Abstract
The Type Six Secretion System (T6SS) is required for Bordetella bronchiseptica cytotoxicity, cytokine modulation, infection, and persistence. However, one-third of recently sequenced Bordetella bronchiseptica strains of the predominantly human-associated Complex IV have lost their T6SS through gene deletion or degradation. Since most human B. bronchiseptica infections occur in immunocompromised patients, we determine here whether loss of Type Six Secretion is beneficial to B. bronchiseptica during infection of immunocompromised mice. Infection of mice lacking adaptive immunity (Rag1-/- mice) with a T6SS-deficient mutant results in a hypervirulent phenotype that is characterized by high numbers of intracellular bacteria in systemic organs. In contrast, wild-type B. bronchiseptica kill their eukaryotic cellular hosts via a T6SS-dependent mechanism that prevents survival in systemic organs. High numbers of intracellular bacteria recovered from immunodeficient mice but only low numbers from wild-type mice demonstrates that B. bronchiseptica survival in an intracellular niche is limited by B and T cell responses. Understanding the nature of intracellular survival during infection, and its effects on the generation and function of the host immune response, are important to contain and control the spread of Bordetella-caused disease.
Introduction
The ability of bacteria to persist inside host cells shields them from antibodies, complement, and other-extracellular host defenses and thus represents an effective strategy for survival during infection. Many bacteria, including Mycobacterium tuberculosis, Salmonella enterica and Francisella tularensis, employ a variety of different intracellular survival mechanisms to achieve long-term persistence [1–3]. Until recently, Bordetella species were generally considered exclusively extracellular respiratory pathogens [4], but in vitro studies suggest that the Bordetella may be able to survive intracellularly [5–12]. However, whether the bacteria utilize this intracellular survival strategy during the infection process remains unclear.
The three Classical Bordetellae, Bordetella bronchiseptica, B. pertussis, and B. parapertussis, cause a variety of respiratory diseases ranging from asymptomatic infection to fatal pneumonia [13]. B. pertussis and B. parapertussis are the etiological agents of whooping cough in humans and are believed to have diverged independently from a B. bronchiseptica-like ancestor [14]. B. bronchiseptica infects a wide range of mammalian hosts including mice, providing a natural-host infection model that can allow molecular manipulation of both pathogen and host. B. bronchiseptica infection induces a significant Th1-type T-lymphocyte cytokine response that is characterized by high levels of IL-2, IFN-γ, and TNF-α, but low levels of IL-5 and no IL-4 [15,16], and is generally associated with an immune response to intracellular pathogens [17,18]. Furthermore, the bordetellae were shown to be able to survive intracellularly in vitro in epithelial cells, dendritic cells (DCs) and macrophages [6–8]. There have been reports of the recovery of B. pertussis and B. bronchiseptica from bronchiolar lavage of mice, murine nasal cavity dendritic cells, and alveolar macrophages from HIV-infected patients [19–21], suggesting that intracellular survival is a potential mechanism employed by B. bronchiseptica during infection. However, the relevance of these observations to natural infection is unclear. Defining the role of intracellular survival in Bordetella disease has important implications for the development of vaccines and therapeutics.
The Type Six Secretion System (T6SS), which is widely distributed amongst Gram-negative bacteria [22], has been shown to be involved in intracellular survival of several species [23]. Further, up-regulation of most T6SS genes is dependent on contact with or intracellular growth inside the host cell [24]. In fact, many bacteria persist during infection by utilizing their T6SSs for intracellular survival and replication, including S. enterica [25], F. tularensis [26], Aeromonas hydrophila [27], and Yersinia pseudotuberculosis [28]. The T6SS in B. bronchiseptica has been reported to have a function in persistence and immunomodulation during infection [29], but its contribution to intracellular survival has yet to be characterized.
