Abstract
Background:
Moderately vigorous physical activity (MVPA) provides a protective affect against cognitive decline and cardiovascular risk factors. Less is known about sedentary pastimes or non exercise physical activity (NEPA) and cognitive performance.
Method:
125 healthy adults 65 or older with no clinical evidence of cognitive impairment were enrolled. The CogState computerized neurocognitive battery was administered. Leisure activities were measured using the Community Health Activity Program for Seniors (CHAMPS).
Results:
Sedentary pastimes were associated with executive dysfunction (P = 0.01); MVPA with high memory scores (P = 0.05) and NEPA with improved working memory (P = 0.05). Only sedentary pastimes and executive dysfunction retained significance after correction for multiple comparisons. Smoking and alcohol confounded the association of memory with sedentary pastimes and MVPA.
Conclusions:
Study highlights: negative impact of sedentary pastimes on executive function, need for additional investigation of sedentary behavior, NEPA, the impact of addictions upon activity in late life.
Keywords: cognition, exercise, sedentary, aging, cardiovascular risk factors, physical activity
Introduction
With the rising proportion of older adults in the population, there has been a growing interest in maintaining “brain fitness” in later life. 1 Compelling evidence from prospective studies has shown that engagement in stimulating cognitive activities is associated with a decrease in the rate of cognitive decline 2,3 and a reduced risk of incident dementia. 4 -6 Similarly, physical activity provides a protective effect against cognitive impairment regardless of the form of measurement: ActiGraph accelerometer, self-report, or doubly labeled water. 2,7 -14 In a community-based sample of cognitively unimpaired elderly women, those with the highest exercise frequency and who expended the most kilocalories were least likely to experience cognitive decline. 12 Greater energy expenditure resulting from moderately vigorous physical activity (MVPA) appears to be one key to maintaining brain fitness. 10 -12,15 However, the domain-specific effects of physical activity on cognition are not well understood, with reports suggesting improvements in executive function, memory, processing speed, and attention. 2,7,13,14,16 Physical activity also reduces the rate and severity of cardiovascular risk factors such as hypertension and obesity, each of which are associated with an increased risk of cognitive impairment in late life. 17,18 In a recent study of aging males, nonsurvival was associated with current and past smoking, high alcohol intake, a low physical activity index, and poorer cognitive performance. 19 Alcohol dependence, even when drinking onsets only after 65, is associated with poorer executive function and memory than in a nonalcohol-dependent normative group. 20
Little has been published on nonexercise physical activity (NEPA), sedentary behavior, 21 and cognitive domains. However, NEPA in a 60-year-old population was associated with greater waist circumference, abnormal glucose metabolism, hyperlipidemia, higher risk of cardiovascular events, and all cause mortality. 22
The first goal of this study was to examine the extent to which objectively measured memory and executive function were associated with physical activity. We hypothesized that better performance on measures of memory and executive function would be associated with MVPA. The second goal was to examine the extent to which cognitive functioning was associated with sedentary pastimes. We hypothesized that poorer performance on measures of cognition would be associated with a more sedentary lifestyle. The third goal was to determine whether cardiovascular risk factors would confound the observed associations between cognitive performance and activity levels. We hypothesized that cardiovascular risk factors (smoking, alcohol use, waist hip ratios [WHRs], hypertension, and body mass index [BMI]) could be confounders of sedentary pastimes and cognitive performance specifically for memory and/or executive function.
Methods
Participants
The population consisted of older adults without clinical evidence of cognitive impairment who consented to participate in a longitudinal study of 3 years duration. Here, we report a cross-sectional analysis of the results of the baseline visit. The participants met the following eligibility criteria: (1) age 65 or older; (2) no clinical evidence of cognitive impairment on a measure of global cognitive functioning (Montreal Cognitive Assessment [>26]) 23 ; and (3) no acute or serious medical conditions. Participants were recruited by the University of Pennsylvania Alzheimer’s Disease Center from its “normal control” cohort and in outreach efforts to a suburban residential setting for independent living, from an Older Adult Registry for African Americans interested in research participation, and other community-dwelling individuals in the greater Philadelphia area.
The study population was administered the prescreening Telephone Interview for Cognitive Status to eliminate potential participants who clearly did not meet protocol requirements. Those with scores >28 were eligible per established norms for definitely (range 33-41) and probably nonimpaired (range 26-32). 24 The mean for our population was 35 (standard deviation [SD] = 2.6; range 28-41). A history of medical conditions was taken from the participants and confirmed in their records. A review of medical conditions revealed a multitude of age-related illnesses, controlled by treatment and not exclusionary. One individual had significant Parkinson’s disease that precluded writing, and another was undergoing chemotherapy that affected mental clarity. A total of 150 potential participants were approached and screened; of these, 10 withdrew, 12 declined, and 3 did not meet the eligibility criteria. The final sample was 125. The University of Pennsylvania institutional review board approved the study, and all participants provided informed consent.
