Abstract
Background
Left ventricular (LV) dyssynchrony caused by premature ventricular contractions (PVCs) has been proposed as a mechanism of PVC-induced cardiomyopathy (CM). We sought to understand the impact of different PVC locations and coupling intervals (prematurity) on LV regional mechanics and global function of the PVC beat itself.
Methods and Results
Using our premature pacing algorithm, pentageminal PVCs at coupling intervals of 200–375ms were delivered from the epicardial right ventricular (RV) apex, RV outflow tract (RVOT), and LV free wall, as well as premature atrial contractions (PACs) from the left atrial (LA) appendage at a coupling interval of 200ms in seven healthy canines. LV short axis echocardiographic images, LV stroke volume (SV) and dP/dtmax were obtained during all ectopic beats and VP. LV dyssynchrony was assessed by dispersion of QRS-to-peak strain (earliest – last QRS-to-peak strain) between 6 different LV segments during each of the aforementioned beats (GE, EchoPac). LV dyssynchrony was greater during long- rather than short-coupled PVCs and PVCs at 375ms compared with rapid VP at 400ms (P<0.0001), whereas, no difference was found between PVC locations. Longer PVC coupling intervals were associated with greater SV and dP/dtmax despite more pronounced dyssynchrony (P<0.001).
Conclusions
PVCs with longer coupling intervals demonstrate more pronounced LV dyssynchrony, whereas PVC location has minimal impact. LV dyssynchrony cannot be attributed to prematurity or abnormal ventricular activation alone, but rather to a combination of both. This study suggests that late-coupled PVCs may cause a more severe cardiomyopathy if dyssynchrony is the leading mechanism responsible for PVC-induced CM.
Keywords: left ventricular mechanics, dyssynchrony, premature ventricular contraction arrhythmia, cardiomyopathy, left ventricular dysfunction
Introduction
Frequent premature ventricular contractions (PVCs) have been identified as a reversible cause of non-ischemic cardiomyopathy (CM), referred to as a PVC-induced CM1–7. Abnormal LV mechanics have been implicated as a major mechanism responsible for this CM. We sought to understand the relationship of PVC related changes in left ventricular (LV) mechanics, stroke volume (SV) and contractility (dP/dtmax) at different locations and coupling intervals (prematurity). We hypothesized that shorter PVC coupling intervals would result in greater LV dyssynchrony and that the right ventricular (RV) apex origin would have a higher degree of LV dyssynchrony when compared to an LV or RV outflow (RVOT) origin. To test this hypothesis, we used our novel premature pacing algorithm to simulate PVCs at desired frequency and coupling interval from different epicardial sites and at different coupling intervals7.
Methods
Under general anesthesia with isofluorane, 7 healthy female dogs underwent a left thoracotomy to allow implantation of epicardial bipolar leads (Greatbatch CRT-Myopore, Frisco, TX, USA) in the RV apex, RVOT, LV free wall and left atrial (LA) appendage in order to introduce RV, RVOT and LV PVCs and premature atrial complexes (PACs), respectively. Pacing output was programmed twice voltage threshold at 0.4–0.5ms in each ventricular location and LA appendage. Echocardiographic images, LV SV and dP/dtmax were obtained peri-operatively with open chest animals during a pacing protocol (Supplemental Table 1), consisting of rapid ventricular and atrial pacing at 400ms (150bpm), PVCs in a pentageminal pattern at 200, 250, 300 and 375ms from the LV free wall, RVOT and RV apex, and PACs in pentageminal pattern at 200ms using our premature pacing algorithm7.
Echocardiography
A short-axis view (mid LV at the level of the papillary muscles) was acquired with a commercial system (5MHz probe Vivid-7, Vingmed- General Electric, Fairfield, CT, USA) during the pacing protocol as described above. Radial strain was acquired from the mid-LV short axis view as previously described to assess LV mechanics8. Briefly, frame rates of 70–90 Hz were used for acquisition and endocardial and epicardial borders were manually traced to create a region of interest which was adjusted and re-drawn on playback if required to accomplish optimal tracking (GE EchoPac BT11, Horton, Norway). QRS-to-peak radial strain (ms) was measured in 6 different LV segments at baseline, and during PACs and PVCs only. LV dyssynchrony in the ectopic beat alone was assessed by the dispersion of QRS-to-peak strain between all segments (earliest – last QRS-to-peak strain). Radial strain analysis was performed in at least one PVC beat by a blinded reader.
