Abstract
Objective
Few studies have investigated the epidemiology of systemic lupus erythematosus (SLE) in American Indian and Alaska Native populations. The objective of this study was to determine the prevalence and incidence of SLE in the Indian Health Service (IHS) active clinical population in 3 regions of the US.
Methods
For this population-based registry within the IHS, the denominator consisted of individuals in the IHS active clinical population in 2007, 2008, and/or 2009 and residing in a community in 1 of 3 specified regions. Potential SLE cases were identified based on the presence of a diagnostic code for SLE or related disorder in the IHS National Data Warehouse. Detailed medical record abstraction was performed for each potential case. The primary case definition was documentation in the medical record of ≥4 of the revised American College of Rheumatology criteria for the classification of SLE. Prevalence was calculated for 2007, and the mean annual incidence was calculated for the years 2007 through 2009.
Results
The age-adjusted prevalence and incidence of SLE according to the primary definition were 178 per 100,000 person-years (95% confidence interval [95% CI] 157–200) and 7.4 per 100,000 person-years (95% CI 5.1–10.4). Among women, the age-adjusted prevalence was 271, and the age-adjusted incidence was 10.4. The prevalence was highest in women ages 50–59 years and in the Phoenix Area IHS.
Conclusion
The first population-based lupus registry in the US American Indian and Alaska Native population has demonstrated that the prevalence and incidence of SLE are high. Our estimates are as high as or higher than the rates reported in the US black population.
Systemic lupus erythematosus (SLE) is an autoimmune disease with many potential manifestations and complex classification criteria. Estimates of the overall prevalence of SLE in the US have ranged from 15 to 144 per 100,000 (1,2), and the incidence has ranged from 1.8 to 23.2 cases per 100,000 per year (1). Many epidemiologic studies have documented that SLE is more common in women and in racial/ethnic minority populations, especially blacks (1-5). However, studies of the prevalence and incidence of SLE have been limited by difficulty validating the classification criteria for SLE at a population level without detailed medical record review. Furthermore, most studies have focused on white and black populations. Few epidemiologic studies have focused on the prevalence and incidence of SLE in other racial/ethnic minority populations.
The prevalence and incidence of SLE in American Indian/Alaska Native populations have been estimated in several studies (6,7). In 2 regional studies in the US American Indian/Alaska Native and Canadian Aboriginal populations (8,9), the age-adjusted prevalence ranged from 42 to 112 per 100,000. In both studies, the prevalence of SLE in these populations was significantly higher than that in the general population. In addition, the Canadian study showed that SLE was more severe in Aboriginal patients and was associated with greater mortality (9). A more recent Canadian study using administrative data revealed an increased prevalence of SLE that was most pronounced in First Nations women older than age 45 years (10). Incidence estimates in the American Indian/Alaska Native population have varied, but a study using Indian Health Service (IHS) hospital discharge records without case validation showed that the mean annual incidence ranged from 1.2 to 4.1 per 100,000 person-years in the 1970s and 1980s, with variation by region (7).
In partnership with the Centers for Disease Control and Prevention (CDC), we developed the IHS lupus registry. This registry and 4 other CDC-funded registries were designed to address the limitations of data on the prevalence and incidence of SLE in racial/ethnic minority populations in the US (3). The objective of this population-based registry was to determine the prevalence (in 2007) and average annual incidence (from 2007 to 2009) of SLE in the IHS active clinical population in 3 regions of the US.
PATIENTS AND METHODS
Study population
This population-based registry was developed as a public health surveillance project within the IHS in partnership with the CDC. The protocol was reviewed by the following institutional review boards (IRBs) and was determined to be a public health activity and not research: IHS National IRB, Alaska Area IRB, Phoenix Area IRB, and Oklahoma City Area IRB. Staff of the CDC IRB previously judged these registries to be public health surveillance and not research (3). Permission was obtained from each IHS region or facility included in the registry for access to the medical records according to local policies and procedures for public health activities and accounting for disclosures.
Any individual included in the IHS active clinical population in 2007, 2008, and/or 2009 and residing in a community of interest was eligible for inclusion in the registry. Active clinical population was defined based on the following criteria: 1) must be alive at the beginning of the calendar year; 2) must have 2 or more visits to an IHS-funded clinic in the past 3 years, at least 1 of which must be to a core medical clinic (which includes clinics in primary care, women’s health, pediatrics, diabetes, and urgent care); and 3) must not be a non-Indian beneficiary. The active clinical population is based on the definitions used in Government Performance and Results Act (GPRA) reporting and is more restrictive than the IHS user population, which captures anyone with at least 1 visit to any IHS-funded clinic (including dental and specialty clinics) or at least 1 inpatient stay funded by the IHS in the last 3 years and who does not require a visit to a core medical clinic (11). The purpose of restricting the population for this project and for GPRA reporting is to include a population receiving ongoing medical care at IHS-funded facilities.