Leisure activity measure
The Community Health Activity Program for Seniors (CHAMPS) questionnaire assesses the weekly frequency and duration of 40 different activities undertaken by older adults. (See Table 1 for a detailed description of the items included in each type of activity.) These include NEPA (eg, watering houseplants and walking while shopping), MVPA (eg, cycling, tennis, dancing, brisk walking, golf, brisk walking, and aerobics), and sedentary pastimes (eg, computer time, playing cards, or dinner with friends). 25 The CHAMPS has moderate 6-month stability, good test–retest reliability, and acceptable predictive validity. 26 The CHAMPS also showed moderate correlations with the Physical Activity For The Elderly and the Yale Physical Activity Scale. 27 Stewart et al assigned a standard metabolic equivalent (MET) value to each activity and adjusted the intensity for older adults. In our study, metrics for physical exercise included frequency, hours, and energy expenditure (METS*hours). Energy expenditure was computed using the algorithm developed by Stewart et al consistent with the guidelines of the American College of Sports Medicine. 25 All participants were provided assistance during scale completion.
Table 1.
Distribution of Leisure Activities in this Community-Dwelling Population.a,b
| Leisure Activities | Mean, hours | Standard Deviation (SD) | Minimum | Maximum |
|---|---|---|---|---|
| Sedentary pastimes | 47.97 | 22.63 | 4 | 140 |
| Cognitive | 27.13 | 13.41 | 0 | 64 |
| Social | 20.84 | 14.12 | 0 | 82 |
| Nonexercise physical activity | 19.01 | 11.82 | 0 | 66 |
| Moderately vigorous physical activity | 5.00 | 5.38 | 0 | 31 |
Abbreviations: CHAMPS, Community Health Activity Program for Seniors; NEPA, nonexercise physical activity; MVPA, moderately vigorous physical activity;
aN = 125.
bVariables were generated for cognitively stimulating activities by taking the relevant items (6, 8, 17, and 18) from the CHAMPS multiplying the times per week by the hours spent in each activity and adding all the items to get a total score for cognitive activity. Similarly variables were generated for social (1, 2, 3, 4, 5, 11, and 12), NEPA (10, 20, 22, 27, 28, 34, 25, and 39), and moderately vigorous MVPA (7, 9, 14, 15, 16, 19, 21, 24, 25, 26, 29, 30, 31, 32, 33, 36, 38, and 40). The table gives the mean time spent by the cohort in each type of activity. Cognitive activities include computer time (e-mail, surfing the net, and games), watching television, and reading. Social activities involved dinner out with friends, attending church, visiting grandchildren, and travel. Cognitive and social scores were combined to create the variable sedentary pastimes. NEPA includes watering house plants, walking around the grocery shop or pharmacy, light housekeeping such as dusting, stretching in place, and being out on a golf course in a golf cart. MVPA refers to tennis, heavy gardening, aerobic or strength training, and yoga.
Cognitive testing
The CogState computerized battery was chosen because it is easily administered, valid, 29 reliable, 30 and capable of detecting subtle changes in cognition. 31,32 The cognitive tests used in this study assess psychomotor speed (Detection test [DET]), visual attention (Identification [IDN]), visual recognition memory (One Card Learning [OCL] test), verbal learning and memory (International Shopping List [ISL] test, immediate recall and ISL test, delayed recall [ISRL]), working memory (One Back test [ONB]; Two Back test [TWOB]), and problem solving and reasoning (Groton Maze Learning test [GMLT]). Participants were administered the CogState tests over a 35-minute period. 33
Statistical Analysis
Descriptive statistics were used to characterize the study population. Histograms were generated for each outcome variable. Of the 125 participants, 118 had complete data for the CogState Tests. Outliers that were 2 or more SDs from the mean were not included in the analysis (1 subject for CPAL and 1 subject for GMLT). Four participants had missing data for the ISL and were removed from the analysis for this test. Given the sample size and the number of neurocognitive tests administered, formal analyses were performed using 3 composite outcomes based on the following neurocognitive domains: processing speed/attention (DET and IDN); memory (OCL, ISL, ISRL, and CPAL); and executive function (ONB, TWOB, and GMLT). To create the composite variables, each individual variable within a cognitive domain was transformed into a z-score using the mean and SD of the total sample in this study. These z-scores were then averaged across tests resulting in a single composite score for processing speed/attention, memory, and executive function for each participant. Higher scores on these composites indicate better performance. We report P values without adjustment for multiple comparisons. 34 -36 However, given that 3 outcomes are of interest: processing speed/attention, memory, and executive function, a Bonferroni adjusted P value of .0167 will be used as the threshold for statistical significance.
