Abstract
The abuse of opiates such as morphine in synergy with HIV infection accelerates neurocognitive impairments and neuropathology in the CNS of HIV infected subjects, collectively referred to as HAND. To identify potential pathogenic markers associated with HIV and morphine in perturbing the synaptic architecture, we performed quantitative mass spectrometry proteomics on purified synaptosomes isolated from the caudate of two groups of rhesus macaques chronically infected with SIV differing by one regimen- morphine treatment. The up regulation of heat shock 70 kDa protein 5 in the SIV+morphine group points to increased cellular stress during SIV/Morphine interaction thus leading to CNS dysfunction.
Keywords: HSPA5, CNS, SIV, Morphine, Synaptosomes, Proteomics
INTRODUCTION
Chronic HIV infection is frequently accompanied by different neuropathologies, known collectively as HIV-associated neurocognitive disorders (HAND) which vary in severity and include HIV-associated dementia (HAD) and HIV encephalitis (HIVE) (Crews et al, 2009; Shapshak et al, 2011). A characteristic hallmark associated with these maladies includes neuroinflammation and cognitive decline. The prevalence of HAND is becoming a major concern given the aging population of individuals affected by HIV, who are already at risk for age-related neurocognitive impairment and neurodegenerative diseases. Due to effective management of HIV disease with the advent of combination antiretroviral therapy (cART), there has been a significant increase in ageing of HIV-1-positive population in the United States (Hellmuth et al, 2014). While the mortality has been greatly reduced, morbidity is still present affecting the quality of life. To further aggravate this aspect is the abuse of illegal drugs by these individuals thus leading to comorbidity. Not only is HIV frequently transmitted via drug use, drug abusers infected with HIV are also more likely to be affected by HAND than infected non-users (Bokhari et al, 2011; Hollenbach et al, 2014).
There are compelling reasons to investigate HIV and opioid interactions and their role in exacerbating neuropathogenesis as previous studies in HIV-infected opiate abusers demonstrate severe neuropathology compared to infected non-drug users (Anthony et al, 2008; Bell et al, 2006; Bell et al, 1998). While detailed mechanisms elucidating the role of morphine in exacerbating HAND is still under investigation, studies have documented evidence for increased activity of glial cells such as microglia and astrocytes in the presence of morphine and HIV proteins (Bruce-Keller et al, 2008; Turchan-Cholewo et al, 2009). In addition, opioid-mediated injury in synergy with HIV infection has documented a role for μ-opioid receptor (MOR) in an in vitro model to further enhance morphine-Tat mediated neurotoxicity (Turchan-Cholewo et al, 2008).
Despite these emerging mechanisms associated with HIV and morphine synergy in exacerbating HAND, a significant gap exits in understanding neurodegeneration mediated by these two entities at the synapse, a key structure involved in neurotransmission and neuroplasticity. Thus, to ascertain changes in expression of synaptic proteins associated with HIV and morphine, we isolated purified synaptosomes (isolated synapses containing the pre and postsynaptic components) from the caudate nucleus of rhesus macaques that were chronically infected with SIV and differing by one regimen, morphine treatment. We chose the caudate nucleus, as it is a target of both HIV/SIV and morphine. Quantitative mass spectrometry based proteomics identified several proteins to be differentially expressed between the treatment groups. One potential lead identified was the heat shock 70 kDa protein 5 (HSPA5) to be up regulated in the SIV+Morphine group. Further validation in vivo and in vitro confirmed this finding suggesting a potential role for HSPA5 in SIV/morphine induced changes at the synapse.
MATERIAL AND METHODS
Ethics Statement
All animal protocols were approved by the local animal care committee (IACUC) at the University of Kansas in accordance with the Guide for the Care and Use of Laboratory Animals. All efforts were made to reduce suffering of the animals and included the use of anesthetics ketamine and medetomidine at the time of necropsy.