Despite the important role of the T6SS during B. bronchiseptica infection, a subset of recent B. bronchiseptica isolates from the predominantly human-associated Complex IV are missing the T6SS. Since most B. bronchiseptica human infections occur in immunocompromised individuals [30–35], we aimed to determine whether loss of the T6SS might be beneficial for B. bronchiseptica during infection of immunocompromised hosts. Here we compare the wild-type B. bronchiseptica strain RB50 with RB50ΔclpV, an isogenic B. bronchiseptica mutant with an in-frame deletion in the clpV ATPase of the T6SS, during infection of mice lacking adaptive immunity (B and T cells). We show that loss of T6SS function results in a hypervirulent phenotype characterized by early host lethality of immunodeficient mice due to high numbers of predominantly intracellular bacteria in systemic organs. In contrast, wild-type B. bronchiseptica kill their cellular hosts via a T6SS-dependent mechanism and are therefore not recovered from systemic organs. A more careful examination revealed an intracellular stage in the lungs of both wild-type and immunodeficient mice, demonstrating that B. bronchiseptica can occupy an intracellular niche during natural host infection. These results reveal the ability of B. bronchiseptica to survive intracellularly and demonstrate that both the T6SS and adaptive immune components contain bacteria within the respiratory tract and limit B. bronchiseptica intracellular survival during infection.
Materials and Methods
Analysis of clinical strains
The T6SS locus in the genomes of 58 B. bronchiseptica strains isolated from humans (17 strains), a variety of different mammals (31 strains), turkeys (9 strains) and from an unidentified host (1) were analyzed [36–39]. All genomes were compared against the genome of strain RB50, and the presence of the T6SS locus (BB0793-BB0818) [29] in the individual genomes as well as the presence of pseudogenes was assessed visually using the Artemis Comparison Tool (ACT) [40].
Bacterial Strains and growth
Bordetella bronchiseptica strain RB50 and its isogenic, in-frame deletion mutant RB50ΔclpV have been previously described [29,41]. RB50 and RB50ΔclpV were grown and maintained on Bordet-Gengou (BG, Difco) agar supplemented with 10% defibrinated sheep’s blood (Hema Resources) and 20 ug/ml streptomycin (Sigma). For infections, bacteria were grown overnight at 37°C to mid-log phase in Stainer Scholte liquid broth (SS).
Mouse Experiments
Four- to six- week old C57Bl/6 and Rag1-/- mice were ordered from Jackson laboratories (Bar Harbour, ME) and were bred in a pathogen-free facility at the Pennsylvania State Laboratory (University Park, PA). All experiments were conducted following institutional guidelines, and all animal experiments were conducted as previously described [29,42,43]. Briefly, the number of bacterial colony units in liquid SS culture was determined by the optical density measured by absorbance of light at 600 nm. The bacteria were diluted to 1x107 CFU/mL in phosphate buffered saline (PBS), and inocula were confirmed by plating dilutions on BG agar and counting resultant colonies after incubation for two days at 37°C. For inoculation, mice were lightly sedated with 5% isofluorane (IsoFlo, Abbott Laboratories) and were inoculated with 5x105 CFU bacteria by gently pipetting 50 μL of the inoculum onto their external nares. To quantify bacterial numbers in respiratory tract and systemic organs, mice were euthanized via CO2 inhalation, and the indicated organs were excised. Tissues were homogenized in one mL PBS, serially diluted and plated on BG agar containing 20 μg/mL streptomycin, and colonies were counted after incubation at 37°C for two days. Mice with lethal bordetellosis indicated by ruffled fur, hunched stature, and limited responsiveness were euthanized to prevent unnecessary suffering. For survival curves, Rag1-/- mice were lightly sedated with 5% isofluorane and inoculated with 5x105 CFU RB50 or RB50ΔclpV. Mice were monitored over the course of the experiment and any mice that were hunched over, had labored breathing, or displayed unresponsiveness or lack of motility were removed from the experiment and euthanized to prevent unnecessary suffering.
