Abstract
Elevated plasma concentrations of coagulation factor XI may increase risk of venous thromboembolism (VTE), but prospective data are limited. We studied prospectively the associations of plasma factor XI and a key F11 genetic variant with incident VTE in whites and African Americans. We measured factor XI in 16,299 participants, initially free of VTE, in two prospective population cohorts. We also measured the F11 single nucleotide polymorphism rs4241824, which a genome-wide association study had linked to factor XI concentration. During follow-up, we identified 606 VTEs. The age, race, sex, and study-adjusted hazard ratio of VTE increased across factor XI quintiles (p<0.001 for trend), and the hazard ratio was 1.51 (95% CI 1.16, 1.97) for the highest versus lowest quintile overall, and was 1.42 (95% CI 1.03, 1.95) in whites and 1.72 (95% CI 1.08, 2.73) in African Americans. In whites, the F11 variant was associated with both factor XI concentration and VTE incidence (1.15-fold greater incidence of VTE per risk allele). In African Americans, these associations were absent. In conclusion, this cohort study documented that an elevated plasma factor XI concentration is a risk factor for VTE over extended follow-up, not only in whites but also in African Americans. In whites, the association of the F11 genetic variant with VTE suggests a causal relation, but we did not observe this genetic relation in African Americans.
Keywords: deep vein thrombosis, factor XI, prospective study, pulmonary embolism
Introduction
The balance between the endogenous procoagulant and anticoagulant systems contributes to the etiologies of venous thromboembolism (VTE), that is, venous thrombosis (DVT) and pulmonary embolism (PE). On the procoagulant side, a few studies have reported that elevated circulating intrinsic coagulation factor XI concentrations may increase VTE risk [1, 2], but prospective data are limited. In a small prospective nested-case control study in the Longitudinal Investigation of Thromboembolism Etiology (LITE), we reported that the incidence of VTE was 1.8 fold higher (95% CI, 1.3–2.7) for participants in the fifth compared with the first quintile of factor XI [3]. Another study reported that patients with severe factor XI deficiency have reduced incidence of VTE [4]. Moreover, a clinical trial showed that inhibition of factor XI prevents post-surgical VTEs [5].
Supporting a potential etiologic role for factor XI, two [6, 7] of four [6–9] genome-wide association studies (GWAS) in whites and some candidate-gene studies [10–13] have linked SNPs in the structural F11 gene to VTE risk in whites or blacks [12]. Our GWAS for VTE found the top SNP was rs4253399 [6], which is in high linkage disequilibrium (LD) with other F11 variants linked to VTE, namely, rs3756008, rs2036914, and rs2289252. These latter three SNPs have been associated with factor XI concentrations, and adjustment for factor XI concentrations attenuated the SNP associations with VTE [10, 13]. A separate GWAS of plasma factor XI levels in individuals of European ancestry recently identified and replicated two SNPs significantly associated with factor XI: rs710446 in the kininogen 1 (KNG1) gene and rs4241824 in F11 [14]. According to 1000 Genomes, phase 1 data, F11 rs4241824 and rs4253399 are moderately correlated in individuals of European ancestry (CEU, r2 = 0.60) but virtually uncorrelated in Americans of African ancestry (ASW, r2 = 0.09). In summary, prior research suggests an underlying F11 or KNG1 locus might increase risk of VTE by elevating factor XI.
Because F11 was important in our VTE GWAS, we recently expanded factor XI measurement to the entire LITE study population in order to examine the associations between factor XI concentration and VTE occurrence. We also explored whether VTE is associated with the top F11 SNP (rs4241824) related to factor XI concentration [14].
Methods
Study population
The Longitudinal Investigation of Thromboembolism Etiology (LITE) study is a prospective study of VTE occurrence in 2 pooled, multi-center, longitudinal population-based cohort studies: the Atherosclerosis Risk in Communities (ARIC) Study [15] and the Cardiovascular Health Study (CHS) [16]. We reported the LITE study design, methods, and VTE incidence rates in detail elsewhere [17, 18]. In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC study in 1987–1989, and had subsequent examinations in 1990–92, 1993–95, 1996–98, and 2011–13, with annual telephone contact in between. In CHS, 5,201 men and women aged ≥65 years enrolled in 1989–1990. CHS recruited an additional 687 African Americans using similar methods in 1992–1993. CHS contacted participants every six months for follow-up, alternating between a telephone interview and clinic visit for the first 10 years and by telephone interview only after that. The institutional review committees at each study center approved the methods and staff obtained informed participant consent.