Rhesus macaques, morphine treatment and SIV infection
A detailed regimen of morphine treatment, SIV infection and information on viral load and other parameters has been described in our previous publication (Bokhari et al, 2011). Briefly, 2- to 3-year-old, Indian rhesus macaques (Macaca mulatta) were randomly divided into two groups: SIV only and morphine+SIV. For all animal studies, clinical grade morphine was purchased from the University of Kansas Pharmacy. Morphine was administered intramuscularly 4 times daily, at 6 hour intervals, at a dose of 3 mg/kg with a 1 ml syringe (27.5 G needle). The macaques were gradually acclimated to morphine by starting with 1mg/kg for one week and escalating to a final dose of 3 mg/kg in 1 mg/kg increments per week. Morphine administration was maintained throughout the study to avoid withdrawal effects. The SIV only group received saline injections at the same time intervals. Post morphine and saline treatments, animals were infected with SIVmacR71/17E (Raghavan et al, 1999) for 12 months. At necropsy, tissues were harvested following PBS perfusion under lethal anesthesia.
Isolation of Synaptosomes
Purified caudate syanptosomes from the two groups of monkeys were isolated using a discontinuous sucrose density gradient by differential centrifugation as described in our previous studies (Pendyala et al, 2012a; Pendyala et al, 2014) and further subjected to quantitative mass spectrometry based proteomics.
Quantitative Mass Spectrometry based Proteomics
We used the Isobaric Tag for Relative and Absolute Quantitation (iTRAQ) labeling as described in our earlier study (Pendyala et al, 2012a) to identify differentially expressed synaptic proteins between the two groups of monkeys. Briefly, 100 µg of purified synaptosomal protein from each individual sample and the pool (equal amount of protein obtained from synaptosomal fractions of all samples) was digested overnight with trypsin at 37°C. Digested peptide fraction from each sample was labeled separately using the iTRAQ (Applied Biosystems, Foster City, CA) standard protocols for the 8-plex kit as per the manufacturer’s instructions. Subsequently, all samples were combined and purified using a Waters Oasis MCX cartridge (Waters, Milford, MA). Purified peptides were then subjected to OFFGEL fractionation using the 3100 OFFGEL Fractionator (Agilent Technologies, Santa Clara, CA) as per the manufacturer’s protocol. Using a 13-cm-long IPG gel strip with a linear pH gradient ranging from 3–10 purified peptides were separated into 12 fractions and subsequently concentrated by vacuum centrifugation. Each of these fractions was then spotted onto a MALDI plate and further subjected to LC-MS/MS analysis.
Data Analysis
TOF MS and MS/MS spectra generated were analyzed using Protein Pilot v.2.0.1 software that utilizes the Paragon scoring algorithm (Shilov et al, 2007) and the following search parameters were included: iTRAQ 8plex (peptide labeled) sample type, iodoacetemide cys alkylation, trypsin digest, biological modifications ID focus and thorough search effort. The protein FASTA database used for protein searches was a concatenated “target-decoy” version of Macaca subset of the NCBInr database (08312009).
Cell culture
SH-SY5Y neuroblastoma cells were cultured in DMEM/F12 (1:1) media containing 10% FBS and penicillin/streptomycin (Invitrogen, Carlsbad, CA). For assessing RNA and protein level expression by real time PCR and western blot analysis respectively, cells were plated at a density of 2 X 105 per well onto a 6-well plate. Cells were allowed to adhere overnight and were then subjected to treatment with either 50 ng/ml Tat or 0.1 µM morphine alone or in combination for 24 h. Post treatment, media was carefully removed, cells washed in ice-cold phosphate buffered saline and were harvested for subsequent analysis by real-time PCR or western blot analysis.
Real Time PCR
Total RNA from the basal ganglia of the rhesus macaques and SH-SY5Y neuroblastoma cells was extracted with Total RNA was extracted with TRIzol reagent (Invitrogen) according to the instructions of the manufacturer. The conditions for reverse transcription (RT) and real-time PCR assays have been described previously (Yao et al, 2011). Real-Time PCR primers for rhesus and human HSPA5 (Forward - GGGCCCTGTCTTCTCAACAT, Reverse – ACTTTCTGGACGGGCTTCAT) and β-actin (Forward – CCAACCGCGAGAAGATGA, Reverse – CCAGAGGCGTACAGGGATAG) were obtained from SA Biosciences and quantitative analyses of mRNA were conducted using ABI 7500 Fast Real-Time PCR system (Applied Biosystems). Amplifications were performed for 40 cycles (denaturation, 30 s at 95° C; annealing, 1 min at 60° C). Relative units (2−ΔΔCT) were calculated and used here as a measure of mRNA expression.
