Abstract
OBJECTIVES
To understand the natural history of frailty after an aggressive surgical intervention, kidney transplantation (KT).
DESIGN
Prospective cohort study (December 2008–March 2014).
SETTING
Baltimore, Maryland.
PARTICIPANTS
Kidney transplantation recipients (N = 349).
MEASUREMENTS
The Fried frailty score was measured at the time of KT and during routine clinical follow-up. Using a Cox proportional hazards model, factors associated with improvements in frailty score after KT were identified. Using a longitudinal analysis, predictors of frailty score changes after KT were identified using a multilevel mixed-effects Poisson model.
RESULTS
At KT, 19.8% of recipients were frail; 1 month after KT, 33.3% were frail; at 2 months, 27.7% were frail; and at 3 months, 17.2% were frail. On average, frailty scores had worsened by 1 month (mean change 0.4, P < .001), returned to baseline by 2 months (mean change 0.2, P = .07), and improved by 3 months (mean change −0.3, P = .04) after KT. The only recipient or transplant factor associated with improvement in frailty score after KT was pre-KT frailty (hazard ratio = 2.55, 95% confidence interval (CI) = 1.71–3.82, P < .001). Pre-KT frailty status (relative risk (RR) = 1.49, 95% CI = 1.29–1.72, P < .001), recipient diabetes mellitus (RR = 1.26, 95% CI = 1.08–1.46, P = .003), and delayed graft function (RR = 1.22, 95% CI = 1.04–1.43, P = .02) were independently associated with long-term changes in frailty score.
CONCLUSION
After KT, in adult recipients of all ages, frailty initially worsens but then improves by 3 months. Although KT recipients who were frail at KT had higher frailty scores over the long term, they were most likely to show improvements in their physiological reserve after KT, supporting the transplantation in these individuals and suggesting that pretransplant frailty is not an irreversible state of low physiological reserve.
Keywords: frailty, kidney transplantation, physiological reserve
With advances in transplant surgery, including better surgical technique and immunosuppression, kidney transplantation (KT) has become the preferred treatment option for appropriate individuals with end-stage renal disease (ESRD).1 KT reverses the physiology of ESRD, restoring the kidneys’ function of removing excess fluid and waste from the blood without the burden of hemodialysis, but involves an initial invasive procedure.
The surgical insult that occurs during KT is profound, and recovery is potentially challenging for vulnerable recipients. During this time, recipients are at high risk of postsurgical complications and acute rejection episodes.2 Thus, there is an interesting duality after KT; recipients recover physically and psychosocially because of restored kidney function and gained independence, but they must undergo major surgery, after which they are at risk of early postoperative complications. It is hypothesized that people initially get worse after the physical insult of transplantation and then will continue down one of two paths: continued improvement or progressive decline.
Frailty, a measure of physiological reserve initially described and validated in geriatric populations,3 is emerging as an important risk factor for adverse KT outcomes, including delayed graft function (DGF),4 early hospital readmission,5 and mortality6 in adult recipients of all ages. Although previous studies suggest that older adults become more frail as they age,7–9 little is known about changes in frailty after surgery and in particular after KT. Although it is likely that increased frailty would certainly be the natural history of ESRD, it is unclear whether frailty status worsens initially after the physical insult of surgery and whether recipients continue to worsen or improve in the months after KT. Furthermore, it is unclear whether being frail at the time of KT represents an irreversible state of low physiological reserve in which recipients are unable to return to their baseline frailty status or whether they are able to increase their physiological reserve after kidney function is restored. The goal of this study was to characterize the change in frailty over time after transplantation and identify predictors of long-term changes in frailty score.