Factor XI plasma measurements and F11 genotyping
ARIC and CHS exhausted most baseline citrated plasma previously; therefore we measured Factor XI concentrations on fasting citrate plasma that had been collected at ARIC in 1993–95 (6 years after baseline) and CHS in 1992–93 (3 years after baseline for the original cohort and at baseline for the African American cohort) and stored unthawed at −70°C until analysis in 2014. The Laboratory for Clinical Biochemistry Research at the University of Vermont assayed factor XI by sandwich ELISA with affinity-purified polyclonal antibodies from Affinity Biologicals (Ancaster, Ontario, CAN). The coefficient of variation for control samples during this study averaged 9.6%. Blind analysis of 74 pairs of ARIC samples split at the time of blood draw and stored until 2014 yielded a coefficient of variation of 10.8% and an intra-class reliability coefficient of 0.81.
Each study performed genotyping using the IBC genotyping array [19], from which we obtained information on F11 SNP rs4241824, the F11 SNP most strongly associate with FXI concentration in whites [14]. The rs4241824 call rate was 98% in both whites and African Americans, and it was in Hardy-Weinberg equilibrium for both groups. Blind analysis of 196 pairs of ARIC samples split and genotyped separately yielded >99% agreement on rs4241824. In order to control for population stratification in African Americans, we used exome chip data [20] to derive ten principal components of ancestry using EIGENSTRAT [21].
Measurement of risk factors
We analyzed risk factors measured at the ARIC or CHS visits in which factor XI was measured. We calculated body mass index as weight (kg)/height (m)2. We defined diabetes as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, a self-reported physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks.
VTE occurrence
Staff contacted ARIC and CHS participants annually or semi-annually by phone and asked about all hospitalizations in the previous year. They retrieved hospital records for possible VTE events through 2011 in ARIC and through 2001 in CHS. To validate VTE events, two physicians reviewed the records using standardized criteria [17], requiring positive imaging tests for diagnosis of DVT and PE. We restricted DVTs for this analysis to those in the lower extremity or vena cava, because upper extremity DVTs were relatively few and almost always the result of venous catheters. The reviewers sub-classified VTEs as unprovoked (no obvious cause) or provoked (associated with cancer, major trauma, surgery, marked immobility).
Statistical analysis
Of the 12,887 ARIC and 5,265 CHS participants who attended the relevant exam, we excluded those not white or African American (n = 38 ARIC, 34 CHS), those with a VTE prior to the blood draw (n = 302 ARIC, 323 CHS), those taking anticoagulants (n = 124 ARIC, 107 CHS), those without citrate plasma specimens or factor XI measurements (n = 299 ARIC, 624 CHS), and those with no further follow-up (n = 2 ARIC). This left a maximum of 16,299 participants (12,122 in ARIC and 4,177 in CHS) for the present analyses. Time at risk for VTE was computed from the date of biomarker measurement to the earliest of the following: date of hospital admission for incident VTE, date of death, date of last follow-up contact, or end of follow-up.
Our main hypothesis was that factor XI concentration is associated positively with VTE incidence. We used Cox proportional hazards models to calculate hazard ratios (HR) and 95% confidence intervals of incident VTE. For analyses, factor XI was analyzed as quintiles of the entire sample or a continuous variable. We ran analyses separately for ARIC and CHS and pooled them only after verifying associations appeared similar for both studies. We performed a test for trend in VTE occurrence across factor XI quintiles using the median factor XI value to represent each quintile. We verified the proportional hazards assumption of the Cox models by testing an interaction of factor XI with log follow-up time. Model 1 associating factor XI with VTE included adjustment for age (continuous), sex, race, and study (ARIC, CHS); Model 2 adjusted additionally for characteristics previously associated with VTE in this cohort: diabetes status (yes or no) and body mass index.
In ARIC, we also examined the race-specific associations of the F11 SNP rs4241824 with VTE. Of the ARIC sample (n = 12,122), 11,411 gave permission for DNA use and had rs4241824 data. For each participant, we coded the SNP as having 0, 1, or 2 risk alleles and used an additive genetic model. For the analysis in African Americans, we also adjusted for ten principal components of ancestry.
