mixture of innate and adaptive immune cells, many of which are recognized as playing a role in cancer immune surveillance. This local immune system could provide a first line of defense against early precancerous lesions and could potentially be exploited for immune-based therapies.
Keywords: fallopian tube, immune system, intraepithelial lymphocytes, dendritic and NK cells, digital microscopy
INTRODUCTION
In addition to its critical role in fertilization1, the fallopian tube, acts as a conduit for a variety of pathogens entering the pelvis. It, therefore, contains populations of immune cells that provide surveillance against these pathogens2 and previous studies have shown that both the innate and the adaptive arms of the immune system are involved. Thus, attention has been directed to the immunology of the fallopian tube, in so far as infection3 and tolerance towards the fetus and pregnancy-related diseases4 are concerned, but the details of the immune system in this location remain poorly understood. Recent studies have implicated the fallopian tube as the site of origin of ovarian low-grade5–6 and high-grade serous carcinoma7–10. These observations as well as studies showing that the density and composition of the immune cell infiltrate in human ovarian cancer, including T- and B-cells, NK-cells and macrophages, have prognostic significance in predicting response to chemotherapy11–14, prompted us to undertake a comprehensive analysis of the immune cells in the fallopian tube as we reasoned that the immune system could potentially play a role in cancer immune surveillance. Accordingly, the present study was undertaken to characterize the immune cell repertoire of the normal human fallopian tube using immunohistochemistry (IHC) and flow cytometry, with a panel of markers that identify the most important adaptive and innate immune cells. The findings in this study lay the groundwork for subsequent studies of the various immune cell populations in potential ovarian cancer precursor lesions which may provide insight into the role of immune surveillance in the early phases of malignant initiation, transformation and progression of ovarian cancer.
MATERIAL AND METHODS
Human Tissues
Ten formalin-fixed paraffin-embedded (FFPE) tissue blocks of fallopian tube were retrieved from the surgical pathology files of the Department of Pathology, Spedali Civili of Brescia (Brescia, Italy). Patients underwent surgical removal of the fallopian tubes for non-cancer related conditions. Clinical details of the patient cohort are shown in Table 1. Haematoxylin & eosin (H&E) stained slides were reviewed by a pathologist (LA) in order to exclude the presence of any neoplastic or inflammatory conditions. Representative sections of the fallopian isthmus and fimbriae were available from each patient. In addition, three fragments of fresh fallopian tube were collected from two pre-menopausal and one post-menopausal patient who underwent total hysterectomy with bilateral salpingo-oophorectomy for surgical treatment of uterine leiomyoma. The study was approved by the local Ethics Committee.
Table 1.
Clinical details of the patients cohort
| Patient ID | Age (yrs) | Menopausal status | Surgical Procedure/Indication |
|---|---|---|---|
| #1 | 63 | Post | TAH-BSO/Leiomyomas |
| #2 | 67 | Post | TAH-BSO/Leiomyomas |
| #3 | 67 | Post | TVH-BSO/Prolapse |
| #4 | 73 | Post | TVH-BSO/Prolapse |
| #5 | 63 | Post | TVH-BSO/Leiomyomas |
| #6 | 32 | Pre | SA/Sterilization |
| #7 | 35 | Pre | SA/Sterilization |
| #8 | 30 | Pre | SA/Sterilization |
| #9 | 26 | Pre | SA/Sterilization |
| #10 | 35 | Pre | SA/Sterilization |
Post: post- menopausal; TAH-BSO: total abdominal hysterectomy with bilateral salpingo-oophorectomy; TVH-BSO: total vaginal hysterectomy with bilateral salpingo-oophorectomy; Pre: pre-menopausal; SA: salpingectomy
Immunohistochemistry and immunofluorescence
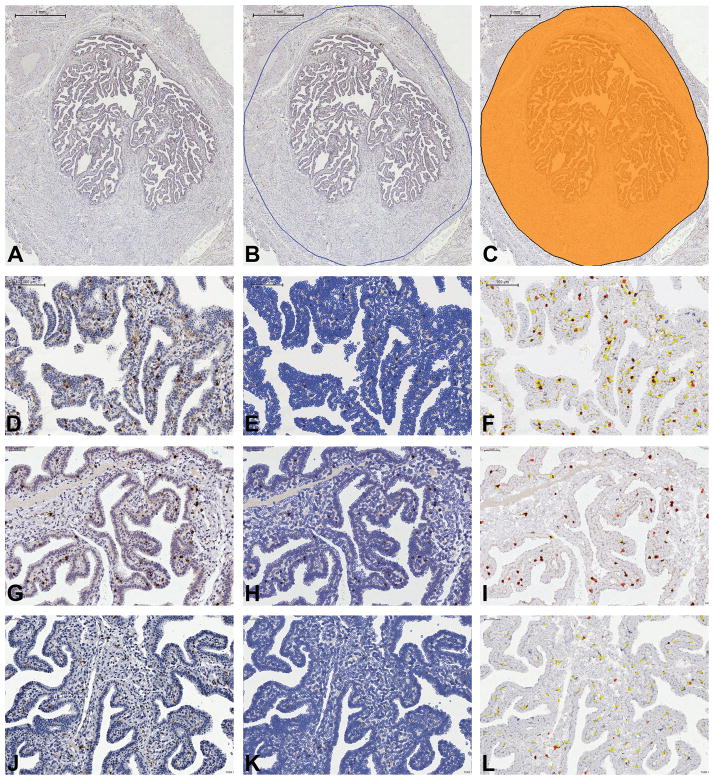
Immunohistochemistry was performed on four microns FFPE tissue sections. The list of primary antibodies and detection systems is shown in Table 2. For single staining, diaminobenzidine (DAB, Dako, Glostrup, Denmark) was used as the chromogen and sections were counterstained with Meyer’s Haematoxylin. For double immunohistochemistry, after completing the first immune reaction using DAB, the second immune reaction was visualized using Mach 4 MR-AP (Biocare Medical, Concord, CA, USA), followed by Ferangi Blue (Biocare Medical) as chromogen. Double immunofluorescence sections were visualized with fluorescent labeled Texas Red- and FITC-conjugated isotype-specific secondary antibodies (Table 2). In order to compare the immune cell distribution in different parts of the fallopian tube, two pathologists (LA and WV) evaluated the intraepithelial [IE], lamina propria [LP] and muscular wall [MW], separately (Table 3). Immune cell density of dominant populations was initially determined using digital microscopy. The absolute cell count was quantified automatically using a custom-programmed script in Cognition Network Language based on the Definiens Cognition Network Technology platform (Definiens AG, Munich, Germany). In brief, immunostained slides of fallopian tube sections from the isthmus were digitalized using an Aperio ScanScope CS Slide Scanner (Aperio Technologies, Vista, CA, USA) at 40× magnifications and analyzed using Tissue Studio 2.0 (Definiens AG). The quantitative scoring algorithm was customized using commercially available templates and was created to match the requirements of the staining parameter in the fallopian tube including Manual ROI (region of interest) Selection (Draw Polygon). Immune cell counts are expressed as the number of positive cells/mm2 of fallopian tube area and as a ratio between positive cells and the total nucleated cells identified in the fallopian tube (Table 4). For immune cells showing intraepithelial localization, an additional cell count was performed at 40x magnification and expressed as a ratio of immune cells/epithelial cells, by counting at least 500 epithelial cells and excluding areas where there was epithelial overlay (Table 5).
