Abstract
Primary sclerosing cholangitis (PSC) is a pre-malignant biliary tract disease that confers a significant risk for the development of cholangiocarcinoma (CCA). The chronic biliary tract inflammation of PSC promotes pro-oncogenic processes such as cellular proliferation, induction of DNA damage, alterations of the extracellular matrix and cholestasis. The diagnosis of malignancy in PSC can be challenging as inflammation-related changes in PSC may produce dominant biliary tract strictures mimicking CCA. Biomarkers such as detection of methylated genes in biliary specimens represent non-invasive techniques which may discriminate malignant biliary ductal changes from PSC strictures. However, conventional cytology and advanced cytologic techniques such as fluorescence in situ hybridization (FISH) for polysomy remain the practice standard for diagnosing CCA in PSC. Curative treatment options of malignancy arising in PSC are limited. For a subset of patients selected using stringent criteria, liver transplantation following neoadjuvant chemoradiation is a potential curative therapy. However, most patients have advanced malignancy at the time of diagnosis. Advances directed at identifying high risk patients, early cancer detection, and development of chemopreventive strategies will be essential to better manage the cancer risk in this pre-malignant disease. A better understanding of dysplasia definition and especially its natural history is also needed in this disease. Herein, we review recent developments in our understanding of the risk factors, pathogenic mechanisms of PSC associated with CCA, as well as advances in early detection and therapies.
Keywords: Biliary dysplasia, chemoprevention, interleukin-6, perihilar cholangiocarcinoma
Introduction
Chronic inflammation and cholestasis in primary sclerosing cholangitis (PSC) promote carcinogenesis of the biliary tract by fostering pro-survival signaling pathways and development of genetic aberrations.1 Inflammatory pathways are not only strongly associated with carcinogenesis but are often retained by CCA cells to facilitate tumor invasion and migration; an observation which is not surprising as inflammation is critical in tissue remodeling and cancer mimics dysregulated tissue remodeling. The risk of cholangiocarcinoma (CCA) among patients with PSC is increased 400-fold when compared to the general population.2 CCA remains one of the leading causes of liver related mortality in this population.3, 4 Perihilar cholangiocarcinoma (pCCA) is the CCA subtype most often seen in the context of PSC.5 Although there have been reports of a dysplasia-carcinoma sequence in PSC, the characteristics of pre-malignant lesions and prevalence of biliary dysplasia in PSC is incompletely understood.6–8 These facts result in several different questions for the clinician caring for the PSC patient. Is my PSC patient at risk for developing CCA and if so, what is the surveillance strategy? If I detect dysplasia what are the therapeutic strategies? If my patient has CCA what are the treatment options? We review recent advances addressing these pertinent and difficult questions. We also provide our perspectives reflecting on these advances and our vision for research-driven advances to answer these questions. Finally, although PSC usually co-exists with inflammatory bowel disease, and the risk of colorectal cancer is increased in these patients, guidelines for colon cancer surveillance in IBD have been published elsewhere and will be not discussed in this review.9
Epidemiology & Risk Factors
Population-based studies suggest that the annual risk for CCA is approximately 2% with a 10 and 30-year cumulative incidence of 6–11% and 20%, respectively.2, 3, 10 The development of CCA can be the heralding event which brings patients with undiagnosed PSC to the attention of clinicians. For example, population-based studies have reported that 27–37% of biliary cancers are detected within the first year of a diagnosis of PSC.2, 10 Consequently, it is important to have a high index of suspicion for CCA around the time of the diagnosis of PSC. However, clinicians should maintain vigilance throughout the disease course as the majority of biliary cancers will develop more than a year after the initial PSC diagnosis.
Our knowledge concerning risk factors for CCA in PSC is limited. An older age at the time of PSC diagnosis, history of colorectal cancer or dysplasia, longer duration of IBD, variceal hemorrhage, smoking and alcohol consumption have been reported to have a positive association with biliary cancer and PSC.2, 4, 10–15 An older age at the time of PSC diagnosis and a history of colorectal cancer have been implicated in more than one study.2, 4, 10, 15 However, the evidence is insufficient to utilize these factors to risk stratify PSC patients who are more likely to benefit from a screening program.
There are several PSC subgroups which appear to have a lower risk of CCA. Among longitudinal studies of small duct PSC, biliary cancer has not been reported unless there is progression to large duct PSC.16 Hence, we do not routinely screen for CCA among asymptomatic patients with small duct PSC. In addition, the risk of CCA among pediatric patients appears to be lower when compared to their adult counterparts. For example, in a case series conducted over a 25-year period at a large tertiary institution that is a referral center for CCA and PSC, there were no cases of biliary cancer among pediatric PSC patients less than 18 years old.17 Similarly, among 29 pediatric PSC patients from a population-based study in North America, only 1 person (3%) was diagnosed with CCA before the age of 18 years.18 Consequently, routine CCA screening of pediatric PSC patients would likely be low-yield. There is emerging evidence to suggest that patients with PSC and a lower alkaline phosphatase are more likely to have improved outcomes.19 While a study found no cases of CCA among PSC patients with a persistent reduction in serum alkaline phosphatase less than 1.5 times the upper limit of normal, this observation was not confirmed in a subsequent study.19, 20 Therefore, there is insufficient evidence to suggest alkaline phosphatase could be utilized as a serologic marker to risk stratify PSC patients who should undergo routine CCA screening.
In our opinion, it is likely that various genetically driven subsets of PSC exist, some of which are susceptible to CCA and some which are not. Hopefully, future studies of PSC patients using a precision/individualized medicine genetic approach will address this topic.
Mechanisms of Inflammation-Induced Biliary Tract Cancer
Although the precise etiology of PSC is ambiguous, recent studies have highlighted an immune-mediated basis. In approximately 25% of cases, PSC is seen in the context of at least one other autoimmune disease outside the gastrointestinal tract.21 PSC has robust associations within the HLA complex, which provides further credence to the notion that immunity plays a role in its pathogenesis.22 Haplotypes with well-established associations with PSC include HLA-DRB1*1301-DQB1*0603, HLA-A1-B8-DRB1*0301-DQB1*0201, and HLA-DRB1*1501-DQB1*0602.23, 24 Overall, there are 16 known genome-wide significant loci in PSC, including the HLA complex on chromosome 6.25–27 Of these 16 loci, 12 were recently identified using the Immunochip, a genotyping array with marker coverage across a number of loci from 12 immune-mediated diseases.25 A stronger association with PSC than with IBD is seen with 6 of these 12 loci, which denotes that although there is some overlap, the genetic architecture for these two diseases is distinct.25 To date, no germ-line oncogenic genetic mutations have been described in PSC. These findings reinforce the association between PSC and other immune-based diseases, highlight an immune-mediated pathophysiological basis for PSC, and suggest CCA development is a secondary event related to inflammation, and not a primary genetic process.25
Biliary tract cancer is a prototype of malignancies occurring in the context of inflammation. PSC promotes chronic inflammation of the biliary tree, predisposing to the development of CCA.28 Chronic inflammation facilitates oncogenesis via induction of DNA damage, promotion of cellular proliferation, and inhibition of apoptosis. For example, inflammatory cytokines activate inducible nitric oxide (iNOS) with excess production of nitric oxide (NO) and consequent nitrosative stress.29 iNOS is not present in normal biliary epithelia but its expression has been demonstrated in PSC as well as CCA. Oxidative DNA lesions are the primary mechanism of DNA damage in inflammation and the most abundant oxidative DNA lesion is 8-oxodeoxyguanine. These lesions are typically excised by DNA repair processes. NO inhibits 8-oxodeoxyguanine base excision DNA repair processes with resultant accumulation of this oxidative lesion in PSC.30 The failure to repair 8-oxodeoxyguanine is mutagenic and fosters cancer development and progression.30 Thus, NO has an integral role in mediating DNA damage in biliary tract inflammation and carcinogenesis.30
Sublethal pro-apoptotic signaling has recently been mechanistically linked to the genesis of chromosal instability, a hallmark of cancer.31, 32 The pro-apoptotic death receptor agonist, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), has been implicated in both PSC and cancer development, presumably by this mechanism of inducing chromosomal instability.33, 34 The role of TRAIL and sublethal cell injury in the development of PSC-associated CCA merits further study.