Cytokine ELISA
Cytokine analysis was conducted as previously described [44]. Briefly, mice were inoculated with 5x105 CFU RB50 or RB50ΔclpV and spleens and lungs were collected and homogenized on day 21 for cytokine analysis. Interleukin 1β (IL-1β), Interleukin 6 (IL-6), Interleukin 10 (IL-10), Interleukin 17 (IL-17), Tumor Necrosis Factor α (TNF-α), and Interferon Gamma (IFN-γ) concentrations were determined via ELISA according to manufacturer’s instructions (R&D Systems).
Pathology
Twenty-one days following inoculation with RB50 or RB50ΔclpV, mice were euthanized and the lungs were inflated with 1.5 ml 10% formalin in PBS. Spleens were excised and placed in 10% formalin in PBS. Tissues were processed and stained with hematoxylin and eosin (H&E) at the Animal Diagnostic Laboratory at the Pennsylvania State University in University Park, PA. Tissue sections were analyzed and scored on a qualitative scale as previously described [29] by one of the authors (M.J. Kennett) who is experienced in rodent pathology and was blinded to the experimental treatments. Descriptive evaluations of lesions were recorded, and lung and spleen lesions were graded on a scale ranging from 0 to 5: A score of 0 was given for sections with no lesions and no inflammation; 1 for sections with few lesions (less than 10% tissue affected) and slight inflammation; 2 indicated mild lesions (11–20% tissue affected); 3 was given for moderate lesions (21–30% of the lung/spleen tissue affected); 4 had extensive lesions with marked inflammation (31–50% tissue affected); and a score of 5 was given for samples exhibiting extensive lesions with high inflammation (>51% lung / spleen tissue affected).
Intracellular Survival Assay
Murine RAW 264.7 macrophages obtained from ATCC (ATCC TIB-71) were grown in Dulbecco’s modified Eagles medium (DMEM, Difco) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% nonessential amino acids, and 1% sodium pyruvate. Cells were grown to 80% confluency in 5% CO2 in 96-well tissue culture treated plates (Greiner Bio-One) at 37°C. Cells were primed for two hours with 1000 U/mL recombinant IFNγ in DMEM in 5% CO2 at 37°C, and then bacterial suspensions of RB50 or RB50ΔclpV were added to wells at a multiplicity of infection (MOI) of 100. Plates were centrifuged at 5000 RPM for 5 min at room temperature (RT) and were incubated at 37°C. After 1 hour, 100 μL of 0.1% triton X solution (Sigma) in PBS was administered to a subset of wells, followed by a 5 minute incubation at RT and vigorous pipetting to lyse open cells. 10 μL dilutions were serially diluted and plated on BG to quantify total bacteria (intracellular and extracellular) present after 1 hour. At one hour, supernatant was removed from remaining wells and replaced with 100 μL of 100 μg/mL gentamicin solution (Sigma-Aldrich) in DMEM to remaining sample wells. Plates were incubated in 5% CO2 at 37°C, and then at 1, 24, and 48 hours post-gentamicin addition, appropriate wells were washed 3x with DMEM and treated with 100 μL 0.1% triton X as described above for enumeration of intracellular bacteria. At 24 hours, the supernatants were replaced with 10 μg/mL gentamicin solution in DMEM to prevent uptake of gentamicin into intracellular compartment of RAW264.7 cells.
Modified Intracellular Survival Assay
A modified intracellular survival assay conducted on homogenates has been previously described [19]. Briefly, C57Bl/6 and Rag1-/- mice were inoculated with 5x105 CFU RB50 or RB50ΔclpV and euthanized on day 21 post-inoculation (p.i.). Nasal cavities, lungs, spleens and livers were excised and homogenized in one mL PBS. 100 μL of the homogenate was removed and was serially diluted and plated on BG agar for quantification of total bacteria. Gentamicin (100 μg/mL) was added to remaining homogenates and samples were incubated for 1 hour at 37°C. After three washes to remove the antibiotic, remaining bacteria were resuspended in 0.1% triton X, serially diluted, and plated on BG supplemented with 20 μg/ml streptomycin for intracellular bacterial enumeration. As a control to test for gentamicin effectiveness in each organ, spleens, livers, lungs, and nasal cavities excised from naïve Rag1-/- mice were homogenized and 107 CFU RB50 or RB50ΔclpV were added to each organ. Gentamicin was shown to be 99.999% effective at killing extracellular bacteria introduced to lungs, nasal cavity, and spleen tissues, and 99% effective at killing extracellular bacteria in the liver (data not shown). Samples were incubated at 37°C for one hour, followed by washes to remove antibiotic, and the remaining viable bacteria were enumerated via serial dilution and plating on BG agar.