Western blot analysis
10 μg each of caudate synaptosomal protein from the two monkey groups and SH-SY5Y cell lysate from four groups were subjected to western blot analysis as described in our previous publication (Pendyala et al, 2012b). To prevent any nonspecific antibody binding, membranes were blocked in 5% nonfat dried milk for 1 h with constant shaking at room temperature. Using antibodies HSPA5 (1:2000, BD Transduction Laboratories, San Jose, CA) and β-actin (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA) membranes were probed overnight at 4° C on an orbital shaker. The next day, blots were washed three times in TBS-T followed by incubation in secondary antibody (1:5000 HRP conjugated anti Rabbit, Santa Cruz Biotechnology, Santa Cruz, CA) with constant shaking at room temperature for 1 h. The blots were further washed three times in TBS-T and developed using the 1:1 solution of Super Signal West Pico Chemiluminescent Substrate and Luminol/Enhancer (Thermo Fisher Scientific, Rockford, IL, USA). Band density from individual samples was quantified using the NIH Image J software.
Statistics
Rhesus data represented is from all the animals used in the current study. For the in vitro studies, data represented are from 6–8 independent experiments using the tests described in the text and figure legends. Differences were considered significant at p<0.05 and respective statistical tests were performed using Prism software (GraphPad Software Inc., San Diego, CA).
RESULTS
To identify changes in the synaptic proteome of rhesus macaques chronically infected with SIV and differing by morphine vs saline treatment, purified synaptosomes from the two groups were subjected to a global quantitative mass spectrometry based proteomics. Synaptosomes are subcellular membranous structures comprising of the complete presynaptic terminal including synaptic vesicles and mitochondria along with the post synaptic membrane and are ideal model to understand changes occurring at the synapse during a neurological manifestation. Given the biochemical preparation associated with their isolation, a critical attribute is the purity that can be assessed using enzyme markers or can be inspected morphologically under electron microscopy as described in our previous publication (Pendyala et al, 2014) .
Using an iTRAQ-based approach coupled to LC-MALDI-TOF-MS/MS, purified synaptosomes from the two groups of monkeys to global quantitative mass spectrometry based proteomics (Fig 1) that identified a total of 810 proteins (Table S1). Since the differences observed in this study were subtle yet significant, we employed a cutoff based on the p-values at p<0.05. This criterion identified a total of ten proteins of which six were up regulated and four down regulated in the SIV+Morphine treated group (Table 1). Among the differentially expressed proteins, we found a member of the heat shock protein 70-kDa family, HSPA5 (a.k.a. Grp78 or BiP) to be up regulated (+1.29 fold) in the SIV+Morphine group.
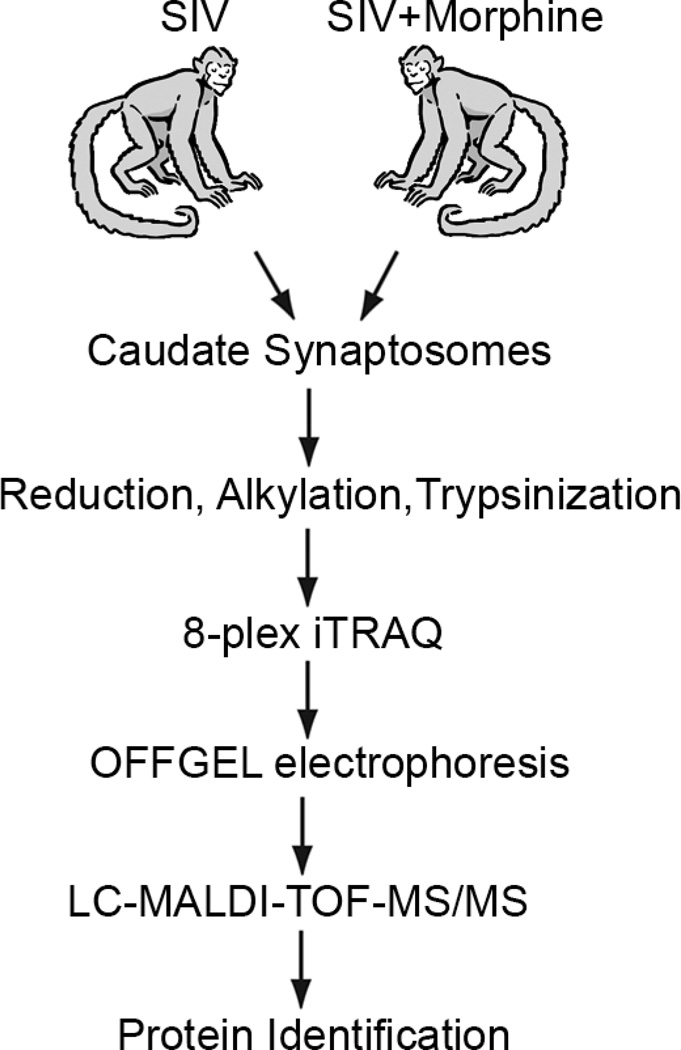
Figure 1.