Results
Among the 16,299 participants initially free of VTE, the distribution of plasma factor XI concentration was normal and similar for the Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS). As shown in Table I, the proportions who were African American, women, or had diabetes increased across quintiles of factor XI, as did mean body mass index.
Table I.
Participant Characteristics [Mean ± SD or %] in Relation to Quintiles of Factor XI, ARIC, 1993–1995; CHS, 1992–1993
| Quintile of Factor XI (%) | |||||
|---|---|---|---|---|---|
| Characteristic | 8.0–92.2 | 92.2–105.1 | 105.1–117.3 | 117.3–133.4 | 133.4–400.0 |
| Pooled | |||||
| N | 3,259 | 3,260 | 3,260 | 3,261 | 3,259 |
| Age, years | 64.4 (8.7) | 63.1 (8.3) | 63.2 (8.2) | 63.1 (8.1) | 64.0 (8.2) |
| African American | 19% | 20% | 21% | 23% | 25% |
| Women | 39% | 49% | 56% | 64% | 72% |
| Diabetes | 11% | 12% | 14% | 16% | 24% |
| BMI, kg/m2 | 27.2 (5.0) | 27.6 (4.9) | 28.1 (5.6) | 28.3 (5.4) | 28.9 (5.6) |
| ARIC | |||||
| N | 2,390 | 2,526 | 2,484 | 2,461 | 2,261 |
| Age, years | 60.4 (5.9) | 59.8 (5.7) | 59.8 (5.7) | 59.7 (5.6) | 59.9 (5.6) |
| African American | 21% | 21% | 22% | 25% | 28% |
| Women | 39% | 48% | 55% | 64% | 72% |
| Diabetes | 10% | 12% | 13% | 17% | 25% |
| BMI, kg/m2 | 27.6 (5.2) | 27.9 (5.0) | 28.5 (5.8) | 28.8 (5.5) | 29.6 (5.8) |
| CHS | |||||
| N | 869 | 734 | 776 | 800 | 998 |
| Age, years | 75.3 (5.4) | 74.2 (5.4) | 74.1 (5.0) | 73.7 (4.9) | 73.3 (5.0) |
| African American | 14% | 16% | 16% | 19% | 18% |
| Women | 38% | 52% | 57% | 66% | 74% |
| Diabetes | 14% | 11% | 16% | 16% | 19% |
| BMI, kg/m2 | 26.1 (4.3) | 26.6 (4.7) | 26.9 (4.7) | 27.0 (4.6) | 27.2 (4.9) |
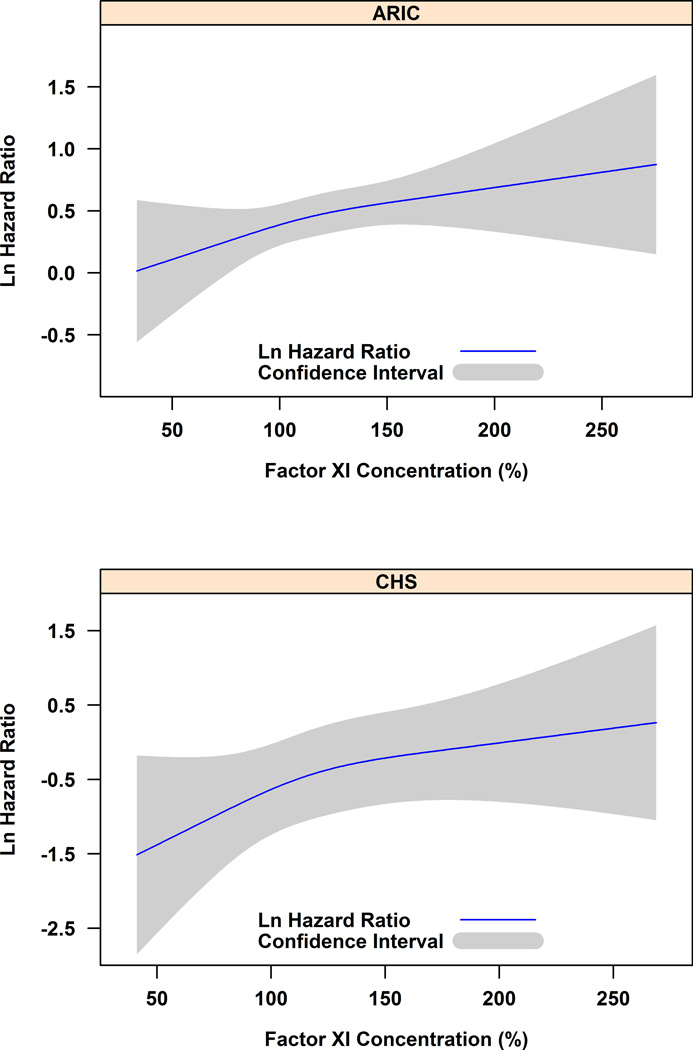
Over a median of 17 years of follow-up in ARIC and 9 years in CHS, we identified 606 (n = 523 ARIC, 83 CHS) DVTs of the lower extremity or PE. The incidence rates of VTE per 1,000 person-years rose similarly across factor XI quintiles in ARIC (2.5, 2.5, 2.8, 3.0, and 3.4) and CHS (1.7, 2.7, 2.2, 2.8, and 3.5). The hazard ratios of VTE increased steadily across factor XI concentration in both studies, without evidence that VTE risk was limited to the highest concentrations (Figure 1). We pooled ARIC and CHS for the remaining analyses.