LV SV and dP/dtmax
An impedance-based multipolar catheter (Ventricath 507 5Fr, Millar Inc. Houston, TX, USA) was introduced into the LV through a right carotid artery cutdown to assess acute changes in SV and dP/dtmax during pacing protocol. A continuous hemodynamic recording allowed us to obtained SV and dP/dtmax in at least 10 PVC beats. All hemodynamic measurements were made under general anesthesia.
All procedures were approved by the McGuire Institutional Animal Care and Use Committee (IACUC) in accordance with the provisions of the USDA Animal Welfare Act Regulations and Standards, PHS Policy, the Guide for the Care and Use of Laboratory Animals and VA Policy.
Statistical analysis
Repeated measures ANOVA models were used for each outcome (dispersion of QRS-to-peak strain, SV, and dP/dtmax) and all models included the coupling interval (200, 250, 300, 375, 400 ms), location (LV, RV, RVOT, LA), and the interaction between both variables. These models were used to estimate the mean and 95% confidence intervals (95% CI) for every combination of the PVC coupling interval and PVC location. Differences in the estimated means and 95% CIs at each PVC coupling interval and location are reported in the Supplemental Tables 2 – 7. In these comparisons, a Bonferroni adjustment was made to control the Type-I error rate in each overall comparison. Omnibus tests were performed over all PVC coupling intervals for each PVC location and vice versa. Statistical significance on all omnibus tests were determined at the 0.05 level. Statistical analysis was performed using SAS/STAT® Software (SAS Institute, Inc. Cary, NC).
Results
LV mechanics
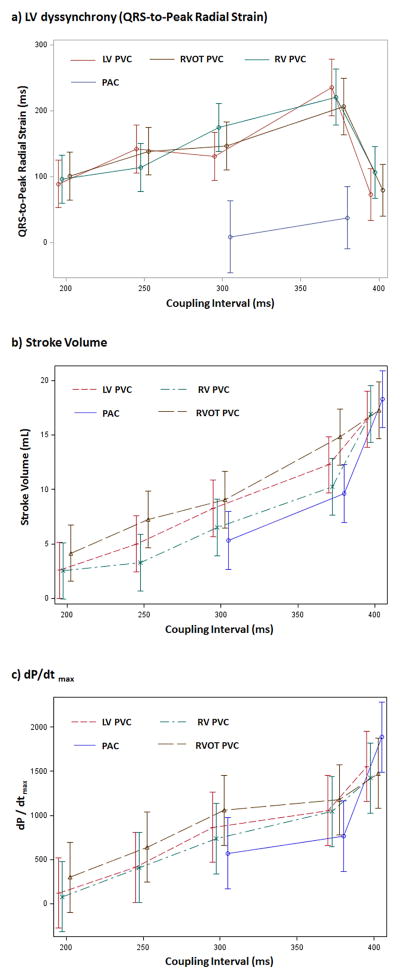
The dispersion of QRS-to-peak radial strain (LV dyssynchrony) in the premature ventricular beat (PVC) was significantly related to the coupling intervals (P=0.0002). LV dyssynchrony increased from all PVC locations as the PVC coupling interval was increased from 200 to 375ms (Table 1, Figure 1a). Thus, at longer PVC coupling intervals greater LV dyssynchrony was noted during the ectopic beat itself. This is visually apparent as shown in representative samples (Supplemental videos 1 and 2). All Bonferroni-adjusted pairwise comparisons of the coupling intervals at each location are included in Supplemental Table 2.
Table 1.
LV dyssynchrony (assessed by dispersion of QRS-to-peak strain), stroke volume and dP/dtmax in rapid ventricular pacing (VP) beats, and ectopic ventricular and atrial beats (PVCs and PACs, respectively) at different locations and coupling intervals.
