Abstract
Vascular wall endothelial cells control several physiological processes and are implicated in many diseases, making them an attractive candidate for drug targeting. Vascular-targeted drug carriers (VTCs) offer potential for reduced side effects and improved therapeutic efficacy, however, only limited therapeutic success has been achieved to date. This is perhaps due to complex interactions of VTCs with blood components, which dictate VTC transport and adhesion to endothelial cells. This review focuses on VTC interaction with blood as well as novel ‘bio-inspired’ designs to mimic and exploit features of blood in VTC development. Advanced approaches for enhancing VTCs are discussed along with applications in regenerative medicine, an area of massive potential growth and expansion of VTC utility in the near future.
Endothelial cells (ECs) are implicated in a number of conditions, including inflammation, atherosclerosis, sepsis, thrombosis, ischemia, pulmonary hypertension, diabetes and some cancers [1,2]. ECs line blood vessels throughout the body and act as gatekeepers that control the transport of nutrients from blood to bodily tissues, blood fluidity, vascular signaling, vascular permeability, angiogenesis and blood cell trafficking to all surrounding tissues. Thus, ECs represent an important target for drug delivery. Many intravenously (IV) administered drug carriers are designed with active targeting approaches that employ ligands with high affinity to EC-specific receptors. Such vascular-targeted drug delivery approaches are a promising avenue to improve therapeutic efficacy and minimize side effects associated with non-targeted therapeutics. Despite this promise, these delivery systems have achieved limited therapeutic success to date.
Vascular targeting originally emerged from observation of indirect effects of cancer treatments. In particular, the interest in attacking the tumor vasculature arose from early observations by Denekamp and Hobson that the tumor endothelium has high proliferation rates that maintain tumor growth relative to the healthy endothelium [3]. A follow-up study by the same group observed that cancer treatments such as radiation and chemotherapy designed to directly kill tumor cells also caused damage to the tumor vasculature, and this led to the suggestion of ‘vascular attack’ as a potential strategy to halt tumor growth [4,5]. Initially, a major stumbling block to this vascular targeting approach was identifying appropriate molecular targets, which has been partially surmounted via technological developments, such as phage display. With these techniques, libraries have since been developed to identify differences in genes and protein expressions between healthy and disease tissues [6]. Given the knowledge of shared molecular pathways in diseases regarding ECs [7], the vascular targeting approach has since been extended to various human diseases, including cardiovascular diseases. Recently, a wealth of research has focused on developing vascular-targeted particles, as they offer promise of high targeting efficiency with multivalency, drug protection/resistance and tunable loading and release properties [8].
Several challenges currently facing vascular-targeted drug delivery arise from the complexities of the vascular environment. Blood itself is a complex fluid, composed of erythrocytes (or red blood cells [RBCs]), leukocytes (or white blood cells [WBCs]), platelets, and plasma fluid (a high concentration solution of proteins, clotting factors, sugars and electrolytes). Each of these components can interact with vascular-targeted drug carriers (VTCs) and dramatically affect targeting efficiency.
In order for a VTC to successfully reach the vascular endothelium from circulation, it must navigate the complex branching vasculature, avoid systemic immune recognition and clearance, exit the bulk blood flow (marginate) and interact with the target endothelium and dock on the endothelium via specific receptors (Figure 1). Each of these steps presents different design challenges for VTCs, which must be addressed sequentially. Ultimately, the transportation of the drug carrier and its specific interaction with all blood components are just as critical as the choice of targeting ligand itself. If the VTC is not able to navigate the vasculature, exit blood flow, and interact with the appropriate ECs, all drug-targeting abilities will be rendered useless.
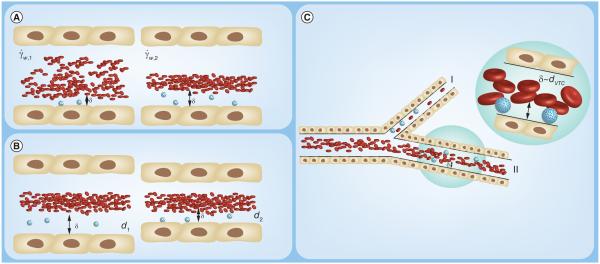
Figure 1.
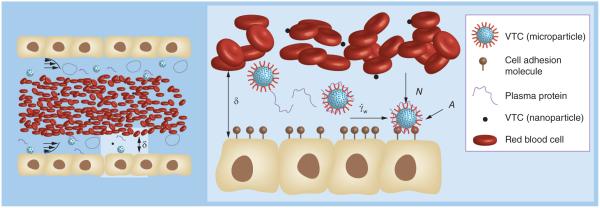
Blood is a complex fluid composed of red blood cells (RBCs), leukocytes, plasma proteins and platelets. (B) In order for a vascular-targeted drug carriers to bind to its target receptor on the inflamed vascular wall, it must overcome shear forces (ÝW) via adequate ligand-receptor bond formation, providing the adhesive force (A). Adhesion may be increased by RBC collisions, which provide a normal force toward the wall (N), while decreased by plasma protein adsorption. Adhesion of nanoparticles is less efficient than microparticles due to entrapment in the red blood cell core and, thus, limited deflection into the cell free layer δ.
VTC: Vascular-targeted drug carrier.
This review focuses on interactions of VTCs with blood components and the essential optimization of the transport step from human blood flow to the vascular wall for enhanced VTC binding efficiency. Design of targeted drug delivery particles has been reviewed extensively [9–12]. We have taken the inverse approach in this review by highlighting recent work identifying VTC interactions with blood components, which in turn inspire successful VTC particle designs. We review the current understanding of VTCs in the context of blood and identify novel approaches to improve VTC interactions with blood components for enhanced EC-targeting. We discuss the utility and mimicry of blood cell features in assisting drug delivery vehicles to the endothelium, as well as the role of the plasma protein corona as a promising drug delivery tool. Finally, we identify promising future directions for improving EC-targeting as well as discuss the application of VTCs in tissue engineering.
Impact of blood flow dynamics & blood cell interactions on VTC targeting efficiency & optimization
Blood is a shear-thinning, anisotropic suspension that, when flowing, is characterized by a RBC-rich core in the center of the vessel. A corresponding RBC-depleted layer, or cell-free layer (CFL), forms near the vascular wall which contains WBCs, plasma and platelets [13,14]. The presence of this RBC core and CFL drives a number of physical interactions that impact VTC-targeting.
RBCs comprise 40–45% of blood volume, are biconcave in shape, and are highly deformable [15,16]. These RBC features, along with the hemodynamic, heterogeneous collisions with other blood components, are a key reason for blood cell segregation in flow [17]. Formation of the CFL leads to a decrease in hydrodynamic resistance, which allows leukocytes to marginate to the vessel wall more efficiently. Leukocyte margination also relies on heterogeneous collisions with the more flexible and discoidal RBCs, leading to their preferential localization in the CFL, called near wall excess (NWE) [18]. Originally, the term ‘margination’ described the ability of circulating leukocytes and platelets to localize to the CFL and associate with ECs in the presence of shear forces. Localization to the vascular wall allows these cells to constantly sample the ECs and respond rapidly to any changes in vascular permeability or surface protein expression. As with leukocytes, the presence of the RBC core drives VTC distribution and directly impacts VTC transport within a blood vessel. The significance of these effects will depend on parameters such as particle geometry, mechanical and material properties, as well as the specific vessel geometry, which defines the CFL and RBC core.
Physical interactions in the RBC core & CFL impact VTC transport to ECs
For VTCs, margination from bulk blood flow to ECs can be a major transport challenge. As aforementioned, heterogeneous collisions between RBCs and less deformable WBCs cause leukocyte margination to the vascular wall, creating the RBC core and CFL [17]. These collisions also occur with VTCs, which can experience deflection out of the RBC-core and increased exposure to ECs. While further work is needed to determine the specific role of changing RBC geometry in RBC interactions with VTCs, as RBC geometry can vary depending on capillary size, shear stress, RBC cell age and disease state (such as sickle-cell anemia) [15,19], some general guidelines have emerged on the impact of VTC size, shape and density on their interactions with RBCs based on recent studies [20–30].
The size of a particle is important in determining its margination dynamics and efficiency. Margination studies in simple flow scenarios have indicated benefits of small nanoparticles (NPs) over large microparticles [31], however, the addition of RBCs in in vitro and in vivo studies as well as in simulation generally yields higher margination efficiency of micron-sized particles [23–28,30]. In particular, studies from our group, which measured binding efficiency of VTCs under physiological conditions using human blood, show that NPs (100–500 nm) exhibit reduced adhesion relative to 2–3 μm VTCs in varied vessel sizes [22,23]. Furthermore, VTCs targeted to atherosclerotic plaques in ApoE −/− mice aorta revealed that 2 μm spheres adhered significantly more than 500 nm particles [24]. Similarly, a theoretical model by Lee et al. demonstrated that NPs 100 nm and smaller distributed uniformly throughout a blood vessel, whereas larger particles marginated more efficiently [30]. The general consensus is that NPs in the 100–500 nm size range mostly co-localize with the RBC rich core while microparticles with diameter larger than 1 μm exhibit NWE. However, in vivo work by Muro et al. using anti-ICAM coated polystyrene (PS) beads observed a lower targeting efficiency of large microparticles (particularly, 5 and 10 μm) relative to submicron 100 nm particles, explained by the higher pulmonary capillary entrapment for the micron-sized, untargeted control [32]. Namdee et al. similarly found that 5 μm spheres exhibited significantly lower adhesion to wall compared with 2 μm spheres with blood flow in channels on the order of arterioles and venules (10–50 μm diameter) [16], which was attributed to the small height of the CFL in small channels leading to RBC collisions with large microparticles to negatively impact the adhesion. This highlights an important paradox for VTCs; VTCs in the large micrometer dimensions are superior at marginating to the vascular wall in large vessels, yet there are sometimes ill-suited to sustain adhesion, and in some cases not able to pass through, small capillaries [33].