METHODS
Study Design
This was a prospective, longitudinal study of 349 KT recipients seen at Johns Hopkins Hospital, Baltimore, Maryland, between December 2008 and March 2014. (Of 349 participants with more than one visit after KT, 228 had visits within 3 months of KT, and 116 had a visit at exactly 3 months.) Mean follow-up was 14 months, and the last follow-up visit occurred 5.9 years after KT. Participants were KT recipients who were seen at transplantation, agreed to participate in this longitudinal study, and were followed at the transplant center (as opposed to those who followed up with their nephrologist or at an outside centers and those from out of the Baltimore, Maryland, area) through May 2014. Research assistants approached all eligible KT recipients at the time of admission for KT and performed a frailty assessment. The same research assistants then performed follow-up assessments during routine clinical follow-up at Johns Hopkins. Frailty (as described below) was measured at the time of KT and during clinical follow-up. Recipient and KT factors (age, sex, race, donor type, pre-KT frailty status) did not differ according to number of post-KT frailty measures, duration of follow-up, or whether the recipient had a visit at exactly 3 months. Recipient and transplant factors (sex, age, race, body mass index (BMI), diabetes mellitus, time on dialysis, donor type, previous transplant, human leukocyte antigen mismatch, peak panel reactive antibody, DGF, acute rejection) were ascertained from medical records. The Johns Hopkins institutional review board approved the study.
Frailty Measurement
Frailty was measured as Fried and colleagues defined and validated it3,10–19 and as has previously been validated in ESRD and KT populations.4,5,20–22 The phenotype was based on five components: shrinking (self-report of unintentional weight loss of >10 pounds in the past year based on dry weight), weakness (grip strength below an established cutoff based on sex and BMI), exhaustion (self-report), low activity (kcal/wk below an established cutoff), and slow walking speed (time to walk 15 feet below an established cutoff according to sex and height).3 Each of the five components was scored as 0 or 1, representing the absence or presence of that component. The aggregate frailty score was calculated as the sum of the component scores (range 0–5); nonfrail was defined as a score of 0 or 1, intermediately frail as a score of 2, and frail as a score of 3–5, as previously determined.4,5,20
Change in Frailty Score After KT
Mean change in frailty score was assessed 1, 2, and 3 months after KT, and whether there was a change in frailty score after KT was determined using a single-sample t-test with a two-sided P-value. Then, participants were classified each month as being equally frail (no change in frailty score), less frail (≥1-point decrease in frailty score between KT and follow-up visit), or more frail (≥1-point increase in frailty score between KT and follow-up visit) than at the time of KT. The transition between nonfrail, intermediately frail, and frail at KT and 3 months after KT was estimated. Next, predictors of frailty score changes after KT were identified using a multilevel mixed-effects Poisson model for unbalanced design. Recipient age, recipient sex, recipient race, donor type, BMI, previous transplant, recipient diabetes mellitus, peak panel reactive antibody, number of human leukocyte antigen mismatches, and time on dialysis were considered as potential pre-KT predictors. Factors identified during or after KT were also considered, including DGF and acute rejection as predictors of frailty score changes. Using an adjusted Cox proportional hazards model, predictors of becoming less frail (decrease in frailty score) were also identified.
Change in Frailty Components
For participants who became less frail after KT, which of the five components contributed to the improvement in frailty score 3 months after KT were identified. Similarly, for participants who became more frail after KT, which of the five components contributed to an increase in frailty score 3 months after KT were identified. More than one component could contribute to a participant being more or less frail than at the time of KT.
Statistical Analyses
For all analyses, P < .05 was considered significant. All analyses were performed using Stata version 13.0 (Stata Corp., College Station, TX).
RESULTS
Study Population
The mean age of participants was 53.3 ± 14.2 (range 19–83, median 55.8, interquartile range (IQR) 44.2–63.6, 20.9% aged ≥65); 38.1% were female, 39.8% were African American, the mean BMI was 27.5 − 5.9 kg/m2), and 19.2% had diabetes mellitus. The median number of years on dialysis was 2.1 (IQR 0.4–3.9), 20% were preemptive KT, and 37.3% were live-donor recipients. After KT, 17.8% experienced DGF and 3.2% an acute rejection. Consistent with previous findings,4,5 the prevalence of frailty at the time of KT was 19.8% (Table 1).
Table 1.