Cytotoxicity Assay
Murine RAW 264.7 macrophages from ATCC were grown in DMEM supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% nonessential amino acids, and 1% sodium pyruvate. Cells were grown to 80% confluency in 5% CO2 in 96-well tissue culture treated plates (Greiner Bio-One) at 37°C. DMEM was then replaced with RPMI medium lacking phenol red with 5% fetal bovine serum, 1% L-glutamine, 1% nonessential amino acids, and 1% sodium pyruvate two hours prior to start of the assay. RB50 or RB50ΔclpV were added to plates at an MOI of 100 and the plates were centrifuged at 5000 RPM for 5 min at RT and were incubated in 5% CO2 at 37°C. One hour later, the supernatant was replaced with 100 μg/mL gentamicin in RPMI lacking phenol red to kill extracellular bacteria and the plates were incubated once again in 5% CO2 at 37°C. After one hour or 24 hours, supernatants were collected from wells and lactate dehydrogenase (LDH) release was measured to quantify levels of cytotoxicity using a Cytotox96 kit (Promega) according to the manufacturer’s instructions.
IV Injections
RB50 or RB50ΔclpV grown in SS to mid-log phase were re-suspended in PBS and 200 μL of 5x105 CFU/mL were intravenously injected into the tail veins of mice. Mice were dissected 1 and 7 days post-injection and lungs, livers, spleens, and kidneys were homogenized in one mL PBS, serially diluted and plated on BG agar containing 20 μg/mL streptomycin, and colonies were counted after incubation at 37°C for two days. Blood was collected via retro-orbital bleed, spun down at 5000 RPM for 5 min, and serum was then serially diluted, plated, and incubated at 37°C for two days.
Trypan Blue Exclusion Assay
RAW267.4 macrophage cells were grown in 96 well plates and infected with RB50 or RB50ΔclpV at an MOI of 100 and incubated at 37°C. Supernatants were replaced with 100 μL 100 μg/mL gentamicin solution after one hour, and one and twenty-four hours post-addition of gentamicin a subset of RAW264.7 cells were mixed 1:1 with trypan blue dye (Life Technologies). Total numbers of cells and the number of dead cells were enumerated for each timepoint.
Statistical Analysis
The mean +/- standard error (error bars in figures) was determined for all appropriate data. Two tailed, unpaired student’s t tests were used to determine statistical significance between two normally distributed populations. When more than two groups were analyzed, one- and two-way analysis of variance (ANOVA) tests were used. Survival curves were generated with the Kaplan-Meier method, and Log-Rank test was used to compute significance. Graphpad Prism version 6.04 was used to conduct these statistical tests and to generate figures.
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University at University Park, PA (#46284 Bordetella-Host Interactions). All animals were anesthetized using isoflourane or euthanized using carbon dioxide inhalation to minimize animal suffering.