Resultant transport to the vascular wall following physical interactions with the RBC core are also controlled by particle geometry [9,12,34]. Mathematical models have demonstrated preferential margination of discoidal and ellipsoidal VTCs as compared with spherical VTCs in simple buffer flows. The lateral drift of VTCs to the endothelium is dependent on the Stokes number, and is proportional to aspect ratio, particle volume and density [35–40]. Recent mathematical models account for physiological features of blood flow, such as RBCs, and the increased adhesion of nonspherical microparticles was attributed to increased contact area with the target surface rather than increased localization from the RBC core into the CFL [27]. This is in agreement with in vitro and in vivo data using targeted VTCs to the endothelium [22]. To date, most studies have been limited to oblate, spheroid and spherical VTC geometries due to the ease of fabricating significant quantities of these shapes. Additional work is needed to elucidate the impact of complex shapes and asymmetry on margination for superior VTC efficiency.
Other cellular components of blood, such as leukocytes, physically interact with VTCs, and thus impact targeting efficiency. Leukocytes recognize VTCs by globulin antibodies acquired through opsonization, and subsequently remove or destroy the VTCs. These types of leukocyte-VTC interactions are dependent on size and shape, and spherical microparticles of approximately 2 μm in diameter have been identified as optimal for phagocytosis [33]. In addition to clearing VTCs from blood, leukocytes and VTCs that co-localize to the CFL collide during the adhesion process. Leukocytes can compete with and physically detach bound VTCs in vitro in both human pulsatile and recirculating blood flow [21]. Overall, the competitive removal effects of leukocytes on VTCs occurred at the point of adhesion, and can largely be mitigated by increasing the strength of VTC adhesion [21].
Mathematical treatment of fluid mechanics underlying margination phenomena
As evidenced in the previous section, margination is a complex phenomenon that involves the interplay of several forces and is yet to be completely understood. Owing to limitations in the ability to experimentally image singular particle–cell interactions in bulk blood flow, researchers have turned to computational models to better understand the complexity of both cellular and particle margination. This section aims to describe the underlying fluid dynamics of blood and the significant progress in recent years for modeling of blood cells in flow and their segregation behavior.
In general, blood is modeled as a suspension of vesicles immersed in a viscous fluid that represents plasma. The motion of the vesicles in the fluid depends on their deformation, which is characterized by its membrane stretching elasticity and bending rigidity. The vesicles are modeled such that they mimic the deformation of the membranes of the different kind of blood cells. There are two main approaches to modeling vesicles. The first approach is to use continuum-based methods where the membrane is treated as a 2D viscoelastic interface embedded in a 3D space. These methods use material laws to describe the energy-strain relation of the membrane. A comprehensive review can be found by Li et al. [41] and in more detail, by Vlahovska et al. [42]. Particle-based models are an alternate approach to modeling cells that has gained momentum in recent years. In these models, the membrane is represented as a grid with beads in vertices interconnected through viscoelastic bonds. For a thorough review on these methods the reader is referred to [41,43]. These models are then paired with the fluids equations to simulate blood flow, and the cell interactions within [41–43]. A review by Fedosov and Noguchi on the multiscale modeling of blood flow gives a broader idea of the application of these methods, particularly for the particle-based methods [44].
Generally speaking, a solution of deformable particles (such as RBCs) will migrate away from the wall in simple shearing or pressure driven flow, forming a depletion layer whose thickness is a balance between wall-induced migration and shear-induced diffusion. Margination in blood is typically characterized by heterogeneous collisions where displacement of stiff particles is substantially greater than that of a deformable particle. Models of binary suspensions of rigid and ‘floppy’ capsules have been considered for leukocyte and platelet margination in the presence of RBCs. Crowl and Fogelson analyzed the mechanisms for platelet lateral motion-induced RBCs, and the mechanisms for platelet near-wall excess [45]. They used a Lattice Boltzmann method coupled with the immersed boundary method in two dimensions where they found that the platelet motion is driven by the complex motion of the RBCs. Kumar and Graham proposed a mechanism for margination comprising three main aspects [17]: the single particle migration velocity, collisions of a particle which pushes it toward the wall and the difference in displacement between homogenous and heterogeneous collisions. The overall drift velocity of a particle is composed of the migration velocity, umig, and an additional component that arises from gradients in collision frequency, Vc.
Focusing on the near wall region, the authors observed that the net effect of the collisions is a normal force directed toward the wall and thus this force prevents escape of the particle to the interior, central region of the channel. In opposition to this, the umig component of the drift velocity orients the particle to move toward the interior (center) of the vessel. Stiff particles experience greater displacement when colliding with floppy (deformable) particles. As a result, stiff particles are retained in the near wall region namely as they reach a dilute concentration. On the contrary, floppy particles do not encounter a strong collision force toward the wall, a result of minimal effect from heterogeneous collisions with stiff particles.
Zhao et al. modeled blood flow using the Stokes flow boundary integral method in two and three dimensions to solve for the fluid velocity and hydrodynamic interactions. This approach is convenient for complex suspensions like blood which involve continuous deformation and complex geometry, though it neglects inertia so it is accurate mostly in microvessels [46]. Overall, the model shows good agreement for particle diffusivity compared with experiments with micron-sized beads in a cylindrical tube of similar diameter. The RBC membrane elasticity is modeled by a classic 2D strain energy functional, and has the empirical form:
where ES is the shear modulus of the membrane, ED the dilatational modulus and I1,2 the strain invariants for the in-plane deformation of the unstressed membrane. The amount of RBC deformation is described by the capillary number, which measures a balance of viscous force on the membrane relative to shear elasticity:
Rigid particles in the presence of RBCs were simulated in a suspension undergoing bulk shear motion and flow in a microchannel. The authors observe that shear-induced diffusion and/or a mean lateral velocity determine migration of particles due to hydrodynamic interactions with other particles and RBCs. This mean lateral velocity was observed to push platelets to the wall, resulting in near-wall excess.
Müller et al. employed the dissipative particle dynamics method (DPD) for 2D simulations and smoothed DPD (SDPD) for 3D simulations. Particles are simulated to interact locally via three pair-wise forces and in the case of the SDPD method, these forces are developed via discretization of the Navier–Stokes equation. For the 3D simulations, they used a particle-based model of the RBC membrane. This study analyzed the segregation behavior of drug carriers from the RBC core in terms of size and shape [27].
Vessel geometry & flow profile further complicates interactions between the RBC core, CFL & VTCs
The complex branching network of the vasculature creates an incredibly dynamic and diverse environment for VTCs. Throughout much of the body, blood flow is considered laminar, however, areas of the ascending aorta can become turbulent. Pulsatile flow is common in arteries, as they are subject to large changes in blood pressure. Veins and capillaries, on the other hand, exhibit lower shear rates, minimal pressure fluctuations, and thus, laminar flow [47]. Shear stress, and therefore shear rate, varies widely from large arteries (10–70 dyne/cm2) down to the capillaries (1–6 dyne/cm2) [48]. These variations of flow shear and profile have a direct effect on the RBC core and CFL sizes. The CFL thickness varies throughout the cardiovascular systems due to changes in vessel size, shear rate and hematocrit (Figure 2) [17,49]. Finally, curvature in vessels, bifurcations and areas of disease, such as atherosclerotic plaque development, are associated with disturbed flow profiles, which has been described as “the pattern of flow that is nonuniform and irregular, including recirculation eddies and changes in direction with time (reciprocating flow) and space (flow separation and reattachment)” (Figure 3) [13,50–52]. Given all of these complexities, targeting to a specific region of the vascular wall is often challenging. The immediate environment surrounding a VTC is extremely dynamic and variable depending on the location within the vasculature. Recent works have attempted to better understand changes in local blood flow profiles and their subsequent role on VTC binding efficiency [21,24,53]. It has been observed that disturbed flow enhances VTC adhesion to ECs relative to laminar flow, which could be beneficial in a disease context where these types of profiles are prevalent.
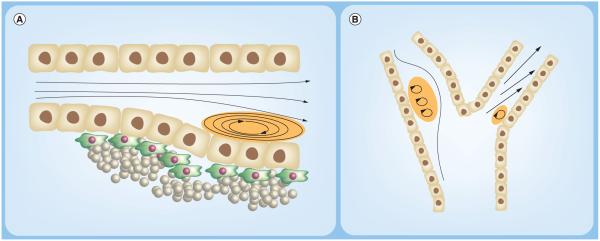
Figure 2.
The cell free layer thickness (δ) depends on (A) shear rate, (B) vessel diameter and (C) hematocrit, or percent volume of red blood cells (RBCs), which is depicted via a vessel bifurcation where the cell free layer is reduced in the bottom daughter vessel (II) relative to the parent vessel. (A) Shows the inverse relationship between wall shear rate (Ýw) and δ, since Ýw,2 > Ýw,1 (B) Shows how reduced vessel diameter results in reduced δ thickness (d1 > d2). In (C), since δ~ dVTC′, adverse VTC adhesion effects may be observed resulting from increased RBC collisions.
VTC: Vascular-targeted drug carrier.
Figure 3.
(A) Plaque development near a vessel constriction, creating by recirculating, low shear blood flow, a common feature of atherosclerosis. (B) The carotid artery bifurcation is a common site of disturbed, low shear flow that is often associated with development of cardiovascular complications. Regions of low/oscillating shear are highlighted in orange.