Overall experimental design used in this study. Purified caudate synaptosomes from the two groups of rhesus macaques were iTRAQ labeled and subjected to LC-MS/MS analysis to identify differentially expressed synaptic proteins.
Table 1.
List of synaptic proteins differentially regulated between the two groups of monkeys. Unpaired student t-test was used to determine the significance.
| Protein identified | Fold change | p-value |
|---|---|---|
| heat shock 70kDa protein A 5, | +1.29 | 0.013 |
| interleukin 1 receptor accessory protein-like 2 | +1.19 | 0.027 |
| lactate dehydrogenase A isoform 5 | +1.28 | 0.028 |
| Syntaxin-1B2 | +1.17 | 0.043 |
| phosphofructokinase, platelet, partial | +1.34 | 0.047 |
| adenylyl cyclase-associated protein | +1.45 | 0.05 |
| GABA(A) receptor-associated protein like 2 | −1.23 | 0.02 |
| oxoglutarate dehydrogenase-like isoform 1 | −1.23 | 0.036 |
| AU RNA-binding protein/enoyl-Coenzyme A hydratase isoform 2 | −1.21 | 0.038 |
| Paralemmin | −1.21 | 0.045 |
The up regulation of HSPA5 was intriguing given the central role of heat shock proteins in proper protein folding and help protect cells from stress. Given the documented role of SIV and morphine in increasing cellular stress (Perez-Casanova et al, 2008), this up regulation in HSPA5 expression especially at the synapse could potentially be associated with a protective mechanism. Since high throughput ‘omics’ approaches generate a vast amount of data sets, it is imperative to perform validation studies to confirm initial findings. Accordingly, we validated our preliminary findings both from the in vivo specimens as well as in vitro, the latter in the human neuroblastoma cell line SH-SY5Y.
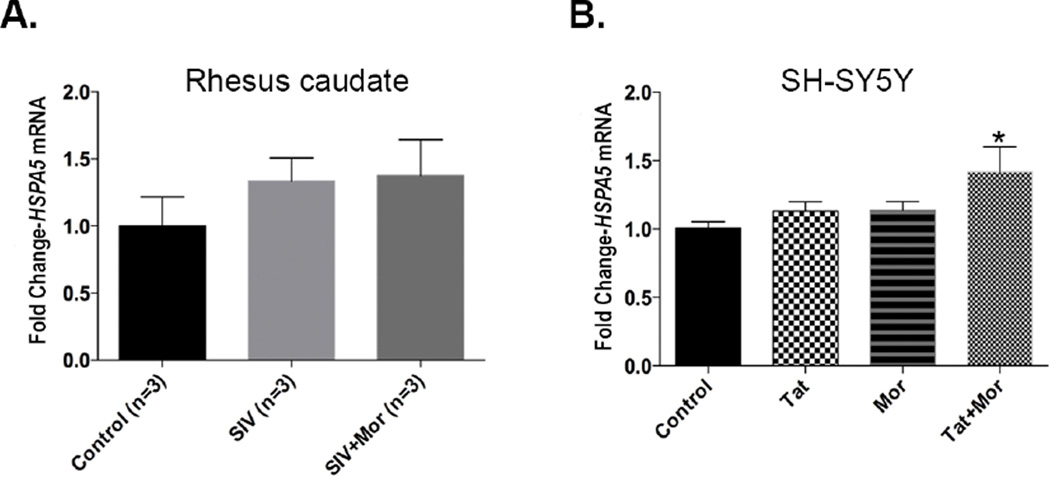
We first validated the expression of HSPA5 at the mRNA level. For in vivo validation we isolated RNA from the basal ganglia of the two groups of monkeys as well as the untreated control monkey group. RT-PCR analysis on the three groups showed an increasing trend in expression of HSPA5, albeit not statistically significant, the highest expression level being in the SIV+Morphine group (Fig 2A). In order to example both factors (the virus and morphine as well as their synergy), we extended our