| Ectopic Coupling Interval | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Ectopic beat Origin | 200ms | 250ms | 300ms | 375ms | VP 400ms | P Value† |
| Dispersion of QRS-to-Peak Strain (ms) | ||||||
|
| ||||||
| PAC (R-R 290–430ms) | - | - | 8.6 (−46, 63) | 38 (−10, 85) | - | 0.42 |
| RV apex PVC | 96 (60, 132) | 114 (78, 151) | 175 (138, 211) | 221 (178, 264) | 106 (67, 146) | <0.0001 |
| RV outflow tract PVC | 101 (65, 136) | 139 (103, 174) | 147 (111, 182) | 206 (163, 248) | 80 (41, 118) | 0.0038 |
| LV free wall PVC | 89 (53, 125) | 142 (105, 178) | 131 (94, 167) | 235 (192, 278) | 72 (33, 111) | <0.0001 |
| P Value * | 0.89 | 0.47 | 0.20 | 0.60 | 0.40 | |
|
| ||||||
| Stroke Volume (mL) | ||||||
|
| ||||||
| PAC (R-R 290–430ms) | - | - | 5.3 (2.7, 8.0) | 9.6 (7.0, 12.3) | 18.3 (15.7, 20.9) | <0.0001 |
| RV apex PVC | 2.5 (−0.1, 5.1) | 3.3 (0.7, 5.9) | 6.5 (3.9, 9.1) | 10.2 (7.6, 12.9) | 16.9 (14.3, 19.5) | <0.0001 |
| RV outflow tract PVC | 4.1 (1.5, 6.7) | 7.2 (4.6, 9.8) | 9.1 (6.5, 11.7) | 14.8 (12.2, 17.4) | 17.3 (14.7, 19.8) | <0.0001 |
| LV free wall PVC | 2.6 (0.0, 5.2) | 5.0 (2.4, 7.6) | 8.3 (5.7, 10.9) | 12.3 (9.7, 14.8) | 16.4 (13.9, 19.0) | <0.0001 |
| P Value * | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0240 | |
|
| ||||||
| dP/dtmax (mmHg/s) | ||||||
|
| ||||||
| PAC (R-R 290–430ms) | - | - | 570 (165, 975) | 763 (363, 1163) | 1886 (1489, 2284) | <0.0001 |
| RV apex PVC | 80 (−318, 478) | 410 (12, 807) | 737 (339, 1134) | 1045 (647, 144) | 1424 (1027, 1821) | <0.0001 |
| RV outflow tract PVC | 299 (−99, 696) | 640 (242, 1038) | 1058 (660, 1455) | 1178 (780, 1575) | 1478 (1081, 1874) | <0.0001 |
| LV free wall PVC | 122 (−276, 520) | 412 (15, 810) | 866 (469, 1264) | 1056 (659, 1453) | 1553 (1156, 1950) | 0.0007 |
| P Value * | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Data expressed in mean (95% CI).
P-values assessing the mean differences between the ventricular ectopic origins do not include PACs (LA appendage).
P-values assessing the difference between the ectopic coupling intervals do not include ROP 400.
Figure 1.
(a) LV dyssynchrony (QRS-to-Peak LV radial strain), (b) stroke volume, and (c) dP/dtmax in premature ventricular beats (PVC) by different coupling intervals (200, 250,300, 375ms) and rapid ventricular paced beats (400ms) from RV, RVOT and LV free wall, as well as premature atrial beats (PACs) at 200ms (R-R interval 290–430ms). RV= RV apex, RVOT= RV outflow tract, LV = LV free wall.
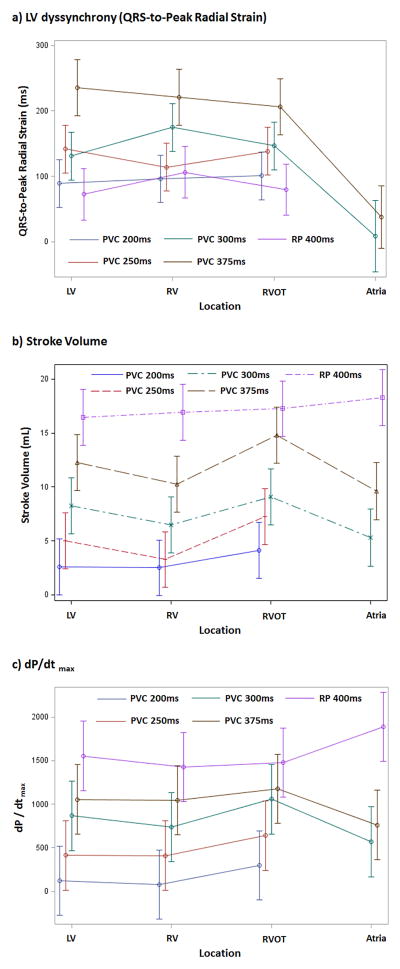
Significant differences in LV dyssynchrony during the PVC were not observed between different sites of origin (Figure 2a). These estimated means are shown in Table 1, while detailed comparisons of the dispersion of QRS-to-peak radial strain between all PVC locations separately by PVC coupling interval are noted in supplemental Table 3.