Change in Frailty Score and State Transition of Frailty Status after Kidney Transplantation (KT)
| Time | Frail, % | Change in Frailty After KT % | |
|---|---|---|---|
| Less Frail Than at KT |
More Frail Than at KT |
||
| At KT | 19.8 | — | — |
| 1 month | 33.3 | 25.6 | 45.1 |
| 2 month | 27.7 | 28.4 | 38.3 |
| 3 month | 17.2 | 44.8 | 25.0 |
The frailty score3 ranges from 0 to 5. Less frail is defined as a 1-point or more decrease in frailty score between KT and follow-up. More frail is defined as 1-point or more increase in frailty score between KT and follow-up.
Change in Frailty Status and Score After KT
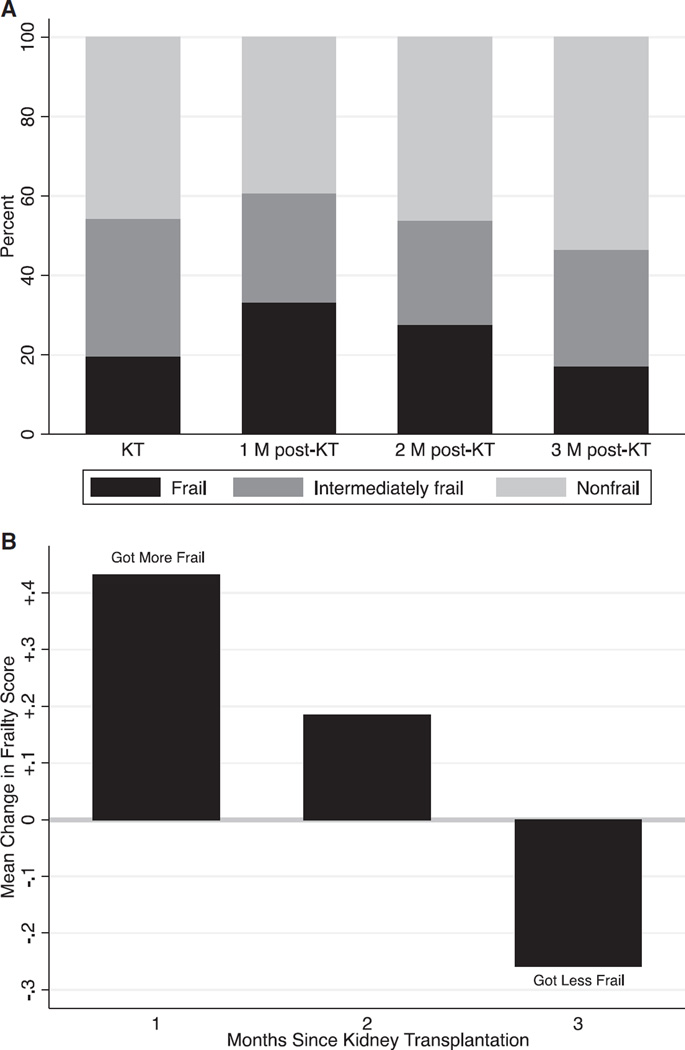
One month after KT, 33.3% of recipients were frail; 2 months after, 27.7% were frail; and 3 months after, 17.2% were frail (Table 1). Each month after KT, there was a higher percentage of KT recipients who were less frail and a lower percentage of those who were more frail than at the time of KT (Figure 1A); at 1 month, 25.6% were less frail than at the time of KT, and 45.1% were more frail; at 2 months, 28.4% were less frail, and 38.3% were more frail; and at 3 months, 44.8% were less frail, and 25.0% were more frail (Table 1). On average, frailty scores were worse than at the time of KT at 1 month (mean change 0.4, P < .001), no different from at the time of KT at 2 months (mean change 0.2, P = .07), and better than at the time of KT at 3 months (mean change −0.3, P = .04) (Figure 1B); results were similar for older adults (mean change at 3 months −0.3).
Figure 1.