To evaluate the relationship between exercise and cognitive performance, linear regression analyses were performed and the results tested for potential confounding by age, sex, race, and education. The CogState composite score was entered as the outcome variable and the leisure activity (moderately intense physical exercise in kilocalories, hours or times per week; sedentary pastimes) entered as the independent variable. Table 2 shows a distinct linear model, with each row representing the association between the outcome measure (CogState composite score) and each of the independent variables. When there were no significant findings on the composite scores, post hoc analysis of the individual memory and executive function tests were done. This applies to NEPA and to the examination of the confounding effects of cardiovascular risk factors. A confounder was defined as any risk factor that impacted the association of interest (ie, executive function and sedentary pastimes) by 10% or more when added to the multivariable model. All analyses were performed using Stata Version 12 (StatCorp, College Station, Texas).
Table 2.
Moderately Vigorous Physical Activity, Sedentary Pastimes, and Cognitive Performance.a,b
| Coefficient | Standard Error | R 2 | 95% Confidence Interval | P | |
|---|---|---|---|---|---|
| Cognitive performance | |||||
| MVPA times/wk | |||||
| Memory | −0.002 | 0.009 | .2290 | −0.021 to 0.016 | .83 |
| Executive function | −0.0003 | 0.008 | .1853 | −0.017 to 0.016 | .97 |
| Processing speed/attention | 0.004 | 0.013 | .1528 | −0.022 to 0.029 | .77 |
| MVPA hours/wk | |||||
| Memory | −0.019 | 0.020 | .2350 | −0.059 to 0.021 | .35 |
| Executive function | −0.020 | 0.018 | .1944 | −0.056 to 0.016 | .27 |
| Processing speed/attention | 0.003 | 0.028 | .1600 | −0.027 to 0.083 | .32 |
| MPVA energy expenditure/wk | |||||
| Memory | −0.00006 | 0.00003 | .2580 | −0.0001 to −1.29e−06 | .05 |
| Executive function | −0.00003 | 0.00003 | .0033 | −0.00008 to 0.00002 | .25 |
| Processing speed/attention | 0.00003 | 0.00004 | .1583 | −0.00003 to 0.0001 | .38 |
| Sedentary pastimes | |||||
| Memory | 0.002 | 0.003 | .2320 | −0.004 to 0.007 | .50 |
| Executive function | 0.006 | 0.002 | .2323 | 0.001 to 0.111 | .01 c |
| Processing speed/attention | −0.003 | 0.004 | .1564 | −0.104 to 0.005 | .46 |
Abbreviation: MVPA, moderately vigorous physical activity.
aN = 118.
bThe composite outcomes memory, executive function, and processing speed/attention are analyzed for their association with MVPA times, hours, and energy expenditure and for sedentary behavior. Sedentary pastimes are associated executive dysfunction and MVPA with high scores on memory tests.
cStatistically significant after adjustment for multiple comparisons using a Bonferroni adjustment of 0.05/3 = 0.017 as the threshold for significance.
Results
Characteristics of the Study Population
There were 125 participants in the study. The mean age of the cohort was 77 (SD = 7.2; range 65-95). The mean years of education was 16 (SD = 2.8; range 6-20). Women constituted 66% of the sample. The racial distribution was African American: 24% and white: 76%. The majority (54%) were married. There were no significant differences in age, gender, or race between the participants and the nonparticipants. Most participants 110 (92%) were employed in jobs with high mental demands (professionals, management, and technology). The participants were frequently diagnosed with adult-onset diabetes, hypertension, and hyperlipidemia. The mean WHR was 0.89 (SD = 0.08; range 0.68-1.07). More men had high-risk WHR (>0.90) than women (>0.85l Pearson χ2 = 4.47, P = .03). The mean BMI was 28.5 placing the majority of the participants in the overweight but not obese class (SD = 6.2; range 18-58). Additional details are provided in Table 3.
Table 3.
Characteristics of the Population.a
| Characteristic | Number | Percentage |
|---|---|---|
| Age, years | ||
| 65-74 | 53 | 42 |
| 75-84 | 49 | 39 |
| 85-95 | 23 | 18 |
| Gender | ||
| Female | 89 | 66 |
| Education, years | ||
| ≤12 | 30 | 22 |
| 13-17 | 58 | 43 |
| >18 | 47 | 35 |
| Race | ||
| Caucasian | 95 | 77 |
| African American | 30 | 24 |
| Marital status | ||
| Married | 73 | 54 |
| Widowed | 32 | 23 |
| Single | 11 | 8 |
| Divorced | 20 | 15 |
| Medical history: cardiovascular risk | ||
| Diabetes | 26 | 22 |
| Hyperlipidemia | 63 | 52 |
| Hypertension | 70 | 58 |
| Cardiac disease | 40 | 33 |
| Blood pressure | ||
| Diastolic > 90 | 21 | 18 |
| Systolic > 140 | 43 | 36 |
| Body mass index | ||
| Normal weight 18.5-24.9 | 34 | 27 |
| Overweight 25-29.9 | 44 | 35 |
| Class1 obesity 30-34.9 | 27 | 21 |
| Class II obesity 35-39.9 | 5 | 4 |
| Class III obesity 40 plus | 9 | 7 |
| Tobacco history | ||
| Never smoked | 11 | 8 |
| Current smoker | 50 | 37 |
| Exsmoker | 73 | 55 |
| Tobacco consumption >1 pack/d | 62 | 46 |
| Duration of smoking ≥20 years | 47 | 35 |
| Alcohol history | ||
| Current drinker | 43 | 39 |
| Exdrinker | 66 | 61 |
| Alcohol consumption ≥3 drinks/d | 7 | 11 |
| Duration of drinking ≥20 years | 30 | 28 |
aN = 125.
