Abstract
Progression of most diseases, such as atherosclerosis, cancer, neurodegenerative disease and osteoarthritis is accompanied with drastic changes in biomechanics of tissue. Hence, non-contact and non-invasive technologies for 3-dimensional mapping of tissue biomechanics are invaluable for diagnostic purposes. Laser speckle Microrheology (LSM) is developed in our lab to enable high resolution mechanical evaluation of tissue. To this end, the tissue sample is illuminated by a coherent and focused laser beam and the back-scattered laser speckle pattern is spatio-temporally processed to extract a color-map of τ, which is the decay time constant of intensity decorrelation at each pixel in the image plane. Time constant, τ, is proven to be closely correlated with tissue mechanical properties. In this paper we validate the theoretical basis for LSM technology and investigate the potential for acquiring depth-resolved information from a light-scattering point of view. The patch analysis approach is introduced and the inter-relation between τ, a number of scaterring events, and penetration depth is explored for each patch. Axial variation of τ is characterized for two sample arterial regions and in-depth changes of mechanical properties are characterized. Finally, the required corrective measures are discussed.
I. Introduction
Progression of most diseases, such as atherosclerosis, cancer, neurodegenerative disease and osteoarthritis is accompanied with drastic changes in tissue biomechanics [1]. Hence, non-contact and non-invasive technologies for 3D mapping of tissue mechanical properties are highly desirable for diagnostic purposes. The extremely heterogeneous complexion of tissue demands high resolution biomechanical characterization. Currently, no such technology exists that can deliver this requirement. Laser Speckle Microrheology (LSM) is a novel non-contact optical technique, capable of probing mechanical properties in biological systems [2-5]. While light scattering techniques such as diffusive wave spectroscopy (DWS) [6] and dynamic light scattering (DLS) [7] demonstrated mechanical testing of homogenous gels and polymers of known optical properties, LSM extends mechanical testing to a whole new stage by applying it to heterogeneous tissue. In this paper, first we describe the proposed LSM setup and the underlying physics of this non-contact mechanical testing realm. Afterwards, the associated post-processing technique for acquiring depth-resolved information, i.e. patch analysis is explained and justified. Later, necessity of an accurate profiling of laser diffusion for a more precise interpretation of patch-analysis is discussed. Next, simulation and experimental results are presented. Finally, possible calibration techniques are suggested for future direction.
II. Laser Speckle Microrheology
In an LSM setup, light from a Helium-Neon laser source (632 nm) is reflected off a pair of scanning mirrors and is brought to focus at the sample site. Light penetrates into the sample and is multiply-scattered before returning back to a high speed CMOS camera. Due to turbid nature of the tissue, light rays travel along paths of slightly different lengths. The constructive and destructive addition of returning rays gives rise to a granular intensity pattern, known as the laser speckle. Since scattering particles in tissue are in constant Brownian motion, light rays’ paths continuously change, leading to “speckle boiling”. The rate of speckle fluctuations is a function of scattering particles movement, which in turn reflects the mechanical properties of surrounding medium [8]. The CMOS camera captures a series of speckle movies and transfers them to a high-speed computer. At the computer, the intensity decorrelation of speckle series, g2(t), is calculated and fitted to a single exponential curve. The time constant of this fitted curve, τ, is then used as a measure of tissue mechanics of the illumination volume [2-5]. Scanning mirrors sweep the focus piont across the sample and facilitate speckle imaging over the surface in order to obtain a lateral map of τ values.
While spatial beam scanning enables superficial mapping of biomechanics in lateral directions, in-depth characterization is hindered due to turbid nature of the tissue. In other words, since the received light is multiply scattered from a number of scattering centers, it carries cumulative contributions from mechanical properties of multiple depths. As a result, proper measures should be taken to decouple the contributions from different depths.
Patch analysis is a post-processing technique used to address this requirement. As described in the next section, patch analysis enables interpreting the speckle pattern emerged at each illumination point and extracting depth information.
III. Theoretical Basis For Patch Analysis
Intensity decorrelation time constant, τ, of laser speckle series reflects the time-scales of particle movements and thus, mechanical properties of the surrounding medium.
For instance, the intensity decorrelation, g2(t), of rays travelled through a more compliant region, such as a lipid pool in a necrotic core fibroatheroma, decays much faster compared to the one associated with a stiffer region, such as the fibrous cap [3].
On the other hand, while back-scattered rays emerging close to the center of the illumination region typically penetrate only into shallow depths, light remitted close to periphery bears information about deeper regions [3].
Patch analysis exploits this fact to extract depth-resolved information and to differentiate between mechanics of different layers of the imaged area.
In patch analysis, intensity decorrelation curve for each pixel of the image series is calculated using circular averaging (averaging over time). In order to improve statistical accuracy, a moving average window is applied at each pixel. This spatial averaging trades the resolution for statistical accuracy. Next, τ value is calculated for each pixel through fitting a single exponential curve to the corresponding g2(t).