Cholestasis occurring in the setting of PSC also confers an enhanced risk of CCA development. Bile acids activate receptor tyrosine kinases such as epidermal growth factor receptor (EGFR). Sustained activation of EGFR in CCA mediates proliferation and induces expression of cyclooxygenase-2 (COX-2) via a mitogen-activated protein kinase (MAPK)-dependent mechanism.35, 36 COX-2 is also induced by various inflammatory cytokines and contributes to carcinogenesis by promoting proliferation and angiogenesis, and inhibiting apoptosis.35 Oxysterols, oxygenated derivatives of cholesterol, are abundant in the bile of patients with biliary tract inflammation.37, 38 Stabilization of COX-2 expression by oxysterols has been implicated in the genesis and promotion of CCA.39 Oxysterols also serve as activators of the hedgehog signaling pathway, a developmental pathway implicated in CCA development.40–43
A recently developed oncogene-driven murine model of CCA highlights the role of inflammatory cytokines in CCA oncogenesis.44 In this model, biliary transduction of constitutively active Akt and yes-associated protein (YAP) coupled with lobar bile duct ligation and systemic interleukin (IL)-33 administration resulted in the development of CCA.44 IL-33 promotes downstream activation of IL-6 signaling in this model. IL-33, an IL-1 family member, is a known biliary mitogen, which promotes inflammation and fibrosis in the biliary tract.45, 46 Inflammatory stimuli promote production of IL-6, an inflammatory cytokine, by cholangiocytes. Activation of IL-6/STAT3 signaling, in turn, promotes growth stimulation of malignant cholangiocytes by activation of p38 or p44/42 mitogen-activated protein kinases (MAPK) signaling pathways in an autocrine or paracrine manner.47 A recent paper also relates IL-6 signaling to YAP activation in mucosal injury of the intestine and perhaps this pathway is also relevant to the biliary tree;48 YAP-mediated epithelial regeneration could also be an initiator of carcinogenesis if sustained and unrelenting.
These mechanistic insights linking chronic inflammation to carcinogenesis provide potential therapeutic avenues for chemoprevention in PSC. For example, inhibitors of STAT3 and Janus kinases (JAK), which activate STAT3 downstream of IL-6, are in clinical development.1 JAK kinase inhibitors are currently being tested for the treatment of inflammatory bowel disease, which co-exists in 85% of PSC patients.49 Thus, the use of JAK inhibitors in these patients may come to fruition, permitting an assessment of their chemopreventive effects in this patient population. In addition to JAK-STAT inhibitors, caspase, iNOS, COX-2, and Hippo pathway antagonists may also be chemopreventive.
Cholangiocarcinoma Screening & Surveillance
Cholangiocarcinoma is divided into perihilar CCA, intrahepatic CCA, and distal subtypes based on the anatomic location of the tumor within the biliary tree.28 pCCA is not only the most common subtype overall but also the subtype primarily seen in the context of PSC.28 pCCA frequently presents with an obstructive biliary stricture without the presence of a mass on cross-sectional imaging (Figure 1A). Inflammatory/fibrotic obstructive biliary strictures in PSC, so called dominant strictures, mimic malignant strictures, and hence, distinguishing a benign inflammatory/fibrotic stricture in PSC from a malignant stricture can be quite challenging and is not possible using noninvasive diagnostic modalities such as magnetic resonance cholangiography (MRC). An endoscopic retrograde cholangiography (ERC) is essential in this setting as it has diagnostic and therapeutic utility.
Figure 1. Imaging features of pCCA in PSC.

(A) ERC image of a dominant common hepatic duct stricture (indicated by white arrow) in a PSC patient with periductal infiltrating pCCA. (B) MRI image of a perihilar mass (indicated by white arrow) with resultant biliary obstruction. ERC, endoscopic retrograde cholangiography; MRI, magnetic resonance imaging; PSC, primary sclerosing cholangitis; pCCA, perihilar cholangiocarcinoma.
Radiologic Imaging
Imaging plays a central role in the detection of CCA and abnormalities seen on imaging often trigger additional investigations aimed at establishing a diagnosis of biliary cancer. Ultrasonography, computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI)/MRC have been investigated as diagnostic imaging modalities.
While inexpensive and noninvasive, ultrasonography may only delineate intrahepatic ductal dilation without providing further detailed information in the absence of a mass lesion.50 Although a CT scan can readily characterize mass lesions and investigate invasion into adjacent structures or metastases, its sensitivity and specificity for CCA detection in PSC is 75% and 85%, respectively.50, 51 A meta-analysis examined 23 studies and the ability of fluorine-18-flurodeoxyglucose (18F-FDG)-PET or PET/CT to detect CCA. The pooled sensitivity and specificity for CCA among those with and without PSC was 81% and 82%.52 Notably, false-positive results can occur secondary to inflammation associated with PSC.53 A recent study examined the role of 18F-FDG-PET/CT and the 18FDG uptake values, normalized to the background liver, at 180 minutes (SUVmax/liver) among 70 patients with PSC and a “dominant stricture.” There were 9 cases of CCA in the cohort and 55% did not have definitive features of cancer on MRI. An analysis of the 18FDG uptake showed that a SUVmax/liver quotient of 3.3 was able to distinguish between CCA and benign strictures with a sensitivity and specificity of 89% and 92% (respectively) while a quotient of less than 2.4 excluded CCA (sensitivity 100%, specificity 78%).54 While these findings warrant confirmation in a larger cohort, these results suggests that the use of 18F-FDG-PET/CT may be helpful in a subset of patients particularly if there is a persistent concern for CCA despite a negative MRI/MRC and negative biliary brushings. Although 18F-FDG-PET/CT and the evolving 18F-FDG-PET/MRI technologies all hold promise for the diagnosis of PSC-associated CCA, many confirmed cases of CCA are 18F-FDG-PET negative. The ultimate role of this approach for the diagnosis of CCA remains to be clarified.
Of these imaging modalities, MRI/MRC is the diagnostic imaging method of choice. A mass lesion with venous phase enhancement is very specific for CCA (Figure 1B).52 However, such definitive features are frequently absent. More commonly, CCA infiltrates along the biliary tree leading to ductal narrowing, thickening and dilation. When present, CCA typically has maximal enhancement on delayed phases.50 Such findings can be subtle and distinguishing benign from malignant strictures in PSC is challenging. Radiographic findings which should raise concern are the development of thickened or nodular bile ducts or a new “dominant stricture”.52 Indeed, inflammatory/obstructive strictures have been reported in up to 50% of patients with PSC which leads to symptoms in approximately 10–30% of individuals. One-quarter of the so-called dominant strictures are malignant.53, 55 However, CCA may be detected in individuals without an obstructive stricture so their absence does not exclude malignancy.56 The presence of perihilar lymphadenopathy by itself should not raise concern as this is commonly seen in patients with PSC.57
Carbohydrate Antigen 19-9
The most widely studied serum biomarker for CCA in PSC is CA 19-9. The synthesis and ability to express CA 19-9 is dependent upon fucosyltransferase-2 & 3 (FUT-2 and FUT-3) activity and individuals who lack FUT-3 activity (Lewis antigen negative) are unable to express CA 19-9 (approximately 7% of the population). 9, 58 Utilizing a CA 19-9 cut-off of 129 U/mL the sensitivity and specificity for CCA detection was 79% and 99% (respectively) and using a threshold of 100 U/mL yielded a similar diagnostic performance. However, only advanced cases of CCA were detected by either cut-off. Furthermore, examining changes in CA 19-9 overtime did not add to the diagnostic yield of a single CA 19-9 value.59 However, other studies have called in to question the high specificity of a CA 19-9 value greater than 129 U/mL. For example, two studies have reported that one-third of PSC patients with a CA 19-9 greater than 129 U/mL do not have underlying CCA.60, 61 Recognizing this assay’s limitations, authors have sought to improve the diagnostic performance of CA 19-9 by utilizing FUT2/3 genotype specific CA 19-9 thresholds. This method has been shown to improve the sensitivity and decrease the number of false positive test results by 43% among a large cohort of PSC patients.58
Biliary Cytology
Conventional biliary cytology can be classified into 5 categories: nondiagnostic, normal, atypical, suspicious or positive for adenocarcinoma.62 The primary advantage of biliary cytology is its high specificity (97–100%) when adenocarcinoma is detected.63, 64 However, suspicious cytology also represents a concerning finding. For example, 34–42% of PSC patients without a mass lesion and suspicious cytology may ultimately be diagnosed with CCA and suspicious cytology is an independent predictor for the development of biliary cancer.56, 65 Atypical cytology is frequently encountered in PSC. This is primarily due to the presence of biliary inflammation and by itself should not raise concern.56 The chief disadvantage of biliary cytology is its limited sensitivity (43%) and potential for false negative results.63 This is secondary to the desmoplastic, paucicellular nature of CCA which can reside in areas that are difficult to access. Therefore, the absence of a positive cytology does not rule out malignancy.
