Abstract
A significant proportion (estimates range from 16–74%) of patients with spinal cord injury (SCI) have concomitant traumatic brain injury (TBI), and the combination often produces difficulties in planning and implementing rehabilitation strategies and drug therapies. For example, many of the drugs used to treat SCI may interfere with cognitive rehabilitation, and conversely drugs that are used to control seizures in TBI patients may undermine locomotor recovery after SCI. The current paper presents an experimental animal model for combined SCI and TBI to help drive mechanistic studies of dual diagnosis. Rats received a unilateral SCI (75 kdyn) at C5 vertebral level, a unilateral TBI (2.0 mm depth, 4.0 m/s velocity impact on the forelimb sensori-motor cortex), or both SCI + TBI. TBI was placed either contralateral or ipsilateral to the SCI. Behavioral recovery was examined using paw placement in a cylinder, grooming, open field locomotion, and the IBB cereal eating test. Over 6 weeks, in the paw placement test, SCI + contralateral TBI produced a profound deficit that failed to recover, but SCI + ipsilateral TBI increased the relative use of the paw on the SCI side. In the grooming test, SCI + contralateral TBI produced worse recovery than either lesion alone even though contralateral TBI alone produced no observable deficit. In the IBB forelimb test, SCI + contralateral TBI revealed a severe deficit that recovered in 3 weeks. For open field locomotion, SCI alone or in combination with TBI resulted in an initial deficit that recovered in 2 weeks. Thus, TBI and SCI affected forelimb function differently depending upon the test, reflecting different neural substrates underlying, for example, exploratory paw placement and stereotyped grooming. Concurrent SCI and TBI had significantly different effects on outcomes and recovery, depending upon laterality of the two lesions. Recovery of function after cervical SCI was retarded by the addition of a moderate TBI in the contralateral hemisphere in all tests, but forepaw placements were relatively increased by an ipsilateral TBI relative to SCI alone, perhaps due to the dual competing injuries influencing the use of both forelimbs. These findings emphasize the complexity of recovery from combined CNS injuries, and the possible role of plasticity and laterality in rehabilitation, and provide a start towards a useful preclinical model for evaluating effective therapies for combine SCI and TBI.
Keywords: behavioral analysis, neuronal plasticity, spinal cord injury, traumatic brain injury, ‘dual diagnosis’
Introduction
The coincidence of spinal cord injury (SCI) and traumatic brain injury (TBI) has long been acknowledged in trauma patients and such co-morbidity can present a significant problem for determining the best approaches to clinical management and rehabilitation (Arzaga, et al. 2003; Bradbury, et al. 2008; Cooper and Ackland 2005; Davidoff, et al. 1985a; 1985b; Davidoff, et al. 1986; Davidoff, et al. 1988; Hagen, et al. 2010; Holly, et al. 2002; Iida, et al. 1999; Macciocchi, et al. 2008; Macciocchi, et al. 2004; Michael, et al. 1989; O’Malley and Ross 1988; Povolny and Kaplan 1993; Richards, et al. 1991; Ricker and Regan 1999; Soicher and Demetriades 1991; Sommer and Witkiewicz 2004; Stambrook, et al. 1991; Tian, et al. 2009; Watanabe, et al. 1999; Wei, et al. 2008). Published incidence rates range from 16% to 74% (Hagen, et al. 2010); and, in a prospective study, 34% of co-occurring TBIs were mild and 26% were severe (Macciocchi, et al. 2008). TBI complications in military personnel in Iraq are associated with a comorbid incidence of SCI (9.8%) (Bell, et al. 2009). There is a need to identify factors that limit functional gains as well as a need to develop specific treatment strategies for patients with SCI and TBI, but few specific “dual diagnosis” standards of care are available at present (Arzaga, et al. 2003; Ricker and Regan 1999; Sommer and Witkiewicz 2004). The complications associated with “dual-diagnosis” such as cognitive or behavioral dysfunction, are well known in the rehabilitation setting (Arzaga, et al. 2003; Ricker and Regan 1999; Sommer and Witkiewicz 2004), but evidence-based approaches for treatment are lacking. In the clinical setting, mild or moderate TBI is sometimes overlooked in SCI patients because the paralysis is so clinically striking (Arzaga, et al. 2003; Ricker and Regan 1999; Sommer and Witkiewicz 2004). In such SCI patients, TBI may first manifest as the inability to learn their rehabilitation protocols or to accomplish functional tasks expected at their level of injury (Arzaga, et al. 2003). These patients may be labeled as having maladaptive psychological reactions or as being non-compliant (Sommer and Witkiewicz 2004). The cognitive or behavioral disturbances consequent to the TBI need to be evaluated and treated as intensively as the SCI deficits. Without positive findings on computed tomography (CT) or magnetic resonance imaging (MRI), the screening of either mild or moderate TBI can be often excluded from further, more accurate diagnostic evaluation (Ricker and Regan 1999). After the injury, the patient’s communication is often compromised by sedation, intubation, or impaired consciousness, and mild or moderate TBI may be misdiagnosed as depression, denial, psychiatric disorders, or intensive care unit psychosis (Ricker and Regan 1999).
Currently the clinical management of patients with both SCI and TBI is highly variable as in the rehabilitation phase, patients are often assigned to a rehabilitation unit specializing in either SCI or TBI depending on which injury appears more severe. Rehabilitation of one injury is not necessarily well integrated with rehabilitation of the other, and may be complicated by insufficient understanding of their interaction. Hence, there is a mounting need to advance our understanding of the mechanistic consequences of combined injury. This is especially true given the apparent importance of cortical plasticity in the recovery of function after partial SCI (Kokotilo, et al. 2009; Nishimura and Isa 2009; 2012). It could be expected that the addition of cortical and other forebrain injury to a SCI might retard recovery by reducing the capacity for cortical plasticity. On the other hand, some studies of unilateral cortical damage, which often occurs in the clinical setting, have suggested that such damage can actually enhance forelimb function by promoting plasticity in the contralateral cortex (Allred, et al. 2010; Jones, et al. 2009; Jones, et al. 1996; Jones, et al. 2012).
There is at present no preclinical model of combined SCI and TBI to enhance our mechanistic understanding of the interactions between these comorbidities. Thus, the biological and behavioral effects invoked by concurrent mild, mild-complicated, or moderate TBI in the outcome of SCI are largely unknown. This argues for: 1) capturing more information on concurrent SCI and TBI in clinical practice and in clinical trials, and 2) using this clinical information to guide the establishment of reliable and useful animal models of dual diagnosis. We have formed a translational partnership between basic scientists and clinicians to develop the first animal model of combined brain and spinal cord injury. In this first iteration of that model, we used unilateral contusion lesions of the cervical cord and the somatomotor cortical surface to examine these interactions. Rats were given controlled cortical contusion injury followed immediately by a unilateral cervical spinal cord contusion injury using a balanced experimental design (Fig. 1). We monitored forelimb behavioral recovery using standardized scales for 6 weeks, and terminal histopathology was characterized. The data revealed novel interacting features of TBI and SCI that emphasize the complexity of these interactions, including a dissociation of apparent retardation and enhancement of recovery from SCI depending upon the laterality of the TBI. This model can now be experimentally probed using clinically relevant therapeutics.
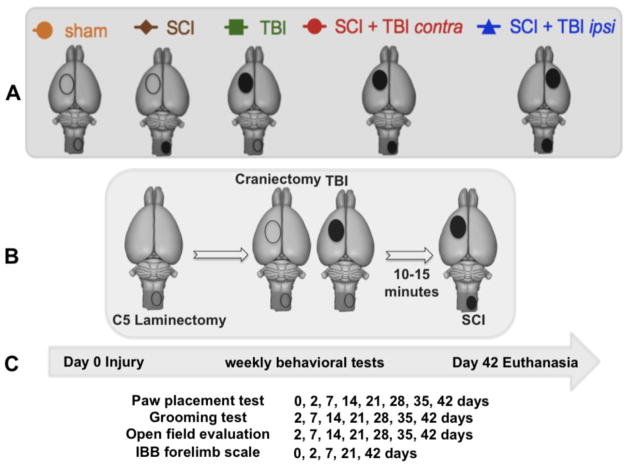
FIG. 1.
(A) Injury groups: sham (craniectomy + laminectomy) (n = 9); spinal cord inury (SCI) + craniectomy (n =10), traumatic brain injury (TBI) + laminectomy (n = 10), SCI + contralateral TBI (n = 10), SCI + ipsilateral TBI (n=10). (B) Sequence of surgical procedures for producing the combined SCI + TBI model. (C) Behavioral testing was done 2, 7, 14, 21, 28, 35 and 42 days after the injury, and then rats were euthanized.