analysis in vitro using the SH-SY5Y neuroblastoma cell line. To mimic this synergy, we treated SH-SY5Y cells alone with 50 ng/ml Tat or 0.1 µM morphine and in combination of these two factors. Since both Tat and morphine in high doses can induce cell death, we chose these concentrations based on the minimal cell death observed with these concentrations based on our previous studies (Buch et al, 2007; Gurwell et al, 2001; Hauser et al, 2009; Khurdayan et al, 2004). Compared to the control group, a similar increasing trend in HSPA5 mRNA expression was seen in the Tat only and morphine only groups, while a significant increase was observed in the Tat and morphine treated group (Fig 2B).
Figure 2.
(A) Real time PCR analysis showing an increase in HSPA5 expression in the basal ganglia of SIV+Morphine group compared to the SIV only and control groups (B). Increasing trend in HSPA5 expression in SH-SY5Y neuroblastoma cells treated alone with 50 ng Tat, 0.1 µM or in combination of Tat and morphine for 24 h. in vitro data represented as Mean ± SEM of 6 independent experiments. One-way ANOVA with Dunnett’s multiple comparison test was used to determine the significance for both in vivo and in vitro data. *p<0.05 versus control.
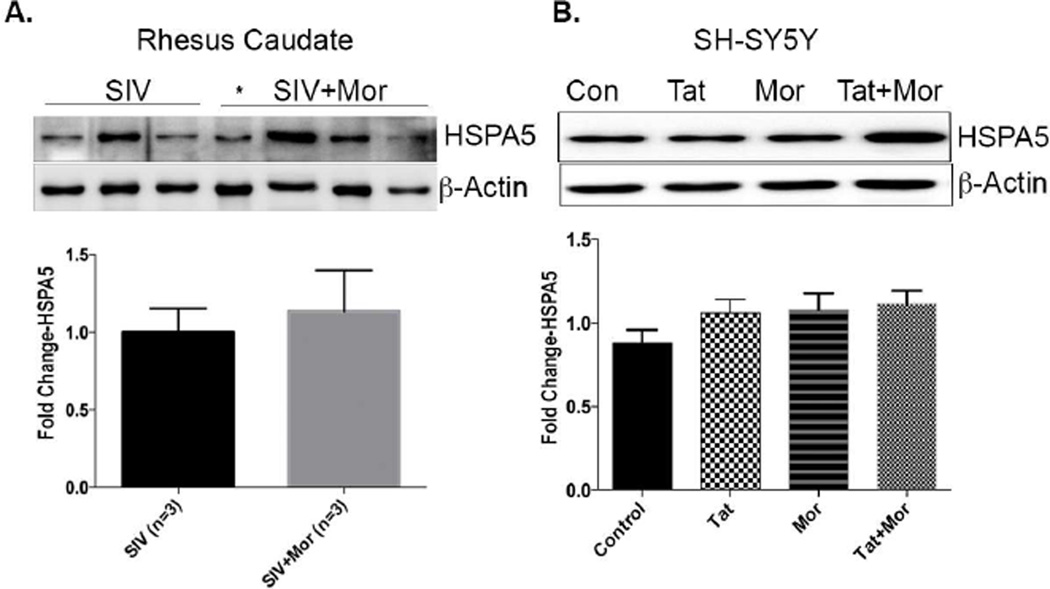
Since our study design was initiated to assess changes occurring at the level of the proteome, we further extended our validation studies to examine change in HSPA5 expression at the protein level. Western blot analysis on the purified caudate synaptosomes showed an increase in HSPA5 expression in the SIV+Morphine group versus the SIV only group. This increase in HSPA5 expression was however not significant (Fig 3A). We then examined protein lysates from SH-SY5Y cells treated in the presence of Tat, morphine and combination of Tat and morphine. Similar to the mRNA changes, we saw a similar increasing trend in HSPA5 protein expression, the maximum being in the Tat and morphine treated group compared to the controls (Fig 3B). Together these data imply that the up regulation in HSPA5 expression at the synapse could point to increased cellular stress during SIV/morphine interaction and the ensuing downstream events eventually leading to CNS dysfunction.