Figure 1.
Logarithm hazard ratio of venous thromboembolism in relation to factor XI concentration*, ARIC and CHS.
* Analyzed by restricted cubic splines with knots at the fifth, fiftieth, and ninety-fifth percentiles of the study-specific factor XI distribution.
Overall, the age, race, sex, and study-adjusted hazard ratios of VTE (Model 1) increased across factor XI quintiles (p<0.001 for trend), and the hazard ratio was 1.51 (95% CI 1.16, 1.97) for the highest versus lowest quintile (Table II). Additional adjustment for diabetes and BMI weakened the association (p = 0.007 for trend). The association between factor XI and VTE appeared somewhat stronger for unprovoked than provoked VTE, for PE than DVT, and in African Americans than whites, although the race by factor XI interaction was nonsignificant (p = 0.31).
Table II.
Incidence Rates and Hazard Ratios (HRs) of Venous Thromboembolism in Relation to Factor XI, ARIC, 1993–2011, CHS 1992–2001
| Quintile of Factor XI (Ag %) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 8.0–92.2 | 92.2–105.1 | 105.1–117.3 | 117.3–133.4 | 133.4–400.0 | p-trend | Per 1-SD increment in FXI* |
p-value | |
| Pooled | ||||||||
| Total VTE | ||||||||
| N of VTEs | 101 | 113 | 121 | 129 | 142 | |||
| Person years | 42,040 | 44,224 | 44,127 | 43,713 | 41,656 | |||
| Incidence rate (per 103 py) | 2.4 | 2.6 | 2.7 | 3.0 | 3.4 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.09 (0.83, 1.43) | 1.19 (0.91, 1.55) | 1.31 (1.00, 1.70) | 1.51 (1.16, 1.97) | <0.001 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.09 (0.83, 1.43) | 1.13 (0.86, 1.47) | 1.24 (0.95, 1.62) | 1.40 (1.07, 1.82) | 0.007 | 1.10 (1.02, 1.19) | 0.02 |
| Unprovoked VTE | ||||||||
| N of VTEs | 40 | 45 | 42 | 45 | 57 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.11 (0.73, 1.71) | 1.06 (0.69, 1.64) | 1.19 (0.77, 1.83) | 1.60 (1.05–2.42) | 0.02 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.11 (0.72, 1.70) | 0.99 (0.64, 1.54) | 1.10 (0.71, 1.70) | 1.49 (0.98, 2.26) | 0.06 | 1.15 (1.01, 1.30) | 0.03 |
| Provoked VTE | ||||||||
| N of VTEs | 61 | 68 | 79 | 84 | 85 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.08 (0.77, 1.53) | 1.27 (0.91, 1.78) | 1.39 (0.99, 1.94) | 1.48 (1.05, 2.07) | 0.009 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.08 (0.76, 1.53) | 1.21 (0.87, 1.70) | 1.33 (0.95, 1.86) | 1.35 (0.96, 1.90) | 0.05 | 1.07 (0.97, 1.19) | 0.19 |
| PE | ||||||||
| N of PEs | 43 | 48 | 64 | 73 | 72 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.07 (0.71, 1.61) | 1.42 (0.96, 2.10) | 1.66 (1.13, 2.43) | 1.72 (1.17, 2.53) | <0.001 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.06 (0.70, 1.61) | 1.32 (0.90, 1.96) | 1.54 (1.05, 2.27) | 1.58 (1.07, 2.33) | 0.006 | 1.17 (1.05, 1.30) | 0.003 |
| DVT | ||||||||
| N of DVTs | 58 | 65 | 57 | 56 | 70 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.12 (0.79, 1.60) | 1.01 (0.70, 1.46) | 1.05 (0.72, 1.52) | 1.38 (0.96, 1.97) | 0.11 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.12 (0.78, 1.60) | 0.97 (0.67, 1.40) | 1.00 (0.69, 1.46) | 1.27 (0.88, 1.82) | 0.28 | 1.03 (0.91, 1.16) | 0.65 |