Materials and Methods
For the purposes of the current paper the term ipsilateral refers to the side ipsilateral to the SCI (right side of the animal) and contralateral refers to the side contralateral to the SCI (the left side of the animal).
Animals
Female Long-Evans hooded rats (Simonsen Laboratories, Gilroy, CA, USA) with a mean age of 77 days (range; 75–80) and mean weight of 230 g (range: 198–257) were used in this study. Rats were housed individually in plastic cages, maintained on a 12 hour light/dark cycle, and had access to food and water ad libitum. All animal experiments were approved by the Institutional Laboratory Animal Care and Use Committee of the University of California at San Francisco and were performed in compliance with NIH guidelines and recommendations. Surgical procedures were carried out aseptically under deep anesthesia induced and maintained by inhalation of isoflurane (IsoFlow, Abbott Laboratories, North Chicago, IL, USA; 2–3%), and anesthetic plane was monitored using withdrawal to foot pinch. Animals were administered cefazolin (Ancef, Novation, LCC, Irving, TX) 25 mg/kg, prior to surgery and for 3 days postoperatively. Lacrilube ophthalmic ointment (Allergan Pharmaceuticals, Irvine, CA, USA) was applied to the eyes prior to surgery and body temperature was monitored using a rectal thermal probe and maintained at 37.5 ± 0.5°C using a heating pad.
Combined injury models
The following experimental groups (see Fig. 1) were compared: sham (craniectomy + laminectomy; n = 9), SCI + craniectomy (n =10), TBI + laminectomy (n = 10), SCI + contralateral TBI (n = 10), SCI + ipsilateral TBI (n=10). Group comparisons of both initial deficit and long term recovery were made. During surgery, a spinal laminectomy was made first, then a craniectomy, followed by a TBI for those groups with TBI, and then a SCI for those groups with SCI. Due to procedural limitations on transferring animals between the two injury devices, SCI was performed approximately 10+ minutes after TBI in the dual injury groups: SCI + contralateral TBI and SCI + ipsilateral TBI (Fig. 1B).
Traumatic Brain Injury (Controlled cortical impact (CCI)) injury model
We used a well-validated controlled TBI device that has been described in detail elsewhere (Dennis, et al. 2009; Igarashi, et al. 2007; Lu, et al. 2011). Briefly, the rats were mounted in a Kopf stereotaxic frame under isoflurane anesthesia. A unilateral craniectomy (6.0 mm diameter) was produced in the skull between 3.0 mm posterior and 3.0 mm anterior to bregma and between 1.0 and 7.0 mm lateral to bregma using a high-speed drill. CCI was produced using the electric CCI device with a 5.0 mm diameter impactor with a convex tip (Custom Design & Fabrication, Inc., Sandston, VA), which perpendicularly compressed the curvature of the sensori-motor cortex to a depth of 2.0 mm at 4.0 m/s velocity with a dwell time of 150 msec (Igarashi, et al. 2007). The CCI machine was switched on when the top of the convex tip touched the pia. Contusion is one of the most common manifestations of head trauma in humans (Strich 1970), making CCI a valuable and clinically relevant model of TBI. Heart rate and blood oxygenation was monitored with a Mouse Ox™ pulse-oximeter (Torrington, Connecticut); temperature was monitored and maintained at 37.5 °C. The injury sites were closed and the animals were recovered in Thermocare®, Intensive Care Unit with Dome Cover (Thermocare, Inclined Village, NV).
Spinal cord injury model
We used the Infinite Horizon (IH) SCI device (Infinite Horizons, Inc., Lexington KY), which had a special impactor tip that was 2 mm in diameter (Scheff, et al. 2003). Briefly a dorsal midline incision was made, the skin was dissected from underlying fascia, and the trapezius muscle was cut just lateral to the midline from C1/C2 to T2. Underlying muscle layers were blunt-dissected to expose the spinous processes from C3 to T1. A small animal retraction system (Fine Science Tools Inc., North Vancouver, BC, Canada) was used to hold muscle layers apart. A C5, dorsal laminectomy was performed to expose the entire right side and most of the left side of the spinal cord, leaving the dura matter intact. Rats were then secured in place with vertebral clamps at C4 and C6. The impactor rod was centered over the right side of the C5 laminectomy site with the medial edge of the 2 mm impounder aligned on midline to induce a unilateral injury similar to our earlier NYU/MASCIS device unilateral cervical contusion injuries (Gensel, et al. 2006; Gruner 1992; Mihai, et al. 2008; Nout, et al. 2009). Peak force was pre-set at 75 kdyn.
Behavioral Testing
Behavioral testing was done by two observers blind to the lesion conditions, and rats were videotaped before surgery and at 2, 7, 14, 21, 28, 35 and 42 days after the injury (Fig. 1C). Motor performance was measured using the paw preference test (Dunham, et al. 2010; Gensel, et al. 2006; Schallert, et al. 2000), the grooming test (Bertelli and Mira 1993; Gensel, et al. 2006), locomotion in an open field (Martinez, et al. 2009), and the Irvine, Beatties, Bresnahan (IBB) cereal eating test (Irvine, et al. 2010). All behavioral analyses were conducted by personnel blind to experimental group.
Paw preference test
Animals were placed in a clear plastic cylinder with two mirrors placed at angles such that both sides of the rat were clearly visible. The rats were recorded with a digital camera for 3 min, and slow motion high definition (HD) playback was used to determine the number of the times the animal placed its left, right, or both forepaws against the side of the cylinder during weight supported movements. Individual placements were scored as either “left” or “right” when 0.5 sec or more passed without the other limb contacting the side of the cylinder. If both forepaws were used for weight-supported movements within 0.5 sec of each other, a score of “both” was given. During lateral exploration, a “both” score was given for every two-step “walking” sequence, during which, both paws changed position on the side of the cylinder. If one paw remained anchored while the other was placed on different parts of the cylinder, no score was given until the anchored forepaw was lifted. Scoring was performed using video playback by trained raters who were blind to experimental condition. Animals were tested before surgery and on day 2, 7, 14, 21, 28, 35 and 42 days after SCI.
Grooming test
This test was originally developed to examine recovery in a rat brachial plexus reconstruction model (Bertelli and Mira 1993) and was adapted for cervical SCI by Gensel et al. (2006). Animals were placed in a clear plastic cylinder with two mirrors placed at angles such that the rat’s head was always clearly visible. Cool tap water was applied to the animal’s head and back with soft gauze prior to placing the animal in the cylinder, and the animal was recorded with a digital camera for 3 min. Slow motion HD playback was used to score each forelimb independently to identify the maximal contact made while performing any part of the grooming sequence. A six-point scoring system was used in which 0 indicates the animal is unable to contact any part of the face or head; 1 indicates the animal’s forepaw makes contact with the underside of the chin and/or mouth area; 2 indicates the animal’s forepaw contacts the area between nose and eyes, but not the eyes; 3 indicates the animal’s forepaw contacts the eyes and the area up to, but not including, the front of the ears; 4 indicates the animal’s forepaw contacts the ears, but not the area of the head behind the ears; and 5 indicates the animal’s forepaw contacts the area of the head behind the ears. Animals were tested at 2, 7, 14, 21, 28, 35 and 42 days after injury.
Irvine, Beatties, Bresnahan (IBB) forelimb rating scale
Skilled forelimb function was assessed using an updated version of the IBB cereal eating test as described in Irvine et al (2010). Briefly, rats were individually placed in their home cages and given spherical- and doughnut- shaped pieces of cereal that were of a consistent size and shape prior to the initiation of eating; rats were not scored when eating cereal pieces that were broken prior to testing initiation. Each trial was recorded to allow slow motion HD playback and evaluation of paw use. Videos of animals eating the cereal were evaluated using standardized scoring of common forelimb behaviors (including joint position, object support, digit movement and grasping technique) used while consuming both cereal shapes. An IBB score was assigned using the 10 point (0–9) ordinal scale for each shape, and the highest score i.e. the one reflecting the greatest amount of forelimb recovery, was assigned.
Open field locomotor test
The forelimb and hindlimb locomotor deficits were assessed during spontaneous locomotion using an open-field scoring derived from the Basso, Beattie and Bresnahan (BBB) scale (Basso, et al. 1996) and designed for an accurate evaluation of the behavioral consequences of cervical SCI (Martinez, et al. 2009). Rats were tested in pairs for a 4 min period in a circular plexiglas arena (95 cm diameter, 40 cm wall height) with an anti-skid floor. Open field locomotor function was assessed by two examiners, who consulted with one another to complete a scoring grid that gave a forelimb and hindlimb functional score to each animal in each session. Behavioral deficits affecting the limbs ipsilateral to the cervical SCI were categorized by evaluating the articular movement amplitude, weight support, fine distal positioning and stepping abilities. The scaling grid yielded final scores (maximum grade, 20) for the affected forelimb and hindlimb.