Overview of Fluorescence in-situ Hybridization
Because of the limitations of biliary cytology, fluorescence in-situ hybridization (FISH) has been employed as a second generation test to enhance clinicians’ ability to risk-stratify PSC patients. The FISH assay examined in patients with PSC utilizes 3 centromeric probes that target chromosomes 3, 7 and 17 and a locus specific probe to 9p21 from samples obtained by biliary brushings. FISH detects abnormal gains or losses of chromosomes (aneusomy) and the results can be categorized as normal, trisomy (10 or more cells with 3 copies of chromosome 7, and 2 or fewer copies of the other 3 probes), tetrasomy (10 or more cells show 4 copies of all probes) or polysomy (5 or more cells show gains of 2 or more of the 4 probes).62 FISH polysomy indicating duplication of more than one chromosome is a marker for chromosomal instability, a hallmark of cancer.
When compared to a normal FISH result, trisomy/tetrasomy are not independent predictors of CCA. In contrast, polysomy is strongly associated with a diagnosis of CCA.56, 65–67 A meta-analysis examined the performance of FISH testing among 690 PSC patients and the pooled sensitivity, specificity, positive and negative likelihood ratio for polysomy and CCA was 51%, 93%, 6.8 and 0.6, respectively.68 This study did not distinguish between other factors that can increase the probability of biliary cancer when polysomy is present and it is important for FISH results to be interpreted in the clinical context of each individual patient (see below).
i. Polysomy Associated with a “Dominant Stricture” or Elevated CA19-9
Polysomy in the presence of a dominant stricture may increase the probability of cancer. For example, in a study that included 235 PSC patients (with and without a mass lesion), 55% of patients with polysomy were diagnosed with biliary cancer. However, if a “dominant stricture” was present in the setting of polysomy, 73% of PSC patients were diagnosed with CCA.66
An elevated CA-19-9 in the setting of polysomy is also concerning for cancer. For example, among PSC patients (without a mass lesion at baseline) suspected of CCA with equivocal cytology (atypical or suspicious) and polysomy, 100% of patients with a CA 19-9 ≥129 U/mL compared to 24% of subjects with a CA 19-9 < 129 U/mL were diagnosed with CCA within 3 years.65 In a separate study, 71% of patients with polysomy (regardless of cytology) and a CA 19-9 ≥129 U/mL who lacked a mass lesion on imaging were diagnosed with CCA compared to 37% of individuals with a CA 19-9 < 129 U/mL.56 When polysomy was incorporated in the multivariable analyses in both of these studies, CA 19-9 was no longer statistically significant. However, given the high proportion of individuals with an elevated CA 19-9 and polysomy who were diagnosed with cancer, an increased CA 19-9 should raise concern.
ii. Serial or Multifocal Polysomy
When compared to the PSC population at large, those with polysomy in the absence of definitive features of CCA at the time of the initial assessment (i.e., lack a positive cytology or definitive mass lesion) are likely rare (estimated to be less than 5% of total PSC population seen at one institution over a 7 year period).56 While uncommon, this important subgroup of patients represents a diagnostic challenge. Consequently, a study examined the performance of polysomy when it is detected on subsequent examinations (serial polysomy) among those without definitive radiographic features of biliary malignancy. The 3-year cumulative incidence of CCA was 75% among those with serial polysomy and 18% of subjects with non-serial polysomy (polysomy detected only once at the index examination). After 1 year of follow-up the incidence of CCA did not increase among those with non-serial polysomy.67 These results reinforce the importance of repeating an ERC with brushings for cytology and FISH if polysomy is detected in the absence of definitive features of CCA.
The largest study which examined FISH in PSC included 371 patients without a mass lesion on imaging and investigated the natural history of polysomy when it was detected in multiple areas of the biliary tree (multifocal polysomy) when compared to unifocal polysomy, serial polysomy and other FISH subtypes. In the adjusted analysis, multifocal polysomy was the strongest predictor of a diagnosis of CCA (regardless of whether a dominant stricture or serial polysomy was present) and the 1- and 3-year cumulative incidence of CCA among those with multifocal polysomy was 65% and 83%, respectively. Among those with polysomy and a positive cytology, 71% had polysomy detected at another region of the biliary tree where adenocarcinoma was not detected by routine cytology. These findings reinforce the role of FISH and indirectly supports the hypothesis that CCA in PSC may arise from a field defect beyond the primary site of malignancy. It also suggests that it may be important to brush multiple areas of the biliary tree, regardless of whether and where a dominant stricture is located, and place the specimens in separate vials when CCA is suspected. However, individuals with unifocal polysomy should not be ignored as this was also a risk factor for CCA when compared to a normal FISH result.56
Advanced Endoscopic Biliary Imaging Techniques
As conventional cytology has limited sensitivity and propensity for false-negative results and the availability of FISH is limited, advanced techniques for endoscopic biliary imaging have become a recent research focus. These techniques, however, have largely been examined in non-PSC patients. These techniques include Spyglass Spyscope which enables direct endoscopic visualization of bile ducts and directed biopsies of suspicious lesions. In a retrospective analysis, Spyglass Spyscope had a 77% accuracy in detection of CCA in patients with diagnostic uncertainty after conventional cytology of endoscopically obtained biliary brushings and EUS-FNA.69 Intraductal ultrasound (IDUS) of the biliary system provides evaluation of the periductal tissues during an ERCP. In a prospective comparative analysis, ERCP supplemented with IDUS allowed differentiation of benign from malignant biliary strictures in 88% of patients (n = 33).70 Moreover, ERCP plus IDUS had a significantly greater accuracy in differentiating between malignant and benign strictures compared to MRCP.70 Another promising diagnostic approach which can supplement conventional ERCP is probe-based confocal laser endomicroscopy. Mucosal imaging was conducted in 14 patients utilizing a confocal laser scanning miniprobe introduced via the accessory channel of a conventional cholangioscope.71 Subsequently, targeted biopsy specimens were obtained under visual cholangioscopic guidance in all patients.71 By detecting a specific pattern of neovascularization, confocal laser microscopy enabled prediction of CCA with a sensitivity of 83% and specificity of 88%.71 In comparison, standard histopathology had a sensitivity and specificity of 50% and 100%.71 Finally, we note that narrow-band imaging in combination with cholangioscopy is disappointing in its ability to identify biliary dysplasia in the setting of PSC.72 These advanced biliary imaging techniques hold promise; however, their utility in PSC patients remains unstudied, a patient population more complex than those with de novo CCA. Moreover, in our own experience, technical failure with poor visualization of the PSC biliary tract is common with Spyglass Spyscope technology, diminishing sensitivity and specificity on an “intent-to-diagnose” assessment.