Communities of interest selected for inclusion in the registry were those where access to rheumatology specialist consultation was available within the IHS system (direct care) at the time of development of the registry rather than as an external consultation requiring funding from Contract Health Services (contract health care). Across the US, the IHS services are administered by 12 Area offices. At the time of the development of this registry, 3 of the 12 Areas had rheumatologists available for provision of care at IHS facilities (direct care), including the Alaska, Phoenix, and Oklahoma City Areas. In these 3 regions, the distribution of rheumatology clinics varied, with direct rheumatology care available to all of the active clinical population in Alaska and only to residents of certain communities in the other Areas.
Although all 3 Areas had rheumatologists available for direct care at the time of the development of the registry, the duration of availability varied by Area, with full direct care access since 1976 in Alaska, since 2005 in Phoenix, and not until 2011 (after the time period of registry data collection) in the Oklahoma City Area. Communities of interest included in the registry are grouped into 3 regions: 1) Alaska (encompassing the entire IHS Alaska Area); 2) Phoenix (encompassing the majority of communities in the Phoenix Area); and 3) Oklahoma (encompassing 2 Service Units [groupings of communities] in the Oklahoma City Area). Separate denominator files were created for each year, based on the active clinical population residing in communities of interest in each included region in 2007, 2008, and 2009.
Case ascertainment
Potential SLE cases were ascertained from the IHS National Data Warehouse (NDW). The NDW is a central repository of limited clinical and administrative data needed for mandatory reporting by IHS and tribal facilities. Data are transmitted electronically from IHS and tribal health care facilities to the NDW. A probabilistic matching strategy is used to assign each individual patient in the national IHS database a unique identifier to be linked across all facilities. The NDW contains demographic data (including historic addresses), administrative encounter data, and limited clinical data needed for reporting on conditions such as diabetes.
The following criteria were applied to each denominator file for each region to select potential cases from the NDW: any encounter at any IHS facility from 2006 through the first half of 2010 for any of the following International Classification of Diseases, Ninth Revision (ICD-9) codes: 710.0, 710.8, 710.9, 695.4, 710.1, and 710.4. These codes include codes for SLE, undifferentiated connective tissue disease, discoid lupus, systemic sclerosis, and polymyositis, in order to capture a broader range of possible connective tissue disease diagnoses that might include SLE. All potential cases were entered into a secure IHS database, and demographic information was collected from the NDW to begin populating the abstraction database. Medical record abstraction was initiated for all individuals in the database.
Field data abstraction
Field medical record abstraction to obtain the detailed information needed for SLE classification was performed for all potential cases after they were entered in the database. Trained abstractors reviewed medical records, both paper and electronic, for each individual at each facility where medical records existed for that individual within our regions of inclusion. Data elements included all elements necessary for confirmation of classification criteria, date of diagnosis, as well as additional elements related to medication use, other potential manifestations of SLE, and complications possibly related to SLE. A standardized data dictionary and chart abstraction forms were used, based on those developed by the Georgia Lupus Registry (GLR) (4). Abstractors underwent training by the Principal Investigator (EDF) that began with an overview of SLE disease manifestations and criteria. The training included a detailed review of the definition of all data elements in the data dictionary, with examples. Practice charts were used for training. To become certified to perform field abstraction, abstractors were required to demonstrate 95% agreement with the gold standard for elements constituting the American College of Rheumatology (ACR) revised criteria for SLE (12,13) and clinical manifestations and 90% agreement for all other elements. After every 100 charts reviewed by an individual abstractor during field medical record abstraction, quality assurance audits were performed for 5 charts to ensure agreement between abstractors, using the same criteria described above for percent agreement.
Case definitions
Our primary case definition for SLE was documentation of the presence of ≥4 of the 11 ACR revised criteria for SLE in the medical record. This primary case definition was identical to that in the 2 other CDC-funded lupus registries with published results (4,5). We used an alternate definition for sensitivity analysis, which included cases meeting the primary definition plus those cases with documentation of 3 of 11 ACR criteria in whom the final diagnosis of SLE was made by the treating rheumatologist. This alternate definition was selected to allow for the clinical judgment of the treating rheumatologist in diagnosing this complex disease and account for the potential of missing data in prevalent cases with a long duration of disease. The alternate definition was identical to that used in the GLR (4). The ACR criteria were determined according to the standard definitions (12,13), including renal disorder (proteinuria >0.5 gm/day or >3+ on at least 2 separate occasions or cellular casts present). End-stage renal disease (ESRD) was considered present if chronic dialysis or a history of renal transplantation in the medical record was documented by a physician.
Statistical analysis
Prevalence was calculated using the number of cases meeting the primary or alternate definition with a date of diagnosis of 2007 or earlier divided by the number of individuals in the 2007 denominator, expressed as the rate per 100,000 population. Prevalence was calculated overall, by sex, by region (Alaska, Phoenix, Oklahoma), and by age (using the following age groups: 0–11 years, 12–19 years, 20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years, and 80 years and older). Age-adjusted rates were calculated overall and for each sex and region using the 2000 projected US population (14). For each proportion, 95% confidence intervals (95% CIs) were calculated (15,16).