Histopathological Analysis
At 42 days after surgery, animals were anesthetized with xylazine (TranquiVedTM, Vedco Inc., St. Joseph, MO; 10 mg/kg IP) and ketamine (ketamine HCl, Abbott Laboratories, N. Chicago, IL; 80 mg/kg IP), and transcardially perfused with 0.9% NaCl and 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains and spinal cords were postfixed in 4% paraformaldehyde for 2h and then cryoprotected in 30% sucrose in PBS for 48–72 hours (until the tissue sank). The tissue was frozen at 80°C until further analysis. The brains were sectioned transversely on a cryostat at 30 μm, and spinal cords at 20 μm. Sections were stained with hematoxylin and eosin. The remaining spared area of the brain at the epicenter, and at 2.5 mm anterior and 2.5 mm posterior from the center were calculated as a proportion of the ipsilateral brain at the each section. For the spinal cord, the remaining spared areas were measured as a proportion of the ipsilateral hemi-cord areas at the lesion epicenter. Areas of tissue damage were determined by the presence of large cystic cavities, aggregates of microcysts in the white matter, and dense gliosis. Motor neuron loss was assessed by counting large ventral horn motor neurons in sections 120 μm apart through 1.2 mm of cord centered on the lesion epicenter (10 sections per animal). All cells in the ventral horn ranging from 30 to 70 μm in diameter with a discernable nucleolus were counted.
Statistical Analysis
Quantitative behavioral data and histopathological measurements are reported as mean +/− standard error (SE) for each injury group. Factorial repeated measures analysis of variance (ANOVA) was used to analyze all behavioral data. For analyses that compared ipsilateral and contralateral limbs in the same animal, both time and limb were treated as repeated measures. The null hypothesis was rejected at α = 0.05. Significant differences identified by the ANOVA were isolated using the Tukey’s procedure for pairwise multiple comparison post-hoc test on group means in accordance with highly-cited best-practices from the statistical literature, (Keppel and Wickens 2004). Spearman correlation was used to assess the relationship between behavior and histology. All statistics were performed with SPSS v.19 (IBM).
Upon publication of these primary data, all data will be entered into an NIH-supported centralized preclinical spinal cord injury database repository for later inclusion in advanced multivariate meta-analytic studies by the SCI research community (Ferguson, et al. 2013).
Results
General Health
All animals survived the full 42 days duration of the study. Subjects showed no obvious respiratory distress or bladder dysfunction at any time post injury and easily accessed water and food. There was a slight drop in body weight after surgery but this recovered in all groups by post-op day 14, and no animals dropped below 90% of pre-operative body weight during the post-operative period. No differences between groups were observed.
Behavioral Results
All behavioral results are depicted over time (Figs. 2–4), and the full set of behavioral outcomes are shown as collapsed means in Fig. 4.
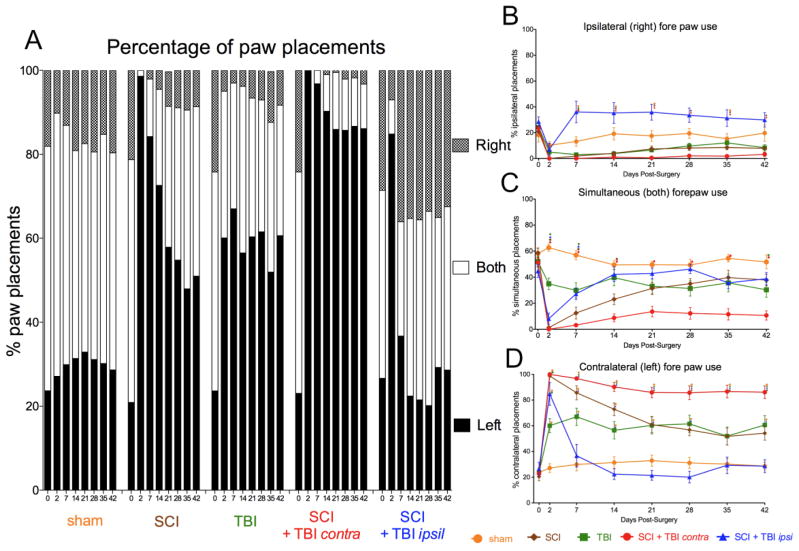
FIG. 2.
Paw placements in the cylinder. (A) The percentage of total paw placements (left, right, or both) is shown for each group. (B) The proportion of total paw placements made by the ipsilateral (right) paw is shown. (C) The proportion of total paw placements made simultaneously by both paws is shown. (D) The proportion of total paw placements made by the contralateral (left) paw is shown. (A–D) The performance of sham rats did not significantly differ from pre-injury at any time post-operatively. SCI alone rats showed a profound deficit that recovered to the level of TBI alone rats over 42 days weeks. TBI produced a stable deficit in paw placement that did not recover over 6 weeks. SCI + contralateral TBI produced a profound deficit that failed to recover over 42 days, showing an almost complete preference for the limb contralateral to the SCI. SCI + ipsilateral TBI rats initially did not use the right forepaw (2 days after the injury) but they then significantly increased right limb use (i.e. ipsilateral to the SCI). (B) SCI + ipsilateral TBI significantly increased ipsilateral forepaw use compared to SCI + contralateral TBI rats 7 days after surgery. (C) SCI + contralateral TBI significantly reduced simultaneous forepaw use compared to sham. (D) SCI + contralateral TBI significantly enhanced contralateral forepaw use compared to both sham and SCI + ipsilateral TBI rats 7 days after surgery. SCI: spinal cord injury, TBI: traumatic brain injury, ○: significant difference compared to sham group, ◇ :significant difference compared to SCI group, □ :significant difference compared to TBI group, ▲ :significant difference compared to SCI + ipsilateral TBI group
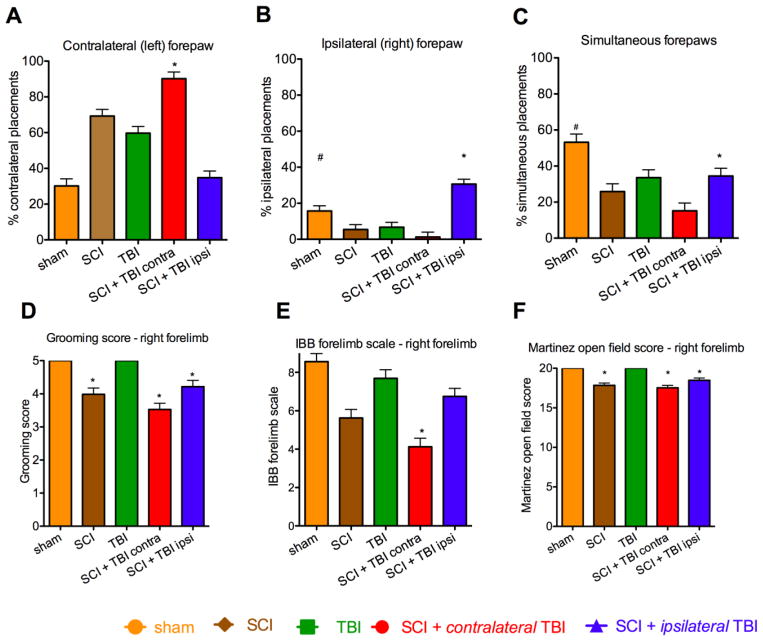
FIG. 4.
Performance over all days. (A) Contralateral (left) forepaw placements: The SCI + contralateral TBI group used their contralateral (left) forepaw more than other injury groups (p* < 0.05). (B) Ipsilateral (right) forepaw placements: SCI + ipsilateral TBI rats used their right forepaw more as compared to the other injury groups (p* < 0.05). The Sham group used the ipsilateral forepaw more than SCI alone, TBI alone, and SCI + contralateral TBI (p# < 0.05). (C) Simultaneous (both) paw placements: Shams used simultaneous forepaw placements more than the injury groups (p#<0.05). SCI + ipsilateral TBI group used simultaneous forepaw placement more than SCI + contralateral TBI group (p*<0.05). (D) Grooming test: Sham and TBI alone groups had better grooming scores than groups with SCI (p* < 0.05). (E) Irvine, Beatties, Bresnahan (IBB) forelimb scale: The SCI + contralateral TBI group had worse performance than other injury groups (p* < 0.05) (F) Open field forelimb locomotor test: Sham and TBI alone groups performed better than SCI alone, SCI + contralateral TBI, and SCI + ipsilateral TBI groups (p* < 0.05). SCI: spinal cord injury, TBI: traumatic brain injury. Error bar shows standard error of the mean.