Table 2.
Details of primary antibodies and detection systems
| Primary antibody | Source | Clone | Dilution | Company | Detection system |
|---|---|---|---|---|---|
| CD2AP | Ms IgG1 | B-4 | 1:1000 | SC | E |
| CD3 | Rb mAb | SP7 | 1:100 | TS | E |
| CD3 (IF) | Rb mAb | SP7 | 1:100 | TS | Rb FITC |
| CD4 | Ms IgG1 | 4B12 | 1:50 | NL | NP |
| CD8 | Ms IgG1 | C8/144B | 1:30 | DK | E |
| CD11c | Ms IgG2 | 5D11 | 1:50 | Mo | E |
| CD20 | Ms IgG2a | L26 | 1:250 | DK | E |
| CD45 | Ms IgG1 | X16/99 | 1:500 | NL | NP |
| CD56 | Ms IgG1 | 123C3.D5 | 1:30 | TS | E |
| CD56 (IF) | Ms IgG1 | 123C3.D5 | 1:30 | TS | Ms biot/Sav-TXR |
| CD66b | Ms IgM | G10F5 | 1:200 | Bi | NP |
| CD68 | Ms IgG3 | PG-M1 | 1:200 | DK | E |
| CD117 | Rb pAb | polyclonal | 1:50 | DK | E |
| CD123 | Ms IgG2a | 7G3 | 1:50 | BD | NP |
| CD138 | Ms IgG1 | MI15 | 1:100 | DK | E |
| CD163 | Ms IgG1 | 10D6 | 1:50 | TS | E |
| CD207 (Langerin) | Ms IgG2b | 12D6 | 1:150 | VL | E |
| CD303 (BDCA2) | Ms IgG1 | 124B3.13 | 1:75 | De | NP |
| Calretinin | Rb pAb | polyclonal | 1:200 | I | E |
| Neutrophil Elastase | Ms IgG1 | NP57 | 1:150 | DK | NP |
| Estrogen Receptor | Rb mAb | SP1 | 1:100 | TS | E |
| Foxp3 biot | Rat IgG2a | FJK-16s Set | 1:200 | eB | RP |
| Granzyme B | Ms IgG2a | 11F1 | 1:100 | NL | NP |
| Perforin | Ms IgG1 | 5B10 | 1:20 | NL | E |
| Progesterone Receptor | Ms IgG1 | PgR | 1:200 | DK | E |
| T-bet | Ms IgG1 | 4B10 | 1:50 | SC | NP |
| TCRβF1 | Ms IgG1 | 8A3 | 1:30 | TS | NP |
| TCRCγM1 | Ms IgG1 | γ3.20 | 1:400 | TS | NP |
IF: immunofluorescence; Rb mAb/pAb: rabbit monoclonal or polyclonal antibody; Ms: mouse antibody; biot: biotinylated antibody.
Detection system: E: EnVision (Dako); Rb FITC: Swine anti-rabbit FITC (Dako); NP: Novolink polymer (Novocastra Laboratories); Ms biot: goat anti-mouse biotynilated IgG1, Sav-TXR: Streptavidin TXR-conjugated (Southern Biotecnology*); RP: Rat-on-mouse HRP-polymer (Biocare Medical).
Company addresses: *SouthernBiotech, Birmingham, AL, USA; SC: Santa Cruz Biotechnology, Santa Cruz, CA, USA; TS: Thermo Scientific, Fremont, CA, USA; NL: Novocastra Laboratories, Newcastle upon Tyne, UK; DK: Dako, Glostrup, Denmark; Mo: Monosan, Uden, The Netherlands; Bi: BioLegend, San Diego, CA, USA; BD: BD Biosciences, San Jose, CA, USA; VL: Vector Laboratories, Burlingame, CA, USA; De: Dendritics, Lyon, France; I: Invitrogen, Carlsbad, CA, USA; eB: eBioscience, San Diego, CA, USA.
Table 3.