Figure 2.
(a) LV dyssynchrony (QRS-to-Peak LV radial strain), (b) stroke volume and (c) dP/dtmax in premature ventricular beats (PVC) arranged by different origin (RV, RVOT and LV) at 200, 250, 300 and 375ms and rapid pacing (RP) at 400ms, in addition to premature atrial beats (PAC) at 200ms (R-R interval 290–340ms). RV= RV apex, RVOT= RV outflow tract, LV = LV free wall.
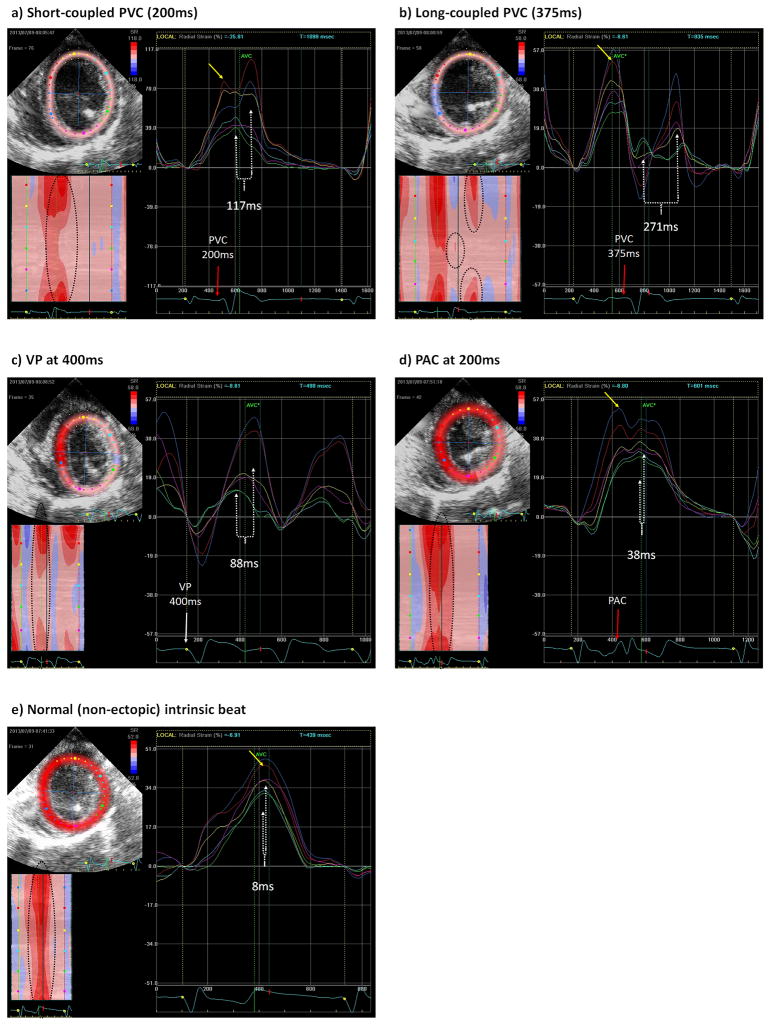
Furthermore, the QRS-to-peak radial strain during rapid VP beats at 400ms demonstrated a significantly lower LV dyssynchrony when compared to PVCs at a coupling interval of 375ms regardless of site of origin (P<0.0001, Figure 1a, Supplemental Table 2). Examples are shown in Figure 3A–C and supplemental video 1–3.
Figure 3.