Paw placement test
The results on paw placement in the cylinder are shown in Fig. 2, and are reported as a percentage of either ipsilateral (right), contralateral (left), or simultaneous versus total paw placements. Some animals showed slight postural instability, but this did not inhibit their ability to explore the cylinder. Pre-operatively, all animals used both paws simultaneously for the majority of weight supported wall movements in the cylinder (percentage of simultaneous vs. total placements 53.1±1.7 %; mean±standard error of the mean (SEM)), and there was no significant overall preference for the use of either limb independently (left vs. total placements = 23.4±1.4%; right vs. total 23.5±1.5%; mean±SEM).
The effects of SCI, TBI and the combination of these injuries is shown in Figure 2. Simultaneous paw placements are shown in Fig. 2C, whereas independent ipsilateral (i.e. ipsilateral to the SCI, hence right) and contralateral (left) placement proportions are shown in Fig. 2B and D respectively. As can be seen in Fig. 2A, the patterns of recovery of paw use following the various lesions differed between groups. Use of the paw contralateral to the SCI (i.e. the left paw, solid bars in Fig. 2A) increased after SCI alone, TBI alone and especially after SCI + contralateral TBI. This additive effect is also evident in Fig 2D; compare SCI alone (brown line) to TBI alone (green line) to SCI + contralateral TBI (red line). Use of the paw ipsilateral to the SCI was almost abolished by the combined lesion (Fig. 2A gray and white, SCI + contralateral TBI; Fig. 2B, red line). Interestingly, adding an ipsilateral TBI to the SCI, reduced use of the left paw to near normal by week 1 following injury (Fig. 2A, black bars; Fig. 2D: compare the blue [SCI + ipsilateral TBI] and yellow [Sham] lines). And, independent and simultaneous use of the paw ipsilateral to the SCI (i.e. right forepaw) returned to a more equal balance with the left forepaw after injury (Fig. 2A, gray and white bars respectively; Fig. 2B blue line).
Statistical analysis of the scored results revealed a differential pattern of effects on the ipsilateral and contralateral limb, confirmed by 3-way, double repeated measures ANOVA (effect of injury condition with limb and time as repeated measures), F = 6.08, p < 0.001. Follow-up, 2-way repeated ANOVAs were performed for each limb separately. For the contralateral (left) paw placement data (Fig. 2D), a mixed repeated measures ANOVA revealed a significant main effect of injury condition, F = 43.29, p< 0.0001. In addition there was a significant effect of time and a time by injury condition interaction, all F = 7.39, all p < 0.0001. A posthoc Tukey’s on the group means revealed that SCI + contralateral TBI was higher than either single injury group (p < 0.05; Fig. 4A). For the ipsilateral (right) paw placement data (Fig. 2B), mixed repeated measures ANOVA revealed a significant main effect of injury condition, F=18.52, p< 0.0001. In addition there was a significant effect of time and a time by injury condition interaction, both F > 2.92, p < 0.0001. A Tukey’s post hoc test on the group means revealed that SCI + ipsilateral TBI had less lateralization of function than the other injuries (p < 0.05; Fig. 4B). Analysis of the raw response numbers demonstrated that the SCI + ipsilateral TBI had a significantly lower overall number of responses than SCI alone, p < .05; no other group differences were significant, p > .05 (Supplementary Fig. 1). To test the extent to which the change in overall response number influenced the lateralization results we used analysis of covariance (ANCOVA) to correct for total response number. Response number was not a significant covariate for either right or left paw preference, both p > .05, indicating that response number did not significantly influence the results (Supplementary Fig. 2). Together, the statistical results indicated that both SCI and contralateral TBI produced a lateralization of paw preference that was additive in the combined injury condition. However, subjects with SCI + ipsilateral TBI had a more balanced ipsilateral and contralateral forelimb use.
Grooming test
All animals showed normal grooming with the contralateral (left) forelimb at all time-points post-injury (data not shown). TBI alone did not affect grooming performance (Fig. 3A, green line), but rats with SCI (either SCI alone or combined SCI and TBI) showed significant deficits in grooming (Fig. 3A, brown, red and blue lines). Two days after injury, the majority of animals with SCI were only able to touch the bottom of the snout (score of 1), but improved from day 2 to 7 post-injury (SCI alone, 4.2±0.28, SCI + contralateral TBI, 3.6±0.30, SCI + ipsilateral TBI; 4.1±0.23; mean±SEM). By twenty-one days after the injury, only the SCI + contralateral TBI group exhibited sustained deficits in the ability to groom (SCI + contralateral TBI, 4.0±0.33; mean±SEM).
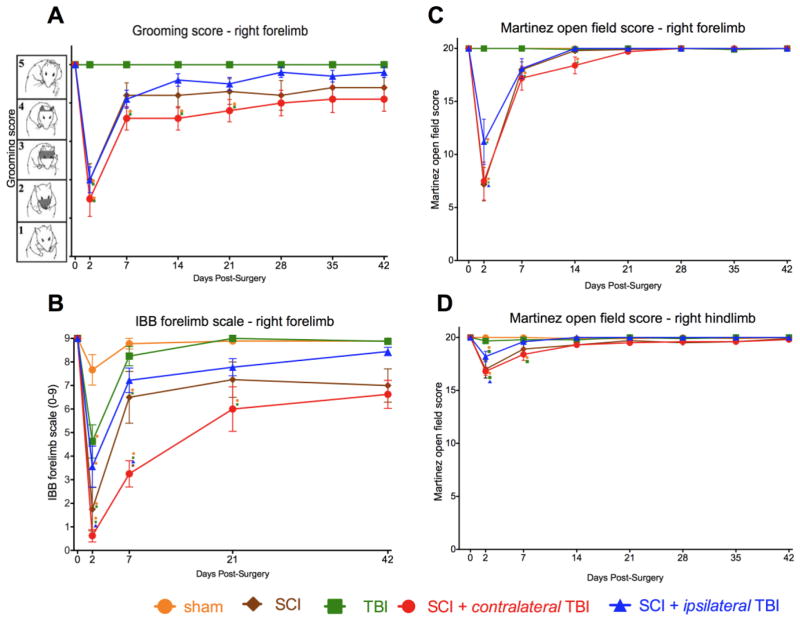
FIG. 3.
Grooming, paw use (IBB) and open field locomotor performance over time after injury. (A) Both sham lesions and TBI alone did not affect grooming, but rats with SCI (SCI alone and combined SCI and TBI) produced deficits in grooming. Two days after injury, the majority of animals with SCI were only able to touch the bottom of the snout (score of 1), but they remarkably improved from 2 days to 7 days post-injury. Twenty-one days after the injury, only SCI + contralateral TBI rats demonstrated significantly lower grooming scores than sham and TBI only groups (score of 4). Forty-two days after injury, no statistically significant differences between groups were evident. (B) Irvine Beatties Bresnahan (IBB) forelimb scale. (Irvine, et al. 2010). Before the injury, the rats could eat any type of cereal without any deficit in forelimb function (score of 9); but 2 days after the injury, rats with both SCI and TBI showed a significant decrease in ipsilateral (right) forepaw function. Seven days after the injury, only the SCI and SCI + contralateral TBI rats showed deficits in forelimb function. (C) Open field forelimb locomotor test using the Martinez score (Martinez, et al. 2009). Neither sham nor TBI alone affected forelimb locomotor scores, but rats with SCI (SCI alone and combined SCI and TBI) exhibited deficits in locomotor function. Rats with SCI showed severe impairments of forelimb movements and postural abilities at 2 days post-injury but rapidly recovered within the first 7 days. SCI + contralateral TBI rats recovered more slowly than SCI alone and SCI + ipsilateral TBI rats, but finally reached a similar level of motor skills. (D) Openfield hindlimb locomotor test. During the first 7 days after the injury, rats with SCI showed hindlimb deficits, which were mainly characterized by poor stepping. SCI: spinal cord injury, TBI: traumatic brain injury, ○: significant difference compared to sham group, □ :significant difference compared to TBI group, ▲ :significant difference compared to SCI + ipsilateral TBI group
Statistical analysis revealed a differential effect of TBI and SCI on grooming. A 2-way mixed repeated measures ANOVA revealed a significant main effect of injury condition, F=11.40, p< 0.0001. In addition there was a significant effect of time and a time by injury condition interaction, both F > 9.77, p < 0.0001. A posthoc Tukey’s performed on the group means revealed that Sham and TBI alone were better than SCI alone, SCI + contralateral TBI, and SCI + ipsilateral TBI groups (p < 0.05; Fig. 4D), but the groups with SCI did not differ from each other. The results confirm that SCI, but not TBI produced a deficit on the grooming task.