Immunohistochemical profile and localization of the main immune cell types of the fallopian tube
| ADAPTIVE | ||
|---|---|---|
| Immune cell type | Immunophenotype | Tissue localization |
| T-cells | CD3+CD20− | IE, LP, MW |
| Cytotoxic T-cells | CD8+CD3+ | IE, LP, MW |
| T-helper cells | CD3+CD4+ | IE, LP, MW |
| Regulatory T-cells | CD3+Foxp3+ | LP, MW |
| TCRγδ+ T-cells | TCRCγM1+ | IE, LP, MW |
| B-cells | CD3−CD20+ | IE, LP, MW |
| Plasma cells | CD138+ | LP, MW |
| INNATE | ||
| Macrophages | CD68+CD163+ | IE, LP, MW |
| NK cells | CD3−CD56+ | IE, LP, MW |
| Plasmacytoid DCs | CD303(BDCA2)+ | LP*, MW* |
| Langerhans cells | CD207(Langerin)+ | IE, LP |
| Other type of DCs | CD11c+/−CD163+/− | IE, LP, MW |
| Mast cells | CD117+ | LP, MW |
| Neutrophils | CD66b+Neutr El+ | LP*, MW* |
DCs: dendritic cells; IE: intraepithelial; LP: lamina propria; MW: muscular wall; *: almost exclusively intravascular localization; Neutr El: neutrophil elastase
Table 4.
Digital Microscopy cell analysis of immune cells of the fallopian tube
| IHC Marker | Positive cells/mm2 (range) | Positive cells/total nucleated cells (range) |
|---|---|---|
| CD45 | 433 (147–743) | 1/18 (1/49–1/11) |
| CD3 | 120 (110–136) | 1/69 (1/83–1/56) |
| CD4 | 35 (15–75) | 1/175 (1/555–1/82) |
| CD8 | 160 (94–196) | 1/47 (1/76–1/36) |
| CD11c | 278 (200–364) | 1/21 (1/29–1/17) |
| CD20 | 27 (3–58) | 1/270 (1/3333–1/135) |
| CD68 | 254 (178–320) | 1/31 (1/45–1/24) |
| CD163 | 306 (204–368) | 1/30 (1/50–1/25) |
| TCR CγM1 | 11 (10–21) | 1/555 (1/833–1/370) |
IHC: immunohistochemistry
Table 5.
Ratio between different types of immune cells and epithelial cells in the IE compartment
| Immune cell type | OA Ratio | Pre- vs Post-menopause (p-value) | Fimbriae vs Isthmus (p-value) |
|---|---|---|---|
| T-cells | 1/16 | 0.53 | 0.41 |
| Cytotoxic T-cells | 1/15 | 0.79 | 0.07 |
| T-helper cells | 1/400 | 0.0027* | 0.94 |
| B-cells | 1/64 | 0.0003* | 0.14 |
| TCRγδ+ T-cells | 1/120 | 0.93 | 0.46 |
| NK cells | 1/70 | 0.67 | 0.0001* |
| Macrophages | 1/12,5 | 0.034* | 0.08 |
| Langerhans cells | 1/625 | 0.62 | 0.88 |
IE: intraepithelial; OA: overall average; Pre-; premenopausal; vs: versus; post-: post-menopausal; F: fimbriae; I:isthmus:
statistically significant
Flow cytometry
Fresh fragments of human fallopian tubes were initially washed with saline solution to eliminate the large majority of circulating mononuclear cells. Then they were finely dissected, minced and digested using 1 mg/mL collagenase IV (Sigma–Aldrich, Dorset, UK) in PBS for 2 h at 37 °C. Digested tissue was filtered through 70 μm and 40 μm filters. Filtered material containing the fallopian tube single cell suspension was centrifuged twice for 10 min at 1500 rpm and stained with a mixture of the following monoclonal antibodies (mABs): iNKT-FITC, CD16-PE, CD8-PerCP-Cy5.5, CD3-PE-Cy7, CD45RA-APC-H7, CD4-V450 and CD45-V500 (all from Becton Dickinson, Franklin Lakes, NJ, USA) and CD56-APC (Miltenyi Biotec, Bergisch Gladbach, Germany). At least 50000 lymphocytes were acquired from each sample using a FACSCanto II system (Becton Dickinson), while analyses were performed with FlowJo software version 8.8.7 (TreeStar, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using the GraphPrism program. For comparison of the number of the different types of intraepithelial immune cells according to menopausal status (pre- vs post-) and to tubal site (fimbriae vs isthmus), a Student’s t-test was used. P < 0.05 was considered statistically significant.