Results are interpreted noting the fact that τ values associated with patches close to center typically reflect mechanical properties of superficial layers, whereas by moving away from the center, contributions from lower layers become more pronounced.
IV. Monte-Carlo Ray Tracing
While patch analysis provides invaluable information about depth mechanical properties, even more accurate evaluation is viable by accounting for the effects of multiple scatterings. Multiple scattering accelerate intensity decorrelation for the rays penetrating deeper into the medium and traveling through relatively longer paths.
For example, in the simple case of a purely viscous medium, intensity decorrelation function is given by:
| (1) |
where n is the number of scattering events, g is the asymmetric parameter of light scattering and is typically between 0.8 and 0.95 for tissue, and τD is a parameter related to the particle diffusion coefficient [8]. It is evident from eq (1) that at each patch on the receiver plane, g2(t) decays at a speed governed by the number of scattering events of remitted light and the mechanics of the regions that the rays have spanned.
Thus accurate profiling of light path through a multiple-scattering medium is essential to compensate for scattering-dependent weighting of τ and a more precise depth-resolved mechanical assessment,
To this end, a Monte-Carlo Ray Tracing (MCRT) approach is devised. A Laser beam of 633 nm wavelength and 5μm beam waist is considered. Optical properties of a typical atherosclerotic fibrous plaque are plugged into the ray tracing algorithm (μs= 440 cm−1, μa = 3.6 cm−1, g = 0.8) [3] and the light path inside the illumination volume is tracked.
A field of view (FOV) of 1 mm is assumed and for the photons returning to the receiver plane, penetration depth, and number of scattering events are recorded, among other parameters.
The mean-maximum penetration depths as well as the number of scattering events are calculated for each pixel. Moreover, using eq (1), the expected value of τ is calculated assuming a typical known τD value.
V. Simulation Results
Speckle intensity decorrelation is simulated using an MCRT code followed by the patch analysis.
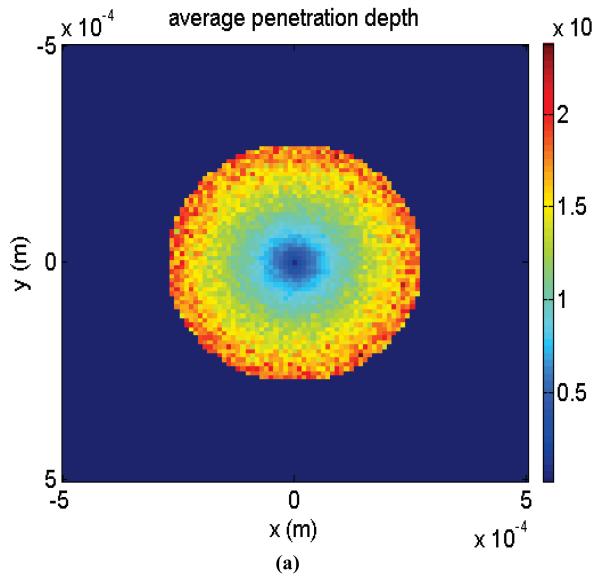
Fig. 1 (a), (b), and (c) demonstrate color-maps of mean-maximum laser penetration into the scattering medium, average number of scattering events, and the expected value of τ associated with each patch. From Fig. 1 (a) it is evident that the light exiting from the center is coming from shallower depths. However, by moving away from the origin, contribution from deeper regions increases. Also, Fig. 1 (b) demonstrates the fact that light remitted from outer rings is scattered more than the light emerging close to center. Therefore, as expected, intensity decorrelation decay is more accelerated close to periphery, as shown in Fig. 1 (c).
Fig. 1.
(a) Color-map of mean-maximum penetration depth for each pixel, (b) Color-map of average number of scatterings for each pixel, and (c) Expected value of τ for each pixel for diffusion time constant of τD = 4.65 s.
VI. Experimental Results
In order to validate the potential of LSM for producing high resolution maps of mechanical properties, laser speckle time series acquired from atherosclerotics plaque are processed using a patch analysis.