Irvine Beatties Bresnahan (IBB) forelimb scale
The IBB cereal eating test was employed to evaluate injury effects on skilled forelimb function and digital control (Irvine, et al. 2010). After injury while eating either the doughnut or spherical shaped cereals, the impaired forelimb was engaged for food manipulation. At 2 days after the injury, both SCI and TBI alone produced a significant impairment in ipsilateral (right) forepaw function compared to sham injuries (Fig. 3B).
Statistical analysis confirmed these effects. A 2-way mixed repeated measures ANOVA revealed a significant main effect of injury condition, F=15.74, p< 0.0001. In addition there was a significant effect of time and a time by injury condition interaction, all F = 6.90, all p < 0.0001. A posthoc Tukey’s test performed on the group means revealed that SCI + contralateral TBI was significantly worse than all groups (p < 0.05), but except for SCI alone (p = .14) (Fig. 4E). To test for transient deficits, we performed further post-hoc testing of early time-points. We found that rats in the SCI + contralateral TBI group had significantly poorer ipsilateral forepaw use than the SCI + ipsilateral TBI group on 2 and 7 days after injury. The SCI alone group fell consistently between the SCI + contralateral TBI and SCI + ipsilateral TBI groups, but only differed significantly from the SCI + contralateral TBI group on 7 days after injury (Fig. 3B).
Forelimb and hindlimb open field locomotor tests
To evaluate forelimb and hindlimb locomotor function we used the scaling system described by Martinez et al. (2009). TBI alone affected neither forelimb nor hindlimb locomotor tests, but rats with SCI (SCI alone and combined SCI and TBI) had locomotor deficits. Qualitative patterns were as follows:
1. Forelimb function recovery
Rats with SCI showed severe impairments of forelimb movements and postural abilities at 2 days post-injury (Fig. 3C). The injuries lead to a flaccid paralysis of the forelimb, characterized by a lack of movements of the distal joints and restricted movements of the proximal ones. These impairments resulted in a lack of postural support. Rats with SCI alone exhibited the most prominent and rapid recovery of the affected forelimb locomotor abilities within the first 7 days. SCI + contralateral TBI rats recovered more slowly than SCI alone and SCI + ipsilateral TBI rats, but all recovered.
Statistical analysis confirmed these results. A 2-way mixed repeated measures ANOVA revealed a significant main effect of injury condition, F = 16.44, p< 0.0001. In addition there was a significant effect of time and a time by injury condition interaction, both F > 14.74, all p < 0.0001. Posthoc Tukey’s test of the group means revealed that Sham and TBI alone groups performed better than SCI alone, SCI + contralateral TBI, and SCI + ipsilateral TBI groups (p < 0.05; Fig. 4F).
2. Hindlimb function recovery
During the first 7 days after the injury, rats with SCI showed moderate hindlimb deficits, which were mainly characterized by poor stepping (Fig. 3D). Rats with SCI alone (19.1±0.88, Martinez scale) and SCI + contralateral TBI (19.0±0.38. Martinez scale) showed a similar pattern of loss and recovery. SCI + ipsilateral TBI produced significantly better recovery than SCI + contralateral TBI rats within the first 7 days (p<0.05), and also recovered slightly but not significantly better than SCI alone (p>0.05). A 2-way mixed repeated measures ANOVA revealed a significant main effect of injury condition, F=8.43, p< 0.0001. In addition there was a significant effect of time and a time by injury interaction, both F > 4.76, all p<0.0001. A posthoc Tukey’s test on the group means revealed that the SCI + contralateral TBI group were worse than Sham, TBI alone, and SCI + ipsilateral TBI groups (p < 0.05). The SCI alone was worse than TBI alone and sham groups (p < .05).
Histological outcomes
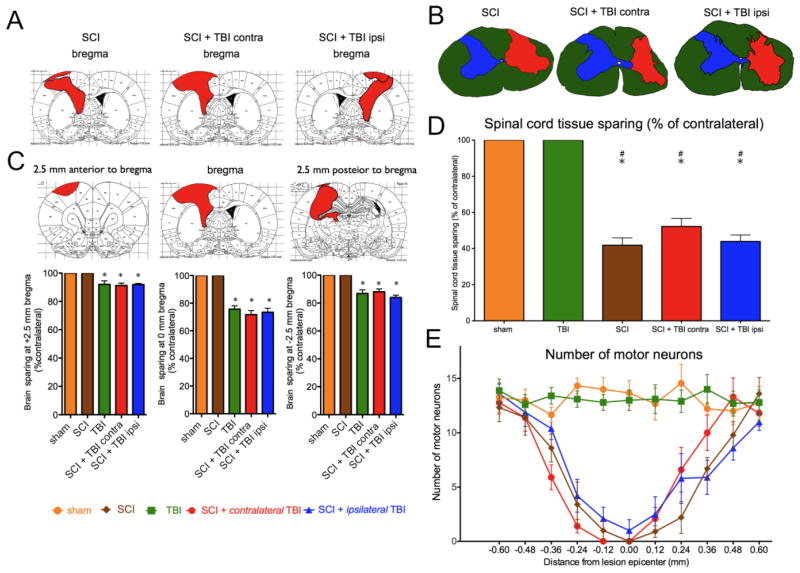
Spinal cord and brain lesion extents are depicted for the median size of the lesions with TBI (Fig. 5A) and the groups with SCI (Fig. 5B). There were no statistically significant differences in the size or location of the lesions between injury groups. Statistical analysis of the TBI histology at lesion epicenter using oneway ANOVA and Tukey’s posthoc tests revealed that the TBI alone, SCI + ipsilateral TBI and SCI + contralateral TBI+ groups had lower brain sparing than either SCI or sham groups, F = 44.75, p < .0001. The size of the TBI lesions did not differ between groups with TBI, all p > .05 (Fig. 5C). Analysis of spinal cord sparing using oneway ANOVA and Tukey’s posthoc tests showed a significantly lower sparing in SCI alone, SCI + ipsilateral TBI, and SCI + contralateral TBI relative to TBI alone and sham, F = 44.03, p < .0001. However there were no significant differences between the groups with SCI, all p > .05 (Fig. 5D). Analysis of motor neuron number was performed by 2-way repeated measures ANOVA (distance from epicenter as a repeated measure). Motor neuron counts throughout the extent of the lesion are shown in Fig. 5E. Groups with SCI showed also showed significant effect of group by distance on motor neuron loss, relative to Sham and TBI groups, F = 6.27, p < .0001 (Fig. 5E; SCI groups vs. sham, p<0.05; SCI groups vs. TBI, p<0.05).
FIG. 5.
Both brain (A) and spinal cord (B) lesions were not significantly different in size or location between groups. The median lesions are shown for each group (A, B), and for each location.. (C). The median tissue sparing at 2.5 mm anterior to bregma, at bregma, and 2.5 mm posterior to bregma is shown below (*p<0.05 vs. Sham or SCI alone). (D) Spinal cord sparing at the epicenter of the lesion is shown (#p<0.05 vs. sham; *p<0.05 vs. TBI). (E) Motor neuron counts throughout the extent of the lesion show no significant differences between injury groups. SCI: spinal cord injury, TBI: traumatic brain injury.
Behavior and histology
To evaluate the relationship between behavior and histology we used Spearman correlations (Table 1). Brain lesion sparing significantly predicted performance on paw preference but not other tasks. Left-sided brain sparing was inversely correlated with left limb use and positively correlated with right limb use. The converse pattern was observed for right-sided brain sparing. In contrast, the right-sided spinal cord injury lesion size did not generate systematic lateralization in the full combined injury dataset correlating with the number of bilateral paw placements but not the left and right limbs individually. It should be noted that spinal cord lesion size correlated with other bilateral outcome tasks, including grooming, IBB, forelimb openfield, and hindlimb openfield performance.
Table 1.
Spearman correlation (rs) between behavior test and tissue sparing
| Tissue Sparing at Lesion Epicenter
|
|||
|---|---|---|---|
| Spinal Cord | Brain | ||
|
| |||
| Right | Left | Right | |
|
| |||
| rs | rs | rs | |
| Paw Preference, Left | −0.31** | −0.56 *** | 0.41 * |
| Paw Preference, Right | - | 0.46 ** | −0.61 *** |
| Paw Preference, Both | 0.43 ** | 0.34 * | - |
| Grooming | 0.85 *** | - | - |
| Cereal | 0.54 *** | - | - |
| Forelimb Openfield | 0.84 *** | - | - |
| Hindlimb Openfield | 0.60 *** | - | - |
- : not significant
p < .01
p< .001
p < .0001
Of the functional measures taken, only the total number of bilateral placements correlated with both brain and spinal cord sparing. In particular left-sided brain sparing and right-sided spinal cord sparing both positively correlated with bilateral paw placement. Together, the correlational analysis reinforces the concept that in combined SCI + TBI, an activated left hemisphere helps compensate laterality in paw preference that is produced by an intact left hemisphere.