Distribution of Leisure Activities
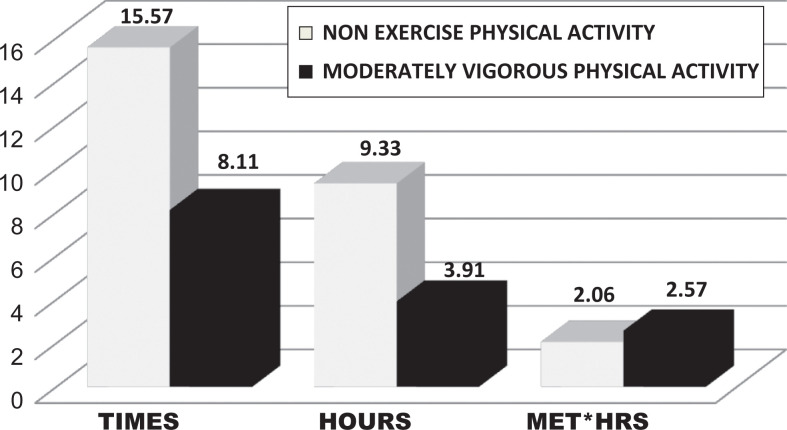
Socially engaging (eg, dinner with the spouse and visit with the grandchildren) and cognitively stimulating activities (eg, computer time surfing the net, e-mailing, and games) were the most commonly selected activities from the CHAMPS items. Social and cognitive items were combined to create a composite variable for sedentary pastimes. The CHAMPS has numerous options for NEPA, and these were popular. Fewer participants elected MVPA (Table 1). The mean number of hours participants were involved in MVPA was 3.91 hours over 8 sessions with an average energy expenditure of 2.57 kcal/wk. The mean number of hours spent in NEPA was 9.4 hours over as many as 16 sessions with an average energy expenditure of 2.06 kcal/wk (Figure 1).
Figure 1.
Moderately vigorous activity and nonexercise physical activity per week (means). Older adults engaged in nonexercise physical activity (NEPA) with a high frequency and for many hours per week resulting in an energy expenditure of 2.06 MET*hours quite similar to that of moderately vigorous physical activity (MVPA) 2.57.
Sedentary Pastimes and Cognitive Performance
Sedentary pastimes are associated with performance on the Executive Function composite score. Specifically, lower scores were significantly associated with more sedentary behaviors (Table 2). This result retained statistical significance even after the Bonferroni correction of the P value.
Moderately Vigorous Physical Activity and Cognitive Performance
Table 2 shows significant and nonsignificant findings of the linear regression analysis with the outcome variables being the CogState composite scores and the independent covariates being the frequency of exercise, the hours spent, and the energy expended. Energy expended in MVPA was positively associated with cognitive activity, but the frequency and duration of MVPA were not. The memory composite scores were higher for those involved in MVPA, but this did not reach significance at the lowered threshold P = .017.
Cardiovascular Risk Factors as Confounders of the Association of Activity Level and Cognitive Performance
Sedentary pastimes
None of the cardiovascular risk factors (smoking, alcohol, BMI, WHR, or hypertension) met the criteria for confounding at the 10% level of the association between executive function and sedentary pastimes. The largest impact was observed with the addition of hypertension to the multivariable model, which changed the association between sedentary pastimes and executive function by only 5%.
In our evaluation of immediate verbal memory, the marginal association we observed with sedentary pastimes was confounded by reported smoking (13.5%) and alcohol use (63%).
Moderately vigorous physical activity
Smoking appeared to confound the relationship between delayed verbal memory and energy expenditure during MVPA. The association was stronger increasing by 10% (coefficient −0.00025; standard error 0.00009; 95% confidence interval [CI] −0.0004 to −0.00009, P = .004) after adjusting for smoking than without the adjustment (Table 2). High alcohol use was also a confounder of the association between delayed verbal memory and energy expenditure. The association was stronger after adjusting for alcohol increasing by 11% (coefficient −0.0002516; standard error 0.00009; 95% CI −0.0004 to −0.00008; P = .005) than without adjustment (coefficient −0.0002251; standard error 0.00009; 95% CI −0.0003947 to −0.00006; P = .010). In our evaluation of delayed verbal memory, the marginal association we observed with MVPA was confounded by reported smoking (10%) and alcohol use (11%). Other cardiovascular risk factors did not influence the associations of interest.
Nonexercise Physical Activity and Cognitive Performance
With respect to NEPA, there were no significant findings on the CogState composite scores. However, more hours spent in NEPA were associated with a trend toward better performance on the one back card test, a measure of working memory (coefficient 0.019; standard error 0.009; 95% CI −4.7e−06 to 0.038; P = .05).