RESULTS
Occurrence, density and distribution of immune cells in normal fallopian tube
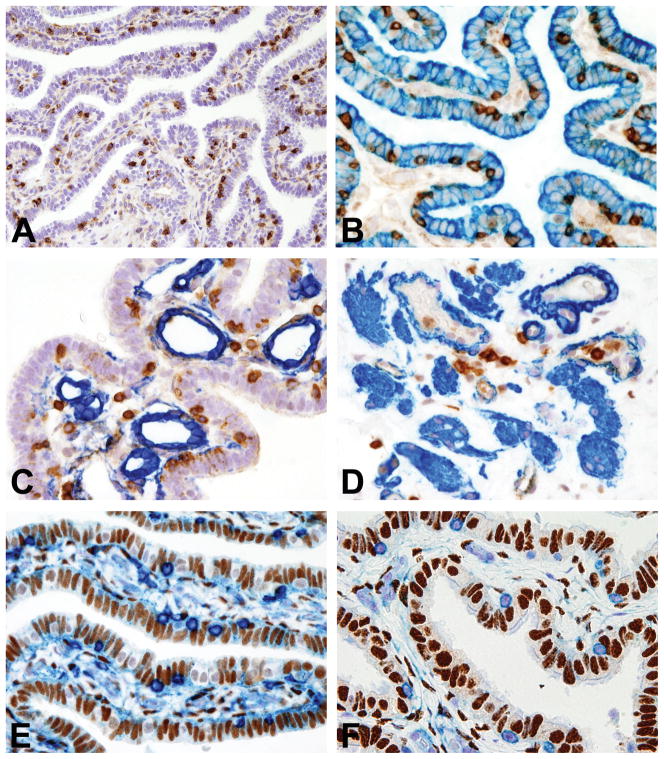
Prior to this study we had observed that normal human fallopian tube regularly contains CD45+ immune cells (Figure 1A). Based on a digital microscopy (DigMic) approach using Definiens Tissue Studio (Figure 2), we determined a density of CD45+ immune cells ranging from 147 to 743/mm2 (Table 4). In all samples we observed that CD45+ cells were distributed in the epithelium, lamina propria and muscular wall (Figure 1B–D). In the epithelium the CD45+ cells were intermingled with secretory and ciliated cells lying just above the basement membrane. In contrast, in the lamina propria and wall of the tube they were sparse or occasionally formed small aggregates that frequently surrounded blood vessels. Significantly, double immunohistochemistry for CD45 coupled with either estrogen or progesterone receptor showed no expression of hormones receptors by immune cells (Figure 1E–F). The morphology of CD45+ cells was extremely variable, including round to oval cells admixed to cells with irregular morphology (i.e spindle or dendritic-like), thus indicating cell heterogeneity. To shed light on the identity and function of the immune cells in the epithelium, lamina propria and muscular wall of the fallopian tube, we undertook a comprehensive investigation using immunohistochemistry, immunofluorescence and flow cytometry.
Figure 1. Localization and hormone receptor expression of resident leucocyte populations in normal fallopian tube.
Sections are from FFPE normal fallopian tube (FT) (A–F). A illustrates CD45+ immune cells in a FT isthmic section; B illustrates CD45(brown)/CK7(blue) double immunostaining showing CD45+ immune cells intermingled with CK7+ epithelial cells; C illustrates CD45(brown)/CD34(blue) double immunostaining showing CD45+ immune cells in close proximity to CD34+ vessels of the FT lamina propria; D illustrates CD45(brown)/smooth muscle actin (blue) double immunostaining showing CD45+ immune cells in close proximity to smooth muscle actin+ smooth muscle fibers/vessels of the FT muscular wall; E–F illustrate double immunostaining with CD45 (E–F, blue) and ER (brown, E) and PgR (brown, F) showing no expression of hormones receptors by immune cells. Sections are counterstained with Meyer’s haematoxylin. Magnification: 20× (A) and 40× (B–F).
Figure 2. Digital Microscopy analysis of the main immune cell populations in the normal fallopian tube (FT).
Sections are from FFPE normal fallopian tube and are digitalized by Aperio Scanscope and processed by Tissue Studio Definiens. A–C illustrate ROI (region of interest) selection: A illustrates the digital image of whole isthmic section of FT; B illustrates the manual selection of the ROI; C indicates the ROI (orange color) submitted to Tissue Studio Definiens for image analysis (Bar scale: A–C 1mm). D–F, G–I, J–L are matched native (D, G and J) and processed for nuclear identification (E, H and K) and signal detection (F, I and L; white cells are negative cells whereas yellow, orange and red cells correspond to positive cells with different signal intensity) images (Bar scale: D–F 100μm; G–L: 50 μm).
CD8+ intraepithelial T-cells represent the dominant lymphoid subset
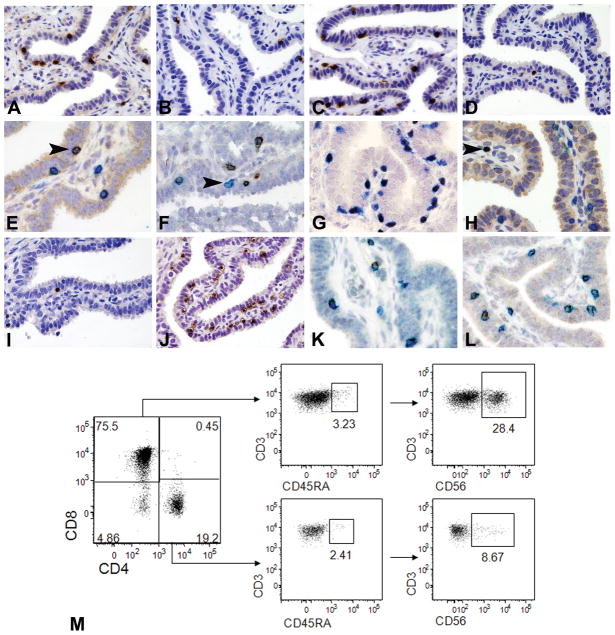
Among B- and T-lymphoid cells, CD3+ T-cells represented the dominant population, as validated by DigMic (Table 4 and Figure 3A). They were evenly distributed in all samples and localized in the epithelium as single cells intermingled between epithelial cells just above the basement membrane. In contrast CD20+ B-cells were less frequently detected in the epithelium, lamina propria or wall (Tables 4–5 and Figure 3B). In the epithelium the average ratio of immune cells to epithelial cells was 1/16 for CD3+T-cells and 1/64 for CD20+B-cells respectively (Table 5) and the ratio between intraepithelial B-cells and T-cells was approximately 1/4. In the lamina propria and muscular wall, T- and B-cells were found as single scattered cells; in the muscular wall they also presented as perivascular lymphoid aggregates. Among T-cells, CD8+ cells were the most abundant subset, especially in the epithelium, while CD4+ cells were poorly represented (Tables 4–5 and Figure 3C–D). Accordingly, the average ratio of immune cells to epithelial cells was 1/400 for CD4+ T-cells and 1/15 for CD8+ T-cells respectively (Table 5), with a CD4+/CD8+ ratio of 1/25.