DISCUSSION
In light of the clinical evidence that SCI is often accompanied by TBI ‘dual diagnosis’ (Macciocchi, et al. 2008), the principle purpose of the present study was to begin the development of a rat preclinical model of combined TBI and SCI. For practical reasons as well as experimental design concerns, we chose to employ a well-characterized unilateral cervical contusion injury (Gensel, et al. 2006) and a controlled cortical impact injury (Igarashi, et al. 2007). We were able to determine the effects of individual and combined injuries on a variety of neurological outcome measures focused on forelimb function. In addition, this paradigm revealed a laterality of TBI effect that we suggest reflects underlying dynamic mechanisms of neurological dysfunction that have important implications for repair and rehabilitation after CNS injuries.
Effects of SCI or TBI alone
SCI and TBI alone each produced significant changes in paw placement preference, as predicted from prior work (Gensel, et al. 2006; Schallert, et al. 2000). As expected, this occurred ipsilateral to the right-sided SCI only lesion, and contralateral to the CCI-only lesion. The moderate SCIs used in this study produced a profound initial lack of use of the ipsilateral (right) forepaw in the cylinder, but this recovered substantially over the 6 weeks of the post-injury period. The CCI-TBI injury produced somewhat of a lesser decline in paw placement by the right (contralateral to the lesion), but this decline was permanent over the post-operative course. The grooming test showed quite different results. As predicted by prior work (Gensel, et al. 2006), the 75 kdyne IH unilateral cervical injury produced a moderate deficit in the ability of the rats to use their right (ipsilateral) forelimb to reach the back of the head during grooming, and this ability recovered somewhat over the 6 weeks after SCI. CCI-TBI on the contralateral (left) side, which damages cortical and other projections to the spinal cord controlling the right (ipsilateral) forelimb, produced essentially no deficit at all in right sided (or left sided) grooming. Thus, performance on the grooming test must reflect abilities mediated by the circuits damaged by the cervical SCI, but not by those damaged by the CCI-TBI. The IBB cereal test was sensitive to both injuries, and recovered partially after moderate SCI alone, and almost completely after TBI alone. The open field locomotor test was only modestly and transiently affected by either lesion alone. The differences in sensitivity of these outcome measures to SCI vs. TBI, and the differences in degree and rate of recovery, no doubt reflect differences in the neural substrates underlying these behaviors and their recoveries after CNS injury.
Based on substantial evidence for an important role of the forebrain and cortex in mediating plasticity and recovery of function after SCI, we reasoned that a concurrent TBI using the cortical contusion injury method would have deleterious effects on neurological outcome and recovery when compared to SCI alone. In rats receiving a TBI contralateral to the SCI, this rather obvious prediction was strongly supported by the data, although the degree of interaction between TBI and SCI varied depending upon the measure used. A contralateral (left) TBI given at the same time as the right-sided SCI produced a profound reduction in ipsilateral paw placement, with almost all paw placements in the cylinder being made with only the left paw. The combination lesion resulted in a sustained and more significant deficit than either SCI or TBI alone. This is not surprising given the evidence that SCI and CCI produce paw placement deficits by somewhat different mechanisms (i.e. ‘lower motor’ spinal deficits and ‘cortical sensorimotor neglect’) (Schallert and Woodlee 2003). In addition, plasticity in cortical/forebrain mechanisms is likely to be involved in mediating recovery of function. While contralateral (left) CCI-TBI injuries alone had no effect on the grooming response, they appeared to retard the time course and degree of recovery from a right-sided SCI (Fig. 4). Similarly, the combined SCI plus a contralateral CCI-TBI produced an enhanced initial deficit and retarded recovery on the IBB cereal eating measure, and on the initial recovery of the Martinez open-field score. Thus, the addition of a contralateral CCI-TBI appeared to be additive to the SCI-produced deficits for each of the forelimb tests used, but perhaps with different degrees of effects. This all provides additional evidence suggesting that contralateral cortical/forebrain plasticity is involved in recovery of forelimb function after unilateral cervical SCI. Note that there is also evidence for a role of the spared, ipsilateral cortex in recovery after unilateral cervical SCI (Rosenzweig, et al. 2010; Strong, et al. 2009).
The initial rationale for including a group with an ipsilateral (right sided) CCI-TBI was to provide a CNS injury control that would evaluate the effects of loss of forebrain-cortical systems not directly involved in the mediation of the movements used to measure recovery. However, examination of the effects of the ipsilateral CCI-TBI on the paw placement measure reveals effects that suggest interactions between the two sides of the brain after unilateral spinal cord or brain injuries. SCI alone results in a nearly complete loss of use of the ipsilateral right paw on day 2, and a recovery on day 7 to less than 20% (combining the independent and ‘both’ columns, Fig. 2A). Adding a contralateral CCI-TBI makes this even worse, with almost no recovery over 6 weeks. However, adding a CCI-TBI to the ipsilateral brain results in less of a deficit at 2 days, and a return to a more normal balanced proportion of ipsilateral and contralateral forepaw use by 7 days, continuing until 6 weeks. Thus, the ipsilateral CCI-TBI appears to both reduce the initial SCI deficit and promote equal use of the forepaws compared to SCI alone, while the contralateral CCI-TBI produces a profound worsening of the deficit.
What can explain the apparent release of function by an ipsilateral brain injury? How can additional damage enhance performance and recovery? Inhibitory interactions between ipsilateral and contralateral cortical and subcortical structures have been described in a number of experiments over the years. For example, complete ablation of the striate and extrastriate cortex in cats produces a profound and apparently permanent visual hemianopsia resulting in lack of orientation to visual stimuli in the contralateral visual field. However, ablation of the contralateral superior colliculus ‘released’ visual orientation immediately, suggesting that the cortical lesion had produced an inhibition of the ipsilateral colliculus that masked the residual capacity for orientation (Sprague 1966). Damage to the cortex in humans and animals, induces a ‘compensatory reliance’ on the ipsi-lesional limb (Jones, et al. 2012). In addition, unilateral cortical damage actually appears to enhance the skilled reaching ability of the ipsi-lesional limb (Hsu and Jones 2005; 2006; Jones, et al. 2009; Jones, et al. 1996; Jones, et al. 2012; Luke, et al. 2004), and this increased motor skill is accompanied by evidence of plasticity, including increased numbers of synapses on neurons in the contra-lesional cortex. Humans with cortical damage, especially on the parietal regions of the right side, exhibit a contralateral spatial inattention (or neglect) that appears to reflect a lack of awareness rather than an inability to see or move on the contralateral side. Whether our results depend upon contralateral inattention or ipsilateral enhancement, or how these effects are related, will require additional experiments. However, it may be reasoned that ipsilateral enhancement and contralateral neglect after cortical lesions or TBI are related by cortical plasticity and synaptogenesis (Hsu and Jones 2005; 2006; Jones, et al. 2009; Jones, et al. 2012).
Deficits in paw placement in the cylinder have been seen repeatedly with cortical injuries, and have been ascribed to ‘sensorimotor neglect’ (Schallert, et al. 2000). Thus, cortical TBI lesions by themselves may reduce the use of the forepaw in the cylinder test by reducing the initiation or production of the paw movement without necessarily disrupting the motor apparatus needed to perform the movement. This is in contrast to the complete lack of effect of TBI/cortical damage on the grooming response, which nevertheless recruits activation and coordination of some of the same spinal motoneuron pools and muscles involved in paw placement (McKenna, et al. 2000). The C5 contusion lesion, on the other hand, is located in a position to both reduce motoneuron and spinal circuits directly involved in lifting the limb and partially damages corticospinal and other descending tracts rostral to distal forelimb motoneuron pools, and therefore could be expected to reduce paw placement and grooming function by a combination of effects on both. Both paw placement and grooming show partial recovery after unilateral SCI alone, and this likely reflects reorganization and plasticity at the spinal level as well as in the brain. The release of ipsilateral paw placement by the ipsilateral cortical TBI suggests that the residual capacity for lifting and placing the paws is relatively preserved after the C5 contusion (vertebral level), and that part of the deficit seen after a unilateral cervical SCI alone is due to damage to descending (e.g. corticospinal) fiber tracts. Thus, the superimposition of a contralateral inattention or neglect by the ipsilateral cortical lesion might release, or force, the residual capacity of the partially damaged ipsilateral cord. This view suggests that the cortical lesion is ‘dominant’ in this particular combination of CNS deficits, and raises the possibility that the release of, or altered balance, of circuits due to combined lesions might in some cases actually provide enhanced recovery. The corollary for rehabilitation strategies is that suppression of activity from unbalanced inputs might be useful for improving function, such as is seen in forced-use protocols after stroke (Willis, et al. 2002; Wolf, et al. 1989).