Discussion
The results suggest that a higher Memory Composite Score is associated with greater energy expended with MVPA, and this finding is concordant with some previous work. 2,10 -12 In the Baltimore Longitudinal Study of Aging, cardiorespiratory fitness was associated with improved performance in the memory domain. 37 In one magnetic resonance imaging study, total energy expenditure was associated with better performance in verbal and episodic memory and increased cingulate gray matter. 38 Others have found that more physical activity 14,39 or aerobic fitness 40 is associated with improved executive function. Thus, this article has not clarified the domain-specific effects of MVPA.
The most robust results support our second hypothesis that sedentary pastimes were associated with a poorer Executive Function composite score. To our knowledge, only one published longitudinal study examines the relationship between sedentary behavior and cognitive performance in older adults. They report that watching television was associated with lower scores on executive function tests while computer use with better performance on executive function and verbal memory tests. 21 This suggests that not all sedentary behaviors have negative implications.
None of the cardiovascular risk factors were confounders of the association of sedentary pastimes and executive function. This speaks to the health of our sample and their compliance with medical treatment (data not shown). However, a pattern emerged that both smoking and alcohol use confounded the association of memory with both sedentary pastimes and energy expenditure during MVPA. This is consistent with the smoking and drinking habits of our participants (Table 3). In fact, 37% were current smokers, 55% exsmokers, 46% smoked 1 pack/d, and 35% smoked for ≥20 years. Similarly, 39% were current drinkers, 61% exdrinkers, 11% consumed ≥3 drinks/d, and 28% had been drinking for ≥20 years. Alcohol dependence with an onset in late life is understudied. Yet alcohol dependence with an onset in late life has as an important role in causing cognitive impairment as for the earlier onset, long duration subset. 20 The contribution of late life factors to longevity was reported for 3 groups: nonsurvivors, healthy, and unhealthy survivors. Those who were current or exsmokers or abused alcohol fell into the nonsurvival group. 19 These represent modifiable risk factors. 41
In our population, the mean time spent in sedentary behaviors per week was 48 hours compared with NEPA (16 hours) and MVPA (5 hours). The accelerometer data from the National Health and Nutritional Examination Survey (NHANES) collected from 2630 adults 60 years and older were examined for the prevalence of sedentary behavior and MVPA. 42 The MVPA decreased with each successive age-group (60-69, 70-79, and ≥80). In the NHANES, the mean number of hours per day spent in sedentary behavior was 8.5 hours and higher for those older than 80 years. The mean number of hours per day spent in MVPA is 1.78 hours. Similarly, our population spent more hours per day being sedentary (mean = 6.9 hours) and much fewer hours engaged in MVPA (mean = 0.7 hours).
The American College of Sports Medicine Association recommends 2.5 to 5 hours/wk of MVPA for a total of 30 to 60 minutes/d with an energy expenditure (>500-1000 MET*min/wk) for health benefit in adults. 28 Only a small proportion of our participants were involved in MVPA, but they met these requirements: 4 hours/wk for a total of 34 minutes/d or 533.4 MET*minutes or 257 MET*hours (Figure 1). Guidelines recommended for adults in the general population seem applicable to our “fitness conscious” subset to assure maximizing cognitive performance benefits.
For participants “sedentary by choice,” among the oldest of old (≥85) or who have medical constraints, our findings suggest that a personalized exercise prescription would be of value. For the “sedentary by choice,” the strategy would be a step-wise increase in aerobic exercise, resistance training, balance, and flexibility regimes. For those among the oldest of old or having medical constraints, the focus would be on increasing NEPA. Assessment programs could be low cost, noninvasive, and easily executed. Shortcuts for calculations (target heart rate zones, heart rate variability, 43 rate of perceived exertion, maximum oxygen consumption [VO2max], body fat composition, 44 balance, and flexibility) and prototype exercise prescriptions are in Appendices A and B. 45
This article involves community-dwelling adults in late life with a focus on their physical exercise, sedentary pastimes, and cognitive performance. This study is among the few to examine sedentary behavior among seniors and its association with cognitive performance. It highlights the domain-specific effects of inactivity on executive dysfunction. The results also support previous findings of the association between MVPA and better performance on memory tests. Our analysis of cardiovascular risk factors highlights the importance of alcohol and smoking addictions in late life and their impact on memory performance. To our knowledge, previously unreported, increased NEPA is associated with better performance on a test of working memory.
Some limitations should be considered in interpreting our results. Because this is a relatively small sample of well-educated, professionally successful, reasonably healthy participants with a greater involvement of women and CAUCASIANS, the generalizability of these results might be limited. We compensate for this by adopting nonrestrictive eligibility criteria and employing statistical adjustments for potential confounders. The exercise duration and frequency were self-reported during an interview at a single time point and the METs* hours were then calculated. However, our results are broadly consistent with studies that use more rigorous measures (accelometers), suggesting that self-report was an adequate means of collecting the information for this study.