Results
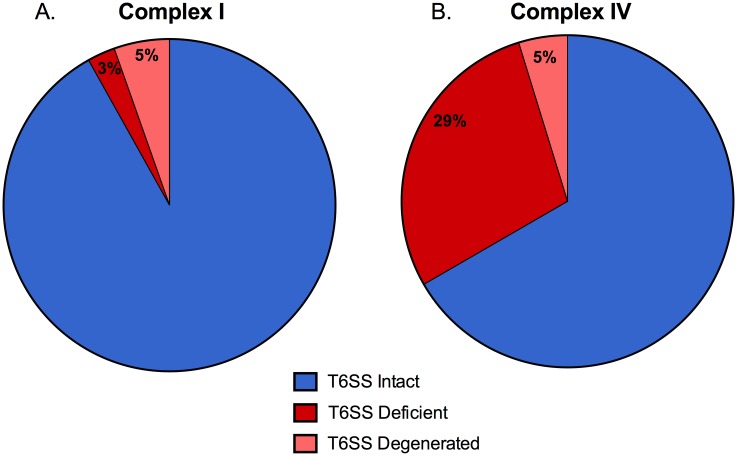
29% of Complex IV B. bronchiseptica isolates have lost their Type Six Secretion System
The T6SS is a crucial virulence factor for B. bronchiseptica that has been shown to increase pathology and cytotoxicity, affect cytokine induction, and aid in bacterial persistence during infection of wild-type mice [29]. However, an analysis of 58 recently sequenced B. bronchiseptica isolates revealed that 7 of the total isolates (12%) have lost their entire T6SS locus. In addition, 3 isolates (5%) contain pseudogenes (referred to in this paper as T6SS-degenerate) in their T6SS locus, implying that their T6SS may be non-functional. 6 of the 7 T6SS-deficient strains isolated and one T6SS-degenerate strains are from Complex IV, which is comprised mainly of human-isolated B. bronchiseptica strains [14]. Thus, 29% of Complex IV strains are T6SS-deficient and 5% are T6SS-degenerated (Fig 1A). In contrast, only 3% of Complex I B. bronchiseptica strains analyzed (1/37) are T6SS-deficient and 5% (2/37) are T6SS-degenerated (Fig 1B). This data suggests that loss of the T6SS may be linked to B. bronchiseptica survival in the human population, and may contribute to infection of immunocompromised patients.
Fig 1. Clinical B. bronchiseptica strains that have lost their T6SS locus are aggregated in Complex IV.
Genomes of 58 B. bronchiseptica clinical isolates were compared to prototypical RB50 genome. Presence of T6SS loci, as well as presence of pseudogenes in T6SS loci, was determined for all clinical isolates. Clinical strains containing an intact T6SS (blue), strains lacking a T6SS locus (red), and strains containing a pseudogene in the T6SS locus (pink) were divided based on whether they come from Complex I (A) or Complex IV (B).
Deleting the clpV component of T6SS results in hypervirulence in immunodeficient mice
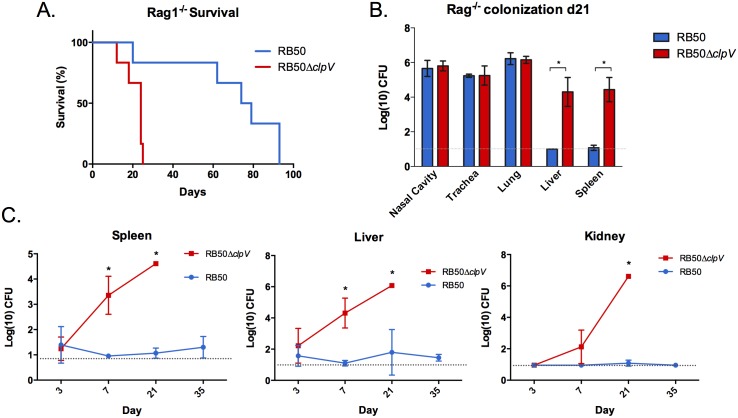
Since most B. bronchiseptica human infections occur in immunocompromised patients [30–35,45,46], we hypothesized that T6SS-deficient B. bronchiseptica may be able to infect and persist in immunodeficient mice. To test this, mice lacking T cells, B cells, and antibodies (Rag1-/- mice) were inoculated with RB50 or RB50ΔclpV, an isogenic mutant lacking the clpV ATPase gene of the T6SS [29]. While most Rag1-/- mice infected with RB50 survived beyond day 60, those infected with RB50ΔclpV succumbed to lethal bordetellosis by day 24 p.i. (p<0.05)(Fig 2A). Quantification of RB50 and RB50ΔclpV in respiratory tract organs of Rag1-/- mice on day 21 (prior to death with RB50ΔclpV infection) revealed similar numbers of Bordetella (Fig 2B) indicating that the hypervirulence of the ΔclpV strain is not caused by increased bacterial load in the respiratory tract. We therefore hypothesized that the early host death caused by RB50ΔclpV may result from systemic bacterial infection. Rag1-/- mice inoculated with either RB50 or RB50ΔclpV were thus assessed for bacterial numbers in the respiratory tract (nasal cavity, trachea, and lungs) or systemic organs (liver and spleen) 21 days post-infection. While similar numbers of RB50 and RB50ΔclpV were recovered from respiratory tract organs, only RB50ΔclpV was recovered from systemic organs (Fig 2B). These data indicate that a functional T6SS prevents B. bronchiseptica from colonizing systemic organs of Rag1-/- mice.