Figure 3. Cellular populations of the adaptive immune system in the normal fallopian tube.
Sections from FFPE normal fallopian tube are analyzed by immunohistochemistry (A–L) whereas single cell suspension of fresh normal fallopian tube is analyzed by flow cytometry (M). A–D illustrate the main lymphoid populations detected in the intraepithelial compartment (IE) including CD3+ T-cells (A), CD20+ B-cells (B), CD8+ cytotoxic T-cells (C) and CD4+ T-helper cells (D). E illustrates CD3(blue)/CD56(brown) double immunostaining showing co-expression of CD56 NK marker by a CD3+ T-cells (arrow head). F illustrates CD3(brown)/CD56(blue) double immunostaining showing the presence of CD3+CD8+ T-cells (prevalent subtype) and CD3−CD8+ cells (arrow head), likely representative of CD8+ NK subset. G illustrates CD3(blue)/T-bet (brown) double immunostaining showing the prevalence of Tbet+CD3+ T-cells. H illustrates CD3(blue)/Foxp3(brown, nuclear) double immunostaining showing absence of Foxp3+ regulatory T-cell in the IE compartment; arrow head indicates a single CD3+Foxp3+ regulatory T-cells in the lamina propria. I–J show comparison between IE TCRγδ+ (I) and TCRαβ+ T-cells (J). K–L illustrate double immunostaining with CD3 (K–L, blue) and Granzyme B (K, brown) and Perforin (L, brown) showing the presence of those cytotoxic molecules in CD3+ T-cells. Sections are counterstained with Meyer’s haematoxylin. Magnification: 40× (A–D, I–J) and 60× (E–H, K–L). M shows flow cytometry characterization of T lymphocytes on digested tubal tissue. Left plot: CD4/CD8 ratio; central and right plots: percentage of CD45RA+ and CD56+ cells, respectively, in the CD8+CD4− and CD4+CD8− gates (The panel is representative of three different samples).
The prevalence of CD8+ cells was confirmed by a flow cytometric analysis on single cell suspensions of fallopian tube (Figure 3M). The “global” CD4+/CD8+ ratio on flow cytometry was similar to the one found on the whole tubal sections analyzed with DigMic but significantly lower than the ratio observed in the epithelium on the tissue sections (1/4 to 1/5 versus 1/25), suggesting that CD8+ T-cells preferentially home within the epithelium. Significantly, the vast majority of T-cells lacked CD45RA expression, suggesting a memory phenotype. According to double immunohistochemistry (Figure 3E) and immunofluorescence analysis (not shown) we identified in the epithelium a fraction of intraepithelial CD3+ cells expressing the NK cell marker CD56. Flow cytometric characterization of CD3+CD56+ cells demonstrated that their percentage was highly variable from sample to sample (10 to 60% of CD3+ cells), with most of the cells displaying an immunophenotype characteristic of effector memory cytotoxic T cells (CD8+CD45RA−). On the other hand, the presence of NKT cells in the CD3+CD56+ population was excluded by negativity for the NKT specific invariant Vα24-JαQ TCRα chain15 (not shown).
By flow cytometry we also observed a subset of CD3−CD8+ cells (not shown). This finding was confirmed by double immunohistochemistry on FFPE sections (Figure 3F). These cells represent a fraction of CD16hiCD56dim NK cells with low density surface expression of CD816.
On FFPE sections, the large majority of fallopian tube CD3+ T-cell population was positive for T-bet (Figure 3G) and negative for nuclear Foxp3 (Figure 3H), indicating that virtually all of them were characterized by a type 1 polarization17, 18 and that regulatory T-cells19 are almost completely absent in the normal fallopian tube. Recently a new antibody has been shown to be capable of identifying TCRγδ+ T-cells on FFPE20. We were able to confirm this data using this reagent to assess the presence of intraepithelial γδ+ T-cells in the small bowel (unpublished data). In the fallopian tube TCRγδ+ T-cells were rare and distributed as single intraepithelial T-cells (Figure 3I). Accordingly, the average ratio with epithelial cells was of 1/120, whereas the ratio of TCRγδ+/CD3+ T-cells was approximately of 1/8. This finding was confirmed by immunohistochemistry for TCRαβ that marked the large majority of intraepithelial lymphocytes (Figure 3J). Scattered TCRγδ+ T-cells were also present in the lamina propria or in vessels of the wall of the tube. Based on the finding that most T-cells were represented by the CD8+ subset, we extended the analysis by performing double immunohistochemistry for CD3 coupled with Granzyme-B or Perforin and could demonstrate that a significant proportion of intraepithelial T-cells contained these cytotoxic molecules (Figure 3K–L).
Occasional CD138+ plasma cells were identified as scattered single cells in the lamina propria and tubal wall (not shown).
CD11c+ dendritic cells and CD163+ macrophages represent the predominant innate cell populations
On CD45 stained sections, we observed that a large fraction of positive cells showed irregular, non-lymphoid morphology, suggesting that they may be resident cells of the mononuclear phagocyte system. Based on the DigMic analysis, CD11c, CD68 and CD163 reactivity was frequently observed (Table 4). In tissue sections, this set of markers can identify human macrophages (CD11c, CD68 and CD163[Figure 4A–C]) and dendritic cells (CD11c and CD68). By double immunohistochemistry for CD163 and CD11c we could identify distinct cell populations that were differentially distributed in the epithelium, lamina propria and wall of the tube (Table 6). In the epithelium, the highest fraction was represented by CD11c+CD163− cells (Figure 4D), while single positive CD11c−CD163+ cells and CD11c+CD163+ double positive cells were more rare. CD11c+CD163− cells were regularly and evenly distributed between epithelial cells above the basal lamina and showed long intraepithelial cytoplasmic projections (Figure 4D, insert). In the lamina propria and wall, the most abundant population was represented by double positive CD11c+CD163+ cells, while single positive CD11c−CD163+ and CD11c+CD163− cells were less frequently observed (Figure 4E). We extended the analysis of dendritic cells by staining for the Langerhans cells marker CD207(Langerin) and the plasmacytoid dendritic cells marker CD303(BDCA2). CD207(Langerin)+ Langerhans cells were very rare but were regularly detected in the epithelium and lamina propria (Figure 4F), whereas CD303(BDCA2)+ plasmacytoid dendritic cells were only occasionally found within the lumen of blood vessels in the lamina propria and tubal wall (Figure 4G).