Incidence was calculated using the number of cases meeting the primary or alternate definition with a date of diagnosis from 2007 to 2009 divided by the number of person-years of individuals at risk from 2007 to 2009. Mean annual incidence is reported per 100,000 person-years. Incidence was calculated overall, by sex, by region, and by age using the same age groups as used for prevalence. Age-adjusted rates were calculated overall and for each sex and region using the 2000 projected US population (14). For each proportion, 95% CIs were calculated. When fewer than 15 cases were available, the incidence was calculated, but age-adjusted estimates were not.
The prevalence of individual ACR criteria in prevalent cases meeting our primary case definition was calculated overall and by region. Differences between the prevalence of ACR criteria by region were analyzed by chi-square or Fisher’s exact test as appropriate. Two-sided P values less than 0.05 were considered significant. Statistical analyses were performed using OpenEpi version 3.01 (17), SAS version 9.3, and Stata.IC version 11.2 for Windows.
RESULTS
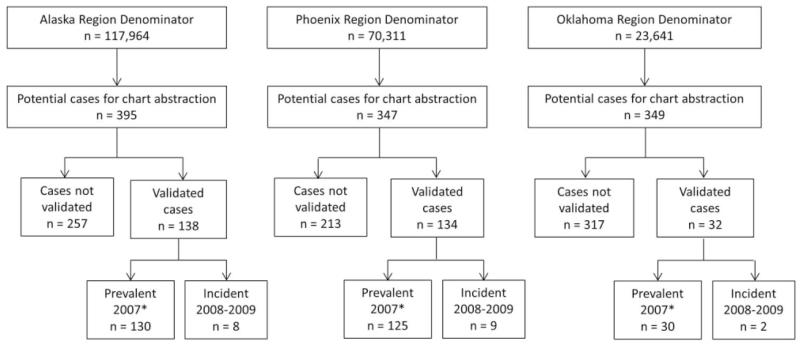
The flow chart for inclusion in the registry is shown in Figure 1. The denominators for each region represent the active clinical population for all communities of interest included in the registry in 2007. Potential cases were identified from these denominators for chart abstraction, as described above. The number of validated cases according to the primary case definition is shown, further subdivided into prevalent cases in 2007 and incident cases in 2008–2009. The total number of prevalent cases validated by the primary case definition was 285. The cases not validated included miscoded cases, cases with alternate diagnoses, and cases with insufficient ACR criteria for classification as SLE. The percentage of cases validated was lowest in the Oklahoma region (<10%) but was similar in the Alaska and Phoenix regions (35–40%). For all field abstraction quality assurance audits, each abstractor passed with 95–96% agreement on key elements and 96–98% overall agreement.
Figure 1.
Flow diagram for inclusion of potential cases in the population-based systemic lupus erythematosus (SLE) registry in the US American Indian and Alaska Native population. The denominator for each region represents the active clinical population in 2007 for the communities of interest included in the registry. Potential cases in the denominator identified as having an International Classification of Diseases, Ninth Revision code associated with lupus or other connective tissue disease were flagged for medical record abstraction. Potential cases are classified as either not validated (includes miscoded cases, alternate diagnoses, and insufficient American College of Rheumatology [ACR] criteria for classification as SLE) or validated (documentation in the medical record of ≥4 of the ACR classification criteria for SLE). Validated cases are subdivided into prevalent cases (2007) or incident cases (2008–2009). Incident cases in 2007 were included in the number of prevalent cases in 2007 (*).
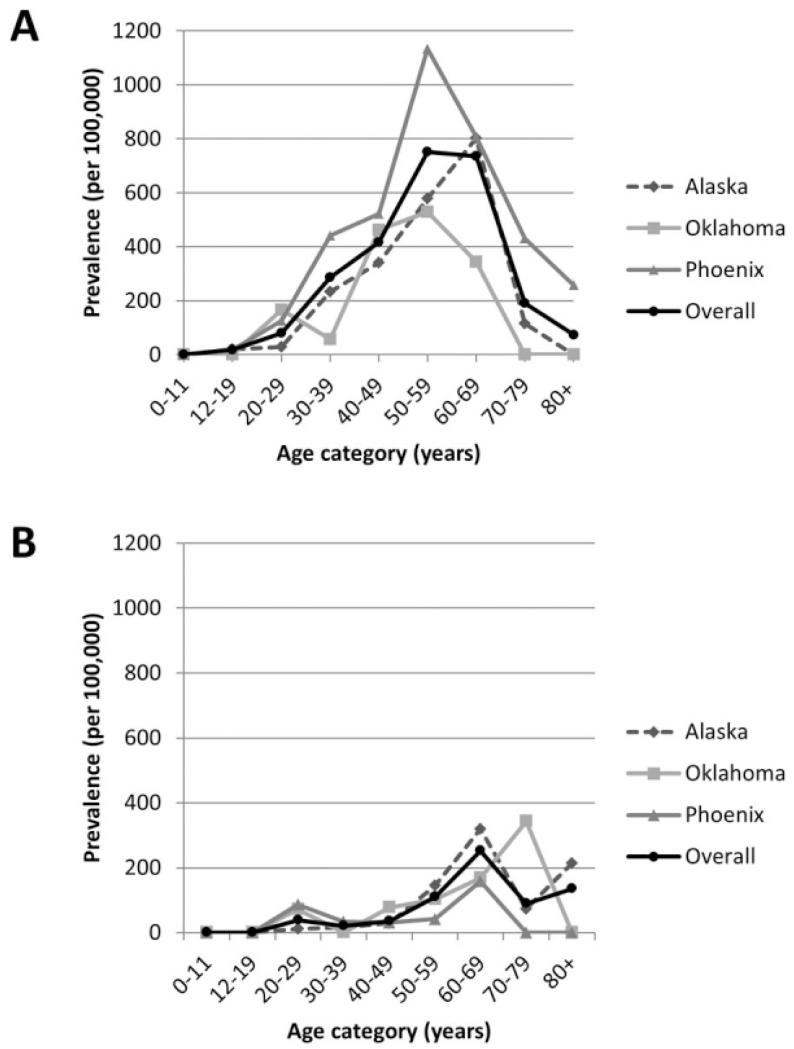