Hematocrit in most vessels ranges between 30 and 45%, but hematocrit in capillaries is known to be lower due to the Fahraeus–Lindqvist Effect and much more variable [54,55]. While collisions with RBCs in larger vessels promote micron-sized VTC NWE, increased collisions with RBCs in small vessels may produce negative adhesion effects [22]. Increasing local hematocrit from 30 to 45% resulted in negative adhesion effects for larger, 5 μm VTCs in straight channels, explained by the smaller CFL associated with the expansion of the RBC core at higher hematocrit and the subsequent disruptive blood cell-VTC collisions [21]. Increases in hematocrit minimally impacted VTC adhesion in vertical step flow channel (VSFC) with recirculating flow, typical of bifurcations and region of sudden expansion in vivo, perhaps due to unique particle-RBC collision dynamics present in recirculation flow [21]. Müller et al. performed 3D simulations of blood flow and with increasing hematocrit (20–40%), 2 μm carriers increasingly distributed within the CFL with increasing hematocrit levels [27], in agreement with increased adhesion in vitro with 2 μm particles at higher hematocrit [21]. Thus hematocrit, dependent on vessel and VTC size, is a key determinant on the margination and binding efficiency of VTCs.
Work from our group has identified a size dependent critical shear rate, above which adhesion decreased due to higher disruptive hemodynamic forces, depending on the ligand type and surface density [23]. Thus, shear rate is an important factor in determining VTC adhesion efficiency, but since arteries exhibit high pulsatile shear, it is critical to investigate more complex flow environments. Sohrabi et al. theoretically investigated NP deposition using three different models and found that pulsatility enhances NP deposition relative to steady flows. In this study, blood was modeled as a laminar, Newtonian fluid, and thus, the impact of blood cell interactions was not taken into account [56]. Further work from our group investigated the effect of oscillating pulsatile and disturbed recirculation flow on VTC binding efficiency in blood flow containing RBCs and plasma. Overall, binding efficiency in pulsatile flow containing RBCs and plasma was size dependent with approximately two- to three-fold improved adhesion observed for microparticles relative to laminar profiles of the same average shear rate, owed to the increased residence time of the particles near the ECs [24].
Intricacies within the vasculature result in complexities in blood flow, which has a direct effect on VTC efficiency. Bifurcation flow is a common feature of blood vessel geometry and often results in disturbed, pulsatile flow features, similar to that featured in the VSFC channel. Mathematical models have simulated NP deposition in bifurcation flow and found that maximal adhesion of NPs occurred more at the bifurcation region relative to the straight vessel entrance region [57]. In another study from our group, Charoenphol et al. characterized VTC binding efficiency to inflamed endothelium in a VSFC, where disturbed flow was recreated featuring a recirculation eddy. VTC adhesion efficiency was size-dependent, favoring microparticles over NPs, and peaked at the flow reattachment point, owed to low shear and wall-directed velocity [24].
Overall, the investigation of blood flow profile, which is directly linked to vessel geometry, has generally revealed that VTC adhesion is favored in regions of complex/disturbed flow regimes. This provides supporting evidence for targeting vascular diseases with VTCs, particularly for cardiovascular complications, where oscillating shear stress, periods of high/low shear and recirculation eddies are common. Many groups are working toward physiological simulations of systemic blood flow dynamics, but are often limited to one case or one area in the body due to complexity and computational costs [58–60]. Furthermore, the theoretical models that investigate single cells dynamics are not scalable to the entire vasculature. Future studies and computational advances are needed to further elucidate VTC targeting efficiency in complex microvasculature networks and in capillary-sized vessels.
Bio-inspired VTCs mimicking blood component features: approaches for optimizing VTC targeting efficiency
The previous section discussed the critical impact that blood cells have on VTC targeting efficiency. ‘Bio-inspired’ drug carrier designs have emerged as a viable route to improve transport of VTCs to the vascular wall [61]. Over the past decade, there have been attempts to mimic the adhesion targeting properties of leukocytes and physical properties of blood cells. Leukocyte adhesion is highly efficient due to multiple surface ligands which enable capture, firm arrest and transmigration through the endothelial cells postinjury [62]. Leukocytes use two different adhesion mechanisms: PSGL-1 terminated with Sialyl–Lewisx, which binds to P-selectin during initial capture to the endothelial cell, and integrins which, when activated by the initial capture step, result in adhesion to cell adhesion molecules (CAMs) [63]. This adhesion cascade results in firm adhesion of the leukocyte under shear stress and subsequent transmigration into the inflamed tissue space. Numerous studies have investigated VTCs targeted to various adhesion molecules utilized by leukocytes [2,64,65], such as vascular cell adhesion molecule-1 (VCAM) [66–68], intercellular adhesion molecule-1 (ICAM) [32,69–71], platelet endothelial cell adhesion molecule-1 (PECAM), vascular endothelial (VE)-cadherin and selectins. Multi-receptor targeting approaches more closely mimic leukocytes and hold great potential as a design parameter for increasing specificity in drug delivery. This has been explored in several studies, where multi-CAM targeting aided the optimization of certain therapeutic nanocarriers, and targeting performance could be modulated via combination and multiplicity of targeting ligands [72–74]. An earlier study by Eniola et al. directly measured the binding efficiency of dual-targeted microspheres (Sialyl–Lewisx and anti-ICAM-1) perfused over receptor-coated surfaces using a parallel-plate flow chamber. The study showed that firm adhesion, hence targeting, occurred only when both ligands interact with their receptors, mimicking the leukocyte adhesion cascade [74]. This concept was later applied for MRI imaging of atherosclerosis in ApoE −/− mice, where dual-targeted iron-oxide microparticles (PV-MPIO) specific to P-selectin and VCAM-1 showed high specificity to activated endothelial cells in atheroprone regions [73]. Furthermore, the PV-MPIO bound only to the endothelial cells, not the macrophages, and adhesion density scaled closely with plaque size and lesion macrophage content [73].
Platelets, similar to leukocytes, marginate to the vascular wall in response to injury, particularly in diseases such as thrombosis [75]. One of the key functions of platelets is to form clots that seal injured vessels and arrest bleeding. Platelets are discoidal, 2–4 μm in diameter, and contain various surface ligands that are essential for clot formation. The development of synthetic materials that mimic biophysical and biochemical platelet attributes has recently opened new doors in the treatment of diseases like thrombosis. Doshi et al. fabricated synthetic platelets that mimic the size, morphology and deformability of natural platelets and functionalized the VTCs with ligands specific to endothelial cells and platelets themselves [76]. Synthetic platelets were perfused at 1500 s−1 in human blood in a 127 μm parallel-plate flow chamber assay, and exhibited significantly higher co-localization with platelet thrombi relative to the rigid, targeted PLGA NP control. A similar study by Anselmo et al. investigated platelet-like NPs (PLNs), and used a triple-ligand strategy to simultaneously target platelet aggregates and the endothelial surface via adhesion to von Willebrand factor (VWF) and collagen [77]. The researchers showed that VWF/collagen targeted PLNs specifically bind to targeted surfaces with minimal off-target effects. An in vitro wound model utilized perfusion of PLNs and activated platelets through a parallel-plate flow chamber over VWF/collagen surfaces, and revealed that triple-targeted PLNs actively recruited platelets from flow and were the most effective in adhering to the VWF/collagen surfaces, validating the power of synergistic effects between the three ligands [77]. Lastly, these triple-targeted PLNs were tested in vivo and reduced the bleeding time by 65% in a BALB/c mouse transection model. However, rigid, triple-targeted NPs did not restore the extent of the effect of flexible PLNs. In vitro, larger PLNs (1–2 μm in diameter) were less efficient in targeting compared with 200 nm PLNs in terms of their adhesion to anti-OVA glass in buffer flow [77], which is in contrast to aforementioned analyses with VTCs in RBC containing buffer or blood flows. The 200 nm PLNs more efficiently instigated hemostasis relative to larger micron-sized PLNs in vivo to which the authors suggested is due to shorter circulation time of the larger particles.
In addition to synthetically targeted VTCs, a few researchers have coated particles with cellular membranes. Hu et al. coated PLGA NPs with RBC membranes and observed superior circulation half-life by the RBC-membrane-coated NPs compared with control particles coated with synthetic stealth materials, such as polyethylene glycol (PEG). The initial half-life (i.e., 50% particle clearance) for PEGylated PLGA carriers was 6.5 h, whereas RBC-membrane-coated PLGA was 9.6 h, demonstrating the superior in vivo immune evasion properties of biomimetic PLGA NPs over current stealth strategies, such as PEG [78]. Along the same line, a recent work explored coating of silicon NPs with membranes purified from leukocytes and showed these NPs can evade the immune system and exert targeting capabilities similar to natural leukocytes [79]. Specifically, these leukocyte-membrane-coated NPs showed increased circulation time as well as improved tumor localization relative to uncoated silicon NPs, which rely on passive targeting via the EPR effect.
The emerging trend of VTC design using blood cell mimicry with both targeting ligands and physical property imitation holds immense potential in both extending in vivo circulation by immune system evasion and enhanced margination due to blood cell-VTC collision dynamics. Collectively, these strategies have high potential in personalized medicine. However, there is no strong evidence to date that longer circulation time for VTCs is more important in vivo than rapid and high margination and adhesion.
Rethinking the traditional view of the plasma protein corona – a potential tool for drug delivery
The noncellular component of blood, plasma, plays a major role in IV particle drug delivery, especially in particle uptake and clearance by the reticuloendothelial system [80–83]. When a VTC is exposed to blood plasma, protein adsorption onto the particle surface occurs in seconds [84]. The dynamic plasma protein corona is a function of many parameters, including particle size, shape, hydrophobicity, material, exposure time and other physicochemical properties [85]. The corona is classically understood via the Vroman effect, where highly abundant (low affinity) proteins such as albumin are replaced over time by scarce (high affinity) proteins, however recent works have suggested this may not entirely explain the dynamics of corona formation [86]. Protein coronae on VTCs cause opsonization, a process defined by the adsorption of ‘opsonins’, typically immunoglobulins and complement, which initiate phagocytosis and clear- ance of VTCs from the blood. Thus, the protein corona has classically been viewed as an undesirable and unavoidable consequence of particulate VTCs in the human body due to shielded ligand-receptor interaction and reduced NP targeting efficiency in the presence of serum [87]. Additionally, the uptake specificity of functionalized NPs by cells is reduced in the presence of serum proteins [88]. The adhesion of VTCs to inflamed endothelium is negatively impacted by plasma protein adsorption, but this was shown to be a function of plasma donor and particle material type [89]. As a result, what a cell ‘sees’ in blood is not the pristine NP surface, but rather the corona-adsorbed NP population (Figure 4) [90].