Approach to Biliary High Grade Dysplasia
Presence of high-grade dysplasia of the biliary tract may herald CCA development in PSC patients.7, 8, 73, 74 Liver explants from patients with concomitant PSC and CCA are more likely to harbor high-grade dysplasia compared to PSC explants without CCA.6 Moreover, cytogenetic assessment of liver explants with biliary dysplasia from patients with PSC has demonstrated that patients with previous or current CCA are more likely to have FISH polysomy in dysplasia than patients without.7 In this study, FISH polysomy was detected in 58% of the areas with high-grade dysplasia compared to 11% of areas with low-grade dysplasia.75 Thus, improved cytogenetic techniques can detect high-grade dysplasia prior its progression to CCA.7 The presence of FISH polysomy in the absence of other diagnostic features of CCA (cross-sectional imaging with mass or dominant stricture +/− positive cytology) may indicate underlying high-grade dysplasia.
Management of PSC patients with dysplasia remains complex and unclear. On one hand, definitions and stratification into low and high-grade dysplasia are not standardized. The natural history is also unknown. On the other hand, a dysplasia-carcinoma sequence has been reported73 and most patients with dysplasia develop CCA (personal observation). Hence, one can argue equally vociferously for careful observation and surveillance or an aggressive approach with liver transplantation (see below). In countries where organ allocation is favorable to these patients, liver transplantation has been suggested as the treatment of choice.74 In the U.S., a conservative approach is the sole option as only patients with overt CCA are eligible for transplantation.
Such patients should be enrolled in intensive surveillance for CCA detection. PSC patients with biliary dysplasia would be ideal candidates for chemopreventive strategies, once potential agents are identified.
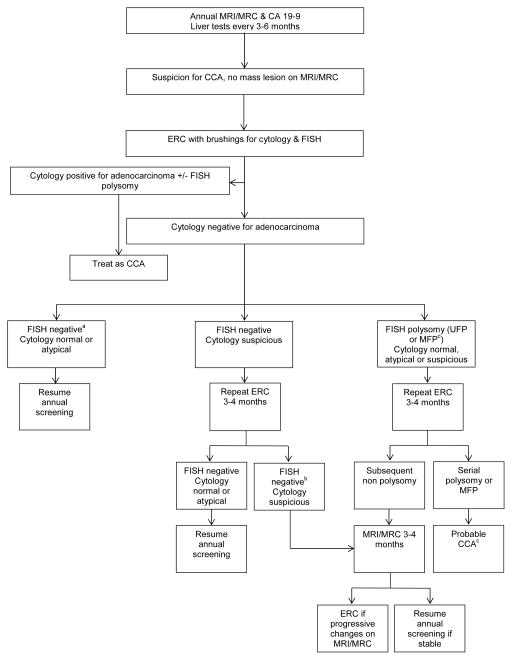
A Rationale Approach to Cholangiocarcinoma Screening & Surveillance
Among adults over the age of 20 with large duct PSC it is our practice to obtain liver tests every 3–6 months and an annual MRI/MRC and CA-19-9.52 If a suspected CCA is detected, a transperitoneal biopsy of the primary mass lesion via endoscopic ultrasound or a percutaneous approach should not be performed due to the high risk of peritoneal seeding which would make patients ineligible for a liver transplant.76 Although the cost-effectiveness of this surveillance strategy and its effect on long-term outcome are unclear, patients and physicians desire a surveillance approach given the disastrous consequences of diagnosing CCA at a time when it is symptomatic. In this regard, non-invasive imaging of the biliary tree and determination of cancer-associated biomarkers is a pragmatic approach until further information is available.52
An ERC with brushings for cytology and FISH is typically performed among individuals with suspicious features on imaging (a new dominant stricture or the development of focal bile duct thickening, irregularity or enhancement), symptoms that suggest biliary obstruction or worsening laboratory tests (including new elevations of CA 19-9 greater than 100 U/mL in the absence of cholangitis). Subsequent follow-up is based on the results of this initial assessment (Figure 2). Currently, there is no evidence to support or refute this practice and it is unclear if this strategy improves patient outcomes or is cost-effective. However, given the prevalence of CCA among PSC patients and the possibility to undergo a curative therapy if detected early, we believe this strategy is rationale and pragmatic.
Figure 2. Screening and Surveillance in CCA.
Approach to cholangiocarcinoma screening and surveillance among adults with large duct primary sclerosing cholangitis. CCA, cholangiocarcinoma. Modified from Eaton et al.62
aNormal FISH, trisomy/tetrasomy are considered a negative FISH study.
bIf FISH positive for polysomy, repeat ERC in 3–4 months and follow polysomy algorithm
cEstimated proportion of patients with cancer 3 years after diagnosis of multifocal polysomy or serial polysomy was 83% and 75%, respectively
Abbreviations: CCA (cholangiocarcinoma); FISH (fluorescence in situ hybridization); MRI (magnetic resonance imaging); MRC (magnetic resonance cholangiogram); ERC (endoscopic retrograde cholangiogram); UFP (unifocal polysomy); MFP (multifocal polysomy).
Future Biomarkers
Contemporary methods used to establish a diagnosis of CCA are suboptimal. A better understanding of cancer biology, bile acid composition and key –omics (tumor genomics, epigenomics, transciptomics, proteomics and lipidomics) have paved the way for a series of preliminary studies which have examined the role of novel biomarkers in the diagnosis of CCA. Table 1 highlights key studies which have examined unique markers that also included CCA associated with PSC and PSC controls without CCA (Table 1).
Table 1.
Novel biomarkers for cholangiocarcinoma detection among patients with primary sclerosing cholangitis.
| Biomarker | Source | Study Population | Sensitivity | Specificity | AUCa |
|---|---|---|---|---|---|
|
| |||||
|
DNA methylation
75
CDO1 CNRIP1 SEPT9 VIM |
Biliary brushings | CCA n= 42 (PSC
n=24) Controls n=50 (PSC n=49) |
85% | 98% | 0.94 |
|
| |||||
|
Noncoding RNA82 MicroRNAs in Extracellular vesicles |
Bile | CCA n=46 (PSC
n=4) Controls n=50 (PSC n=13) |
67% | 97% | -- |
|
| |||||
|
Peptides86 Panel of 22 peptides |
Bile | CCA n=25 (PSC
n=10) Controls n=18 (PSC n=18) |
84% | 78% | 0.87b |
|
| |||||
|
VOCs84 Acrylonitrile 3-methyl hexane benzene |
Bile | CCA n=11 (PSC
n=11) Controls n=21 (PSC n=21) |
91% | 73% | 0.89 |
|
| |||||
|
Peptides87 Panel of 42 peptides |
Urine | CCA n=42 (PSC
n=10) Controls n=81 (PSC n=45) |
83% | 79% | 0.87c |
|
| |||||
|
Protein88 Angiopoietin-2 ≥ 5333 pg/mL |
Serum | CCA n=49 (PSC
n=7) Controls n=48 (PSC n=34) |
74% | 94% | 0.85 |
|
| |||||
|
Protein89 Cytokeratin 19 fragments ≥3 ng/mL |
Serum | CCA n=66 (PSC
n=6) Controls n=58 (PSC n=19) |
30% | 97% | --d |
|
| |||||
|
Protein90 Trypsinogen-2 |
Serum | CCA n=41 (PSC
n=8) Controls n=43 (PSC n=43) |
-- | -- | 0.80e |
Reflects diagnostic performance of entire cohort (i.e., patients with and without PSC).
Data from test cohort (discovery cohort not included). Eighty percent of PSC-CCA correctly identified.
Data from test cohort (discovery cohort not included). One-hundred percent of PSC-CCA correctly identified.
Among those only with PSC (n=25) the sensitivity and specificity was 17% and 95%, respectively.