The prevalence of SLE in 2007 according to the primary and alternate definitions is shown in Table 1. The age-adjusted prevalence of SLE in the combined regions according to our primary definition was 178 per 100,000 population (95% CI 157–200) and by our broader alternate definition was 190 per 100,000 population (95% CI 168–213). The prevalence of SLE was significantly higher in women than in men (age-adjusted prevalence by primary definition 271 versus 54, with nonoverlapping 95% CIs). The age-adjusted prevalence was significantly higher in the Phoenix region for both the primary and alternate definitions (248 [95% CI 204–297] and 263 [95% CI 219–315], respectively) compared with those in the Alaska and Oklahoma regions. The age-specific prevalence rates for the primary definition overall and by region and sex are shown in Figure 2. In women, the prevalence was highest in a broader age group 50–69 years, while in men, it was highest in those 60–69 years of age. The highest prevalence by region, sex, and age group was in women ages 50–59 years in the Phoenix region (1,134 [95% CI 830–1,546] by primary definition).
Table 1.
Prevalence of SLE in 2007 according to the primary and alternate definitions, categorized by sex and region*
| Primary definition |
Alternate definition |
||||||
|---|---|---|---|---|---|---|---|
| Denominator | No. of cases |
Unadjusted rate (95% CI) |
Age-adjusted rate (95% CI) |
No. of cases |
Unadjusted rate (95% CI) |
Age-adjusted rate (95% CI) |
|
| Combined regions | 211,916 | 285 | 134 (120–151) | 178 (157–200) | 304 | 144 (128–160) | 190 (168–213) |
| Sex-specific | |||||||
| Female | 116,551 | 251 | 215 (190–244) | 271 (238–307) | 268 | 230 (204–259) | 289 (255–326) |
| Male | 95,365 | 34 | 36 (26–50) | 54 (36–77) | 36 | 38 (27–52) | 57 (39–81) |
| Regional | |||||||
| Alaska | 117,964 | 130 | 110 (93–131) | 149 (124–177) | 139 | 118 (100–139) | 159 (133–189) |
| Phoenix | 70,311 | 125 | 178 (149–212) | 248 (204–297) | 133 | 189 (160–224)) | 263 (219–315) |
| Oklahoma | 23,641 | 30 | 127 (89–179) | 138 (93–200) | 32 | 135 (96–191) | 147 (100–210) |
Rates are per 100,000 population. Age-adjusted rates were calculated using the 2000 projected US population and 10-year age groups (14). The primary definition is documentation in the medical record of ≥4 of 11 American College of Rheumatology (ACR) classification criteria for systemic lupus erythematosus (SLE). The alternate definition is the primary definition or documentation of ≥3 ACR criteria and a final diagnosis of SLE made by the treating rheumatologist. 95% CI = 95% confidence interval.
Figure 2.
Age-specific prevalence of systemic lupus erythematosus (SLE) based on the primary definition (documentation in the medical record of ≥4 of 11 American College of Rheumatology classification criteria for SLE) by region and overall, in women (A) and men (B).
The mean annual incidence of SLE is shown in Table 2. The age-adjusted incidence of SLE in the combined regions according to the primary definition was 7.4 per 100,000 person-years (95% CI 5.1–10.4), and the age-adjusted incidence according to the broader alternate definition was 8.6 per 100,000 person-years (95% CI 6.1–11.8). The age-adjusted incidence was not calculated for men or for the Oklahoma region due to a small number of cases. Differences between remaining regions were not statistically significant. Age-specific incidence and prevalence estimates are shown in Table 3 for the combined regions, including both men and women. For both definitions, the highest incidence was in the age group 40–49 years, and the highest prevalence was in the age group 60–69 years. For the 285 prevalent cases, the mean ± SD age at diagnosis was 39.2 ± 13.5 years. The mean age at diagnosis did not differ significantly by region (P = 0.91). The mean ± SD age at diagnosis for incident cases was 45.5 ± 16.2 years, and this did not differ significantly by region (P = 0.98).