Frailty after kidney transplantation (KT). (A) Prevalence of frailty status according to month (M) since KT. (B) Mean change in frailty score according to month since KT (n = 349 at KT; n = 102 1 month after KT; n = 141 2 months after KT; n = 116 3 months after KT; differing sample sizes reflecting the dynamic nature of the post-KT clinical follow-up).
Transitions in Frailty Status 3 Months After KT
Three months after KT, of those who were nonfrail at KT, 21.6% were intermediately frail, and 11.7% were frail (Table 2); of those who were intermediately frail at KT, 52.0% were nonfrail, and 20.0% were frail; and of those who were frail at KT, 33.4% were nonfrail, and 40.7% were intermediately frail.
Table 2.
Change in Frailty State Between Kidney Transplantation (KT) and 3 Months After KT
| At Time of KT | 3 Months After KT, % | ||
|---|---|---|---|
| Nonfrail | Intermediately Frail | Frail | |
| Nonfrail | 66.7 | 21.6 | 11.7 |
| Intermediately frail | 52.0 | 28.0 | 20.0 |
| Frail | 33.4 | 40.7 | 25.9 |
Nonfrail was defined as a score of 0 or 1, intermediate frailty was defined as a score of 2, and frailty was defined as a score of 3–5. The table displays the percentage of KT recipients who were nonfrail, intermediately frail, and frail 3 months after KT according to frailty status at the time of KT.
Change in Frailty Components
Of those who were less frail after KT, 47% improved (from frail to nonfrail for the component) in grip strength, 14% in weight loss, 55% in physical activity, 25% in exhaustion, and 19% in walk speed (Table 3). Of those who were more frail after KT, 20% worsened (from nonfrail to frail for the component) in grip strength, 36% in weight loss, 43% in physical activity, 50% in exhaustion, and 27% in walk speed.
Table 3.
Frailty Components That Led to Kidney Transplantation (KT) Recipients Becoming Less or More Frail Than at Time of KT
| Frailty Component | Less Frail Than at KT, %a |
More Frail Than at KT, %b |
|---|---|---|
| Weak grip strength | 47 | 20 |
| Unexplained weight loss | 14 | 36 |
| Low physical activity | 55 | 43 |
| Exhaustion | 25 | 50 |
| Slow walk speed | 19 | 27 |
Columns do not sum to 100% because participants could be more or less frail based on one or more components of frailty.
Of those who were less frail after KT.
Of those who were more frail after KT.
Characteristics of Recipients with a Change in Frailty Score over Long-Term Follow-Up
Of the recipient and donor characteristics that were known before KT, the only factor that was associated with change in frailty score after KT was recipient diabetes mellitus (relative risk (RR) = 1.23, 95% confidence interval (CI) = 1.05–1.45, P = .01) in the longitudinal data analysis (Table 4). When frailty at the time of KT was included in the longitudinal model, there was a significant association between the pre-KT measure of frailty and change in frailty score after KT (RR = 1.53, 95% CI = 1.33–1.77, P < .001). Acute rejection was not associated with change in frailty score. The only post-KT factor associated with change in frailty score was DGF (RR = 1.30, 95% CI = 1.10–1.53, P = .003). Recipient diabetes mellitus (RR = 1.26, 95% CI = 1.08–1.46, P = .003), and pre-KT frailty (RR = 1.49, 95% CI = 1.29–1.72, P < .001) remained significantly associated with frailty score change after adjusting for DGF (RR = 1.22, 95% CI = 1.04–1.43, P = .02).
Table 4.