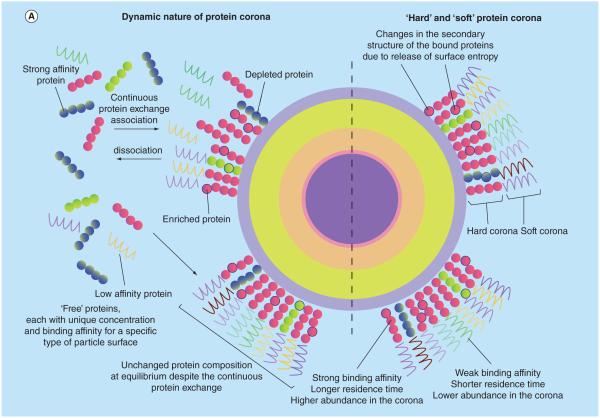
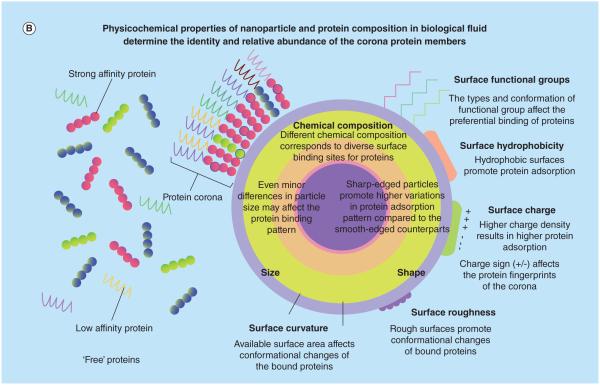
Figure 4. The nanoparticle–protein corona is the effective biological interface ‘seen’ by cells.
(A) The dynamic nature of the protein corona is a result of complex competitive protein–protein interactions and the vast differences among association/dissociation rates of various proteins, which leads to formation of the hard and soft corona. (B) A unique nanoparticle protein corona is formed as a result of particle physicochemical properties and the protein composition of the plasma.
Reproduced with permission from [91], © The Royal Society of Chemistry. For part B, see facing page.
Widespread use of ‘stealth’ mechanisms on particles can limit protein adsorption and thus increase in vivo circulation time and prevent nonspecific interactions with the immune system. Potential avenues for limiting plasma protein targeting effects include the use of PEGylation and zwitterionic functionalization [92–96]. PEGylation has long been known to limit protein adsorption by creating a hydrophilic layer and steric repulsion of proteins [95,97,98]. Thus, it is of interest to explore the possibility that PEG may eliminate, or at least, greatly reduce the negative plasma protein effects on VTCs. However, this may be a challenging because stealth effects are reduced when adding targeting ligands to PEGylated drug carriers. Properties of the targeting ligand and PEG density, molecular weight and conformation can dramatically impact ligand affinity and VTC specificity, as well as corona presence and circulation time [92,99–102]. A debate remains whether PEGylated VTCs are truly free of the plasma effects in vivo [88,92], and PEGylation may never completely limit protein adsorption on VTCs [103]. In contrast, zwitterionic functionality, an alternative to PEGylation, has been shown to result in ‘corona-free’ NPs [93].
Recent works have demonstrated positive features of the protein corona which may be exploited to improve targeting/drug delivery properties of VTCs [104]. Protein-coated VTCs have resulted in cell-specific uptake. In particular, NPs that preferentially adsorb apolipoproteins, such as apoE, resulted in enhanced cellular uptake by brain capillary endothelial cells. This was achieved by coating polybutyl cyanoacrylate NPs with a surfactant coating, polysorbate 80 [105–108]. In addition, uptake of titanium dioxide NPs by A549 human lung epithelial cells was enhanced in the presence of FBS, due to surface adsorbed vitronectin protein [109]. Other studies have also revealed that particles of the same material but different surface properties can adsorb similar coronas but interact with cells by completely different internalization pathways. Specifically, anionic PS and cationic PS NPs incubated with FBS showed nearly identical protein coronas (mainly adsorbed BSA), yet the anionic NPs interacted with monkey kidney epithelial cells via the natural albumin receptor, whereas cationic NPs interacted with scavenger receptors, attributed to a denaturing of BSA by cationic NPs [110]. The redirection of NP-cell interaction as a result of the protein corona has also been observed in comparing uptake of serum-coated versus uncoated PS NPs, where the presence of serum resulted in NP uptake via phagocytosis, rather than clathrin-mediated endocytosis/micropinocytosis for the bare NPs [111]. In general, NPs that adsorb immunoglobulin and complement result in internalization by phagocytes, thus promoting macrophage uptake. The protein corona protects the cell from damage compared with bare NPs, as the corona-adsorbed particle minimally affects cell membrane integrity. However, these effects show protein-specific dependence, as carbon nanotubes coated with γ-globulins resulted in damaged cell membranes whereas fibrinogen-coated carbon nanotubes do not [112]. Thus, the adsorbed NP-protein corona has abilities to modulate cell uptake, adhesion and cell membrane integrity. Of course, other particle parameters such as zeta potential and surface morphology can further confound these results.
A new direction in the field hopes to use the variations of VTC protein corona to assist and improve VTC targeting efficiency. Harnessing the enhanced uptake features of protein-coated NPs could be used to direct the VTC to the cell of interest. This method of thinking considers the protein corona as a natural targeting mechanism, which can be tailored to enhance association with certain cells depending on the physicochemical properties of the NP. In general, hydrophobic NPs adsorb more proteins to mask their surface from water and absorb proteins that induce uptake by macrophages [104]. However, as an example of more directed corona-targeting, the specific corona surrounding N-isopropylacrylamide-co-N-tert-butylacrylamide (NIPAM/BAM) copolymer NPs shows enhanced vitronectin adsorption, which leads to enhanced cellular uptake via αVβ3 integrin, an overexpressed receptor in inflamed endothelium and cancer cells [113]. If one aims to use the corona as a targeting mechanism, then there must be control over the composition in vivo. In this light, the plasma protein corona is a worthy candidate to enhance targeting efficiency of drug carriers and some current studies have begun developing models for predicting cell association based on NP-corona features [114].
The protein corona may also play a fundamental role in development of personalized medicines. Recently, a study revealed that NPs coated with plasma proteins of patients with various diseases resulted in unique corona compositions [115]. The individualized corona may prove to be a useful avenue to pursue for personalized medicine as biological interactions are heavily dependent on the corona formation. Specific NP features may be employed in blood of a certain disease in order to attract specific proteins for enhanced cellular-targeting/uptake. Further research will likely assess different materials and properties of NPs with the goal of predicting the corona profile based on the chosen NP properties and plasma source. Furthermore, optimal VTC targeting and uptake properties can be clarified by investigating these properties for NPs exposed to plasma from a cancer patient.
Advanced approaches to improve VTC-endothelial cell affinity
ECs are stimuli-responsive cells, continually changing the presentation of surface markers, offering novel disease-specific targets. The adhesion molecules and other EC targets established to date represent a rather small subset of potential molecular targets, thus restricting widespread applicability of VTCs. Further identification of the dynamic membrane proteins on ECs in different tissues or disease conditions will yield a diverse array of potential EC targets. Novel proteomics approaches to quantify the dynamic ‘membranone’ of ECs are working to overcome traditional challenges of membrane protein purification and analysis, including the low frequency and hydrophobicity of membrane proteins [116–118]. Additional work may find value in establishing targets of tissue specific markers in the interstitial extracellular matrix in addition to targeting ECs themselves [119].
To address the growing repertoire of EC targets, novel ligand moieties will need to be translated to VTC designs. Antibodies will continue to be a mainstay in targeting approaches due to their versatility and low immunogenicity. However, alternative protein scaffolding approaches have become increasingly attractive for their smaller size and may enable ease of conjugation to potential VTCs. The most common of these include affibodies, adnectins, anticalins and DARPins [120,121]. Affibodies have been utilized on targeted NPs for tumor applications [122–124], yet the majority of these targeting approaches have yet to find application in particle therapeutics or vascular targeting. Similarly, aptamers, oligonucleotides of RNA or single-stranded (ss)DNA, represent an increasingly effective targeting moiety which have yet to be widely translated to VTCs [125]. Aptamers are developed using a process called systematic evolution of ligands by exponential enrichment SELEX [126], which leads to high affinity targeting moieties. Aptamers have been developed for relevant applications, targeting molecules such as EC αvβ3 integrin [127], thrombin for anticoagulation [128,129] and inflamed ECs in aortic mouse atherosclerosis plaques [130]. Some groups have successfully translated these molecules into VTC moieties targeted to tumor ECs [131,132], but many more opportunities remain to use these high affinity ligand approaches toward EC targets.
Combined with novel targeting ligands to highly specific EC targets, the next generation of VTC design will likely evolve to more complex designs with regard to VTC geometry, mechanical stiffness and asymmetric presentation of ligands to increase transport and adhesion at the vascular wall. For example, Janus particles, which are two-faced, asymmetrically-coated particles, may offer combinatorial targeting and stealth functionalities not achievable by uniform VTCs (Figure 5) [133]. Recent studies have explored how controlled, anisotropic presentation of ligands on Janus particles impacts cellular uptake, finding that macrophage uptake depended on ligand patch size and varied compared with uniformly-coated controls [134,135]. Moreover, such approaches could yield controlled release for a multitude of therapeutics [136]. Overall, these emerging, novel design parameters will likely shape the future of VTC delivery strategies and greatly enhance targeting efficiency.