Table 2.
Mean annual incidence of SLE from 2007 through 2009 according to the primary and alternate definitions, categorized by sex and region*
| Primary definition |
Alternate definition |
||||||
|---|---|---|---|---|---|---|---|
| Person-years of observation |
No. of cases |
Unadjusted rate (95% CI) |
Age-adjusted rate (95% CI) |
No. of cases |
Unadjusted rate (95% CI) |
Age-adjusted rate (95% CI) |
|
| Combined regions | 649,302 | 38 | 5.9 (4.2–8.0) | 7.4 (5.1–10.4) | 44 | 6.8 (5.0–9.0) | 8.6 (6.1–11.8) |
| Sex-specific | |||||||
| Female | 357,137 | 30 | 8.4 (5.8–11.8) | 10.4 (6.6–14.6) | 36 | 10.1 (7.2–13.8) | 12.1 (8.3–17.1) |
| Male | 292,165 | 8 | 2.7 (1.3–5.2) | – | 8 | 2.7 (1.3–5.2) | – |
| Regional | |||||||
| Alaska | 357,419 | 17 | 4.8 (2.9–7.5) | 6.1 (3.4–10.2) | 20 | 5.6 (3.5–8.5) | 7.2 (4.2–11.5) |
| Phoenix | 221,770 | 18 | 8.1 (5.0–12.6) | 10.7 (6.2–17.5) | 21 | 9.5 (6.0–14.2) | 12.7 (7.6–20.1) |
| Oklahoma | 70,113 | 3 | 4.3 (1.1–11.7) | – | 3 | 4.3 (1.1–11.7) | – |
Rates are per 100,0000 person-years. Age-adjusted rates were calculated using the 2000 projected US population and 10-year age groups (14). The primary definition is documentation in the medical record of ≥4 of 11 American College of Rheumatology (ACR) classification criteria for systemic lupus erythematosus (SLE). The alternate definition is the primary definition or documentation of ≥3 ACR criteria and a final diagnosis of SLE made by the treating rheumatologist. 95% CI = 95% confidence interval.
Table 3.
Overall age-specific incidence of SLE from 2007 to 2009 and age-specific prevalence of SLE in 2007 according to the primary and alternate definitions*
| Incidence |
Prevalence |
|||||||
|---|---|---|---|---|---|---|---|---|
| Primary definition |
Alternate definition |
Primary definition |
Alternate definition |
|||||
| Age group, years |
No. of cases |
Unadjusted rate (95% CI) |
No. of cases |
Unadjusted rate (95% CI) |
No. of cases |
Unadjusted rate (95% CI) |
No. of cases |
Unadjusted rate (95% CI) |
| 0–11 | 0 | 0.0 (0.0–2.1) | 0 | 0.0 (0.0–2.1) | 0 | 0.0 (0.0–6.5) | 0 | 0.0 (0.0–6.5) |
| 12–19 | 2 | 2.0 (0.6–7.4) | 2 | 2.0 (0.6–7.4) | 3 | 9.1 (3.1–26.7) | 3 | 9.1 (3.1–26.7) |
| 20–29 | 6 | 5.8 (2.6–12.6) | 8 | 7.7 (3.9–15.2) | 21 | 63.1 (41.2–96.4) | 24 | 72.1 (48.4–107.2) |
| 30–39 | 5 | 6.7 (2.9–15.8) | 5 | 6.7 (2.9–15.8) | 42 | 174.7 (129.3–236.1) | 44 | 183.0 (136.4–248.0) |
| 40–49 | 13 | 16.5 (9.6–28.2) | 16 | 20.3 (12.5–32.9) | 67 | 253.6 (199.7–321.9) | 73 | 276.3 (219.8–347.2) |
| 50–59 | 7 | 11.9 (5.8–24.5) | 7 | 11.9 (5.8–24.5) | 89 | 471.8 (383.6–580.1) | 93 | 493.0 (402.6–603.5) |
| 60–69 | 3 | 9.5 (3.2–27.9) | 3 | 9.5 (3.2–27.9) | 53 | 526.2 (402.5–687.5) | 55 | 546.0 (419.8–710.0) |
| 70–79 | 1 | 6.0 (1.1–34.2) | 2 | 12.1 (3.3–44.1) | 8 | 148.8 (75.4–293.4) | 10 | 186.0 (101.1–342.1) |
| 80+ | 1 | 15.4 (2.7–87.0) | 1 | 15.4 (2.7–87.0) | 2 | 94.9 (26.0–345.4) | 2 | 94.9 (26.0–345.4) |
Rates are per 100,0000 person-years. The primary definition is documentation in the medical record of ≥4 of the American College of Rheumatology (ACR) classification criteria for systemic lupus erythematosus (SLE). The alternate definition is the primary definition or documentation of ≥3 ACR criteria and a final diagnosis of SLE made by the treating rheumatologist. 95% CI = 95% confidence interval.