Fig 2. The T6SS modulates virulence and bacterial dissemination.
(A) Groups of Rag1-/- mice (n = 8) were inoculated with 5x105 CFU of RB50 (blue) or RB50ΔclpV (red) and were monitored for survival. (B) Groups of Rag1-/- mice (n = 4 per group) were inoculated with 5x105 CFU of RB50 (blue) versus RB50ΔclpV (red) and dissected on day 21 p.i. for bacterial enumeration in respiratory tract and systemic organs. The experiment was performed three times with similar results and a representative data set is shown. (C) Rag-/- mice were inoculated with 5x105 CFU RB50 (blue) versus RB50ΔclpV (red) and bacteria were enumerated from the spleen, liver, and kidney on days 3, 7, 21, and 35. With the exception of RB50ΔclpV on day 21 (n = 1), three mice were sacrificed per group per timepoint. * denotes p value <0.05. Grey dotted line indicates the limit of detection.
In order to determine how rapidly RB50ΔclpV reaches systemic organs, we enumerated bacterial numbers on days 3, 7, and 21. RB50ΔclpV was recovered from the liver and spleen of Rag1-/- mice as early as day 3 p.i., and additionally from the kidneys by day 7. RB50ΔclpV numbers then increased in all three organs by day 21 (Fig 2C). In contrast, RB50 was only recovered from systemic organs on day 3, but was then completely absent or present in only small numbers on subsequent days (Fig 2C). A human B. bronchiseptica isolate naturally missing a T6SS locus [36] (D445) was also recovered systemically from Rag1-/- mice by day 3 p.i. and on subsequent days (S1 Fig and data not shown). Importantly, both RB50 and RB50ΔclpV were recovered from systemic organs of immune-competent mice (wild-type C57Bl/6) on day 3 p.i., but only RB50ΔclpV was recovered from systemic organs by day 7 p.i., suggesting that the T6SS limits B. bronchiseptica persistence in systemic organs irrespective of host immune status (S2 Fig). Thus, a functional T6SS limits B. bronchiseptica survival and growth in systemic organs of immunodeficient and wild-type mice during infection.