Optimal cut-off for trypsinogen-2 concentration not reported. Among those only with PSC (n=51) the AUC was 0.76.
Abbreviations: AUC (area under the curve); CCA (cholangiocarcinoma); PSC (primary sclerosing cholangitis); VOCs (volatile organic compounds); -- (not reported).
Compared to non-neoplastic tissue, tumor cells often have a higher proportion of aberrant DNA methylation which can in turn serve a useful biomarker for cancer detection.77, 78 Among the studies which have examined new biomarkers in PSC-associated CCA, a 4 methylated gene panel (CDO1, CNRIP1, SEPT9 and VIM) obtained from biliary brushings among patients with and without PSC has the best diagnostic performance reported to date (sensitivity 85% and specificity 98%).75 However, among the cases of CCA in patients with PSC, 83% had advanced cancers who would have been ineligible for a liver transplant and the role of this biomarker panel to detect early-stage CCA among patients with PSC who are under a longitudinal surveillance program is unclear but warrants further study.
In addition to DNA methylation, noncoding RNAs have been examined as markers in malignant conditions.79, 80 Indeed, among patients with CCA (not associated with PSC) and PSC controls, the measurement of U2 small nuclear RNA fragments in bile was able to distinguish CCA from PSC without cancer, area under the curve (AUC) 0.86.81 A subsequent study examined the role of another group of noncoding RNAs (microRNAs) and found that a panel of biliary vesicle microRNAs among those with and without PSC had a sensitivity of 67% and specificity of 96% for the diagnosis of CCA.82
Volatile organic compounds (VOCs) represent a gas-phase biomarker which has been examined as a potential diagnostic modality in a variety of conditions.83 Examining the presence of VOCs in bile or urine to distinguish benign from malignant biliary strictures has also been investigated.84 For example, among a dedicated PSC cohort, a model adjusted for age and gender plus VOC levels obtained from bile was able to identify CCA with a sensitivity and specificity of 91% and 73%, respectively.84 In addition to VOCs, a small pilot study examined the bile lipid profile among those with de novo CCA and benign biliary conditions including PSC and found that a combination of two phosphatidylcholines was able to distinguish benign from malignant strictures with a sensitivity and specificity of 100% and 83%, respectively.85
To date, much of the published work to improve methods of CCA detection have centered on proteomics either obtained from bile, urine or serum. Indeed, there appears to be a varying protein composition of bile between those with benign and malignant strictures and a panel of 22 peptides was able to identify 80% of CCA associated with PSC.86 Similarly, a panel of 42 urine peptides was able to accurately identify all of the PSC patients with CCA.87 A variety of studies have examined protein markers in serum. One such study investigated the performance of circulating angiopoietin-2. This protein was noted to have a better diagnostic accuracy compared to CA 19-9 (AUC 0.85 versus 0.77) and a sensitivity and specificity for the detection of CCA of 74% and 94%, respectively.88 An increase in serum cytokeratin 19 fragments was associated with an increase in mortality and it demonstrated an excellent specificity (≥ 95%) even when limited to a PSC only subgroup but the sensitivity remained poor (≤ 30%) when a cutoff of ≥3 ng/mL was used.89 Trypsinogen-2, another serum protein, is increased in CCA and the use of trypsinogen-2 was able to differentiate between PSC with and without CCA (AUC 0.76).90 Lastly, an additional study combined a variety of serum proteins (CA 19-9, leucine-rich α-2-glycoprotein and IL-6) among patients with CCA and conditions associated with benign biliary strictures and reported an AUC of 0.98. However, it did not appear that PSC patients with CCA were examined.91 These studies highlight the evolving methods which are being investigated to distinguish benign from malignant strictures among patients with PSC.
Management
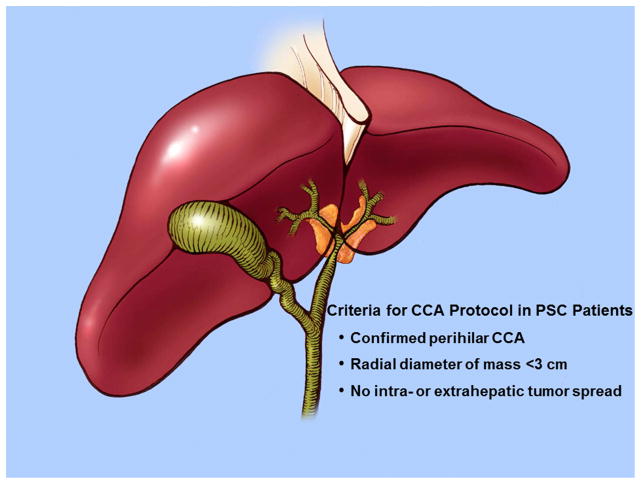
Outcomes of surgical resection for pCCA rising in the setting of PSC have been quite disappointing.92, 93 Furthermore, curative surgical resection is often not an option for pCCA occurring in the setting of PSC due to the underlying parenchymal liver disease, the field defect, and the predilection for skip lesions.5 These tumors are also usually unresectable either due to local tumor extent or the underlying disease itself.94 Liver transplantation appeared to be an optimal treatment for pCCA in PSC patients as it would address the underlying disease as well as hepatic or vascular invasion.94 However, despite this logical basis the outcomes were subpar with one study noting a three-year survival of 30% in PSC patients incidentally diagnosed with CCA.95 Efficacy of palliative radiotherapy lead to the development of a protocol combining chemoradiation followed by liver transplantation. Selection criteria for this protocol is quite rigorous and includes the following prerequisites in a PSC patient: confirmed diagnosis of pCCA, radial tumor diameter less than 3 cm, and absence of intra- or extrahepatic metastasis (Figure 3).28
Figure 3.
Criteria for Liver Transplantation in PSC Patients with pCCA.
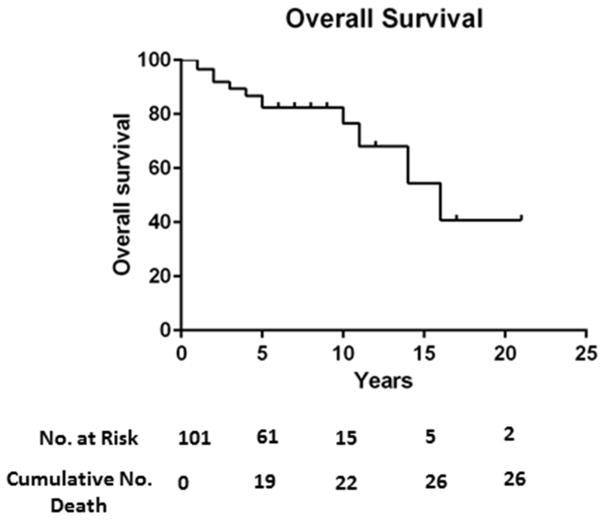
According to a retrospective analysis of 191 patients enrolled in the neoadjuvant chemoradiotherapy followed by liver transplantation protocol, 16 patients underwent transperitoneal fine-needle aspiration of the primary tumor (13 percutaneous, 3 endoscopic ultrasound).96 A total of 6 patients had biopsies demonstrating malignancy. Five of these patients (83%) were found to have direct metastasis into the peritoneum at operative staging, suggesting peritoneal seeding after transperitoneal biopsy.96 Thus, patients with a prior transperitoneal biopsy of the primary tumor are excluded from this protocol. After judicious selection, patients undergo a combination of radiosensitizing chemotherapy with 5-fluorouracil, external beam radiation therapy, brachytherapy with endoscopically placed iridium-192 beads, and maintenance chemotherapy with capecitabine.97 Prior to orthotopic liver transplantation, patients undergo a staging laparotomy to assess for the presence of metastasis.97 pCCA patients who underwent neoadjuvant chemoradiation in anticipation of liver transplantation at 12 U.S. centers had a 65% recurrence free survival at 5 years.97 The overall 10-year survival for PSC patients with pCCA who underwent neoadjuvant chemoradiation in anticipation of liver transplantation at our center is approximately 75% (Figure 4). Approximately 30% of patients drop out of the protocol prior to liver transplantation, primarily due to cancer progression.98 Small case series of combined orthotopic liver transplantation and en bloc Whipple procedure with chemoradiation alone99 or chemoradiation plus brachytherapy100 have been reported for treatment of hilar cholangiocarcinoma. This is a rational approach for potentially curative therapy of distal cholangiocarcinoma. However, in the setting of hilar cholangiocarcinoma without any involvement of the distal bile duct by malignant or pre-malignant lesions, this method may be overly aggressive.