Segmental LV radial strain during a (a) short-coupled LV PVC at 200ms, (b) long-coupled LV PVC at 375ms, (c) LV rapid VP at 400ms, (d) PAC at 200ms, (e) normal intrinsic beat. Direct visualization of contraction and radial strain in a short-coupled PVC (Panel a) demonstrate those segments near the origin of an early or short-coupled PVCs (LV free wall in green and light blue) have their peak maximum contraction immediately after peak contraction of the preceding intrinsic beat, which appears as a long fused peak contraction between intrinsic and PVC beat in segments near PVC origin. Few milliseconds later, the peak contraction of segments away from PVC origin (septal segments in red and dark blue) is noted while the first segments (near PVC origin) start relaxation (Supplemental Video 1). In contrast, segments near the origin of a late-coupled PVC (Panel b) have their peak radial strain after relaxation of the preceding intrinsic beat is almost completed, causing dyskinesia of the opposite segments, while the segments away from PVC origin have their peak contraction when the segments near PVC origin have already completed relaxation, leading to a visually apparent LV dyssynchrony (Supplemental Video 2). Yellow dot marks QRS of a normal intrinsic beat with a peak radial strain (yellow arrow) that occurs at least around 250ms after beginning of QRS. Red arrow marks QRS initiation of LV PVC in panel “a” and “b” (200, 375ms coupling interval) and PAC (200ms) in panel “d”. White arrow in panel “c” denotes the initiation of VP beat at 400ms. White dotted line marks dispersion of QRS-to-peak strain in all panels. Left lower quadrant in all panels is a representation of radial strain in all 6 segments (Y axis) plotted by time (X axis), with red color representing the peak radial strain (contraction) and blue color indicating the lowest radial strain (greatest relaxation).
Premature atrial beats did not cause LV dyssynchrony when compared with sinus rhythm (P>0.05; mean dispersion of QRS-to-peak radial strain in PAC: 38ms (95% CI: −10, 85) vs. sinus rhythm: 25.1ms (95% CI: 3.4, 46.9) (Figures 3D & E). In contrast, PVCs from any origin at a 375ms coupling interval caused a statistically greater degree of LV dyssynchrony when compared to PACs (P<0.0001) and sinus rhythm (P=0.0001) (Table 1, Figure 3B & 3D, Supplemental Table 3 and Video 2 & 4).
Stroke Volume and dP/dtmax
The stroke volume (SV) and dP/dtmax were significantly different between different PVC coupling intervals and PVC locations (p<0.0001, Table 1, Figure 1b–c and 2b–c). The longer the PVC coupling interval the greater the increase in SV and dP/dtmax. After adjusting for multiple comparisons, there were significant differences in SV and dP/dtmax between different coupling intervals at each PVC location (Table 1, Supplemental Table 4 and 6). Likewise, for a given coupling interval, significant differences in the SV and dP/dtmax were observed between different PVC locations, with the greatest SV and dP/dtmax in the RVOT origin PVCs (Table 1, Supplemental Table 5 and 7).
Regardless of location, PVCs at a coupling interval of 375ms demonstrated significantly lower SV and dP/dtmax when compared to rapid VP at a similar CL of 400ms (P<0.0001, Figure 1b–c or Supplemental Table 4 and 6). Similarly, a PAC (200ms coupling interval) with R-R interval of 290–430ms had a significantly lower SV and dP/dtmax when compared to rapid atrial pacing at 400ms (P<0.0001).
Discussion
The present study provides an understanding of the acute changes in LV mechanics and LV dyssynchrony during ectopic beats of different prematurity and different origin (PACs and PVCs from several locations) in structurally normal canine hearts. The acute hemodynamic effects of PACs and PVCs from different locations (RV and LV apex and LV free wall) and coupling intervals have been studied in isolated canine hearts9, however, acute changes in LV mechanics during PVCs have never been studied in the intact animal.
Our main findings include: 1) LV dyssynchrony, SV and dP/dtmax increase with premature ventricular beats at longer coupling intervals regardless of the site of origin; 2) LV dyssynchrony is similar between ventricular ectopic beats from different origins (RV apex, RVOT and LV free wall) at identical coupling intervals; and 3) premature atrial beats (R-R interval 290–430ms) and rapid atrial/ventricular paced beats at 400ms have significantly better LV mechanics, SV and dP/dtmax than PVCs at 375ms regardless of PVC origin.
These findings show that LV dyssynchrony during ventricular ectopic beats in the intact heart is primarily dependent on the coupling interval (prematurity) rather than the site of origin. In addition, the lower LV mechanics and hemodynamics with PVCs at 375ms when compared to rapid VP beats at 400ms and premature atrial beats, suggests that these adverse changes caused by premature ventricular ectopic beats are not solely due to an abnormal activation sequence (also found with rapid VP at 400ms) or prematurity (also found with PACs), but rather a combination of both.