The frequency of the individual ACR criteria in cases in the registry overall and by region is shown in Table 4. The presence of antinuclear antibodies (ANAs) was the most common criterion (98.2% overall) and did not differ by region. Criteria with significant differences between regions included arthritis, immunologic disorder, photosensitivity, renal disorders, oral ulcers, and discoid rash. The least frequent criterion was neurologic disorder, which was present in 2.8% of cases overall. Renal disorder was present in 39.6% of cases. From medical record abstraction, ESRD was noted in 5.6% of prevalent cases, with no statistically significant difference by region (P = 0.33).
Table 4.
Frequency of the individual ACR criteria for the classification of systemic lupus erythematosus among 285 prevalent cases meeting the primary definition, overall and categorized by region*
| ACR classification criterion* | Combined regions (n = 285) |
Alaska (n = 130) |
Phoenix (n = 125) |
Oklahoma (n = 30) |
|---|---|---|---|---|
| Presence of ANAs | 98.2 | 99.2 | 97.6 | 96.7 |
| Hematologic disorder | 89.8 | 90.0 | 90.4 | 86.7 |
| Arthritis† | 80.4 | 71.5 | 94.4 | 60.0 |
| Immunologic disorder† | 60.7 | 71.5 | 51.2 | 53.3 |
| Photosensitivity† | 53.0 | 56.9 | 55.20 | 26.7 |
| Serositis | 48.1 | 44.6 | 54.4 | 36.7 |
| Renal disorder† | 39.6 | 51.5 | 28.0 | 36.7 |
| End-stage renal disease‡ | 5.6 | 6.9 | 5.6 | 0 |
| Oral ulcers† | 35.1 | 30.8 | 44.8 | 13.3 |
| Malar rash | 31.6 | 27.7 | 35.2 | 33.3 |
| Discoid rash† | 8.4 | 3.1 | 15.2 | 3.3 |
| Neurologic disorder | 2.8 | 4.6 | 0.8 | 3.3 |
The primary definition is documentation in the medical record of ≥4 of the American College of Rheumatology (ACR) classification criteria for systemic lupus erythematosus. Values are the percent. ANAs = antinuclear antibodies.
Significant (P < 0.01) differences between regions.
Defined by abstraction only.
DISCUSSION
In this population-based lupus registry of American Indian/Alaska Native people within the IHS active clinical population in 3 regions of the US, we determined that the prevalence and incidence of SLE are high. By our primary case definition, the age-adjusted prevalence of SLE in the combined regions was 178 per 100,000 population, with a prevalence of 271 in women and 54 in men. Prevalence according to the alternate definition was 190 per 100,000 for the combined regions, with a prevalence of 289 in women and 57 in men. The age-adjusted overall incidence was 7.4 per 100,000 person-years according to the primary case definition and 8.6 per 100,000 person-years by the alternate definition. The small number of incident cases precluded comparison of age-adjusted incidence rates, but the crude incidence was higher in women than in men (8.4 versus 2.7 per 100,000 person-years according to the primary definition and 10.1 versus 2.7 per 100,000 person-years by the alternate definition).
The age-adjusted prevalence of SLE in American Indian/Alaska Native women (271 per 100,000) is similar to that in black women, the population with the highest prevalence of SLE described (196 and 186 per 100,000 in the recently published GLR and the Michigan Lupus Epidemiology and Surveillance Program [MILES], respectively) (4,5). The age-adjusted prevalence in American Indian/Alaska Native men was also higher than that in black men (54 per 100,000 for American Indian/Alaska Native men, compared with 24 and 19 per 100,000 in the GLR and MILES, respectively) (4,5). In addition, we observed a higher prevalence of SLE compared with previous regional estimates in indigenous populations in Southeast Alaska (112 per 100,000) (8) and Manitoba (42 per 100,000) (9). In a recent study of the prevalence of SLE among Medicaid-enrolled adults, using administrative data, the estimated prevalence in American Indian/Alaska Native people (166 per 100,000 overall, 213 in women and 49 in men) (2) was comparable with that in our registry.
Our estimates of incidence are less precise than our prevalence estimates because of the small size of the population included in the IHS registry. However, the age-adjusted incidence in the combined regions (7.4 per 100,000 person-years according to the primary definition) is higher than most estimates of incidence in the general US population and similar to the age-adjusted incidence reported in blacks in the GLR and MILES (8.7 and 7.9 per 100,000 person-years) (4,5).
The annual incidence of SLE was previously described in the IHS population using hospital discharge data from 1971 to 1975 (7). In that older study, incidence was reported by tribe. In 3 tribes in the northern US, the annual incidence of SLE was increased (16.6–27.1 per 100,000/year) compared with that in other tribes (0–6.9 per 100,000/year) (6). Those cases were validated using the ACR 1971 criteria for the classification of SLE (18). An additional study using IHS hospital discharge billing codes from 1980 to 1990 investigated incidence by IHS administrative Area (7) and demonstrated the highest incidence of SLE in the Aberdeen, Alaska, Billings, and Phoenix Areas (4.1, 3.3, 3.2, and 3.0 per 100,000, respectively). We did not include the Aberdeen or Billings Area in this registry. It is no longer acceptable to report rates by tribe, without permission from the tribe, but the regions included in our registry do not include significant numbers of persons from the tribes noted to have the highest incidence of SLE in the study from the 1970s.