Figure 4.
Kaplan-Meier curve for overall survival in PSC patients with pCCA undergoing neoadjuvant chemoradiation followed by liver transplantation.
For advanced stage pCCA, liver transplantation following neoadjuvant chemoradiation is not an option. In this setting, the combination of gemcitabine and cisplatin remains the pragmatic standard of care.101 Genetic analysis of a tumor can identify potentially actionable events such as mutations which may be candidates for targeted therapy. Recent reports have highlighted evidence of disease regression with chemotherapy directed at the aberrant pathway.102 For instance, patients with fibroblast growth factor receptor 2 (FGFR2) gene fusions had stable disease with ponatinib, an FGFR inhibitor.102 However, FGFR2 gene fusions and other mutations such as isocitrate dehydrogenase 1 and 2 mutations tend to occur more frequently in intrahepatic CCA then pCCA. The genetic aberrations of PSC-associated CCA have yet to be identified. Hopefully, advances in genomic characterization of tumors will allow detection of driver mutations and druggable targets associated with perihilar tumors.
Future Directions
The majority of patients with PSC do not develop CCA and thus it remains unclear which patients have a higher propensity of developing CCA. Recognizing this high-risk subset is imperative and will aid not only in earlier detection but also selecting patients for intensive screening and potential chemopreventive strategies in the future. Currently, there is a lack of chemopreventive therapies and further pre-clinical studies are needed to identify such agents. Detecting early-stage CCA is another challenge in PSC patients. Continued development of tumor biomarkers in biological specimens will be crucial in this regard. Curative nonsurgical treatment of malignancy arising in the context of PSC remains a therapeutic conundrum, partly due to the genetic heterogeneity of these tumors and rapid development of therapeutic resistance with genetic evolution of the tumor. Although significant progress has been made in recognizing oncogenic pathways and mutational changes in CCA, such studies focusing on CCA in PSC remain to be performed. We look forward to these and other advances so that we can prevent and better manage this devastating complication of PSC.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grants DK59427 (G.J.G.), T32DK007198, the American Liver Foundation and International Liver Cancer Association (S.R.), and the Mayo Foundation.
Abbreviations
- AUC
area under the curve
- Carbohydrate antigen 19-9
- CCA
cholangiocarcinoma
- COX-2
cyclooxygenase-2
- CT
computed tomography
- EGFR
epidermal growth factor–receptor
- ERC
endoscopic retrograde cholangiography
- 18F-FDG
fluorine-18-flurodeoxyglucose
- FGFR
fibroblast growth factor receptor
- FISH
fluorescence in situ hybridization
- FUT
fucosyltransferase
- IL
interleukin
- JAK
janus kinase
- MAPK
mitogen-activated protein kinase
- MCL1
myeloid cell leukemia sequence 1
- MRCP
magnetic resonance cholangiopancreatography
- NO
nitric oxide
- pCCA
perihilar cholangiocarcinoma
- PET
positron emission tomography
- PSC
primary sclerosing cholangitis
- STAT
signal transducer and activator of transcription
- SUV
standardized uptake value
- TRAIL
tumor necrosis factor associated apoptosis inducing ligand
- YAP
yes-associated protein
Footnotes
The authors have no commercial relationships or interests to disclose relevant to this manuscript.
Authors’ contributions: Dr. Sumera Rizvi contributed to the outline and drafting of the manuscript, critical revision of the manuscript, and important intellectual content; Dr. John Eaton contributed to the outline and drafting of the manuscript, critical revision of the manuscript, and important intellectual content; Dr. Gregory J. Gores contributed to the outline of the manuscript, critical revision of the manuscript for important intellectual content, and provided manuscript writing supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizvi S, Borad MJ, Patel T, et al. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Seminars in liver disease. 2014;34(4):456–64. doi: 10.1055/s-0034-1394144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boonstra K, Weersma RK, van Erpecum KJ, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58(6):2045–55. doi: 10.1002/hep.26565. Epub 2013/06/19. [DOI] [PubMed] [Google Scholar]
- 3.Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand J Gastroenterol. 1997;32(10):1042–5. doi: 10.3109/00365529709011222. Epub 1997/11/15. [DOI] [PubMed] [Google Scholar]
- 4.de Valle MB, Bjornsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population-based cohort. Liver Int. 2012;32(3):441–8. doi: 10.1111/j.1478-3231.2011.02614.x. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 5.Rizvi S, Gores GJ. Current diagnostic and management options in perihilar cholangiocarcinoma. Digestion. 2014;89(3):216–24. doi: 10.1159/000360791. Epub 2014/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JT, Talwalkar JA, Rosen CB, et al. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34(1):27–34. doi: 10.1097/PAS.0b013e3181bc96f9. [DOI] [PubMed] [Google Scholar]
- 7.Kerr SE, Barr Fritcher EG, Campion MB, et al. Biliary dysplasia in primary sclerosing cholangitis harbors cytogenetic abnormalities similar to cholangiocarcinoma. Hum Pathol. 2014;45(9):1797–804. doi: 10.1016/j.humpath.2014.05.008. Epub 2014/07/17. [DOI] [PubMed] [Google Scholar]
- 8.Fleming KA, Boberg KM, Glaumann H, et al. Biliary dysplasia as a marker of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2001;34(3):360–5. doi: 10.1016/s0168-8278(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 9.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54(5):1842–52. doi: 10.1002/hep.24570. Epub 2011/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36(3):321–7. doi: 10.1016/s0168-8278(01)00288-4. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 11.Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37(10):1205–11. doi: 10.1080/003655202760373434. Epub 2002/11/01. [DOI] [PubMed] [Google Scholar]
- 12.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. The American journal of gastroenterology. 2004;99(3):523–6. doi: 10.1111/j.1572-0241.2004.04067.x. Epub 2004/04/02. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Baluyut A, Ismail A, et al. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31(1):7–11. doi: 10.1002/hep.510310103. Epub 1999/12/29. [DOI] [PubMed] [Google Scholar]
- 14.Bergquist A, Glaumann H, Persson B, et al. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27(2):311–6. doi: 10.1002/hep.510270201. Epub 1998/02/14. [DOI] [PubMed] [Google Scholar]
- 15.Broome U, Lofberg R, Veress B, et al. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22(5):1404–8. doi: 10.1002/hep.1840220511. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 16.Singal AK, Stanca CM, Clark V, et al. Natural history of small duct primary sclerosing cholangitis: a case series with review of the literature. Hepatol Int. 2011;5(3):808–13. doi: 10.1007/s12072-011-9260-4. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 17.Bjornsson E, Angulo P. Cholangiocarcinoma in young individuals with and without primary sclerosing cholangitis. The American journal of gastroenterology. 2007;102(8):1677–82. doi: 10.1111/j.1572-0241.2007.01220.x. Epub 2007/04/17. [DOI] [PubMed] [Google Scholar]
- 18.Deneau M, Jensen MK, Holmen J, et al. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58(4):1392–400. doi: 10.1002/hep.26454. Epub 2013/05/21. [DOI] [PubMed] [Google Scholar]
- 19.Rupp C, Rossler A, Halibasic E, et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment Pharmacol Ther. 2014;40(11–12):1292–301. doi: 10.1111/apt.12979. Epub 2014/10/16. [DOI] [PubMed] [Google Scholar]
- 20.Al Mamari S, Djordjevic J, Halliday JS, et al. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58(2):329–34. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Saarinen S, Olerup O, Broome U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. The American journal of gastroenterology. 2000;95(11):3195–9. doi: 10.1111/j.1572-0241.2000.03292.x. [DOI] [PubMed] [Google Scholar]