Conclusion
Additional research is needed to confirm the association between sedentary pastimes, NEPA, and cognitive performance. The “tipping point” should be identified where the dose of sedentary behavior outweighs the benefit of the physical activity. Sedentary behaviors that are cognitively stimulating should be distinguished from those with negative implications for brain fitness. Even with the current state of our knowledge, clinicians should encourage their older adults to lead more active lives, ranging from more facility with assistive devices to meeting the position stand of the American College of Sports Medicine.
Appendix A
Personalized Assessment: Sedentary by Choice, Oldest of Old, and Medically Restricted
| Target heart rate zone: 60%-80% of her age predicted heart rate max. The formula is (220 − Age = Heart Rate Max × 0.6) and (220 − Age = Heart Rate Max) × 0.8. These 2 numbers are the age-predicted target heart rate zones of 60%-80%. For medically restricted, the target heart rate zone should be between 30% and 40% of age predicted heart rate max. |
| Rate of perceived exertion (RPE) also determines target heart rate zone based on client’s estimate of how hard their body is working on a scale of 1-10. The healthy sedentary adult should be able to achieve a score between 4 and 6 or moderate activity. The oldest of old/medically restricted may score between 3 and 4 in the light physical activity range. |
| Body mass index (BMI): . Find classes by sex at http://www.exrx.net/Calculators/BMI.html |
| Waist hip ratio: . This ratio predicts cardiovascular mortality risk. Quick calculations for age and sex are at http://www.exrx.net/Calculators/WaistHipRatio.html |
|
Balke-Ware treadmill protocol for the sedentary by choice determines VO2max or maximum oxygen consumption. This protocol allows the individual to maintain a constant speed on the treadmill of 3.3 mph while the percentage of incline on the treadmill is increased 1% every minute for a single stage. Heart rate is tracked until the person reaches 80% of the age-predicted heart rate max. http://www.exrx.net/Calculators/Treadmill.html Example: Single-stage protocol to 80%. Actual time 12:45 Convert actual time into number 12:45 = 45/60 = 12.75 Multiply actual time number by estimated max to 100%. 12.75 × 1.2 = 15.30 Convert conversion time number back to time 0.30 × 60 = 18 seconds Add seconds to conversion time minutes = 15:18 Check VO2 chart for appropriate age group at 15:18 = estimated VO2max |
| Polar fitness test for the oldest of old or medically restricted determines VO2max doing a nonexercise aerobic test where the client wears a polar heart rate monitor with a fitness test function. It measures heart rate variability (HRV) for 5 minutes. Reduced HRV is known to be a risk factor for poor cognitive performance. http://www.polar.com/en/support/How_to_perform_Polar_Fitness_Test |
| Skin-fold caliper method for body fat composition: Seven sites are pinched with calipers, separating the fat from the muscle and measuring the skin diameter. The calculations give a total number of millimeters of lean body mass versus fat mass. Chest___ Tricep ____ Midaxillary____ Subscapula____ Suprailiac ____ Ab ____ Thigh___Sum___ Enter sums to receive percentage of body fat considering height, weight, and sex. http://www.exrx.net/Calculators/BodyComp.html |
| Bioelectrical impendence analysis for body fat composition for the morbidly obese: This scale (handheld or scale) measures body composition by sending a low electrical current through the body, which passes freely through muscle but encounters resistance in fat. This resistance, termed “bioelectrical impedance” accurately measures body fat. |
| Flexibility is measured by the sit and reach test on a mat determining the hamstring and lower back flexibility or using a modified chair version. |
| Balance determined by number of seconds standing on 1 foot usually |
Abbreviations: VO2max, maximum oxygen consumption.
VO2max, very low < 16, low 16 to 18, fair 19 to 21, moderate 22 to 24, good 25 to 27, very good 28 to 30, and elite >30; RPE 1: very light activity, 2 to 3: light activity, 4 to 6: moderate activity, 7 to 8: vigorous activity, 9: very hard activity, and 10: maximum effort activity; body composition % fat: low < 21.1, optimal 21.2 to 26.5, moderate 26.6 to 31.0 and 31.1 to 36, and very high > 36; body mass index underweight < 18.5, normal: 18.5-24.9, overweight: 25-29.9, obese class I: 30.0-34.9, obese class II: 35.0-39.9: class III morbidly obese: > 39.9. waist hip ratio: men >0.9 and women >0.85; flexibility with sit and reach: poor < 8.4, fair 8.4 to 10.1, average 10.1 to 12.3, good 12.4 to 14.2, and excellent > 14.1; blood pressure: systolic blood pressure > 140; diastolic blood pressure > 90 balance standing on one foot sedentary 15 seconds; average 20 seconds; and fit 25 seconds. Heart rate variability: HRV is a number ranging from 0 to 100 and most people will have a resting HRV of 50 to 90.