In contrast to SV and dP/dt, LV dyssynchrony did not demonstrate a statistical difference between PVC origins. We can only speculate that the minimal non-statistical difference in LV dyssynchrony between PVC origins may translate into a slightly larger difference in dP/dt and SV that reaches statistical significance in lieu of multiple measures of SV and dP/dt (10–30 PVC beats) that were not performed for LV dyssynchrony (1–2 PVC beats).
Insights into the mechanism of PVC-induced Cardiomyopathy
Some of the postulated mechanism(s) responsible for the contractile LV dysfunction associated with a high PVC burden include: 1) abnormal LV mechanics causing disruption and progression of dysynergy of LV contraction resulting in LV dysfunction4, 10.; 2) post-extrasystolic potentiation (increase in contractility that follows an atrial or ventricular extrasystole) associated with acute intracellular Ca2+ overload and increased myocardial oxygen consumption11, 12, which has an inverse relationship to PVC coupling interval (shorter coupling intervals have a greater intracellular Ca2+and post-extrasystolic potentiation 12,9, 13; 3) autonomic dysregulation; and 4) “tachycardia” due to a short R-to-PVC interval. However, tachycardia as a single mechanism of PVC-induced CM is unlikely, not only because the mean heart rate in our PVC-induced CM model was significantly lower (130±13 bpm) than described in tachycardia-induced CM models (heart rate >180 bpm14, 15), but also due to the absence of histological and mitochondrial abnormalities characteristic of tachycardia-induced CM and other HF models16.
Few small clinical studies have attempted to understand if any specific PVC features, such as PVC burden, coupling interval, origin and QRS duration have any direct association to the development of PVC-induced CM 17–21. Thus far, only PVC burden, epicardial origin and QRS duration have shown to be associated with a higher incidence of PVC-induced CM17–19, while the impact of different PVC origins (LV, RV, outflow) and coupling intervals remain poorly understood. Del Carpio and colleagues20 fail to demonstrate a correlation between PVC coupling interval and LV dysfunction, whereas Sun and colleagues22 found a higher incidence of LV dysfunction in short-coupled PVCs (defined as RR′/RR<0.6) in children and Olgun21 demonstrated that interpolated PVCs correlated independently with PVC-induced CM (despite a higher PVC burden). We postulate that these inconsistent results20–22 derive from the inconsistent assessment of coupling interval, small numbers (50–70) of patients and observational design of these clinical studies along with significant variability in PVC origin (endocardial vs. epicardial; RV vs. RVOT vs. LV), PVC burden and QRS duration between individual patients.
In acute canine studies, coupling interval is known to determine the degree of post-extrasystolic potentiation in the following beats after PVC regardless of location (RV or LV), with a shorter PVC coupling interval associated with a greater post-extrasystolic potentiation after premature atrial or ventricular beats9, 13. In contrast, our findings demonstrate that LV dyssynchrony during the premature ventricular beat itself is significantly greater in late-coupled rather than short-coupled PVCs regardless of the location. These findings provide insights to the possible role of PVC coupling interval in the development of PVC-induced CM: a greater LV systolic dysfunction in late-coupled PVCs would support a primary mechanistic role for LV dyssynchrony, whereas a more severe LV dysfunction with short-coupled PVCs would indicate an alternative mechanism. Our data does not provide concluding evidence that PVC origin (i.e. RVOT, RVA or LV free wall) has no impact on the development of LV dysfunction but if there is any effect, the contribution is likely small. We believe that only a large-scale prospective study of patients with frequent PVCs and PVC-induced CM or the use of established animal models with a strict control of key PVC features may be able to assess the impact of PVC origin and coupling interval in the development of PVC-induced CM.
Limitations
Epicardial PVCs. We assessed LV mechanics of epicardial origin PVCs only. It is conceivable that endocardial origin of PVCs would lead to different vulnerability to LV dysfunction due to different ventricular activation patterns from closer proximity to the His-Purkinje system. Nevertheless, we expect these findings are applicable in humans since canine and human endocardial His-Purkinje conduction system are similar23.
Variability of PAC coupling interval. PACs at a fixed coupling interval will have different R-R intervals depending on AV nodal conduction. Thus, comparing SV and dP/dtmax in PACs and PVCs may have the limitation of not achieving identical prematurity due to the variability in AV conduction with PACs. We believed that this limitation was minimized by assessing PVCS at multiple coupling intervals.