- 22.Eaton JE, Talwalkar JA, Lazaridis KN, et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145(3):521–36. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olerup O, Olsson R, Hultcrantz R, et al. HLA-DR and HLA-DQ are not markers for rapid disease progression in primary sclerosing cholangitis. Gastroenterology. 1995;108(3):870–8. doi: 10.1016/0016-5085(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 24.Spurkland A, Saarinen S, Boberg KM, et al. HLA class II haplotypes in primary sclerosing cholangitis patients from five European populations. Tissue antigens. 1999;53(5):459–69. doi: 10.1034/j.1399-0039.1999.530502.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nature genetics. 2013;45(6):670–5. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138(3):1102–11. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Melum E, Franke A, Schramm C, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nature genetics. 2011;43(1):17–9. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal M, LaRusso NF, Burgart LJ, et al. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer research. 2000;60(1):184–90. [PubMed] [Google Scholar]
- 30.Jaiswal M, LaRusso NF, Shapiro RA, et al. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120(1):190–9. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 31.Ichim G, Lopez J, Ahmed SU, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Molecular cell. 2015;57(5):860–72. doi: 10.1016/j.molcel.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, He Y, Li F, et al. Caspase-3 promotes genetic instability and carcinogenesis. Molecular cell. 2015;58(2):284–96. doi: 10.1016/j.molcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovric MM, Hawkins CJ. TRAIL treatment provokes mutations in surviving cells. Oncogene. 2010;29(36):5048–60. doi: 10.1038/onc.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Kojima Y, Ikejima K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105(31):10895–900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon J-H, Higuchi H, Werneburg NW, et al. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122(4):985–93. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 36.Yoon JH, Gwak GY, Lee HS, et al. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41(5):808–14. doi: 10.1016/j.jhep.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Kuver R. Mechanisms of oxysterol-induced disease: insights from the biliary system. Clin Lipidol. 2012;7(5):537–48. doi: 10.2217/clp.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haigh WG, Lee SP. Identification of oxysterols in human bile and pigment gallstones. Gastroenterology. 2001;121(1):118–23. doi: 10.1053/gast.2001.25513. [DOI] [PubMed] [Google Scholar]
- 39.Yoon JH, Canbay AE, Werneburg NW, et al. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39(3):732–8. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 40.Dwyer JR, Sever N, Carlson M, et al. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. The Journal of biological chemistry. 2007;282(12):8959–68. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 41.El Khatib M, Kalnytska A, Palagani V, et al. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57(3):1035–45. doi: 10.1002/hep.26147. [DOI] [PubMed] [Google Scholar]
- 42.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54(6):2076–88. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nachtergaele S, Mydock LK, Krishnan K, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8(2):211–20. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada D, Rizvi S, Razumilava N, et al. IL-33 Facilitates Oncogene Driven Cholangiocarcinoma in Mice. Hepatology. 2014;60:641a–2a. doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Razumilava N, Gores GJ, et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124(7):3241–51. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marvie P, Lisbonne M, L’Helgoualc’h A, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. Journal of cellular and molecular medicine. 2010;14(6B):1726–39. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30(5):1128–33. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–24. doi: 10.1056/NEJMoa1112168. Epub 2012/08/17. [DOI] [PubMed] [Google Scholar]
- 50.Jhaveri KS, Hosseini-Nik H. MRI of cholangiocarcinoma. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24810. Epub 2014/12/03. [DOI] [PubMed] [Google Scholar]
- 51.Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48(4):1106–17. doi: 10.1002/hep.22441. Epub 2008/09/13. [DOI] [PubMed] [Google Scholar]
- 52.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–78. doi: 10.1002/hep.23294. Epub 2010/01/27. [DOI] [PubMed] [Google Scholar]
- 53.Stiehl A, Rudolph G, Kloters-Plachky P, et al. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36(2):151–6. doi: 10.1016/s0168-8278(01)00251-3. Epub 2002/02/07. [DOI] [PubMed] [Google Scholar]
- 54.Sangfelt P, Sundin A, Wanders A, et al. Monitoring dominant strictures in primary sclerosing cholangitis with brush cytology and FDG-PET. J Hepatol. 2014;61(6):1352–7. doi: 10.1016/j.jhep.2014.07.032. Epub 2014/08/12. [DOI] [PubMed] [Google Scholar]
- 55.Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. The American journal of gastroenterology. 2001;96(4):1059–66. doi: 10.1111/j.1572-0241.2001.03690.x. Epub 2001/04/24. [DOI] [PubMed] [Google Scholar]
- 56.Eaton JE, Barr Fritcher EG, Gores GJ, et al. Biliary multifocal chromosomal polysomy and cholangiocarcinoma in primary sclerosing cholangitis. The American journal of gastroenterology. 2015;110(2):299–309. doi: 10.1038/ajg.2014.433. Epub 2015/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Outwater E, Kaplan MM, Bankoff MS. Lymphadenopathy in sclerosing cholangitis: pitfall in the diagnosis of malignant biliary obstruction. Gastrointest Radiol. 1992;17(2):157–60. doi: 10.1007/BF01888535. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 58.Wannhoff A, Hov JR, Folseraas T, et al. FUT2 and FUT3 genotype determines CA19-9 cut-off values for detection of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol. 2013;59(6):1278–84. doi: 10.1016/j.jhep.2013.08.005. Epub 2013/08/21. [DOI] [PubMed] [Google Scholar]
- 59.Levy C, Lymp J, Angulo P, et al. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50(9):1734–40. doi: 10.1007/s10620-005-2927-8. Epub 2005/09/01. [DOI] [PubMed] [Google Scholar]
- 60.Sinakos E, Saenger AK, Keach J, et al. Many patients with primary sclerosing cholangitis and increased serum levels of carbohydrate antigen 19-9 do not have cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011;9(5):434–9. e1. doi: 10.1016/j.cgh.2011.02.007. Epub 2011/02/22. [DOI] [PubMed] [Google Scholar]
- 61.Venkatesh PG, Navaneethan U, Shen B, et al. Increased serum levels of carbohydrate antigen 19-9 and outcomes in primary sclerosing cholangitis patients without cholangiocarcinoma. Dig Dis Sci. 2013;58(3):850–7. doi: 10.1007/s10620-012-2401-3. Epub 2012/09/26. [DOI] [PubMed] [Google Scholar]
- 62.Eaton JE, Gossard AA, Talwalkar JA. Recall processes for biliary cytology in primary sclerosing cholangitis. Curr Opin Gastroenterol. 2014;30(3):287–94. doi: 10.1097/MOG.0000000000000055. Epub 2014/04/02. [DOI] [PubMed] [Google Scholar]
- 63.Trikudanathan G, Navaneethan U, Njei B, et al. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2013 doi: 10.1016/j.gie.2013.09.015. Epub 2013/10/22. [DOI] [PubMed] [Google Scholar]
- 64.Furmanczyk PS, Grieco VS, Agoff SN. Biliary brush cytology and the detection of cholangiocarcinoma in primary sclerosing cholangitis: evaluation of specific cytomorphologic features and CA19-9 levels. Am J Clin Pathol. 2005;124(3):355–60. doi: 10.1309/J030-JYPW-KQTH-CLNJ. Epub 2005/09/30. [DOI] [PubMed] [Google Scholar]
- 65.Barr Fritcher EG, Voss JS, Jenkins SM, et al. Primary sclerosing cholangitis with equivocal cytology: Fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21331. Epub 2013/07/11. [DOI] [PubMed] [Google Scholar]