Appendix B
Brief Reports and Exercise Prescriptions
| Brief Reports | |||||||
|---|---|---|---|---|---|---|---|
| VO2max, mL/kg/min | Rate Perceived Exertion | Body Composition Fat, % | BMI, kg/m2 | Waist Hip Ratio | Flexibility, in | Blood Pressure | Balance, sec |
| Sedentary by choice | |||||||
| 20 | 4-6 | 28 | 29 | 0.8 | 6 | 142/90 | 10 |
| Morbidly obese | |||||||
| 15 | 3-4 | 39 | 42.8 | 0.95 | 2 | 124/64 | 0 |
| Interpretation of results Morbidly obese: very low VO2max, very high percentage of body fat, obese class III BMI, at risk waist–hip ratio, poor flexibility, and is normotensive with treatment. Sedentary by choice: fair VO2max, moderate percentage if body fat, overweight BMI, a good waist–hip ratio, good flexibility, and blood pressure requiring observation if not treatment. Goals Goal for sedentary by choice is to create a program of exercise that at minimum compensates for the risks of the inactive lifestyle and at best prepares them to follow the adult’s requirement for exercise. Goal for the morbidly obese is to increase the frequency and hours involved in the activities of everyday living in order to expend sufficient energy to achieve weight loss, improve mobility, and mitigate cardiovascular risk. | |||||||
| Exercise Prescriptions | |||||||
| Clients | Aerobic | Resistance | Flexibility | Balance | |||
| Sedentary by choice | Step-wise increases starting with moderate intensity 30 min/d 5×/wk | 2-3 days/wk Use 60%-70% of 1 repetition max for each exercise Train all major muscle groups 1+ sets, 10-15 reps | 2-3 days/wk Mild discomfort Full body 2-3 sets, 10-30 sec | 2-3 days/wk Low intensity Standing on 1 foot 15 sec | |||
| Morbidly obese | Nonexercise physical activity Walk 300 ft to dining room 3×/d | 2-3 days/wk 60%-80% of 1 RM Full body 2-4 sets, 8-12 reps | 2-3 days/wk Low to mild discomfort Full body 2-3 sets, 10-30 sec | 2-3 days/wk Low intensity Eyes closed test/reach test 10 sec | |||
Abbreviations: VO2max, maximum oxygen consumption; RM, repetition max.
Footnotes
Authors’ Note: The article was presented and published at Steinberg Susanne, Livney Melissa, Kling Mitch, McCoubrey Hannah, Yuen Stephanie, Edwards Carol, Louneva Natalia, Xie Sharon, Arnold Steven. Predictors of Resilient Cognitive Aging: Baseline Characteristics. Alzheimer’s Association International Conference, Hawaii, USA, July 16-21, 2011. Steinberg Susanne I, Sammel Mary D, Livney Melissa G, Kling Mitch A, McCoubrey Hannah, Yuen Stephanie, Arnold Steven E. Factors Associated with Cognitive Resilience in Late Life: a Three Sample Comparison. Alzheimer’s Association International Conference, Paris, France, July 14-19, 2012.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Brian Harel, PhD, JD, and Adrian Schembri, PhD, are full-time employees of CogState the company that provided the cognitive tests used in the study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Steven E. Arnold, MD, has grant funding paid to the University of ADC NIH/NIA P30-AG010124, Marian S. Ware Alzheimer’s Program. Susanne Steinberg, MD, MSCE, had a NRSA Postdoctoral Fellowship (217703) from the training grant NIH T32-MH019931.
References
- 1. George D, Whitehouse P. Marketplace of memory: what the brain fitness technology industry says about us and how we can do better? Gerontologist. 2011;51 (5):590–596. [DOI] [PubMed] [Google Scholar]
- 2. Wang HX, Jin Y, Hendrie HC, et al. Late life leisure activities and risk of cognitive decline. J Gerontol A Biol Sci Med Sci. 2013;68 (2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26 (2):144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73 (11):854–861. [DOI] [PubMed] [Google Scholar]
- 5. Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69 (20):1911–1920. [DOI] [PubMed] [Google Scholar]
- 6. Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4 (5):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300 (9):1027–1037. [DOI] [PubMed] [Google Scholar]
- 8. Buchman A, Boyle P, Yu L, Shah R, Wilson R, Bennett D. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78 (17):1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302 (6):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the invade study. Arch Intern Med. 2010;170 (2):186–193. [DOI] [PubMed] [Google Scholar]
- 11. Middleton L, Manini T, Simonsick E. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med. 2011;171 (14):1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yaffe K, Barnes D, Nevitt M, Lui L, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161 (14):1703–1708. [DOI] [PubMed] [Google Scholar]
- 13. Scarmeas N, Luschsinger J, Brickman A, et al. Physical activity and Alzheimer's disease course. Am J Geriatr Psychiatry. 2011;19 (5):471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Luo X, Barnes D, Sano M, Yaffe K. Physical activity and risk of cognitive impairment among oldest-old women [published online July 3, 2014]. Am J Geriatr Psychiatry. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindwall M, Cimino CR, Gibbons LE, et al. Dynamic associations of change in physical activity and change in cognitive function: coordinated analyses of four longitudinal studies. J Aging Res. 2012;2012:493598. doi:10.1155/2012/493598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buchman AS, Boyle PA, Leurgans SE, Barnes LL, Bennett DA. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am J Geriatr Psychiatry. 2011;19 (6):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Staiano AE, Reeder BA, Elliott S, et al. Physical activity level, waist circumference, and mortality. Appl Physiol Nutr Metab. 2012;37 (5):1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah T, Newcombe P, Smeeth L, et al. Ancestry as a determinant of mean population C-reactive protein values: implications for cardiovascular risk prediction. Circ Cardiovasc Genet. 2010;3 (5):436–444. [DOI] [PubMed] [Google Scholar]