Figure 4. Cellular populations of the innate immune system in the normal fallopian tube.
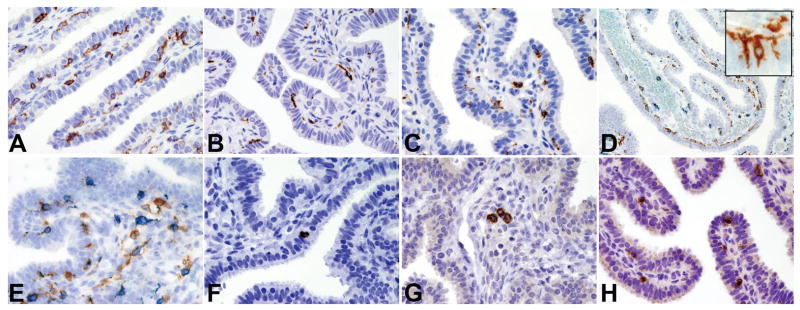
Sections are from FFPE normal fallopian tube (A–H) and stained for CD11c (A), CD68 (B) and CD163 (C). D–E illustrate CD163(blue)/CD11c(brown) double immunostaining showing the presence respectively of CD11c+CD163− cells in the intraepithelial compartment (D; insert showing intraepithelial cytoplasmic projections of dendritic cells) and of CD11c+CD163+ cells admixed to CD11c−CD163+ cells in the lamina propria (E); F illustrates CD207/Langerin+ intraepithelial Langerhans cell; G illustrates occasional CD303/BDCA2+ plasmacytoid dendritic cells in vessels of the lamina propria; H illustrates intraepithelial CD56+ NK cells. Sections are counterstained with Meyer’s haematoxylin. Magnification: 40× (A–C and E–H) and 20× (D).
Table 6.
Immunophenotype and distribution of large stellate/spindle cells in the fallopian tube
| Subsets | IE | non-IE |
|---|---|---|
| CD11c+CD163+ | + | ++ |
| CD11c+CD163− | ++ | + |
| CD11c−CD163+ | + | + |
IE: intraepithelial; +: minor subset; ++: major subset
Among innate immune cells, we also identified CD56+CD3− NK cells in the epithelium, lamina propria and wall; in the epithelium they were evenly distributed between the epithelial cells with an average ratio of immune to epithelial cells of 1/70 (Table 5 and Figure 4H). Interestingly, flow cytometric analysis of fallopian tube fragments showed that at least half of NK cells had a negative/low expression of CD16, suggesting a derivation from the CD56hi compartment (data not shown).
CD117+ mast cells and CD66b+Elastase+ neutrophils were identified respectively as scattered single cells and intravascular cellular groups in the lamina propria and tubal wall (not shown).
Site- and hormone-dependent variation of fallopian tube immune cells
Based on double immunohistochemistry for CD45 coupled with antibodies recognizing estrogen and progesterone receptors, we failed to detect hormone receptor expression on fallopian tube leucocytes, however, the epithelial cells strongly expressed these receptors (Figure 1E–F). Comparison of the intraepithelial number of distinct immune cells according to menopausal status (premenopausal vs postmenopausal status) and localization (fimbriae vs isthmus) is reported in Table 5. We found significant differences in the number of T-helper cells (p<0.0027), B-cells (p<0.0003) and macrophages (p<0.034, according to menopausal status, with T-helper cells and B-cells being more abundant in postmenopausal women while macrophages were more abundant in premenopausal women (data not shown). The only significant difference according to the location in the tube was found for intraepithelial NK cells (p<0.0001) which were more abundant in the isthmus (data not shown).
DISCUSSION
In this study we describe the identity and distribution of immune cells in the normal human fallopian tube by immunohistochemistry, immunofluorescence and flow cytometry, using a large panel of lineage and subset-specific markers. This is a preliminary analysis which lays the groundwork for subsequent studies correlating immune cell populations with the phases of the normal menstrual cycle, various infectious diseases and ovarian serous neoplasia given the emerging role of the fallopian tube as the site of ovarian carcinoma precursor lesions.
We found that the human fallopian tube contains a heterogeneous population of innate and adaptive immune cells including lymphocytes, macrophages, NK cells and dendritic cells. In terms of distribution, CD45+ immune cells are found in the epithelium, lamina propria and muscular wall. By quantitative analysis using digital microscopy, we found that T-cells represent the most abundant lymphoid population. Intraepithelial T-cells are mainly TCRαβ+ and express T-bet and lack Foxp3 in keeping with a Th1 type polarization17–19. As previously reported, we confirmed that intraepithelial T-cells are mostly CD8+21 cells and contain cytoplasmic perforin and granzyme B. Significantly, a minor fraction of intraepithelial CD3+ T-cells expressed TCRγδ. TCRγδ+ T-cells are resident T-cells in skin, small and large intestine, lung and male and female reproductive organs22, 23. In these sites TCRγδ+ T-cells play an important role in maintaining skin and mucosal homeostasis and are involved in regulation of inflammation, tissue repair, protection against infectious agents and elimination of transformed cells24, 25. In the female reproductive organs TCRγδ+ cells have been isolated from the endometrium, including decidua, cervix and vagina23 where they may exert a protective role against viruses26. The percentage of intraepithelial TCRγδ+ T-cells in the fallopian tube is much lower in comparison to other types of mucosae, especially the small intestine, where they constitute up to 60% of intraepithelial lymphocytes27. Similarly, in the endometrium, TCRγδ+ T-cells represent a small fraction of the intraepithelial T-cells28. By double immunohistochemistry and immunofluorescence we found that a fraction of intraepithelial T-cells co-expressed CD56. Although CD3/CD56 co-expression is a typical characteristic of NKT cells29, 30 we excluded the presence of this cell subset by specific flow cytometric staining. In contrast, the vast majority of CD3+CD56+ cells were also CD8+CD45RA−, suggesting that most of the infiltrating lymphocytes are effector memory cytotoxic cells.