- 66.Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010;51(1):174–80. doi: 10.1002/hep.23277. Epub 2009/10/31. [DOI] [PubMed] [Google Scholar]
- 67.Barr Fritcher EG, Kipp BR, Voss JS, et al. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. The American journal of gastroenterology. 2011;106(11):2023–8. doi: 10.1038/ajg.2011.272. Epub 2011/08/17. [DOI] [PubMed] [Google Scholar]
- 68.Navaneethan U, Njei B, Venkatesh PG, et al. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79(6):943–50. e3. doi: 10.1016/j.gie.2013.11.001. Epub 2013/12/24. [DOI] [PubMed] [Google Scholar]
- 69.Siddiqui AA, Mehendiratta V, Jackson W, et al. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol. 2012;10(5):466–71. doi: 10.1016/j.cgh.2011.12.021. quiz e48. [DOI] [PubMed] [Google Scholar]
- 70.Domagk D, Wessling J, Reimer P, et al. Endoscopic retrograde cholangiopancreatography, intraductal ultrasonography, and magnetic resonance cholangiopancreatography in bile duct strictures: a prospective comparison of imaging diagnostics with histopathological correlation. The American journal of gastroenterology. 2004;99(9):1684–9. doi: 10.1111/j.1572-0241.2004.30347.x. [DOI] [PubMed] [Google Scholar]
- 71.Meining A, Frimberger E, Becker V, et al. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol. 2008;6(9):1057–60. doi: 10.1016/j.cgh.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Azeem N, Gostout CJ, Knipschield M, et al. Cholangioscopy with narrow-band imaging in patients with primary sclerosing cholangitis undergoing ERCP. Gastrointest Endosc. 2014;79(5):773–9. e2. doi: 10.1016/j.gie.2013.09.017. Epub 2013/11/12. [DOI] [PubMed] [Google Scholar]
- 73.Lewis JT, Talwalkar JA, Rosen CB, et al. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34(1):27–34. doi: 10.1097/PAS.0b013e3181bc96f9. Epub 2009/11/10. [DOI] [PubMed] [Google Scholar]
- 74.Boberg KM, Jebsen P, Clausen OP, et al. Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2006;45(4):568–74. doi: 10.1016/j.jhep.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Andresen K, Boberg KM, Vedeld HM, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015 doi: 10.1002/hep.27707. Epub 2015/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13(5):356–60. doi: 10.1111/j.1477-2574.2011.00298.x. Epub 2011/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3(4):253–66. doi: 10.1038/nrc1045. Epub 2003/04/03. [DOI] [PubMed] [Google Scholar]
- 78.Shin SH, Lee K, Kim BH, et al. Bile-based detection of extrahepatic cholangiocarcinoma with quantitative DNA methylation markers and its high sensitivity. J Mol Diagn. 2012;14(3):256–63. doi: 10.1016/j.jmoldx.2012.01.014. Epub 2012/03/27. [DOI] [PubMed] [Google Scholar]
- 79.Shigehara K, Yokomuro S, Ishibashi O, et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS One. 2011;6(8):e23584. doi: 10.1371/journal.pone.0023584. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baraniskin A, Nopel-Dunnebacke S, Ahrens M, et al. Circulating U2 small nuclear RNA fragments as a novel diagnostic biomarker for pancreatic and colorectal adenocarcinoma. Int J Cancer. 2013;132(2):E48–57. doi: 10.1002/ijc.27791. Epub 2012/08/22. [DOI] [PubMed] [Google Scholar]
- 81.Baraniskin A, Nopel-Dunnebacke S, Schumacher B, et al. Analysis of U2 small nuclear RNA fragments in the bile differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Dig Dis Sci. 2014;59(7):1436–41. doi: 10.1007/s10620-014-3034-5. Epub 2014/02/01. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Masica D, Ishida M, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60(3):896–907. doi: 10.1002/hep.27050. Epub 2014/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arasaradnam RP, Covington JA, Harmston C, et al. Review article: next generation diagnostic modalities in gastroenterology--gas phase volatile compound biomarker detection. Aliment Pharmacol Ther. 2014;39(8):780–9. doi: 10.1111/apt.12657. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 84.Navaneethan U, Parsi MA, Lourdusamy V, et al. Volatile organic compounds in bile for early diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis: a pilot study. Gastrointest Endosc. 2014 doi: 10.1016/j.gie.2014.09.041. Epub 2014/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navaneethan U, Gutierrez NG, Venkatesh PG, et al. Lipidomic profiling of bile in distinguishing benign from malignant biliary strictures: a single-blinded pilot study. The American journal of gastroenterology. 2014;109(6):895–902. doi: 10.1038/ajg.2014.60. Epub 2014/04/09. [DOI] [PubMed] [Google Scholar]
- 86.Lankisch TO, Metzger J, Negm AA, et al. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology. 2011;53(3):875–84. doi: 10.1002/hep.24103. Epub 2011/03/05. [DOI] [PubMed] [Google Scholar]
- 87.Metzger J, Negm AA, Plentz RR, et al. Urine proteomic analysis differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Gut. 2013;62(1):122–30. doi: 10.1136/gutjnl-2012-302047. Epub 2012/05/15. [DOI] [PubMed] [Google Scholar]
- 88.Voigtlander T, David S, Thamm K, et al. Angiopoietin-2 and biliary diseases: elevated serum, but not bile levels are associated with cholangiocarcinoma. PLoS One. 2014;9(5):e97046. doi: 10.1371/journal.pone.0097046. Epub 2014/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chapman MH, Sandanayake NS, Andreola F, et al. Circulating CYFRA 21–1 is a Specific Diagnostic and Prognostic Biomarker in Biliary Tract Cancer. J Clin Exp Hepatol. 2011;1(1):6–12. doi: 10.1016/S0973-6883(11)60110-2. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lempinen M, Isoniemi H, Makisalo H, et al. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47(5):677–83. doi: 10.1016/j.jhep.2007.05.017. Epub 2007/07/21. [DOI] [PubMed] [Google Scholar]
- 91.Sandanayake NS, Sinclair J, Andreola F, et al. A combination of serum leucine-rich alpha-2-glycoprotein 1, CA19-9 and interleukin-6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer. 2011;105(9):1370–8. doi: 10.1038/bjc.2011.376. Epub 2011/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosen CB, Nagorney DM. Cholangiocarcinoma complicating primary sclerosing cholangitis. Seminars in liver disease. 1991;11(1):26–30. doi: 10.1055/s-2008-1040419. Epub 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 93.Rosen CB, Nagorney DM, Wiesner RH, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213(1):21–5. doi: 10.1097/00000658-199101000-00004. Epub 1991/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rea DJ, Rosen CB, Nagorney DM, et al. Transplantation for cholangiocarcinoma: when and for whom? Surg Oncol Clin N Am. 2009;18(2):325–37. ix. doi: 10.1016/j.soc.2008.12.008. Epub 2009/03/25. [DOI] [PubMed] [Google Scholar]
- 95.Ghali P, Marotta PJ, Yoshida EM, et al. Liver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experience. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(11):1412–6. doi: 10.1002/lt.20512. Epub 2005/10/21. [DOI] [PubMed] [Google Scholar]
- 96.Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2011;13(5):356–60. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98. e3. doi: 10.1053/j.gastro.2012.04.008. quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56(3):972–81. doi: 10.1002/hep.25629. Epub 2012/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nikeghbalian S, Shamsaeefar A, Eshraghian A, et al. Liver transplantation and Whipple surgery combined with chemo-radiotherapy for treatment of hilar cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015 doi: 10.1002/lt.24095. [DOI] [PubMed] [Google Scholar]
- 100.Wu Y, Johlin FC, Rayhill SC, et al. Long-term, tumor-free survival after radiotherapy combining hepatectomy-Whipple en bloc and orthotopic liver transplantation for early-stage hilar cholangiocarcinoma. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14(3):279–86. doi: 10.1002/lt.21287. [DOI] [PubMed] [Google Scholar]
- 101.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 102.Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. Plos Genet. 2014;10(2):e1004135. doi: 10.1371/journal.pgen.1004135. Epub 2014/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]