- 19. Bell CL, Chen R, Masaki K, et al. Late-life factors associated with healthy aging in older men. J Am Geriatr Soc. 2014;62 (5):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kist N, Sandjojo J, Kok RM, van den Berg JF. Cognitive functioning in older adults with early, late, and very late onset alcohol dependence [published online May 15, 2014]. Int Psychogeriatr. 2014. [DOI] [PubMed] [Google Scholar]
- 21. Kesse-Guyot E, Charreire H, Andreeva VA, et al. Cross-sectional and longitudinal associations of different sedentary behaviors with cognitive performance in older adults. PLoS One. 2012;7 (10):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ekblom-Bak E, Ekblom B, Vikström M, de Faire U, Hellénius ML. The importance of non-exercise physical activity for cardiovascular health and longevity. Br J Sports Med. 2014;48 (3):233–238. doi:10.1155/2012/493598. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53 (4):695–699. [DOI] [PubMed] [Google Scholar]
- 24. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 25. Stewart AL, Mills KM, King AC, Haskell WL, Gillis W, Ritter PI. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33 (7):1126–1141. [DOI] [PubMed] [Google Scholar]
- 26. Cyarto EV, Marshall AL, Dickinson RK, Brown WJ. Measurement properties of the CHAMPS physical activity questionnaire in a sample of older Australians. J Sci Med Sport. 2006;9 (4):319–326. [DOI] [PubMed] [Google Scholar]
- 27. Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33 (6):962–970. [DOI] [PubMed] [Google Scholar]
- 28. Garber C, Blissmer B, Deychenes M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43 (7):1134–1359. [DOI] [PubMed] [Google Scholar]
- 29. Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24 (2):165–178. [DOI] [PubMed] [Google Scholar]
- 30. Hammers D, Spurgeon E, Ryan K, et al. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol. 2012;25 (2):89–99. [DOI] [PubMed] [Google Scholar]
- 31. Lim Y, Ellis K, Harrington K, Ames D, Martins R, Masters C. Use of the CogState brief battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian imaging, biomarkers and lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34 (4):345–358. [DOI] [PubMed] [Google Scholar]
- 32. Papp KV, Snyder PJ, Maruff P, Bartkowiak J, Pietrzak RH. Detecting subtle changes in visuospatial executive function and learning in the amnestic variant of mild cognitive impairment. PLoS One. 2011;6 (7):e21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinberg S, Negash S, Sammel M, et al. Subjective memory complaints, cognitive performance and psychological factors in healthy older adults. J Alzheimers Dis Other Dementias. 2013;28 (8):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1 (1):43–46. [PubMed] [Google Scholar]
- 35. Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142 (9):904–908. [DOI] [PubMed] [Google Scholar]
- 36. Poole C. Multiple comparisons? No problem! Epidemiology. 1991;2 (4):241–243. [PubMed] [Google Scholar]
- 37. Wendell CR, Gunstad J, Waldstein SR, Wright JG, Ferrucci L, Zonderman AB. Cardiorespiratory fitness and accelerated cognitive decline with aging. J Gerontol A Biol Sci Med Sci. 2014;69 (4):455–462. doi:10.1093/gerona/glt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flöel A, Ruscheweyh R, Krüger K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49 (3):2756–2763. [DOI] [PubMed] [Google Scholar]
- 39. Eggermont LH, Milberg WP, Lipsitz LA, Scherder EJ, Leveille SG. Physical activity and executive function in aging: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57 (10):1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinstein AM, Voss MW, Prakash RS, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26 (5):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10 (9):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Evenson KB, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older [published online December 15, 2011]. Prev Chronic Dis. 2012. [PMC free article] [PubMed] [Google Scholar]
- 43. Zeki AH, Haan A, Yingzi MN, Neuhaus D, Yaffe J. K. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans. Hypertension. 2014;63 (1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saragat B, Buffa R, Mereu E, et al. Specific bioelectrical impedance vector reference values for assessing body composition in the Italian elderly. Exp Gerontol. 2014;50 (0):52–56. [DOI] [PubMed] [Google Scholar]
- 45. Swain D, ed. American College of Sports Medicine Resource Manual for Guidelines for Exercise Testing and Prescription. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]