| Total VTE, African Americans | ||||||||
| N of VTEs | 29 | 37 | 29 | 41 | 54 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.15 (0.71, 1.88) | 0.91 (0.54, 1.53) | 1.27 (0.79, 2.06) | 1.72 (1.08, 2.73) | 0.01 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.13 (0.70, 1.84) | 0.81 (0.48, 1.36) | 1.18 (0.73, 1.91) | 1.51 (0.94, 2.42) | 0.05 | 1.15 (1.01, 1.29) | 0.03 |
| Total VTE, Whites | ||||||||
| N of VTEs | 72 | 76 | 92 | 88 | 88 | |||
| Model 1 HR (95% CI)† | 1 (Ref.) | 1.07 (0.77, 1.47) | 1.32 (0.97, 1.80) | 1.33 (0.97, 1.82) | 1.42 (1.03, 1.95) | 0.01 | ||
| Model 2 HR (95% CI)‡ | 1 (Ref.) | 1.07 (0.77, 1.47) | 1.27 (0.93, 1.73) | 1.26 (0.92, 1.73) | 1.32 (0.96, 1.82) | 0.06 | 1.07 (0.97, 1.19) | 0.17 |
FXI standard deviation = 28.2.
Age, race, sex, and study adjusted, except race omitted when stratified.
Adjusted further for diabetes and BMI.
To further verify the role of factor XI in VTE, we examined the association with the F11 SNP rs4241824 in ARIC. The frequencies of having 0, 1, or 2 copies of the A risk allele were 25%, 50%, and 25% in whites and 15%, 46%, and 39% in African Americans. For 0, 1, or 2 copies, the mean factor XI values were 106%, 112%, and 119% in whites (p for difference <0.0001), and the variant explained a small amount of factor XI variation (r2 = 0.036). For 0, 1, or 2 copies, in African Americans, factor XI values varied little: means were 115%, 116%, and 118% (p for difference = 0.20, and r2 = 0.001). Table III shows that participants with AA status had 1.33-fold higher VTE risk than GG, among whites, but there was no relation to VTE in African Americans.
Table III.
Race-Specific Incidence Rate and Hazard Ratio (HR) of Venous Thromboembolism in Relation to F11 Genotype, ARIC, 1993–2011, CHS, 1992–2001
| rs4241824 Genotype | |||
|---|---|---|---|
| GG | GA | AA | |
| Whites | |||
| N of VTEs | 70 | 152 | 95 |
| Person years | 33,738 | 67,362 | 34,343 |
| Adjusted VTE rate* | 2.0 | 2.2 | 2.7 |
| Adjusted HR (95% CI) | 1 (Ref.) | 1.09 (0.82, 1.44) | 1.33 (0.98, 1.82) |
| African Americans | |||
| N of VTEs | 31 | 71 | 59 |
| Person years | 5,724 | 17,754 | 14,677 |
| Adjusted VTE rate* | 5.4 | 3.8 | 4.0 |
| Adjusted HR (95% CI) | 1 (Ref.) | 0.71 (0.46, 1.08) | 0.74 (0.48, 1.15) |
Incidence rate per 103 person years, adjusted for age and sex and, in African Americans, for 10 principal components of ancestry.
In Model 2 for VTE incidence, each risk allele copy for this SNP was associated with a 1.15-fold (95% CI 0.99, 1.34) greater hazard of VTE in ARIC whites, but this estimate was 0.87 (95% CI 0.70, 1.09) in African Americans. These hazard ratios changed very little when adjusted for continuous plasma factor XI concentration: 1.14 (95% CI 0.98, 1.34) in whites and 0.87 (95% CI 0.69, 1.09) in African Americans. In contrast, adjusted for rs4241824, the Model 2 hazard ratios for VTE per SD of factor XI concentration in ARIC were 1.03 (95% CI 0.91, 1.16) in whites and 1.09 (95% CI 0.95, 1.26) in African Americans, which are considerably attenuated from the race-specific hazard ratios of 1.07 for whites and 1.15 for African Americans in Table II.