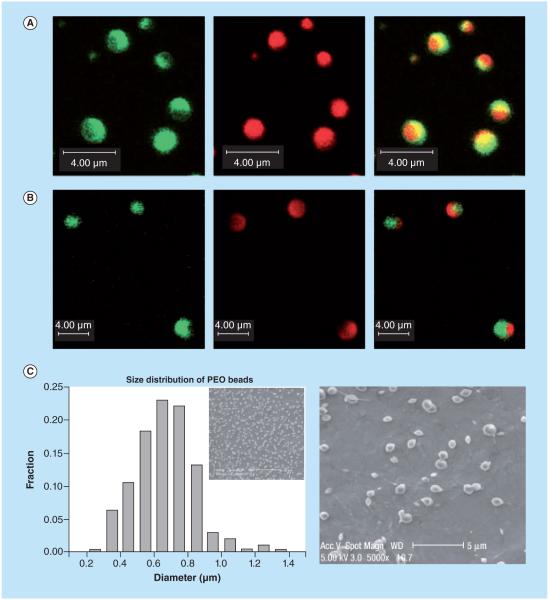
Figure 5. Two distinct polymer systems based on poly(ethylene oxide) (A & C) and poly(acrylic acid) (B & D) were used for biphasic carriers.
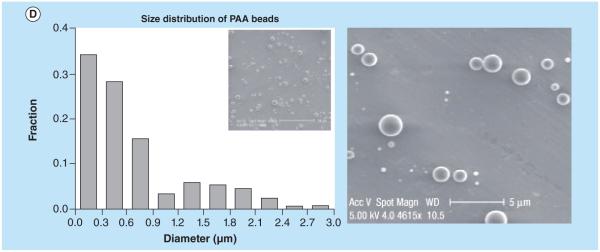
Individual sides were encapsulated with fluorescent biomolecules (FITC-dextran, green, and rhodamine-B-dextran, red). Confocal images of biphasic particles are shown at the fluorescence emission range of FITC and rhodamine B as well as the overlay, revealing the biphasic character of the particles (A & B). The overlays of these two phases reveal the biphasic character of the nanocolloids. Size distributions of the PEO and PAA biphasic particles determined from the scanning electron microscope images are shown (C & D).
PAA: Poly(acrylic acid); PEO: Poly(ethylene oxide); FITC: FITC-dextran.
Reproduced with permission from [137], © Macmillan Publishers Ltd. For part D, see facing page. For color figures, please see online at: http://www.future-science.com/doi/full/10.4155/TDE.15.38
VTC toxicity & immunogenicity
Besides physical interactions with blood cells that impact the distribution and vascular wall binding of VTCs in blood, molecular or chemical interactions between VTCs and blood cells are of high importance as such interactions can lead to toxicity and immunogenicity. While there is still insufficient data to allow full characterization of VTC toxicity in blood, existing evidence suggests this is more of an issue for nanoparticles than it is for microparticles [138]. Similarly, the VTC material type and surface characteristic all affect potential toxicity. VTC-induced hemolysis, in other words, breaking apart of RBCs, tends to be more prevalent for small (~20 nm) nanoparticles relative to larger ones, and occurs for both negatively and positively charged particles. Toxicity issues can also arise with VTC interaction with platelets. Similar to interactions with RBCs, nano-sized VTCs can induce platelet activation to trigger formation of clot within the bloodstream and is a function of several factors including nanoparticle surface characteristic, size and shape. Smaller, positively charged, nonspherical shaped particles may be more likely to cause platelet activation. Readers are referred to Huang et al. for a general discussion about toxicity of VTCs [139]. In addition to toxicity induced by VTC interaction with platelets and RBCs, the presence of VTCs in blood can activate immune cells (white blood cells), leading to inflammation response that if left unchecked could result in severe tissue damage as seen in autoimmune diseases. However, when designed appropriately, VTC interactions with immune cells can be used to elicit a desired and controlled immune response as with vaccines. In certain cases, however, VTC interaction with immune cells may significantly repress their function, i.e. immunosuppression, and lead to increase susceptibility to infection and cancer. Overall, if the immune cells are not the therapeutic targets of the VTC system, strategies to neutralize VTC surfaces, such as pegylation, must be employed to minimize their recognition by the immune system. Readers can find a more comprehensive discussion on VTC-induced immunogenicity in a prior review by Zolnik et al. [140].
Case study: VTC for novel applications in regenerative medicine
The lessons learned as the vascular targeting field has matured from early work in oncology, to targeting inflamed ECs in heart diseases, are now enabling a plethora of alternative therapeutics in tissue engineering, increasing the scope of the field. The utility of VTCs in controlled delivery of growth factors and other vital components to vascularization of tissue grafts is one such avenue of great potential. VTCs are particularly attractive for their ability to provide scheduled delivery of growth factors to the implant region and enhanced vascularization efficiency.
Although nano- and micro-particles have been integrated into scaffolds or combined to form platforms for tissue engineering, the application of injectable, targeted carriers from these particles to enhance angiogenesis and neovascularization for regenerative strategies is in its infancy. In many cases, tissue regeneration takes weeks to months and requires persistent stimulation of growth factors [141]. The production of vascularized grafts for bone, for example, has met limited implementation in clinical practice due to failure to anastomose with the host vasculature and long-term loss of viability [142]. One promising scheme is mimicry of biological cascades by scheduled, sequential delivery of growth factors to enhance tissue regeneration as compared with approaches involving simultaneous growth factor release [142]. However, design of implantable scaffolds to accomplish extended delivery of growth factors with the correct temporal and spatial profile to both stimulate vascularization and enhance matrix production (by osteoblast in tissue engineered bone, for instance) is a complex endeavor [143] and is an unlikely solution to this problem. Injectable, targeted carriers are a potential solution to this challenge, permitting delivery of a temporal sequence of growth factor combinations and, potentially cells, to the implant region. There are several factors that may be suitable for targeting via these carriers to sites of angiogenesis and neovascularization to improve tissue engineering and regenerative outcomes.
It has been generally accepted that after birth, the growth of new blood vessels relies on the sprouting of resident endothelial cells from preexisting vessels (angiogenesis). This is the process that many tissue engineers have tried to replicate in the development of vascularized tissues in vitro [142]. Once implanted, these nascent tissues must establish connection to the host vasculature to survive. The upregulated expression of VEGF, GM-CSF, and HIF-1α in hypoxic environments [144] makes them attractive targets for VTCs, once the implants become ischemic, to deliver growth factor or even cells, such as purified human umbilical vein endothelial cells or endothelial progenitor cells (EPCs).
Recently, it has been shown that adult neovascularization can also occur through recruitment of a heterogeneous mix of myeloid cells from the bone marrow to the blood by VEGF, which is then correctly positioned by the chemokine SDF-1. SDF-1 augments the accumulation of EPCs to the site of neovascularization [145] in ischemic muscle models. In a study examining the possible therapeutic effects of SD-1, the authors showed that increased expression acts as a chemoattractant and homing signal for CXCR4+ EPCs [144]. In another study, SD-1 plasmids were delivered by ultrasound-mediated destruction of microbubbles after injection and localization to the site of neovascularization in a hindlimb ischemia model in rats [146]. The authors demonstrated EPC homing to the area and a significantly increased arteriole density at day 14 post-delivery. For tissue engineering, VTCs targeted to these cytokines, alone or in combinations, can enhance neovascularization and survival of nascent tissues.
Angiogenesis and neovascularization can be enhanced in tissue engineered constructs by delivering growth factors like FGF-2, PDGF and TGF-β or cytokines like SD-1 with dendrimers and micelles functionalized as described above to target areas of overexpression of hypoxic cues. However, hypoxia may also induce apoptosis of the cells in certain types of implants, such as vascularized cardiac tissue [147]. In this case, using hypoxic markers to home VTCs would occur too late to ensure the survival of the graft. Alternative delivery strategies to target VTCs to the implantation site include superparamagnetic NPs or microparticles functionalized with homing sites for target cells. For example, in a study by Bock et al., magnetic scaffolds made of hydroxyapatite and collagen for bone tissue engineering were dip-coated in aqueous solutions containing iron oxide NPs stabilized by various macromolecules [148]. The magnetized scaffold did not affect cell viability and was able to attract particles. Growth factors could potentially be delivered to induce angiogenesis and neovascularization at multiple time points during healing by injection of magnetic carriers and application of a magnetic field gradient. Another application is the isolation of purified populations of endothelial cells. Kim and colleagues recently introduced poly(ɛ-caprolactone) (PCL) microcarriers to stimulate the migration of ECs onto the microcarrier surface from the lumen of the intact aorta within 5 days [149]. Cell isolation techniques such as these can be used to collect desired cell types and co-localize them to sites of tissue engineered implants to enhance vascularization and implant survival.
Conclusion
In this review, we highlighted the importance of blood–VTC interactions in mediating targeting efficiency to the vascular wall. In particular, it is noted that microparticles maintain higher adhesion efficiency over NPs, however, NPs do have advantages in terms of navigating the microvasculature. The exploration of particle density and geometric asymmetry may be useful avenues to improve current transport limitations of VTCs. Vessel geometry and physical interactions with blood cells dictate margination, and thus, VTC adhesion efficiency. Future modeling and experimental studies are needed to clarify adhesion trends in capillary vessels and complex microvasculature to better predict VTC adhesion throughout the body. Going forward, the complexities of blood cannot be ignored when designing VTCs, and should be viewed as another parameter in designing highly efficient drug delivery systems. Using knowledge of blood cells and their interactions with VTCs has in part led to ‘bio-inspired’ VTC design. This has shown great promise in terms of increased circulation time, improved adhesion kinetics and overall targeting efficiency. This is an important avenue that will likely filter into design of next-generation VTCs. Although traditionally viewed as solely unwanted and detrimental for VTCs, the VTC-protein corona has been redefined by recent works to carry great potential for in situ VTC targeting. Continued research efforts will assess the protein corona, and comprehensively evaluate its role in exploitation for targeting as well as toxicity, hopefully resulting in increased translation of VTCs into the clinic. In addition to these considerations, advanced approaches involving high affinity targeting ligands, ligand asymmetry and tuning of the particle modulus are interesting and novel directions for the next generation of VTCs. Lastly, exploration of VTC design for regenerative medicine applications has only recently begun. This represents an area of huge growth and opportunity for vascular-targeted drug delivery strategies. Continued research efforts are critical to greatly expand and reach all of the potential therapeutic benefits and utilities of VTCs. Moving forward, we anticipate the greatest advances in VTC development to be ones that rely heavily on understanding of carrier interactions with blood. It is imperative that researchers take blood components and interactions into consideration during their work and utilize/exploit their key features for optimal VTC drug delivery.