Figure 3.
(A) Western blot analysis on rhesus caudate synaptosomes showing increased HSPA5 expression in the SIV+morphine group compared to SIV only group along with the bar graph (bottom) showing the quantification. * Represents a rapid progressor that was excluded from the analysis. (B) Representative western blot showing an increase in HSPA5 expression in the Tat+morphine treated group compared to the controls along with the bar graph (bottom) showing the quantification. In vitro data represented as Mean ± SEM of 6–8 independent experiments. Unpaired students t-test was used to determine the significance for the in vivo data. For the in vitro data, a Oneway ANOVA with Dunnett’s multiple comparison test was used to determine the significance.
DISCUSSION
Drub abuse in HIV-infected subjects dependent on opiates are more vulnerable to serious neurological complications such as increased cognitive deficits and neuropathology in comparison to HIV-infected non-drug abusers (Anthony et al, 2008; Bell et al, 2006; Bell et al, 1998; Bell et al, 1996; Chiesi et al, 1996; Goodkin et al, 1998; Hellmuth et al, 2014; Nath et al, 2001). Also, earlier studies have elucidated the role of glial cells such as astrocytes and microglial and their activation during HIV and morphine synergy (Bruce-Keller et al, 2008; Turchan-Cholewo et al, 2009) to exacerbate HAND (Hauser et al, 2012; Reddy et al, 2012; Tyor et al, 2012). A previous study using the non-human rhesus macaques showed that morphine exposure increased the severity and rate of HIV disease progression (Kumar et al, 2004). To lend support to this observation, an earlier study from our lab reported that chronic treatment with morphine and SIV infection led to significant increase in neuropathogenesis in rhesus macaques (Bokhari et al, 2011) thus providing evidence that HIV in conjunction with morphine exacerbates HAND,
In the current study, we furthered our analysis to address how chronic SIV and morphine synergy leads to alterations at the synapse that are key regulators of neurotransmission and neuroplasticity. To gain such critical insights, we employed a global unbiased mass spectrometry based proteomics approach using iTRAQ that offers the distinct advantages of quantitation, and importantly multiplexing capability. iTRAQ analysis of purified synaptosomes isolated from the caudate nucleus of the two groups of monkeys identified several synaptic proteins between the two groups. Amongst the proteins that were differentially expressed between the two groups, we focused on HSPA5, a member of the heat shock protein 70 kDa family that was up regulated (+1.29 fold) in the SIV+morphine group which was further validated in vivo and in vitro. However, we do acknowledge that this is a modest increase. A possible explanation for this could be that the up regulation of HSPA5 is an early phenomenon and that it tapers as the disease progresses.
HSPA5 (a.k.a. GRP78 or BiP) is a protein belonging to the HSP70 family and is involved in the folding and assembly of proteins in the endoplasmic reticulum (ER). Earlier studies have documented evidence of increased ER stress in animal models studying the impact of drugs of abuse in the brain. For example an earlier study employing a microarray approach found that Hspa5, along with several other members of the HSP70 family, to be up regulated in the frontal cortex of rats chronically treated with morphine but subsequently reversed on treatment with naloxone (Ammon et al, 2003). Similarly, Jayanthi et al. demonstrated an increase in Hspa5 expression both at the mRNA and protein levels in the rat striatum post treatment with methamphetamine. They further correlate their findings with increased neuronal apoptosis thus suggesting the involvement of ER stress in methamphetamine mediated neurotoxicity in the CNS (Jayanthi et al, 2004). In another study adult male Sprague-Dawley rats exposed to neurotoxic levels of amphetamines led to an increase in Hspa5 mRNA levels in the striatum further lending support to the activation of ER stress in amphetamine mediated CNS damage (Thomas et al, 2010).