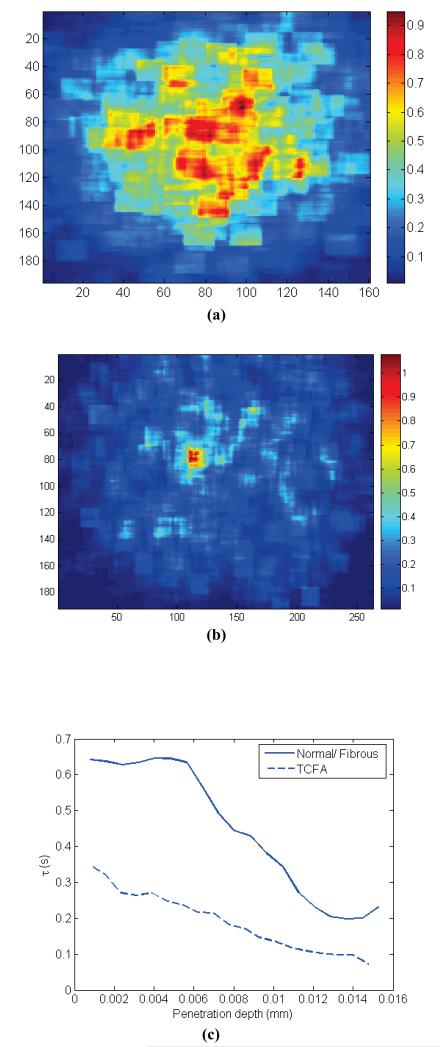
Fig. 2 (a) demonstrates the results of patch-analysis for a Normal-Fibrous atherosclerotic plaque. Fig. 2 (b) displays such results for a Thin Cap Fibroatheroma (TCFA). In Fig. 2 (c) average τ value is displayed versus penetration depth for plaques of Fig. 2 (a) and (b).
Fig. 2.
(a) Patch analysis of τ for Normal/Fibrous plaque, (b) Patch analysis of τ for TCFA plaque, and (c) τ vs. average penetration depth for Normal/Fibrous and TCFA plaques.
While τ values are relatively high for the fibrous plaque and the τ colormap is quite uniform all-over the image area, for the TCFA τ values are low. Moreover, as seen in Fig. 2(c) for the TCFA, a fast decay of τ is observed by moving away from the origin. In other words, while at regions close to the center, which bear information about superficial thin fibrous cap, moderate τ values are measured, a fast decay of τ is observed at peripheral regions associated with underlying lipid pool. In the contrary, for the Normal/Fibrous sample, τ is a relatively constant function of depth. This indicates a more uniform structure of the normal sample.
VII. Discussion
Simulation results clearly show that even for a homogeneous sample, slight variations in patch τ values are expected due to multiple scattering. Thus, exploiting corrective measures are essential in future development of LSM, where 2D scanning is combined with patch processing. Experimental results also demonstrate the effect of multiple scattering and slight reductions of τ values at peripheral regions. Nevertheless, as inferred from Fig. 2, patch-processing is efficient for the cases where drastic changes in mechanical properties occur such as in TCFA. Due to conspicuous difference in mechanical properties of fibrous cap and necrotic core, a noticeable change in τ values is observed, which is clearly distinct from slight alteration of τ observed in fibrous plaque.
While experimental results demonstrate efficacy of patch-analysis for extracting depth-resolved information, they also call for corrective measures to compensate for multiple scattering to improve LSM sensitivity to more subtle changes in biomechanics.
Acknowledgment
This work was funded by the following NIH grants: 5R21HL089203-01 and the ARRA supplement 3R21HL089203-01A1S1.
Contributor Information
Zeinab Hajjarian, Wellman Center for Photomedicine, Harvard Medical School, 40 Blossom St., Boston, MA 02114, USA (zhajjarian@partners.org).
Seemantni K. Nadkarni, Wellman Center for Photomedicine, Harvard Medical School, 40 Blossom St., Boston, MA 02114, USA (phone: 617-726-0183, snadkarni@partners.org).
References
- [1].Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007 Jul;3:413–38. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nadkarni SK, Bouma BE, Helg T, Chan R, Halpern E, Chau A, Minsky MS, Motz JT, Houser SL, Tearney GJ. Characterization of atherosclerotic plaques by laser speckle imaging. Circulation. 2005 Aug 9;112:885–92. doi: 10.1161/CIRCULATIONAHA.104.520098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nadkarni SK, Bilenca A, Bouma BE, Tearney GJ. Measurement of fibrous cap thickness in atherosclerotic plaques by spatiotemporal analysis of laser speckle images. J Biomed Opt. 2006 Mar-Apr;11:21006. doi: 10.1117/1.2186046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nadkarni SK, Bouma BE, D Y, Tearney G, J Laser Speckle Imaging of atherosclerotic plaques through optical fiber bundles. J Biomed Opt. 2008 Sep-Oct;13:054016. doi: 10.1117/1.2982529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hajjarian Z, Xi J, Jaffer FA, Tearney GJ, Nadkarni SK. Intravascular laser speckle imaging catheter for the mechanical evaluation of the arterial wall. J Biomed Opt. Mar-Apr;16:026005. doi: 10.1117/1.3533322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mason TG, Gang H, Weitz DA. Diffusing-wave-spectroscopy measurements of viscoelasticity of complex fluids. J. Opt. Soc. Am. A. 1997;14:11. [Google Scholar]
- [7].Pecora R. Dynamic light scattering: applications of photon correlation spectroscopy. Plenum Press; New York: 1985. [Google Scholar]
- [8].Brown W. Dynamic light scattering: the method and some applications. Clarenson Press; Oxford: 1993. [Google Scholar]