We also detected CD3−CD56+ NK cells, mainly in the epithelium. Using flow cytometry of single cell suspensions, it was possible to demonstrate that CD56hiCD16− represents the dominant subset, as previously suggested31, but that in the CD56dimCD16hi fraction also contains a considerable number of CD8lo expressing cells. Among innate immune cells, the fallopian tube contains numerous CD163+ macrophages and CD11c+ dendritic cells. When analyzed by location using double immunohistochemistry, CD11c+CD163− predominate in the epithelium, whereas CD11c+CD163+ cells were more abundant in the lamina propria and the wall of the tube. In contrast, CD11c−CD163+ macrophages were the least represented subset in the epithelium and non-epithelial compartments. CD11c was originally described as a dendritic cell marker32, however, its specificity on sections of human tissues is questionable since anti-CD11c antibodies show reactivity to various macrophage populations. Significantly, high levels of CD11c can be found on mucosal macrophages33.
In view of these findings we also tested other dendritic cell populations, namely plasmacytoid dendritic cells and Langerhans cells. It was previously reported that plasmacytoid dendritic cells are abundant in the fallopian tube21. We were unable to confirm this finding as we found CD303(BDCA2)+ cells only occasionally in the lumen of blood vessels. Lack of plasmacytoid dendritic cells was also confirmed by the absence of CD123+ and CD2AP+ cells showing plasmacytoid morphology (not shown). Langerhans cells are the predominant antigen-presenting cells in epithelial tissues and selectively express CD207(Langerin) receptor34. The occurrence of Langerhans cells has previously been reported throughout the female reproductive organs, especially in the vagina35, cervix36 and endometrium37. Electron microscopy and immunohistochemistry for CD1a have identified Langerhans cells also in the fallopian tube38. By using the highly specific reagent CD207(Langerin), we could detect rare Langerhans cells, localized predominantly in the epithelium.
In summary, the findings in this study describing the heterogeneous population of immune cells in the fallopian tube lay the ground work for further investigation of the immune system in ovarian serous carcinogenesis given the emerging role of the fallopian tube as the site of putative precursors of ovarian serous carcinoma. This is particularly provocative in view of the theory of incessant ovulation which implicates injury of the ovarian surface epithelium at the time of ovulation as contributing to malignant transformation by virtue of associated inflammation and repair. Conceivably macrophages can be an important component of this process by inducing DNA damage in tubal epithelial cells leading to the development of ovarian carcinoma 39 as borne out by a study reporting that the number of CD68+ macrophages detected in the fallopian tube increases from benign conditions to malignant ones40. Moreover, a recent study indicates that TCRγδ+ lymphocytes can efficiently kill human ovarian potential cancer stem-like cells through IL-17 production. This could represent a promising tool for immunotherapy in ovarian cancer41. Accordingly, further analysis of the identity and composition of immune cells in the fallopian tube and their relationship to ovarian cancer precursors is warranted as this may provide a potential approach to the development of immunotherapy aimed at early neoplastic lesions which are more likely to respond to immunotherapy than established cancers.
Acknowledgments
Sources of Support
This study was supported by “Pathogenesis of Ovarian Serous Borderline Tumors” NCI RO1 CA116184 US National Cancer Institute, the US Department of Defense “Elucidation of Molecular Alterations in Precursor Lesions of Ovarian Serous Carcinoma Consortium” grant OC100517 and by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (2009CKARAL to W.V.) and Associazione Italiana per la Ricerca sul Cancro (AIRC IG 11924 to W.V.). L.A. was supported by Fondazione Beretta (Brescia, Italy) and by the International Society of Gynecologic Pathologists Hernando Salazar Fellowship Award 2011.
Footnotes
Disclosure/Conflict of Interest
The authors declare absence of a conflict of interest.