Discussion
This large prospective study involving two cohorts showed that a higher plasma concentration of factor XI was associated with modestly increased risk of VTE. The association was stronger for unprovoked than provoked VTE, for PE than DVT, and for African Americans than whites. In ARIC whites, risk alleles for F11 SNP rs4241824 were also associated with greater factor XI concentration and greater VTE incidence; yet, plasma factor XI concentration explained little of the SNP association with VTE. In ARIC African Americans, this F11 SNP was associated weakly with plasma factor XI and not associated with VTE risk.
Our previous publication from LITE included factor XI measured on plasma samples from 1987–89 in ARIC and from 1989–90 in CHS (with a few from 1992–93). It included 462 VTEs occurring from 1987 through 2002 in ARIC and 1989 through 2002 in CHS and 1047 participants who remained free of VTE [3]. In contrast, the present study measured plasma factor XI from 1993–95 in ARIC and from 1992–93 in CHS and had 606 incident VTEs, most of which overlapped with those analyzed previously for CHS but few overlapped for ARIC, as ARIC follow-up now went through 2011. The hazard ratios were similar to those we reported previously [3]. With more VTE events, we now were able to confirm that plasma factor XI is a VTE risk factor in African Americans. Previous case-control studies are consistent with plasma factor XI being a VTE risk factor in whites [1, 2] and African Americans [12]. Factor XI has an important role in propagation and stabilization of thrombi [22], and humans with severe factor XI deficiency have reduced incidence of VTE [4].
To support a potential causal association between higher factor XI and VTE, studies in whites have shown that several correlated F11 SNPs are associated with plasma factor XI and VTE in a parallel fashion [6–14]. A recent GWAS found F11 rs4241824 and a KNG1 SNP to be the top SNPs associated with factor XI concentration and they were also associated with activated partial thromboplastin time [14]. We chose to study F11 rs4241824 as our instrumental variable for factor XI because of concerns that KNG1 may affect thrombosis by other pathways besides factor XI. F11 rs4241824 proved to be associated with VTE in ARIC whites. Yet, somewhat surprisingly, in statistical models plasma factor XI did not at all explain the SNP association with VTE in whites. Conversely, the SNP statistically explained a moderate degree of the association between plasma factor XI and VTE. We have no clear explanation for these observations from statistical modeling, but perhaps the SNP may act in ways other than increasing factor XI antigen, such as influencing factor XI activity or by interacting with other coagulation factors. While rs4241824 is located in an intron of F11, it seems to have a role in altering regulatory motifs of other genes according to HaploReg v2 [23], an online annotation of functional non-coding sequences. Additionally, this SNP is in high LD (r2 = 0.9) with another F11 SNP rs3822057 that may influence promoter histone marks, regulatory motifs, and regulatory protein binding. Regardless, both factor XI concentration and F11 were associated with VTE in whites.
In ARIC African Americans, despite the positive association between factor XI concentration and VTE, F11 rs4241824 was unassociated with both factor XI and VTE. Thus, rs4241824 may be a poor instrumental variable for factor XI in African Americans or nongenetic factors may explain their association of factor XI with VTE. A previous case-control study found a different F11 SNP, rs2036914, associated with VTE but not factor XI concentration in African Americans [12]. In a supplemental analysis (not shown), we found the race-specific associations of rs2036914 with factor XI concentrations and VTE to mirror almost identically those for rs4241824. This is not surprising given rs2036914 was highly correlated with rs4241824 in both African Americans (r2 = 0.60) and whites (r2 = 0.88). Yet, our finding is not in accord with the only previous study of African Americans [12], and so the degree to which F11 variation determines factor XI levels and VTE in African Americans remains uncertain.
Some potential limitations of our study warrant consideration. Firstly, we measured factor XI on plasma samples that had been stored for approximately 20 years at −70°C. Previous evidence suggests that factor XI is stable in samples frozen for up to 18 months [24]. Any sample deterioration likely would have weakened the estimated association between factor XI and VTE. Secondly, factor XI was studied at only one ARIC visit. Factor XI concentrations may have fluctuated during the long follow-up, and such fluctuations would tend to weaken the observed association with VTE. Thirdly, we could not determine whether factor XI was associated with VTE independently from other hemostatic factors, since they were measured at different study visits. Fourthly, we identified hospitalized VTE patients only, but pilot data suggest the vast majority of patients with first VTEs in ARIC and CHS were hospitalized.