Pre- and Post-Kidney Transplantation (KT) Factors Associated with Trajectories of Frailty
| Pre-KT Model | Pre-KT Model | |||
|---|---|---|---|---|
| Without Pre-KT Frailty | With Pre-KT Frailty | Without Pre-KT Frailty | With Pre-KT Frailty | |
| Factor | Relative Risk (95% Confidence Interval) | |||
| Frail at KT | — | 1.53 (1.33–1.77) | — | 1.49 (1.29–1.72) |
| Male recipient | 0.97 (0.85–1.12) | 1.01 (0.88–1.15) | 0.95 (0.83–1.10) | 0.99 (0.87–1.13) |
| Aged ≥65 | 1.14 (0.97–1.33) | 1.12 (0.96–1.29) | 1.14 (0.98–1.33) | 1.12 (0.97–1.29) |
| Black | 0.99 (0.86–1.14) | 0.98 (0.86–1.12) | 0.97 (0.84–1.12) | 0.96 (0.84–1.10) |
| Live donor recipient | 0.68 (0.58–0.80) | 0.70 (0.60–0.81) | 0.72 (0.61–0.84) | 0.73 (0.62–0.84) |
| Body mass index (per 5 kg/m2) | 1.01 (0.96–1.08) | 1.01 (0.95–1.07) | 1.01 (0.95–1.07) | 1.00 (0.95–1.06) |
| Previous transplantation | 1.14 (0.95–1.38) | 1.13 (0.95–1.35) | 1.17 (0.97–1.41) | 1.14 (0.96–1.36) |
| Diabetes mellitus | 1.23 (1.05–1.45) | 1.26 (1.09–1.47) | 1.23 (1.05–1.44) | 1.26 (1.08–1.46) |
| Panel Reactive Antibody score 0 | 0.92 (0.80–1.06) | 0.91 (0.80–1.04) | 0.94 (0.81–1.08) | 0.92 (0.81–1.05) |
| No human leukocyte antibody mismatches | 0.85 (0.62–1.17) | 0.90 (0.66–1.22) | 0.87 (0.64–1.19) | 0.91 (0.67–1.23) |
| Time on dialysis (per year) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Delayed graft function | — | — | 1.30 (1.10–1.53) | 1.22 (1.04–1.43) |
| Acute rejection | — | — | 1.10 (0.74–1.64) | 1.14 (0.78–1.67) |
Kidney transplantation recipients who were frail at the time of KT were more than twice as likely (hazard ratio (HR) = 2.55, 95% CI = 1.71–3.82, P < .001) to have improvement in frailty score after KT even after adjusting for all pre- and post-KT factors.
DISCUSSION
In this prospective, single-center cohort with long-term follow-up, frailty was found to be a dynamic state, worsening in the first month after KT, reverting to what it was at the time of KT by the second month after KT, and improving by the third month after KT. By 3 months, 74% of KT recipients who were frail at the time of KT were intermediately frail or nonfrail. KT recipients who were frail at the time of KT were 2.6 times as likely to have better frailty scores even after accounting for recipient, donor, and transplant factors. Frailty status at the time of KT, recipient diabetes mellitus, and DGF were the only factors associated with steeper long-term trajectories of frailty score. These findings suggest that, although KT recipients who are frail at KT had higher scores long-term, they were most likely to show improvements in their physiological reserve after KT, supporting transplantation in these individuals and suggesting that pretransplantation frailty is not an irreversible state of low physiological reserve.
Community-dwelling older adults often transition between nonfrail, intermediately frail, and frail states,8,23,24 but these transitions are most often to states of greater frailty, with more than 40% becoming more frail and fewer than 1% transitioning from frail to nonfrail.8 In older adults, hospitalizations greatly reduces the likelihood of recovering from being intermediately frail or frail.8,23 From these findings, it has been suggested that there is a range of progression in frailty status, with some older adults progressing suddenly because of illness and decline and others progressing more slowly.24 Although these findings in older adults characterize changes in frailty as part of aging, this research characterizes the changes in frailty associated with a major surgical intervention. This study showed that frailty initially worsens, presumably because of the surgical insult, but then improves, presumably because of the restoration of kidney function. In contrast to aging in older adults, KT in adults with ESRD leads to transitions to states of less frailty by 3 months; of those who were frail at KT, 28.0% became nonfrail, and 48.0% became intermediately frail, although similar to the findings in older adults, the likelihood of transitioning between frailty states was highly associated with a recipient’s frailty state at the time of KT. Although the natural history of frailty in individuals with ESRD who do not undergo KT is unclear, it is likely that they have a downward frailty trajectory that may or may not stabilize.