Future perspective
The field of vascular targeting will likely evolve extensively over the next decade. In particular, recent literature describing particles that mimic various features of natural blood cells will likely gain significant research momentum. The complexity of protein-nanoparticle interactions will likely require development of predictive models in attempt to gain a broad and reliable system to understand and account for protein impacts on drug carrier targeting efficiency. In addition, studies have emerged suggesting ways to design particles ‘corona free’ and this could become a feature of the next generation VTCs.
Executive summary.
Impact of blood flow dynamics & blood cell interactions on vascular-targeted drug carrier targeting efficiency & optimization
Vascular-targeted drug carrier (VTC) –blood cell interactions dictate the adhesion efficiency of VTCs in blood flow and complex blood flow profiles tend to result in higher levels of adhesion compared with laminar profiles, which is advantageous for treating diseases such as atherosclerosis.
Stemming from interactions with blood cells, microparticles show superior margination over nanoparticles, an attractive feature from a drug delivery standpoint. However, nanoparticles can navigate the circulation with greater ease and are more likely to evade macrophage uptake than larger carriers. The ideal size may involve deformable and geometrically asymmetric carriers in an effort to optimize margination and circulation behavior.
Bio-inspired VTCs mimicking blood component features: approaches for optimizing VTC targeting efficiency
Designing VTCs that mimic natural blood cell features (multiligands, flexible, discoidal) has shown great promise over rigid VTCs, particularly in studies fabricating ‘synthetic platelets’ to target platelet aggregates and damaged endothelium.
Rethinking the traditional view of the plasma protein corona – a potential tool for drug delivery
The plasma protein corona has traditionally been viewed as an unwanted, undesirable phenomenon that is sought to be mitigated via the use of ‘stealth’ surface functionalities – however, recent works have revealed great potential to exploit the plasma protein corona as a natural mechanism to target cells and enhance uptake efficiency of VTCs.
Advanced approaches to improve VTC–endothelial cell affinity
Advanced, novel approaches to surface design of VTCs include high affinity ligands and asymmetric ligand spacing. These types of design parameters will undoubtedly shape the next generation of VTCs by allowing multiple surface features/functionalities on a single particle, thus producing enhanced targeting efficiency.
Case study: VTC applications in regenerative medicine
Injectable VTCs have been explored as therapies in cardiovascular diseases and cancer but have been minimally explored for applications in regenerative medicine. VTCs can be utilized as a ‘scheduled’ growth factor delivery system that is attracted to the implant region via magnetic scaffolds. This is a promising approach to enhance the neovascularization of nascent tissues and improve their survival.
Acknowledgments
This work was funded by NIH R01 (HL115138) grants to O Eniola-Adefeso.
Key terms
- Vascular-targeted drug delivery
A therapeutic strategy that employs VTCs to specifically adhere to the endothelium or bound blood cells on the endothelium (i.e., platelets) offering potential for limited off-target toxicity, increased drug protection and high targeting efficacy over nontargeted systemic delivery.
- Plasma protein corona
Layer(s) of protein molecules that adsorb onto a drug carrier within seconds of exposure to human serum/plasma. It is a highly dynamic layer, which evolves from competition between proteins for adsorption onto the surface with a wide range of association/dissociation rates of individual proteins
- Margination
Localization of leukocytes or particle to the vascular wall in blood flow.
- Shear stress
A measure of the friction force from a fluid on an object or body in the direction of the fluid. For example, as a VTC travels throughout the bloodstream, it will experience shear stress acting in parallel to the flow direction due to the force of blood flow. The magnitude of this shear stress is dependent on fluid velocity, vessel geometry and fluid viscosity.
- Hematocrit
The percent red blood cell volume in a particular blood vessel.
- Bio-inspired drug carrier designs
A novel approach to designing VTCs and other types of drug carriers by mimicking biophysical and biochemical features of natural blood cells in effort to improve drug carrier performance and targeting efficiency.
- Multireceptor targeting
The use of multiple targeting ligands of a single and/or multiple type(s) on a drug carrier particle surface, resulting in stronger adhesion and more specific targeting.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Nabel EG. Biology of the impaired endothelium. Am. J. Cardiol. 1991;68:6–8. doi: 10.1016/0002-9149(91)90217-9. [DOI] [PubMed] [Google Scholar]
- 2.Brenner JS, Greineder C, Shuvaev V, Muzykantov V. Endothelial nanomedicine for the treatment of pulmonary disease. Expert Opin. Drug Deliv. 2015;12(2):239–261. doi: 10.1517/17425247.2015.961418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denekamp J, Hobson B. Endothelial-cell proliferation in experimental tumors. Br. J. Cancer. 1982;46:711–720. doi: 10.1038/bjc.1982.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metastasis Rev. 1990;9:267–282. doi: 10.1007/BF00046365. [DOI] [PubMed] [Google Scholar]
- 5.Burrows FJ, Thorpe PE. Vascular targeting – a new approach to the therapy of solid tumors. Pharmacol. Ther. 1994;64(1):155–174. doi: 10.1016/0163-7258(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol. Sci. 2002;23(12):576–582. doi: 10.1016/s0165-6147(02)02109-0. [DOI] [PubMed] [Google Scholar]
- 7.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann. NY Acad. Sci. 2001;947(1):271–293. [PubMed] [Google Scholar]
- 8.Gu FX, Karnik R, Wang AZ, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2(3):14–21. [Google Scholar]
- 9.Fish MB, Thompson AJ, Fromen CA, Eniola-Adefeso O. Emergence and utility of non-spherical particles in biomedicine. Ind. Eng. Chem. Res. 2015;54(16):4043–4059. doi: 10.1021/ie504452j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 12.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 13.Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc. Res. 1996;32(4):654–667. [PubMed] [Google Scholar]
- 14.Geislinger TM, Franke T. Hydrodynamic lift of vesicles and red blood cells in flow – from Fåhræus & Lindqvist to microfl uidic cell sorting. Adv. Colloid Interface Sci. 2014;208:161–176. doi: 10.1016/j.cis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Chien S. Red cell deformability and its relevance to blood flow. Annu. Rev. Physiol. 1987;49(1):177–192. doi: 10.1146/annurev.ph.49.030187.001141. [DOI] [PubMed] [Google Scholar]
- 16.Thiriet M. Biology and Mechanics of Blood Flows, Part II: Mechanics and Medical Aspects. Springer; NY, USA: 2008. [Google Scholar]
- 17.Kumar A, Graham MD. Mechanism of margination in confined flows of blood and other multicomponent suspensions. Phys. Rev. Lett. 2012;109(10):108102. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Graham MD. Segregation by membrane rigidity in flowing binary suspensions of elastic capsules. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011;84(6):066316. doi: 10.1103/PhysRevE.84.066316. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi H, Gompper G. Shape transitions of fluid vesicles and red blood cells in capillary flows. Proc. Natl Acad. Sci. USA. 2005;102(40):14159–14164. doi: 10.1073/pnas.0504243102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahidkhah K, Bagchi P. Microparticle shape effects on margination, near-wall dynamics and adhesion in three-dimensional simulation of red blood cell suspension. Soft Matter. 2015;11(11):2097–2109. doi: 10.1039/c4sm02686a. [DOI] [PubMed] [Google Scholar]
- 21.Charoenphol P, Onyskiw PJ, Carrasco-Teja M, Eniola-Adefeso O. Particle-cell dynamics in human blood flow: implications for vascular-targeted drug delivery. J. Biomech. 2012;45(16):2822–2828. doi: 10.1016/j.jbiomech.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Namdee K, Thompson AJ, Charoenphol P, Eniola-Adefeso O. Margination propensity of vascular-targeted spheres from blood flow in a micro fluidic model of human microvessels. Langmuir. 2013;29(8):2530–2535. doi: 10.1021/la304746p. [DOI] [PubMed] [Google Scholar]
- 23.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31(6):1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Charoenphol P, Mocherla S, Bouis D, Namdee K, Pinsky DJ, Eniola-Adefeso O. Targeting therapeutics to the vascular wall in atherosclerosis – carrier size matters. Atherosclerosis. 2011;217(2):364–370. doi: 10.1016/j.atherosclerosis.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials. 2013;34(23):5863–5871. doi: 10.1016/j.biomaterials.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Tan J, Thomas A, Liu Y. Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter. 2012;8:1934–1946. doi: 10.1039/C2SM06391C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller K, Fedosov DA, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 2014;4:4871. doi: 10.1038/srep04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carboni E, Tschudi K, Nam J, Lu X, Ma AWK. Particle margination and its implications on intravenous anticancer drug delivery. AAPS PharmSciTech. 2014;15(3):762–771. doi: 10.1208/s12249-014-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology. 2011;22(11):115101. doi: 10.1088/0957-4484/22/11/115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee T-R, Choi M, Kopacz AM, Yun S-H, Liu WK, Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci. Rep. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J. Nanobiotechnol. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008;25(8):1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavze E, Shapiro M. Motion of inertial spheroidal particles in a shear flow near a solid wall with special application to aerosol transport in microgravity. J. Fluid Mech. 1998;371:59–79. [Google Scholar]
- 36.Lee S-Y, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 37.Filipovic N, Kojic M, Ferrari M. Dissipative particle dynamics simulation of circular and elliptical particles motion in 2D laminar shear flow. Microfluid. Nanofluidics. 2011;10(5):1127–1134. [Google Scholar]