During prolonged ER stress, the expression of chaperones would be activated, protein translation would be attenuated thus activating ER-associated degradation and eventually leading to apoptosis (Trusina et al, 2008). One such important protein is C/EBP homology protein (CHOP), which is involved in ER stress-mediated cell apoptosis (Tagawa et al, 2008; Xu et al, 2010). Studies from our own lab have shown importance of ER stress in modulating cocaine induced microglial toxicity (Costa et al, 2013). While we did not assess the levels of HSPA5 in that study, we did see an increase in CHOP expression. It is thus plausible to hypothesize that cocaine could also have potentially resulted in an increased expression of HSPA5. In a more recent study, we have demonstrated that human brain microvascular endothelial cells exposed to Tat caused an increase in several ER stress mediators including HSPA5 expression (Ma et al, 2014). Such increase in expression of ER stress mediators led to decreased cell viability and increased apoptosis evident by increase in CHOP expression. The current study for the first time further lends distinct strength to the role of ER stress during SIV and morphine synergy at the synapse implicating a role for HSPA5 in HAND. Given the significant role of synapse in regulating neurotransmission and neuroplasticity, the subtle yet significant increase in HSPA5 at the synapse could potentially impact ER stress thus activating a cascade of cell death. This in addition to leading to alterations in dynamics of neurotransmission may also exacerbate cognitive impairment in HAND. Assessing the downstream mechanisms cf. signaling mechanism associated with the up regulation of HSPA5 at the synapse could well form the bases of future studies and can be explored further both in vitro and in vivo.
Supplementary Material
Complete list of synaptic proteins identified between the two groups of monkeys. Unpaired student t-test was used to determine the significance.
ACKNOWLEDGEMENTS
We thank Dr. LeeAnn Higgins of the University of Minnesota Center for Mass Spectrometry and Proteomics for assistance in data acquisition. Funding for this study was supported by grants DA020392, DA023397 and DA024442 to SJB from the National Institutes of Health.
Footnotes
All authors declare no conflict of interest
REFERENCES
- Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Res Mol Brain Res. 2003;112:113–125. doi: 10.1016/s0169-328x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Arango JC, Stephens B, Simmonds P, Bell JE. The effects of illicit drugs on the HIV infected brain. Front Biosci. 2008;13:1294–1307. doi: 10.2741/2762. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121(Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Bell JE, Donaldson YK, Lowrie S, McKenzie CA, Elton RA, Chiswick A, Brettle RP, Ironside JW, Simmonds P. Influence of risk group and zidovudine therapy on the development of HIV encephalitis and cognitive impairment in AIDS patients. AIDS. 1996;10:493–499. doi: 10.1097/00002030-199605000-00007. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, Li Z, Pinson D, Yeh HW, Cheney PD, Buch S. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6:626–639. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: patterns of expression of opioid receptors and relationship to human immunodeficiency virus-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146:1546–1554. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi A, Vella S, Dally LG, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F, et al. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Costa BM, Yao H, Yang L, Buch S. Role of endoplasmic reticulum (ER) stress in cocaine-induced microglial cell death. J Neuroimmune Pharmacol. 2013;8:705–714. doi: 10.1007/s11481-013-9438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, Everall IP, Masliah E. Molecular pathology of neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10:1045–1063. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K, Shapshak P, Metsch LR, McCoy CB, Crandall KA, Kumar M, Fujimura RK, McCoy V, Zhang BT, Reyblat S, Xin KQ, Kumar AM. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83:88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res. 2012;10:435–452. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57:194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J, Milanini B, Valcour V. Interactions between ageing and NeuroAIDS. Curr Opin HIV AIDS. 2014;9:527–532. doi: 10.1097/COH.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbach R, Sagar D, Khan ZK, Callen S, Yao H, Shirazi J, Buch S, Jain P. Effect of morphine and SIV on dendritic cell trafficking into the central nervous system of rhesus macaques. J Neurovirol. 2014;20:175–183. doi: 10.1007/s13365-013-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Yang L, Niu F, Buch S. HIV Tat-Mediated Induction of Human Brain Microvascular Endothelial Cell Apoptosis Involves Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001;7:66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- Pendyala G, Buescher JL, Fox HS. Methamphetamine and inflammatory cytokines increase neuronal Na+/K+-ATPase isoform 3: relevance for HIV associated neurocognitive disorders. PLoS One. 2012a;7:e37604. doi: 10.1371/journal.pone.0037604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala G, Buescher JL, Fox HS. Isolation of synaptosomes from archived brain tissues. In: Xiong HGH, editor. Current Laboratory Methods in Neuroscience Research. New York: Springer-Verlag; 2014. pp. 145–152. [Google Scholar]
- Pendyala G, Ninemire C, Fox HS. Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity. PLoS One. 2012b;7:e38909. doi: 10.1371/journal.pone.0038909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casanova A, Husain K, Noel RJ, Jr, Rivera-Amill V, Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol Cell Biochem. 2008;308:169–175. doi: 10.1007/s11010-007-9625-0. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Cheney PD, Raymond LA, Joag SV, Stephens EB, Adany I, Pinson DM, Li Z, Marcario JK, Jia F, Wang C, Foresman L, Berman NE, Narayan O. Morphological correlates of neurological dysfunction in macaques infected with neurovirulent simian immunodeficiency virus. Neuropathol Appl Neurobiol. 1999;25:285–294. doi: 10.1046/j.1365-2990.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- Reddy PV, Pilakka-Kanthikeel S, Saxena SK, Saiyed Z, Nair MP. Interactive Effects of Morphine on HIV Infection: Role in HIV-Associated Neurocognitive Disorder. AIDS Res Treat. 2012;2012:953678. doi: 10.1155/2012/953678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E, Levine AJ, Minagar A, Novembre FJ, Somboonwit C, Nath A, Sinnott JT. Editorial neuroAIDS review. AIDS. 2011;25:123–141. doi: 10.1097/QAD.0b013e328340fd42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilov IV, Seymour SL, Patel AA, Loboda A, Tang WH, Keating SP, Hunter CL, Nuwaysir LM, Schaeffer DA. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Thomas M, George NI, Saini UT, Patterson TA, Hanig JP, Bowyer JF. Endoplasmic reticulum stress responses differ in meninges and associated vasculature, striatum, and parietal cortex after a neurotoxic amphetamine exposure. Synapse. 2010;64:579–593. doi: 10.1002/syn.20763. [DOI] [PubMed] [Google Scholar]
- Trusina A, Papa FR, Tang C. Rationalizing translation attenuation in the network architecture of the unfolded protein response. Proc Natl Acad Sci U S A. 2008;105:20280–20285. doi: 10.1073/pnas.0803476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Ding Q, Keller JN, Hauser KF, Knapp PE, Bruce-Keller AJ. Cell-specific actions of HIV-Tat and morphine on opioid receptor expression in glia. J Neurosci Res. 2008;86:2100–2110. doi: 10.1002/jnr.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Hwang HY, Fritz-French C. Morphine exposure during HIV encephalitis in SCID mice. Neurochem Res. 2012;37:2836–2841. doi: 10.1007/s11064-012-0877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Han F, Mandal A, Rao GN, Zhang X. Diazoxide attenuates hypothermic preservation-induced renal injury via down-regulation of CHOP and caspase-12. Nephrol Dial Transplant. 2010;25:3859–3867. doi: 10.1093/ndt/gfq298. [DOI] [PubMed] [Google Scholar]
- Yao H, Duan M, Hu G, Buch S. Platelet-derived growth factor B chain is a novel target gene of cocaine-mediated Notch1 signaling: implications for HIV-associated neurological disorders. J Neurosci. 2011;31:12449–12454. doi: 10.1523/JNEUROSCI.2330-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete list of synaptic proteins identified between the two groups of monkeys. Unpaired student t-test was used to determine the significance.