While no studies of concurrent SCI and TBI in rodent models seem to exist, there are a number of relevant studies of sequential lesion effects that also point to important interactions between brain and spinal circuits in recovery (Blanco, et al. 2007). Blanco et al (2007) measured grip strength after unilateral cervical hemisections or sensorimotor cortex lesions in the mouse. Recovery of grip strength occurred over several weeks after either SCI or cortex lesions (compare to the current data from paw placement or IBB score). Recovery after SCI was reversed by contralateral cortical lesions given 26–28 days after the initial injury, suggesting that the contralateral cortex had compensated for the loss of gripping ability from SCI. Lesions of the cortex ipsilateral to the original spinal hemisection did not reverse recovery of the ipsilateral cord deficit. But unexpectedly, those lesions also did not result in any impairment of grip strength of the contralateral forepaw. Thus, the recovery of function from spinal hemisection had somehow induced the capacity to mediate gripping to either the contralateral cortex or to subcortical (or even spinal) circuits. While these experiments involved sequential lesions that allowed time for compensatory plasticity to occur, the issues of how such a transfer takes place, e.g. through forced practice caused by inability or inattention, are similar to those raised by the current findings. One might speculate that the forced practice of the contralateral forepaw due to disuse of the paw ipsilateral to the spinal hemisection provided activity-dependent plasticity sufficient to free the left forelimb from dependence on cortical inputs.
Other work showing that activity in the non-impaired forelimb may actively suppress recovery of the contralateral impaired limb after cortical damage provides additional evidence for the presence of inhibitory or ‘unbalanced’ activity-dependent plasticity that might provide a target for suppressive strategies in rehabilitation (Allred, et al. 2010; Bury and Jones 2004). Indeed, Bury and Jones (2004) provide another example of improving function by inducing CNS damage, in their case, by cutting the corpus callosum to release the contralateral limb from the deleterious effects of activity induced plasticity of the undamaged cortex.
Thus, the concurrent combined injury model investigated in this study provides a first step towards evaluating interactions between TBI and SCI in a preclinical model. While it is acknowledged that purely unilateral CNS traumatic injuries are not common, especially in SCI, this approach has yielded some dramatic evidence of the complexity associated with recovery from dual injuries. Along with previous work on unilateral brain damage and recovery, the findings suggest that therapies for combined injury will need to consider complex interrelations between injuries and treatments to optimize adaptive, and minimize maladaptive, plasticity in recovery (Huie, et al. 2012). Treatments, for example drugs, that positively affect recovery from TBI could adversely affect recovery from SCI, and vice versa, and, the added complication of the problem of balancing inputs after injury provides a strong impetus to continue development of preclinical models to inform clinical practice.
Conclusion
The current study provides strong evidence for complex interactions between SCI and TBI that affect recovery of forelimb function in a model of concurrent combined unilateral injuries in the rat. These data first support the idea that brain damage should exacerbate deficit and reduce recovery after SCI, but add the caveat that balanced activity and potential inhibitory effects of remaining systems will need to be considered in planning for treatments of ‘dual diganosis’ patients.
Supplementary Material
Supplementary FIG. 1. The total number of paw placements for the SCI + ipsilateral TBI group was significantly less than the SCI alone group (p< 0.05) but Analysis of Covariance showed that total response number was not a significant covariate for either right or left paw preference, both p > .05, indicating that response number did not significantly influence the overall distribution of responses.
Supplementary FIG. 2. Actual number of paw placements in the cylinder is shown for each group. (A) total (B) simultaneous (both) (C) contralateral (left) (D) ipsilateral (right). (A–D) (A) Analysis of the raw response numbers demonstrated that the SCI + ipsilateral TBI had a significantly lower overall number of responses than SCI alone, p < .05. No other group differences were significant, p > .05 (B) The injured groups showed significantly reduced number of simultaneous paw placements compared to sham rats 2 days after surgery. (C) SCI + contralateral TBI rats showed a significantly higher number of left paw placements compared to both sham and SCI + ipsilateral TBI rats 42 days after surgery. (D) SCI + ipsilateral TBI significantly enhanced ipsilateral forepaw use compared to SCI + contralateral TBI rats over 42 days after surgery. SCI: spinal cord injury, TBI: traumatic brain injury. Error bar shows standard error of the mean. ○: significant difference compared to sham group, □ :significant difference compared to TBI group, ▲ :significant difference compared to SCI + ipsilateral TBI group
Highlights.
Contralateral traumatic brain injury (TBI) exacerbates the deficit and reduces recovery on forelimb function tests after a unilateral cervical spinal cord injury (SCI) in rats. Ipsilateral TBI however, appears to balance paw use relative to animals without TBI on the paw placement/cylinder test. Grooming behavior and paw placement are differentially sensitive to brain or spinal cord contusion injuries.
Acknowledgments
This work was supported by DoD/CDMRP SCIRP Translational Partnership Award #W81XWH-10-1-0910. T. Inoue was supported by the Uehara Memorial Foundation Research fellowship. The authors would like to acknowledge Ms. Ellie Stuck and Yvette Nout, DVM, PhD for help with some of the behavioral testing, and Jeffrey Sacramento and Jinghua Yao for help with the histological preparations and analysis.
Abbreviations
- ANOVA
analysis of variance
- BBB
Basso, Beattie, Bresnahan Locomotor Rating Scale
- CCI
controlled cortical impact
- CT
computed tomography
- HD
high definition
- IBB
Irvine, Beatties, Bresnahan forelimb rating scale
- IH
Infinite Horizons impactor
- MRI
magnetic resonance imaging
- PBS
phosphate buffered saline
- SCI
spinal cord injury
- SEM
standard error of the mean
- TBI
traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzaga D, Shaw V, Vasile AT. Dual diagnoses: the person with a spinal cord injury and a concomitant brain injury. SCI Nurs. 2003;20:86–92. [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bell RS, Vo AH, Neal CJ, Tigno J, Roberts R, Mossop C, Dunne JR, Armonda RA. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66:S104–111. doi: 10.1097/TA.0b013e31819d88c8. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Mira JC. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J Neurosci Methods. 1993;46:203–208. doi: 10.1016/0165-0270(93)90068-3. [DOI] [PubMed] [Google Scholar]
- Blanco JE, Anderson KD, Steward O. Recovery of forepaw gripping ability and reorganization of cortical motor control following cervical spinal cord injuries in mice. Exp Neurol. 2007;203:333–348. doi: 10.1016/j.expneurol.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Bradbury CL, Wodchis WP, Mikulis DJ, Pano EG, Hitzig SL, McGillivray CF, Ahmad FN, Craven BC, Green RE. Traumatic brain injury in patients with traumatic spinal cord injury: clinical and economic consequences. Arch Phys Med Rehabil. 2008;89:S77–84. doi: 10.1016/j.apmr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Bury SD, Jones TA. Facilitation of motor skill learning by callosal denervation or forced forelimb use in adult rats. Behav Brain Res. 2004;150:43–53. doi: 10.1016/S0166-4328(03)00253-5. [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Ackland HM. Clearing the cervical spine in unconscious head injured patients - the evidence. Crit Care Resusc. 2005;7:181–184. [PubMed] [Google Scholar]
- Davidoff G, Morris J, Roth E, Bleiberg J. Closed head injury in spinal cord injured patients: retrospective study of loss of consciousness and post-traumatic amnesia. Arch Phys Med Rehabil. 1985a;66:41–43. [PubMed] [Google Scholar]
- Davidoff G, Morris J, Roth E, Bleiberg J. Cognitive dysfunction and mild closed head injury in traumatic spinal cord injury. Arch Phys Med Rehabil. 1985b;66:489–491. [PubMed] [Google Scholar]
- Davidoff G, Roth E, Morris J, Bleiberg J, Meyer PR., Jr Assessment of closed head injury in trauma-related spinal cord injury. Paraplegia. 1986;24:97–104. doi: 10.1038/sc.1986.13. [DOI] [PubMed] [Google Scholar]
- Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R. Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil. 1988;69:869–872. [PubMed] [Google Scholar]
- Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, Clark RS, Jenkins LW, Dixon CE, Kochanek PM. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889–899. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham KA, Siriphorn A, Chompoopong S, Floyd CL. Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J Neurotrauma. 2010;27:2091–2106. doi: 10.1089/neu.2010.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Irvine KA, Gensel JC, Nielson JL, Lin A, Ly J, Segal MR, Ratan RR, Bresnahan JC, Beattie MS. Derivation of multivariate syndromic outcome metrics for consistent testing across multiple models of cervical spinal cord injury in rats. PLoS One. 2013 doi: 10.1371/journal.pone.0059712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23:36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- Hagen EM, Eide GE, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta Neurol Scand Suppl. 2010:51–57. doi: 10.1111/j.1600-0404.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg. 2002;96:285–291. doi: 10.3171/spi.2002.96.3.0285. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–2080. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Huie JR, Baumbauer KM, Lee KH, Bresnahan JC, Beattie MS, Ferguson AR, Grau JW. Glial Tumor Necrosis Factor Alpha (TNFalpha) Generates Metaplastic Inhibition of Spinal Learning. PLoS One. 2012;7:e39751. doi: 10.1371/journal.pone.0039751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Potts MB, Noble-Haeusslein LJ. Injury severity determines Purkinje cell loss and microglial activation in the cerebellum after cortical contusion injury. Exp Neurol. 2007;203:258–268. doi: 10.1016/j.expneurol.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Iida H, Tachibana S, Kitahara T, Horiike S, Ohwada T, Fujii K. Association of head trauma with cervical spine injury, spinal cord injury, or both. J Trauma. 1999;46:450–452. doi: 10.1097/00005373-199903000-00018. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Ferguson AR, Mitchell KD, Beattie SB, Beattie MS, Bresnahan JC. A novel method for assessing proximal and distal forelimb function in the rat: the Irvine, Beatties and Bresnahan (IBB) forelimb scale. J Vis Exp. 2010;46:ID2246. doi: 10.3791/2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Allred RP, Adkins DL, Hsu JE, O’Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40:S136–138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733:142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- Jones TA, Liput DJ, Maresh EL, Donlan N, Parikh TJ, Marlowe D, Kozlowski DA. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma. 2012;29:1455–1468. doi: 10.1089/neu.2011.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysys, A researcher’s handbook. 4. Pearson; Prentice Hallm Upper Saddle River, New Jersey: 2004. [Google Scholar]
- Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–2126. doi: 10.1089/neu.2008.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, Zador Z, Yao J, Fazlollahi F, Manley GT. Aquaporin-4 Reduces Post-Traumatic Seizure Susceptibility by Promoting Astrocytic Glial Scar Formation in Mice. J Neurotrauma. 2011 doi: 10.1089/neu.2011.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Macciocchi S, Seel RT, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch Phys Med Rehabil. 2008;89:1350–1357. doi: 10.1016/j.apmr.2007.11.055. [DOI] [PubMed] [Google Scholar]
- Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil. 2004;83:22–26. doi: 10.1097/01.PHM.0000104661.86307.91. [DOI] [PubMed] [Google Scholar]
- Martinez M, Brezun JM, Bonnier L, Xerri C. A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. J Neurotrauma. 2009;26:1043–1053. doi: 10.1089/neu.2008.0717. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Michael DB, Guyot DR, Darmody WR. Coincidence of head and cervical spine injury. J Neurotrauma. 1989;6:177–189. doi: 10.1089/neu.1989.6.177. [DOI] [PubMed] [Google Scholar]
- Mihai G, Nout YS, Tovar CA, Miller BA, Schmalbrock P, Bresnahan JC, Beattie MS. Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J Neurotrauma. 2008;25:1–18. doi: 10.1089/neu.2007.0338. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Isa T. Compensatory changes at the cerebral cortical level after spinal cord injury. Neuroscientist. 2009;15:436–444. doi: 10.1177/1073858408331375. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Isa T. Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Exp Neurol. 2012;235:152–161. doi: 10.1016/j.expneurol.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Nout YS, Mihai G, Tovar CA, Schmalbrock P, Bresnahan JC, Beattie MS. Hypertonic saline attenuates cord swelling and edema in experimental spinal cord injury: a study utilizing magnetic resonance imaging. Crit Care Med. 2009;37:2160–2166. doi: 10.1097/CCM.0b013e3181a05d41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley KF, Ross SE. The incidence of injury to the cervical spine in patients with craniocerebral injury. J Trauma. 1988;28:1476–1478. doi: 10.1097/00005373-198810000-00013. [DOI] [PubMed] [Google Scholar]
- Povolny M, Kaplan S. Traumatic brain injury occurring with spinal cord injury: significance for rehabilitation. J Rehabil Med. 1993;59:23–28. [Google Scholar]
- Richards JS, Osuna FJ, Jaworski TM, Novack TA, Leli DA, Boll TJ. The effectiveness of different methods of defining traumatic brain injury in predicting postdischarge adjustment in a spinal cord injury population. Arch Phys Med Rehabil. 1991;72:275–279. [PubMed] [Google Scholar]
- Ricker JH, Regan T. Neuropsychological and psychological factors in acute rehabilitation of individuals with both spinal cord injury and traumatic brain injury. Top Spinal Cord Inj Rehabil. 1999;5:76–82. [Google Scholar]
- Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT. Brain-dependent movements and cerebral-spinal connections: key targets of cellular and behavioral enrichment in CNS injury models. J Rehabil Res Dev. 2003;40:9–17. doi: 10.1682/jrrd.2003.08.0009. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Soicher E, Demetriades D. Cervical spine injuries in patients with head injuries. Br J Surg. 1991;78:1013–1014. doi: 10.1002/bjs.1800780837. [DOI] [PubMed] [Google Scholar]
- Sommer JL, Witkiewicz PM. The therapeutic challenges of dual diagnosis: TBI/SCI. Brain Inj. 2004;18:1297–1308. doi: 10.1080/02699050410001672288. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Stambrook M, Moore AD, Peters LC, Zubek E, McBeath S, Friesen IC. Head injury and spinal cord injury: differential effects on psychosocial functioning. J Clin Exp Neuropsychol. 1991;13:521–530. doi: 10.1080/01688639108401068. [DOI] [PubMed] [Google Scholar]
- Strich SJ. Lesions in the cerebral hemispheres after blunt head injury. J Clin Pathol Suppl (R Coll Pathol) 1970;4:166–171. doi: 10.1136/jcp.s3-4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MK, Blanco JE, Anderson KD, Lewandowski G, Steward O. An investigation of the cortical control of forepaw gripping after cervical hemisection injuries in rats. Exp Neurol. 2009;217:96–107. doi: 10.1016/j.expneurol.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian HL, Guo Y, Hu J, Rong BY, Wang G, Gao WW, Chen SW, Chen H. Clinical characterization of comatose patients with cervical spine injury and traumatic brain injury. J Trauma. 2009;67:1305–1310. doi: 10.1097/TA.0b013e31819db57c. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Zafonte R, Lairson E. Traumatic Brain Injury Associated with Acute Spinal Cord Injury: Risk Factors, Evaluation, and Outcomes. Topics in Spinal Cord Injury Rehabilitation. 1999;5:83–90. [Google Scholar]
- Wei CW, Tharmakulasingam J, Crawley A, Kideckel DM, Mikulis DJ, Bradbury CL, Green RE. Use of diffusion-tensor imaging in traumatic spinal cord injury to identify concomitant traumatic brain injury. Arch Phys Med Rehabil. 2008;89:S85–91. doi: 10.1016/j.apmr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Willis JK, Morello A, Davie A, Rice JC, Bennett JT. Forced use treatment of childhood hemiparesis. Pediatrics. 2002;110:94–96. doi: 10.1542/peds.110.1.94. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary FIG. 1. The total number of paw placements for the SCI + ipsilateral TBI group was significantly less than the SCI alone group (p< 0.05) but Analysis of Covariance showed that total response number was not a significant covariate for either right or left paw preference, both p > .05, indicating that response number did not significantly influence the overall distribution of responses.
Supplementary FIG. 2. Actual number of paw placements in the cylinder is shown for each group. (A) total (B) simultaneous (both) (C) contralateral (left) (D) ipsilateral (right). (A–D) (A) Analysis of the raw response numbers demonstrated that the SCI + ipsilateral TBI had a significantly lower overall number of responses than SCI alone, p < .05. No other group differences were significant, p > .05 (B) The injured groups showed significantly reduced number of simultaneous paw placements compared to sham rats 2 days after surgery. (C) SCI + contralateral TBI rats showed a significantly higher number of left paw placements compared to both sham and SCI + ipsilateral TBI rats 42 days after surgery. (D) SCI + ipsilateral TBI significantly enhanced ipsilateral forepaw use compared to SCI + contralateral TBI rats over 42 days after surgery. SCI: spinal cord injury, TBI: traumatic brain injury. Error bar shows standard error of the mean. ○: significant difference compared to sham group, □ :significant difference compared to TBI group, ▲ :significant difference compared to SCI + ipsilateral TBI group