Longitudinal strain. The assessment of global longitudinal strain has emerged as an important marker of myocardial function which appears to be additive to ejection fraction24. The animal model in the current study did not permit acquisition of the apical views (due to poor peri-operative apical windows) required for generation of global longitudinal strain. Nevertheless, radial strain alone is considered the most sensitive method to assess timing of peak contraction, which is critical to assess LV dyssynchrony. As these experiments were performed in normal hearts, it is unlikely that significant variation would be present in other cardiac areas.
This study was performed under general anesthesia in structurally normal canine healthy hearts. Thus, we cannot assume that similar findings are expected in abnormal hearts. Further studies are required to understand the impact of PVC coupling interval and origins in other cardiomyopathy models.
This study demonstrate acute changes in LV dyssynchrony during PVCs itself and does not demonstrate a causal effect of LV dyssynchrony in PVC-induced CM. Yet, our findings make the argument of the need to study the effects of different PVCs coupling intervals since LV dysfunction should be different between long- vs short-coupled PVC if LV dyssynchrony were to be a key part of the mechanism of PVC-induced CM.
Numerical convergence for the models assessing dP/dtmax and stroke volume were only possible in the equal correlation and homogenous variance model. An assessment of the standard deviations from the dP/dtmax and stroke volume over the possible combinations of location and coupling intervals show ranges from 105 to ~1000 and 1.8 to 7.2 (respectively). Thus, heterogeneities may exist in this data, however, even though these assumptions may not hold, the issues that arise from ignoring these assumptions will have more of an effect on the standard errors, and thus CIs and p-values, rather than the trends in the means of these outcomes. The Dispersion of the QRS-to-Peak strain was found to sufficiently homogenous when compared to a heterogeneous compound symmetric structure using the AICC as a model selection metric.
Conclusions
Premature ventricular beats with longer rather than shorter-coupling intervals demonstrate a more pronounced LV dyssynchrony in structurally normal hearts, whereas PVC origin has minimal impact on the degree of LV dyssynchrony. LV dyssynchrony during PVCs cannot be attributed to prematurity or abnormal ventricular activation alone, but rather a combination of both. These findings suggest that frequent long-coupled PVCs may result in a more pronounced CM, if LV dyssynchrony is the primary mechanism responsible for PVC-induced CM.
Supplementary Material
Acknowledgments
We wish to acknowledge Katrina Stumpf and Maureen Howren for their unconditional care of these animals and dedication to complete this study.
Funding Sources: Research support was provided by a Scientist Development Grant from the American Heart Association (National Center Award # SDG9310032) to J.F. Huizar and NIH (# UL1TR000058) to VCU Research Incubator for statistical assistance.
Footnotes
Conflict of Interest Disclosures: Dr. Kaszala receives research support from Medtronic, Inc. Dr. Tan receives research support from Boston Scientific Corp. and Biotronik, Inc. Dr. Ellenbogen receives research support from Boston Scientific Corp., Biosense Webster, Medtronic, Inc., St. Jude Medical and NIH; he is a consultant for Boston Scientific Corp., St. Jude Medical., Atricure, and also receives honoraria from Medtronic, Inc., Boston Scientific Corp., Biotronik, Inc., Biosense Webster and Atricure. Dr. Gorcsan III receives research support from GE, Medtronic, and Biotronik. Dr. Huizar received research support from Boston Scientific Corp., Biotronik, Inc. and St. Jude Medical.