We found that the mean age at onset for incident cases was the mid 40s, while the mean age at onset for prevalent cases was 39 years. Previous studies have shown a younger age at onset in indigenous North American populations (~30 years) (9,19). The age at onset in our registry was higher than expected and could possibly be related to the small number of incident cases and long duration of disease in prevalent cases.
Several studies have documented the frequency of individual ACR classification criteria in indigenous North American people. In our registry, the 3 most common criteria met by cases were ANA positivity (98.2%), hematologic disorder (89.8%), and arthritis (80.4%). Discoid rash and neurologic disorder were the least common criteria met (8.4% and 2.8%, respectively). A study of rheumatic diseases in Oklahoma tribal populations found that a significant proportion of patients with SLE had arthritis (>80%), and that discoid lupus was uncommon (20), similar to our findings. The 2 regional studies in Southeast Alaska and Manitoba showed a high frequency of ANA positivity (100%) and a high prevalence of arthritis (≥90%) (8,9).
Renal disorder was documented in 39.6% of cases in this registry, with ESRD documented in 5.6% of cases. Similar to our findings, renal disorder was present in 39% of cases in the previous study in Southeast Alaska (8), and cellular casts and proteinuria were present in 35% and 46% of Aboriginal Manitobans with SLE, respectively (9). The recent data from the GLR and MILES showed that renal disorder was present in 36.7% and 40.5% of blacks with SLE, respectively (4,5); these percentages are higher than those in whites and are similar to the data from the IHS registry. These combined data suggest that renal disease may be as common in American Indian/Alaska Native populations with SLE as in black patients with SLE. The frequency of renal disease may partially explain the increased mortality observed in Aboriginal SLE patients in Manitoba (9). The ESRD data from our study are not directly comparable with the data from the GLR and MILES, because we did not have access to the United States Renal Data System and based the diagnosis of ESRD on medical record review alone. ESRD was noted in 5.6% of patients with SLE in our registry, compared with 8.4% of black patients in the GLR (4) and 15.3% of black patients in MILES (5). Future studies of SLE in American Indian/Alaska Native populations should further investigate ESRD and other measures of disease severity.
There are many possible explanations for the high rates of SLE observed in this registry in comparison with other populations and with previous data from American Indian/Alaska Native populations. First, our registry captured all documented diagnoses of SLE within the IHS system, regardless of the specialty of the provider making the diagnosis. To be included in our denominator, individuals were required to have at least 2 visits to an IHS or tribal facility in the past 3 years. This criterion could have excluded some healthy individuals who do not seek any medical care, leading to higher estimates of prevalence and incidence. However, many persons who are eligible for IHS services choose to receive care elsewhere because of convenience or other insurance options, and we did not have access to records outside the IHS system. Therefore, our denominator was selected to provide the best estimates for this population. Second, the previous studies of prevalence in Alaska and Canada did perform case validation in a manner similar to that used in our registry but included smaller regions and had different methods for case ascertainment (8,9). Third, previous studies of incidence in the IHS either used older SLE classification criteria or did not validate cases (6,7). Fourth, another possible explanation could be the inclusion of the urban location in Phoenix but exclusion of some rural areas. If persons with disease migrate to urban areas for medical care, it could lead to an overestimation of prevalence or incidence in this region. In addition to migration to urban areas by individuals with disease, it is possible that patients with SLE migrate into IHS care as they lose employer-based insurance. However, it is also possible that patients with ESRD migrate out of the IHS given that most IHS facilities do not have direct-care nephrologist access or in-house dialysis, and quantifying the effects of these possible migrations is difficult. Fifth, SLE is an autoimmune disease with strong genetic and environmental associations (21). It is possible that genetic risk factors for SLE are overrepresented in the American Indian/Alaska Native population (22). Finally, differences in environmental exposures such as sun exposure or tobacco use could potentially contribute to the high rates observed in this population and to the regional variation in rates.
Our registry has some limitations. First, we included only 3 of the 12 IHS administrative Areas. This may limit the generalizability to the entire US American Indian/Alaska Native population, especially given the likelihood of variation by region. The rationale for selection of these 3 Areas was the likelihood of better data quality in the Areas where specialist access was readily available. We believe that the exclusion of the Aberdeen and Billings Areas, where previous studies have shown evidence of high rates of SLE, may have led us to underestimate the prevalence of SLE. However, the estimates from this project are higher than previous estimates for American Indian/Alaska Native populations and as high as those reported in black women and men.
Second, we included only select communities within the Phoenix and Oklahoma City Areas, which might limit the generalizability of the estimates to the entire administrative Area. However, the majority of the Phoenix Area was included, and this limitation is more relevant to Oklahoma. Third, this registry was restricted to American Indian/Alaska Native people receiving care within the IHS system. This limits the population to members of federally recognized tribes who live in proximity to IHS-funded facilities and choose to receive care through the IHS. If we had used a broader population denominator of all American Indian/Alaska Native people in these regions, our estimates may have been slightly lower. However, the differences are not readily quantifiable, because potential cases were identified from our denominator, and broadening the denominator could have led to the inclusion of additional cases.