- 38.Mody NA, King MR. Three-dimensional simulations of a platelet-shaped spheroid near a wall in shear flow. Phys. Fluids. 2005;17(11):113302. [Google Scholar]
- 39.Pozrikidis C. Orbiting motion of a freely suspended spheroid near a plane wall. J. Fluid Mech. 2005;541:105. [Google Scholar]
- 40.Broday D, Shapiro M, Fichman M, Gutfinger C. Motion of micron spheroidal particles in vertical shear flows. J. Aerosol Sci. 1997;28:1353. [Google Scholar]
- 41.Li X, Vlahovska PM, Karniadakis GE. Continuum- and particle-based modeling of shapes and dynamics of red blood cells in health and disease. Soft Matter. 2013;9(1):28–37. doi: 10.1039/C2SM26891D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlahovska PM, Barthes-Biesel D, Misbah C. Flow dynamics of red blood cells and their biomimetic counterparts. Comptes Rendus Phys. 2013;14(6):451–458. [Google Scholar]
- 43.Freund JB. Numerical simulation of flowing blood cells. Annu. Rev. Fluid Mech. 2013;46(1):67–95. [Google Scholar]
- 44.Fedosov DA, Noguchi H, Gompper G. Multiscale modeling of blood flow: From single cells to blood rheology. Biomech. Model. Mechanobiol. 2014;13(2):239–258. doi: 10.1007/s10237-013-0497-9. [DOI] [PubMed] [Google Scholar]
- 45.Crowl L, Fogelson AL. Analysis of mechanisms for platelet near-wall excess under arterial blood flow conditions. J. Fluid Mech. 2011;676:348–375. [Google Scholar]
- 46.Zhao H, Shaqfeh ESG, Narsimhan V. Shear-induced particle migration and margination in a cellular suspension. Phys. Fluids. 2012;24(1):011902. [Google Scholar]
- 47.Ku DN. Blood flow in arteries. Annu. Rev. Fluid Mech. 1997;29(1):399–434. [Google Scholar]
- 48.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Johnson PC, Popel AS. Effects of erythrocyte deformability and aggregation on the cell free layer and apparent viscosity of microscopic blood flows. Microvasc. Res. 2009;77(3):265–272. doi: 10.1016/j.mvr.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boryczko K, Dzwinel W, Yuen DA. Dynamical clustering of red blood cells in capillary vessels. J. Mol. Model. 2003;9(1):16–33. doi: 10.1007/s00894-002-0105-x. [DOI] [PubMed] [Google Scholar]
- 51.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 53.Hossain SS, Hughes TJR, Decuzzi P. Vascular deposition patterns for nanoparticles in an inflamed patient-specific arterial tree. Biomech. Model. Mechanobiol. 2014;13(3):585–597. doi: 10.1007/s10237-013-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12(1):5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 55.Dzwinel W, Boryczko K, Yuen DA. A discrete-particle model of blood dynamics in capillary vessels. J. Colloid Interface Sci. 2003;258(1):163–173. doi: 10.1016/s0021-9797(02)00075-9. [DOI] [PubMed] [Google Scholar]
- 56.Sohrabi S, Zheng J, Finol EA, Liu Y. Numerical simulation of particle transport and deposition in the pulmonary vasculature. J. Biomech. Eng. 2014;136(12):121010. doi: 10.1115/1.4028800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J. Control. Release. 2010;146(2):196–200. doi: 10.1016/j.jconrel.2010.04.007. •• Drug carrier adhesion is significantly increased at the bifurcation junction relative to the flat inlet region, confirming the importance of modelling complex flow scenarios to understand drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lafortune P, Aris R, Vázquez M, Houzeaux G. Coupled electromechanical model of the heart: parallel finite element formulation. Int. J. Numer. Method Biomed. Eng. 2012;28(1):72–86. doi: 10.1002/cnm.1494. [DOI] [PubMed] [Google Scholar]
- 59.Greve J, Les A. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Hear. Circ. Physiol. 2006;291(4):1700–1708. doi: 10.1152/ajpheart.00274.2006. [DOI] [PubMed] [Google Scholar]
- 60.Yang W, Vignon-Clementel IE, Troianowski G, Reddy VM, Feinstein JA, Marsden AL. Hepatic blood flow distribution and performance in conventional and novel Y-graft Fontan geometries: a case series computational fluid dynamics study. J. Thorac. Cardiovasc. Surg. 2012;143(5):1086–1097. doi: 10.1016/j.jtcvs.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 61.Howard M, Zern BJ, Anselmo AC, et al. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2015;8(5):4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 63.Omolola Eniola A, Hammer DA. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials. 2005;26(34):7136–7144. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol. Sci. 2003;24(12):640–647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Lobatto ME, Fuster V, Fayad ZA, Mulder WJM. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011;10(11):835–852. doi: 10.1038/nrd3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deosarkar SP, Malgor R, Fu J, Kohn LD, Hanes J, Goetz DJ. Polymeric particles conjugated with a ligand to VCAM-1 exhibit selective, avid, and focal adhesion to sites of atherosclerosis. Biotechnol. Bioeng. 2008;101(2):400–407. doi: 10.1002/bit.21885. [DOI] [PubMed] [Google Scholar]
- 67.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 68.Michalska M, MacHtoub L, Manthey HD, et al. Visualization of vascular inflammation in the atherosclerotic mouse by ultrasmall superparamagnetic iron oxide vascular cell adhesion molecule-1-specific nanoparticles. Arterioscler. Thromb. Vasc. Biol. 2012;32(10):2350–2357. doi: 10.1161/ATVBAHA.112.255224. [DOI] [PubMed] [Google Scholar]
- 69.Rasa G, Muro S. Distinct subcellular trafficking resulting from monomeric vs multimeric targeting to endothelial ICAM-1: implications for drug delivery. Mol. Pharm. 2014;11(12):4350–4362. doi: 10.1021/mp500409y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 2003;85(4):2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McAteer MA, Choudhury RP. Targeted molecular imaging of vascular inflammation in cardiovascular disease using nano- and micro-sized agents. Vascul. Pharmacol. 2013;58(1–2):31–38. doi: 10.1016/j.vph.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Papademetriou I, Tsinas Z, Hsu J, Muro S. Combination-targeting to multiple endothelial cell adhesion molecules modulates binding, endocytosis, and in vivo biodistribution of drug nanocarriers and their therapeutic cargoes. J. Control. Release. 2014;188:87–98. doi: 10.1016/j.jconrel.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McAteer MA, Mankia K, Ruparelia N, et al. A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content. Arterioscler. Thromb. Vasc. Biol. 2012;32(6):1427–1435. doi: 10.1161/ATVBAHA.111.241844. • Demonstrates power of multivalent targeting via leukocyte-mimetic design of vascular-targeted drug carriers (VTCs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Pearson JD. Endothelial cell function and thrombosis. Best Pract. Res. Clin. Haematol. 1999;12(3):329–341. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 76.Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri ZM. Platelet mimetic particles for targeting thrombi in flowing blood. Adv. Mater. 2012;24(28):3864–3869. doi: 10.1002/adma.201200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. doi: 10.1021/nn503732m. •• Employs a unique drug carrier targeting strategy which combines multivalent characteristics with platelet-mimetic properties for highly efficient targeted nanoparticles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parodi A, Quattrocchi N, van de Ven AL, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA. 2008;105(38):14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 83.Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7(12):779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 84.Dell’Orco D, Lundqvist M, Oslakovic C, Cedervall T, Linse S. Modeling the time evolution of the nanoparticle-protein corona in a body fluid. PLoS ONE. 2010;5(6):e10949. doi: 10.1371/journal.pone.0010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem. Rev. 2011;111(9):5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 86.Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 87.Mirshafiee V, Mahmoudi M, Lou K, Cheng J, Kraft ML. Protein corona significantly reduces active targeting yield. Chem. Commun. (Camb.) 2013;49(25):2557–2559. doi: 10.1039/c3cc37307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8(2):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 89.Sobczynski DJ, Charoenphol P, Heslinga MJ, et al. Plasma protein corona modulates the vascular wall interaction of drug carriers in a material and donor specific manner. PLoS ONE. 2014;9(9):e107408. doi: 10.1371/journal.pone.0107408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynch I, Salvati A, Dawson KA. Protein-nanoparticle interactions: what does the cell see? Nat. Nanotechnol. 2009;4(9):546–547. doi: 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- 91.Gunawan C, Lim M, Marquis CP, Amal R. Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J. Med. Chem. B. 2014;2(Issue):2060–2083. doi: 10.1039/c3tb21526a. [DOI] [PubMed] [Google Scholar]
- 92.Dai Q, Walkey C, Chan WCW. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew. Chem. Int. Ed. Engl. 2014;53(20):5093–5096. doi: 10.1002/anie.201309464. [DOI] [PubMed] [Google Scholar]
- 93.Moyano DF, Saha K, Prakash G, et al. Terms of use fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano. 2014;8(7):6748–6755. doi: 10.1021/nn5006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perry JL, Reuter KG, Kai MP, et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012;12(10):5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wattendorf U, Merkle HP. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J. Pharm. Sci. 2008;97(11):4655–4669. doi: 10.1002/jps.21350. [DOI] [PubMed] [Google Scholar]
- 96.Huynh NT, Roger E, Lautram N, Benoît J-P, Passirani C. The rise and rise of stealth nanocarriers for cancer therapy: passive versus active targeting. Nanomedicine (Lond.) 2010;5(9):1415–1433. doi: 10.2217/nnm.10.113. [DOI] [PubMed] [Google Scholar]
- 97.Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG – a versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release. 2014;192:67–81. doi: 10.1016/j.jconrel.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 98.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008;97(10):4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 99.Onyskiw PJ, Eniola-adefeso O. Effect of PEGylation on ligand-based targeting of drug carriers to the vascular wall in blood flow. Langmuir. 2013;29:11127–11134. doi: 10.1021/la402182j. [DOI] [PubMed] [Google Scholar]