We used a factor XI antigen assay. It is likely correlated with factor XI activity assays typically used to detect factor XI deficiency in clinical practice, but we do not know the actual correlation. We hesitate to translate our measurements to those in clinical practice or to suggest that there are current implications of our analysis to clinical practice.
In conclusion, this cohort study documented that a higher plasma factor XI concentration is a risk factor for VTE over extended follow-up, not only in whites but also in African Americans. In whites, an association of a F11 genetic variant with VTE suggests a causal relation, but we did not observe this genetic relation in African Americans. Clinical trial evidence that inhibition of factor XI can prevent VTE further supports its role in VTE etiology [5].
Acknowledgments
The National Heart, Lung, and Blood Institute (NHLBI) supported LITE via HL0597367, ARIC via contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C.
CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
The authors thank the staff and participants of the ARIC Study and CHS for their important contributions, and Elaine Cornell for supervising factor XI measurements.
References
- 1.Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis – current understanding from an epidemiological point of view. Br J Haematol. 2010;149(6):824–833. doi: 10.1111/j.1365-2141.2010.08206.x. [DOI] [PubMed] [Google Scholar]
- 2.Meijers JC, Tekelunburg WL, Bouma BN, et al. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342(10):696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 3.Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114(14):2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomon O, Steinberg DM, Zucker M, et al. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105(2):269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 5.Büller HR, Bethune C, Bhanot S, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232–240. doi: 10.1056/NEJMoa1405760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang W, Teichert M, Chasman DI, et al. A genome-wide association study for venous thromboembolism: The Extended Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium. Genet Epidemiol. 2013;37(5):512–521. doi: 10.1002/gepi.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germain M, Saut N, Greliche N, et al. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One. 2011;6(9):e25581. doi: 10.1371/journal.pone.0025581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trégouët DA, Heath S, Saut N, et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113(21):5298–5303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 9.Heit JA, Armasu SM, Asmann YW, et al. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012;10(8):1521–1531. doi: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Bezemer ID, Rowland CM, et al. Genetic variants associated with deep vein thrombosis: the F11 locus. J Thromb Haemost. 2009;7(11):1802–1808. doi: 10.1111/j.1538-7836.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith NL, Hindorff LA, Heckbert SR, et al. Association of genetic variations with nonfatal venous thrombosis in postmenopausal women. JAMA. 2007;297(5):489–498. doi: 10.1001/jama.297.5.489. [DOI] [PubMed] [Google Scholar]
- 12.Austin H, De Staercke C, Lally C, et al. New gene variants associated with venous thrombosis: a replication study in White and Black Americans. J Thromb Haemost. 2011;9(3):489–495. doi: 10.1111/j.1538-7836.2011.04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezemer ID, Bare LA, Doggen CJ, et al. Gene variants associated with deep vein thrombosis. JAMA. 2008;299(11):1306–1314. doi: 10.1001/jama.299.11.1306. [DOI] [PubMed] [Google Scholar]
- 14.Sabeter-Lleal M, Martinez-Perez A, Buil A, et al. A genome-wide association study identifies KNG1 as a genetic determinant of plasma factor XI level and activated partial thromboplastin time. Arterioscler Thromb Vasc Biol. 2012;32(8):2008–2016. doi: 10.1161/ATVBAHA.112.248492. [DOI] [PubMed] [Google Scholar]
- 15.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. for the Cardiovascular Health Study Research Group (CHS) The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: The Longitudinal Investigation of Thromboembolism Etiology Study. Arch Intern Med. 2002;162(10):1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 19.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3(10):e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid JG, Carroll A, Veeraraghavan N, et al. Launching genomics into the cloud: deployment of Mercury, a next generation sequence analysis pipeline. BMC Bioinformatics. 2014;15:30. doi: 10.1186/1471-2105-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 22.Gailani D, Broze GL., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253(5022):909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 23.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conversation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodhams B, Girardot O, Blanco MJ, et al. Stability of coagulation proteins in frozen plasma. Blood Coagul Fibrinolysis. 2001;12(4):229–236. doi: 10.1097/00001721-200106000-00002. [DOI] [PubMed] [Google Scholar]