Fourth, we used only one data source (the NDW) to identify potential cases. However, the NDW was a robust source of demographic and administrative data that helped facilitate the creation of the registry. Fifth, the proportion of validated cases was low overall and lower in Oklahoma compared with the other regions. We believe the low proportion of validated cases in the Alaska and Phoenix regions may be related to the broad set of connective tissue disease–associated ICD-9 codes included and the lack of more specific coding definitions used in administrative studies, such as the requirement for >1 code or coding by a specialist (23). The lower proportion of validated cases in Oklahoma is likely related to the recent availability of direct care rheumatology services in Oklahoma IHS. Finally, we captured billing codes only from 2006 through the first half of 2010, so it is possible that milder prevalent cases could have been missed.
In summary, we observed a high prevalence and incidence of SLE among American Indian/Alaska Native people in the IHS active clinical population in 3 regions of the US. In American Indian/Alaska Native women, the prevalence of SLE is essentially the same as the prevalence in black women in the US, the group in which the prevalence is highest. These data support a need for increased awareness of SLE by clinicians in IHS and tribal facilities and for research characterizing SLE disease severity and clinical outcomes in American Indian/Alaska Native populations.
ACKNOWLEDGMENTS
We would like to acknowledge the IHS Performance Evaluation System and the field abstractors (Amy Swango-Wilson, Alette Thompson, and Vivian Kelly) for their assistance with this registry. We would also like to acknowledge colleagues from the Georgia Lupus Registry, Michigan Lupus Epidemiology and Surveillance Program, California Lupus Surveillance Program, and the Manhattan Lupus Surveillance Program.
Supported by an interagency agreement award from the CDC to the Indian Health Service (IAA 10FED1003070).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ferucci had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Ferucci, Sumner, Posever, Gordon, Lim, Helmick.
Acquisition of data. Ferucci, Gaddy, Sumner, Posever, Choromanski.
Analysis and interpretation of data. Ferucci, Johnston, Gaddy, Gordon, Lim, Helmick.
REFERENCES
- 1.Lim SS, Drenkard C. Epidemiology of systemic lupus erythematosus: capturing the butterfly. Curr Rheumatol Rep. 2008;10:265–72. doi: 10.1007/s11926-008-0043-4. [DOI] [PubMed] [Google Scholar]
- 2.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65:753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim SS, Drenkard C, McCune WJ, Helmick CG, Gordon C, DeGuire P, et al. Population-based lupus registries: advancing our epidemiologic understanding. Arthritis Rheum. 2009;61:1462–6. doi: 10.1002/art.24835. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the Georgia Lupus Registry. Arthritis Rheumatol. 2014;66:357–68. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66:369–78. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton RO, Gershwin ME, Brady C, Steinberg AD. The incidence of systemic lupus erythematosus in North American Indians. J Rheumatol. 1976;3:186–90. [PubMed] [Google Scholar]
- 7.Acers TE, Acers-Warn A. Incidence patterns of immunogenetic diseases in the North American Indians. J Okla State Med Assoc. 1994;87:309–14. [PubMed] [Google Scholar]
- 8.Boyer GS, Templin DW, Lanier AP. Rheumatic diseases in Alaskan Indians of the southeast coast: high prevalence of rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1991;18:1477–84. [PubMed] [Google Scholar]
- 9.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol. 2000;27:1884–91. [PubMed] [Google Scholar]
- 10.Barnabe C, Joseph L, Belisle P, Labrecque J, Edworthy S, Barr SG, et al. Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res (Hoboken) 2012;64:138–43. doi: 10.1002/acr.20656. [DOI] [PubMed] [Google Scholar]
- 11.Indian Health Service . Government Performance and Results Act (GPRA) Summary Report. Indian Health Service; Rockville (MD): 2009. Accessed at www.ihs.gov/ers/documents/200912AreaReport.pdf. [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2000 Stat Notes. 2001;20:1–9. [PubMed] [Google Scholar]
- 15.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16:791–801. doi: 10.1002/(sici)1097-0258(19970415)16:7<791::aid-sim500>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 17.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. 2013. [Google Scholar]
- 18.Cohen AS, Reynolds WE, Franklin EC, Kulka JP, Ropes MW, Shulman LE, et al. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis. 1971;21:643–8. [Google Scholar]
- 19.Atkins C, Reuffel L, Roddy J, Platts M, Robinson H, Ward R. Rheumatic disease in the Nuu-Chah-Nulth native Indians of the Pacific Northwest. J Rheumatol. 1988;15:684–90. [PubMed] [Google Scholar]
- 20.Gaddy JR, Vista ES, Robertson JM, Dedeke AB, Roberts VC, Klein WS, et al. Rheumatic disease among Oklahoma tribal populations: a cross-sectional study. J Rheumatol. 2012;39:1934–41. doi: 10.3899/jrheum.110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 22.Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009;10:373–9. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38:1612–6. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]