- 100.Liu T, Thierry B. A solution to the PEG dilemma: efficient bioconjugation of large gold nanoparticles for biodiagnostic applications using mixed layers. Langmuir. 2012;28(44):15634–15642. doi: 10.1021/la301390u. [DOI] [PubMed] [Google Scholar]
- 101.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011;63(3):152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 102.Hennig R, Pollinger K, Veser A, Breunig M, Goepferich A. Nanoparticle multivalency counterbalances the ligand affinity loss upon PEGylation. J. Control. Release. 2014;194:20–27. doi: 10.1016/j.jconrel.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 103.Gref R, Lück M, Quellec P. “Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core. Colloids Surfaces B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 104.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int. J. Pharm. 2006;310(1–2):213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 106.Kreuter J, Hekmatara T, Dreis S, Vogel T, Gelperina S, Langer K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Control. Release. 2007;118(1):54–58. doi: 10.1016/j.jconrel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674(1):171–174. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 108.Wagner S, Zensi A, Wien SL, et al. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood–brain barrier model. PLoS ONE. 2012;7(3):e32568. doi: 10.1371/journal.pone.0032568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tedja R, Lim M, Amal R, Marquis C. Effects of serum adsorption on cellular uptake profile and consequent impact of titanium dioxide nanoparticles on human lung cell lines. ACS Nano. 2012;6(5):4083–4093. doi: 10.1021/nn3004845. •• Exploitation of the vitronectin-rich adsorbed protein corona is used to enhance uptake of titanium dioxide nanoparticles by lung epithelial cells. [DOI] [PubMed] [Google Scholar]
- 110.Fleischer CC, Payne CK. Nanoparticle–cell interactions: molecular structure of the protein corona and cellular outcomes. ACS Acc. Chem. Res. 2014;47:2651–2659. doi: 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lunov O, Syrovets T, Loos C, et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano. 2011;5(3):1657–1669. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- 112.De Paoli SH, Diduch LL, Tegegn TZ, et al. The effect of protein corona composition on the interaction of carbon nanotubes with human blood platelets. Biomaterials. 2014;35(24):6182–6194. doi: 10.1016/j.biomaterials.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 113.Caracciolo G, Capriotti AL, Cavaliere C, et al. Cancer cell targeting of lipid gene vectors by protein corona. Nanotechnology: Bio Sensors, Instruments, Medical, Environment and Energy. 2012;3:354–357. [Google Scholar]
- 114.Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–2455. doi: 10.1021/nn406018q. •• Models are developed to predict how the adsorbed protein corona determines how nanoparticles will interact with cells, an area of great interest and potential for future design of highly efficient VTCs. [DOI] [PubMed] [Google Scholar]
- 115.Hajipour MJ, Laurent S, Aghaie A, Rezaee F, Mahmoudi M. Personalized protein coronas: a “key” factor at the nanobiointerface. Biomater. Sci. 2014;2(9):1210. doi: 10.1039/c4bm00131a. [DOI] [PubMed] [Google Scholar]
- 116.Ghosh D, Beavis RC, Wilkins JA. The identification and characterization of membranome components. J. Proteome Res. 2008;7(4):1572–1583. doi: 10.1021/pr070509u. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Yu J, Wang Y, et al. Enhancing identifications of lipid-embedded proteins by mass spectrometry for improved mapping of endothelial plasma membranes in vivo. Mol. Cell. Proteomics. 2009;8(6):1219–1235. doi: 10.1074/mcp.M800215-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Griffin NM, Schnitzer JE. Proteomic mapping of the vascular endothelium in vivo for vascular targeting. Methods Enzymol. 2008;445:177–208. doi: 10.1016/S0076-6879(08)03008-5. [DOI] [PubMed] [Google Scholar]
- 119.Kolonin MG. Tissue-Specific Targeting Based on Markers Expressed Outside Endothelial Cells (1st Edition) Elsevier Inc.; CA, USA: 2009. [DOI] [PubMed] [Google Scholar]
- 120.Gebauer M, Skerra A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr. Opin. Chem. Biol. 2009;13(3):245–255. doi: 10.1016/j.cbpa.2009.04.627. [DOI] [PubMed] [Google Scholar]
- 121.Skerra A. Alternative non-antibody scaffolds for molecular recognition. Curr. Opin. Biotechnol. 2007;18(4):295–304. doi: 10.1016/j.copbio.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 122.Alexis F, Basto P, Levy-Nissenbaum E, et al. HER-2-targeted nanoparticle–affibody bioconjugates for cancer therapy. ChemMedChem. 2008;3(12):1839–1843. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elias DR, Poloukhtine A, Popik V, Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine Nanotechnol. Biol. Med. 2013;9(2):194–201. doi: 10.1016/j.nano.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Narsireddy A, Vijayashree K, Irudayaraj J, Manorama SV, Rao NM. Targeted in vivo photodynamic therapy with epidermal growth factor receptor-specific peptide linked nanoparticles. Int. J. Pharm. 2014;471(1–2):421–429. doi: 10.1016/j.ijpharm.2014.05.063. [DOI] [PubMed] [Google Scholar]
- 125.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu. Rev. Med. 2005;56(1):555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 126.Stoltenburg R, Reinemann C, Strehlitz B. SELEX – a (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007;24(4):381–403. doi: 10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Mi J, Zhang X, Giangrande PH, et al. Targeted inhibition of αvβ3 integrin with an RNA aptamer impairs endothelial cell growth and survival. Biochem. Biophys. Res. Commun. 2005;338(2):956–963. doi: 10.1016/j.bbrc.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 128.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li WX, Kaplan AV, Grant GW, Toole JJ, Leung LL. A novel nucleotide-based thrombin inhibitor inhibits clot-bound thrombin and reduces arterial platelet thrombus formation. Blood. 1994;83(3):677–682. [PubMed] [Google Scholar]
- 130.Ji K, Lim WS, Li SFY, Bhakoo K. A two-step stimulus-response cell-SELEX method to generate a DNA aptamer to recognize inflamed human aortic endothelial cells as a potential in vivo molecular probe for atherosclerosis plaque detection. Anal. Bioanal. Chem. 2013;405(21):6853–6861. doi: 10.1007/s00216-013-7155-z. [DOI] [PubMed] [Google Scholar]
- 131.Ara MN, Matsuda T, Hyodo M, et al. An aptamer ligand based liposomal nanocarrier system that targets tumor endothelial cells. Biomaterials. 2014;35(25):7110–7120. doi: 10.1016/j.biomaterials.2014.04.087. [DOI] [PubMed] [Google Scholar]
- 132.Smith JE, Medley CD, Tang Z, Shangguan D, Lofton C, Tan W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal. Chem. 2007;79(8):3075–3082. doi: 10.1021/ac062151b. [DOI] [PubMed] [Google Scholar]
- 133.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8(1):15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao Y, Yu Y. Macrophage uptake of Janus particles depends on Janus balance. Langmuir. 2015;31(9):2833–2838. doi: 10.1021/la504668c. •• Demonstrates how the ligand patch size (Janus balance) impacts particle phagocytosis, offering a new parameter to tune drug vehicle properties. [DOI] [PubMed] [Google Scholar]
- 135.Zhao Z, Shi Z, Yu Y, Zhang G. Ternary asymmetric particles with controllable patchiness. Langmuir. 2012;28(5):2382–2386. doi: 10.1021/la203654h. [DOI] [PubMed] [Google Scholar]
- 136.Sengupta S, Eavarone D, Capila I, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 137.Roh K-H, Martin DG, Lahann J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005;4:759–763. doi: 10.1038/nmat1486. [DOI] [PubMed] [Google Scholar]
- 138.Nair S, Sasidharan A, Divya Rani VV, et al. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009;20(1):235–241. doi: 10.1007/s10856-008-3548-5. [DOI] [PubMed] [Google Scholar]
- 139.Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol. Membr. Biol. 2010;27(4–6):190–205. doi: 10.3109/09687688.2010.522117. [DOI] [PubMed] [Google Scholar]
- 140.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Minireview: nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Markkanen JE, Rissanen TT, Kivelä A, Ylä-Herttuala S. Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart--gene therapy. Cardiovasc. Res. 2005;65(3):656–664. doi: 10.1016/j.cardiores.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 142.Mercado-Pagán ÁE, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Ann. Biomed. Eng. 2015;43(3):718–729. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chu H, Wang Y. Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther. Deliv. 2012;3(6):693–714. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ho TK, Shiwen X, Abraham D, Tsui J, Baker D. Stromal-cell-derived factor-1 (SDF-1)/CXCL12 as potential target of therapeutic angiogenesis in critical leg Ischaemia. Cardiol. Res. Pract. 2012;2012:143209. doi: 10.1155/2012/143209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamaguchi J-I. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for Ischemic neovascularization. Circulation. 2003;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 146.Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol. Ther. 2011;19(5):895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gu Y, Yu J, Lum LG, Lee RJ. Tissue engineering and stem cell therapy for myocardial repair. Front. Biosci. 2007;12:5157–5165. doi: 10.2741/2555. [DOI] [PubMed] [Google Scholar]
- 148.Bock N, Riminucci A, Dionigi C, et al. A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomater. 2010;6(3):786–796. doi: 10.1016/j.actbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 149.Jin G-Z, Park J-H, Lee E-J, Wall IB, Kim H-W. Utilizing PCL microcarriers for high-purity isolation of primary endothelial cells for tissue engineering. Tissue Eng. Part C. Methods. 2014;20(9):761–768. doi: 10.1089/ten.tec.2013.0348. • The introduction of poly(ɛ-caprolactone) microcarriers in the lumen of the aorta is employed as a new technique for isolation of endothelial cells and results in significantly higher purity over current methods, making an important stride for tissue engineering applications. [DOI] [PMC free article] [PubMed] [Google Scholar]