References
- 1.Taieb JM, Maury P, Shah D, Duparc A, Galinier M, Delay M, Morice R, Alfares A, Barnay C. Reversal of dilated cardiomyopathy by the elimination of frequent left or right premature ventricular contractions. J Interv Card Electrophysiol. 2007;20:9–13. doi: 10.1007/s10840-007-9157-2. [DOI] [PubMed] [Google Scholar]
- 2.Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, Jongnarangsin K, Marine JE, Chugh A, Pelosi F, Oral H, Morady F. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol. 2005;45:1259–1265. doi: 10.1016/j.jacc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 5.Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: A potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–329. doi: 10.1111/j.1540-8167.2000.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Duffee DF, Shen WK, Smith HC. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clin Proc. 1998;73:430–433. doi: 10.1016/S0025-6196(11)63724-5. [DOI] [PubMed] [Google Scholar]
- 7.Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, Mezzaroma E, Chen Q, Kukreja RC, Hoke NN, Thacker LR, 2nd, Ellenbogen KA, Wood MA. Left ventricular systolic dysfunction induced by ventricular ectopy: A novel model for pvc-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:543–549. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J., 3rd Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 9.Takada H, Takeuchi S, Ando K, Kaito A, Yoshida S. Experimental studies on myocardial contractility and hemodynamics in extrasystoles. Jpn Circ J. 1970;34:419–430. doi: 10.1253/jcj.34.419. [DOI] [PubMed] [Google Scholar]
- 10.Topaloglu S, Aras D, Cagli K, Yildiz A, Cagirci G, Cay S, Gunel EN, Baser K, Baysal E, Boyaci A, Korkmaz S. Evaluation of left ventricular diastolic functions in patients with frequent premature ventricular contractions from right ventricular outflow tract. Heart Vessels. 2007;22:328–334. doi: 10.1007/s00380-007-0978-9. [DOI] [PubMed] [Google Scholar]
- 11.Ross J, Jr, Sonnenblick EH, Kaiser GA, Frommer PL, Braunwald E. Electroaugmentation of ventricular performance and oxygen consumption by repetitive application of paired electrical stimuli. Circ Res. 1965;16:332–342. doi: 10.1161/01.res.16.4.332. [DOI] [PubMed] [Google Scholar]
- 12.Cooper MW, Lutherer LO, Lust RM. Postextrasystolic potentiation and echocardiography: The effect of varying basic heart rate, extrasystolic coupling interval and postextrasystolic interval. Circulation. 1982;66:771–776. doi: 10.1161/01.cir.66.4.771. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MW. Postextrasystolic potentiation. Do we really know what it means and how to use it? Circulation. 1993;88:2962–2971. doi: 10.1161/01.cir.88.6.2962. [DOI] [PubMed] [Google Scholar]
- 14.Spinale FG, Holzgrefe HH, Mukherjee R, Arthur SR, Child MJ, Powell JR, Koster WH. Lv and myocyte structure and function after early recovery from tachycardia-induced cardiomyopathy. Am J Physiol. 1995;268:H836–847. doi: 10.1152/ajpheart.1995.268.2.H836. [DOI] [PubMed] [Google Scholar]
- 15.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: A review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 16.Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, Mezzaroma E, Chen Q, Kukreja RC, Hoke NN, Thacker LR, 2nd, Ellenbogen KA, Wood MA. Left ventricular systolic dysfunction induced by ventricular ectopy: A novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:543–549. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F, Jr, Crawford T, Ebinger M, Oral H, Morady F, Bogun F. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Carballeira Pol L, Deyell MW, Frankel DS, Benhayon D, Squara F, Chik W, Kohari M, Deo R, Marchlinski FE. Ventricular premature depolarization qrs duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 19.Yokokawa M, Kim HM, Good E, Crawford T, Chugh A, Pelosi F, Jr, Jongnarangsin K, Latchamsetty R, Armstrong W, Alguire C, Oral H, Morady F, Bogun F. Impact of qrs duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012;9:1460–1464. doi: 10.1016/j.hrthm.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Del Carpio Munoz F, Syed FF, Noheria A, Cha YM, Friedman PA, Hammill SC, Munger TM, Venkatachalam KL, Shen WK, Packer DL, Asirvatham SJ. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: Study of the burden, duration, coupling interval, morphology and site of origin of pvcs. J Cardiovasc Electrophysiol. 2011;22:791–798. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 21.Olgun H, Yokokawa M, Baman T, Kim HM, Armstrong W, Good E, Chugh A, Pelosi F, Jr, Crawford T, Oral H, Morady F, Bogun F. The role of interpolation in pvc-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–1049. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Blom NA, Yu Y, Ma P, Wang Y, Han X, Swenne CA, van der Wall EE. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: An echocardiographic evaluation. Int J Cardiovasc Imaging. 2003;19:295–299. doi: 10.1023/a:1025418531853. [DOI] [PubMed] [Google Scholar]
- 23.Allison JS, Qin H, Dosdall DJ, Huang J, Newton JC, Allred JD, Smith WM, Ideker RE. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18:1306–1312. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 24.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2081. doi: 10.1016/j.jacc.2012.07.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.