References
- 1.Barriere P, Thibault E, Jean M. role of fallopian tube in fertilization. Rev Prat. 2002;52:1757–61. [PubMed] [Google Scholar]
- 2.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 3.Sziller I, Babula O, Ujhazy A, et al. Chlamydia trachomatis infection, fallopian tube damage and a mannose-binding lectin codon 54 gene polymorphism. Hum Reprod. 2007;22:1861–65. doi: 10.1093/humrep/dem107. [DOI] [PubMed] [Google Scholar]
- 4.Basta P, Majka M, Jozwicki W, et al. The frequency of cd25+cd4+ and foxp3+ regulatory t cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol. 2010;8:116. doi: 10.1186/1477-7827-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurman RJ, Vang R, Junge J, et al. Papillary tubal hyperplasia: the putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am J Surg Pathol. 2011;35:1605–14. doi: 10.1097/PAS.0b013e318229449f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Abushahin N, Pang S, et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–99. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 7.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of brca1/2 mutation carriers. Mod Pathol. 2009;22:1133–38. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 9.Przybycin CG, Kurman RJ, Ronnett BM, Shih Ie M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn E, Kurman RJ, Vang R, et al. Tp53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma--evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–26. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen JS, Sahota RA, Milne K, et al. Cd20+ tumor-infiltrating lymphocytes have an atypical cd27- memory phenotype and together with cd8+ t cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 12.Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granot T, Meruelo D. The role of natural killer cells in combinatorial anti-cancer therapy using sindbis viral vectors and irinotecan. Cancer Gene Ther. 2012;19:588–91. doi: 10.1038/cgt.2012.33. [DOI] [PubMed] [Google Scholar]
- 14.Lan C, Huang X, Lin S, et al. Expression of m2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technol Cancer Res Treat. 2013;12:259–67. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. Nkt cells: Facts, functions and fallacies. Immunol Today. 2000;21:573–83. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JP, Guy K, Cosgrove C, Florida-James GD, Simpson RJ. Total lymphocyte cd8 expression is not a reliable marker of cytotoxic t-cell populations in human peripheral blood following an acute bout of high-intensity exercise. Brain Behav Immun. 2008;22:375–80. doi: 10.1016/j.bbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Lipscomb MW, Chen L, Taylor JL, et al. Ectopic t-bet expression licenses dendritic cells for il-12-independent priming of type 1 t cells in vitro. J Immunol. 2009;183:7250–58. doi: 10.4049/jimmunol.0901477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory cd8+ t cell fate coupled by t-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 19.Miyara M, Sakaguchi S. Human foxp3(+)cd4(+) regulatory t cells: Their knowns and unknowns. Immunol Cell Biol. 2011;89:346–51. doi: 10.1038/icb.2010.137. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, et al. Tcr-gamma expression in primary cutaneous t-cell lymphomas. Am J Surg Pathol. 2013;37:375–84. doi: 10.1097/PAS.0b013e318275d1a2. [DOI] [PubMed] [Google Scholar]
- 21.Shaw JL, Fitch P, Cartwright J, et al. Lymphoid and myeloid cell populations in the non-pregnant human fallopian tube and in ectopic pregnancy. J Reprod Immunol. 2011;89:84–91. doi: 10.1016/j.jri.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komori HK, Meehan TF, Havran WL. Epithelial and mucosal gamma delta t cells. Curr Opin Immunol. 2006;18:534–38. doi: 10.1016/j.coi.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Christmas SE, Brew R, Deniz G, Taylor JJ. T-cell receptor heterogeneity of gamma delta t-cell clones from human female reproductive tissues. Immunology. 1993;78:436–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Halary F, Pitard V, Dlubek D, et al. Shared reactivity of v{delta}2(neg) {gamma}{delta} t cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–78. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta t cells and nk cells: Relevance for tumor surveillance. J Immunol. 2006;176:4331–36. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 26.Milligan GN, Dudley-McClain KL, Young CG, Chu CF. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (hsv-2) infection of mice. J Reprod Immunol. 2004;61:115–27. doi: 10.1016/j.jri.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassiliadou N, Bulmer JN. Quantitative analysis of t lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol Reprod. 1996;55:1017–22. doi: 10.1095/biolreprod55.5.1017. [DOI] [PubMed] [Google Scholar]
- 29.Peralbo E, Alonso C, Solana R. Invariant nkt and nkt-like lymphocytes: Two different t cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42:703–08. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 30.O’Reilly V, Zeng SG, Bricard G, et al. Distinct and overlapping effector functions of expanded human cd4+, cd8alpha+ and cd4-cd8alpha- invariant natural killer t cells. PLoS One. 2011;6:e28648. doi: 10.1371/journal.pone.0028648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mselle TF, Meadows SK, Eriksson M, et al. Unique characteristics of nk cells throughout the human female reproductive tract. Clin Immunol. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Corbi AL, Lopez-Rodriguez C. Cd11c integrin gene promoter activity during myeloid differentiation. Leuk Lymphoma. 1997;25:415–25. doi: 10.3109/10428199709039028. [DOI] [PubMed] [Google Scholar]
- 33.Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–64. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoitzner P, Romani N. Langerin, the “catcher in the rye”: An important receptor for pathogens on langerhans cells. Eur J Immunol. 2011;41:2526–29. doi: 10.1002/eji.201141934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hervouet C, Luci C, Rol N, et al. Langerhans cells prime il-17-producing t cells and dampen genital cytotoxic responses following mucosal immunization. J Immunol. 2010;184:4842–51. doi: 10.4049/jimmunol.0901695. [DOI] [PubMed] [Google Scholar]
- 36.Hirbod T, Kaldensjo T, Broliden K. In situ distribution of hiv-binding ccr5 and c-type lectin receptors in the human endocervical mucosa. PLoS One. 2011;6:e25551. doi: 10.1371/journal.pone.0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaldensjo T, Petersson P, Tolf A, Morgan G, Broliden K, Hirbod T. Detection of intraepithelial and stromal langerin and ccr5 positive cells in the human endometrium: Potential targets for hiv infection. PLoS One. 2011;6:e21344. doi: 10.1371/journal.pone.0021344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara H, Ohwada N, Aoki T, Fujimoto T. Langerhans cells in the human oviduct mucosa. Ital J Anat Embryol. 1998;103:253–58. [PubMed] [Google Scholar]
- 39.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–42. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George SH, Milea A, Shaw PA. Proliferation in the normal fte is a hallmark of the follicular phase, not brca mutation status. Clin Cancer Res. 2012;18:6199–207. doi: 10.1158/1078-0432.CCR-12-2155. [DOI] [PubMed] [Google Scholar]
- 41.Lai D, Wang F, Chen Y, et al. Human ovarian cancer stem-like cells can be efficiently killed by gammadelta t lymphocytes. Cancer Immunol Immunother. 2012;61:979–89. doi: 10.1007/s00262-011-1166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]