It is most likely that the improvements in frailty after KT are due to restoration of renal function, although there are other possible benefits of KT, including improved appetite, increased physical activity because of the termination of dialysis, and improved quality of life, that would lead to improvements in frailty status. It is likely that a combination of physiological and psychosocial factors contribute to improvements in frailty after KT. Furthermore, although the majority of people improve their frailty status after KT, there are some who do not, and it is likely that this is because of post-KT complications such as DGF.
Strengths of this study were the prospective, longitudinal measurement of a validated, objective frailty instrument immediately before KT and during routine clinical care after KT. Additionally, ascertainment of recipient, donor, and transplantation factors allowed for identification of factors associated with long-term changes in frailty score after KT. The major limitation of this study is that it was a single-center study with limited follow-up that was ascertained as part of routine clinical care. Additionally, the frailty score ranges from 0 to 5, so participants with a score of 0 could not improve further, and those with a score of 5 could not decline after KT, leading to a possible floor or ceiling effect, but only 14% of the population had a score of 0 and 1% a score of 5, making these floor and ceiling effects less likely.
Frailty is a dynamic process in KT recipients of all ages, initially worsening after the major surgical insult but then improving significantly (even beyond baseline) by the third month after KT. Those who are frail at the time of KT are most likely to improve after KT, possibly because of the restoration of kidney function, even though their frailty scores were higher after KT. Although this work clearly demonstrates that frailty can improve after KT, it remains to be seen whether pretransplantation interventions such as prehabilitation can successfully increase the number of KT recipients whose frailty status improves after KT.
ACKNOWLEDGMENTS
This study was supported by Doris Duke Charitable Foundation Grant 2013071 (PI: Dorry Segev), National Institutes of Health (NIH) Grant K24DK101828 (PI: Dorry Segev), and NIH Grant R01AG042504 (PI: Dorry Segev). Mara McAdams-DeMarco was supported by NIH Grant K01AG043501–01A1, the Carl W. Gottschalk Research Scholar Award from the American Society of Nephrology, and Johns Hopkins University Claude D. Pepper Older Americans Independence Center, National Institute on Aging Grant P30-AG021334. Natasha Gupta and Dorry Segev were supported by the Doris Duke Charitable Foundation Clinical Research Mentorship Grant. Megan Salter was supported by National Institute on Aging (NIA) Grant T32AG000247. Elizabeth A. King was supported by NIA Grant F32-AG044994. We thank the study participants and the research staff at Johns Hopkins, especially Amanda Brennan and Erika Jones for their dedication to this study.
Sponsor’s Role: The sponsor had no role in the design, methods, recruitment, data collection, analysis, or preparation of the paper.
Footnotes
This work was presented at the World Transplant Congress, San Francisco, California, July 26–31, 2014.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors contributed to the concept and design or acquisition of data or analysis and interpretation of the data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. doi: 10.1111/ajt.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco M, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Auyeung TW, Leung J, et al. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15:281–286. doi: 10.1016/j.jamda.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: Characterization in the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2006;61A:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 11.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 12.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: The Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64A:243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng SX, Hung W, Cappola AR, et al. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009;64A:499–502. doi: 10.1093/gerona/gln047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56A:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 16.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 17.Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63A:>984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 18.Chang SS, Weiss CO, Xue QL, et al. Association between inflammatory-related disease burden and frailty: Results from the Women’s Health and Aging Studies (WHAS) I and II. Arch Gerontol Geriatr. 2012;54:9–15. doi: 10.1016/j.archger.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang SS, Weiss CO, Xue QL, et al. Patterns of comorbid inflammatory diseases in frail older women: The Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65A:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-Demarco MA, Law A, Salter M, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-Demarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2014;99:805–810. doi: 10.1097/TP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill TM, Gahbauer EA, Han L, et al. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci. 2011;66A:1238–1243. doi: 10.1093/gerona/glr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]