RB50ΔclpV systemic infection is not the result of inflammatory response
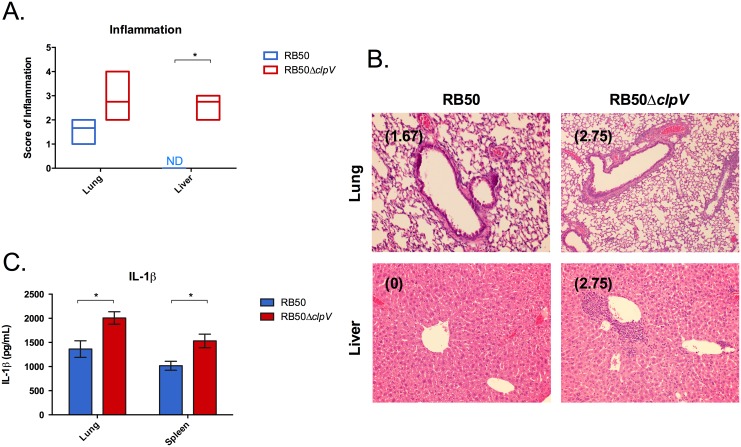
We hypothesized that immunodeficient mice were dying from an overwhelming inflammatory response with infection of the mutant. To test this, we performed histological examination of livers and lungs of infected mice. Rag1-/- mice infected with RB50ΔclpV exhibited higher levels of inflammation in the liver than those infected with RB50, characterized by primarily perivascular neutrophilic infiltrates with foci of necrosis (p<0.05)(Fig 3A). Despite similar levels of colonization in the lungs at this timepoint (Fig 1B), infection with RB50ΔclpV resulted in a trend toward higher inflammatory cell infiltration inflammation and necrosis in the lungs than infection with RB50 (Fig 3A and data not shown). Also, while RB50 caused no significant lesions in the liver, RB50ΔclpV caused high hepatic inflammation with primarily perivascular mixed inflammatory infiltrates by day 21 (Fig 3B).
Fig 3. clpV lowers inflammation and pathology in vivo.
(A) Groups of four Rag1-/- mice were inoculated with 5x105 CFU of RB50 (blue) or RB50ΔclpV (red) and a histopathological analysis was conducted on the lung and liver of infected mice on day 21 p.i. for scoring of inflammation. (B) Representative H&E lung and liver sections from Rag1-/- mice on day 21 p.i. after inoculation with 5x105 CFU of RB50 (blue) or RB50ΔclpV (red) with average pathology scores in parentheses. (C) Groups of Rag1-/- mice were inoculated with 5x105 CFU RB50 (blue) and RB50ΔclpV (red) and elicited IL-1β levels were determined from the lung and spleen on day 21 p.i. This experiment was performed twice with similar results and a representative dataset is shown. ND signifies not detected. * denotes p value <0.05.
To determine whether the T6SS modulates local and systemic cytokine production, cytokine levels were measured in the lungs and spleens of Rag1-/- mice inoculated with RB50 or RB50ΔclpV. Wild-type RB50 induced lower levels of IL-1β than RB50ΔclpV in the lungs and spleens of Rag1-/- mice by day 21, indicating that the T6SS suppresses IL-1β production during infection (p<0.05)(Fig 3C). Together, these data suggest that the B. bronchiseptica T6SS modulates innate immunity by lowering pathology, cell recruitment, and IL-1β production both locally and systemically in Rag1-/- mice.
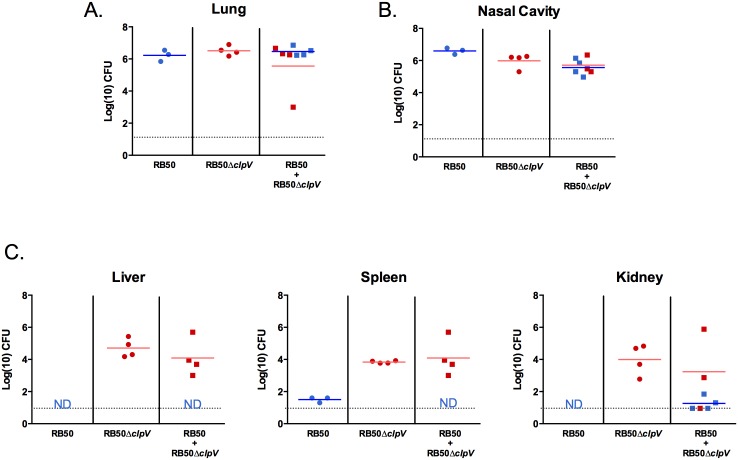
We hypothesized that spread and subsequent survival of RB50ΔclpV in systemic organs could be due to the different immune response induced by the mutant, much like S. aureus lipoprotein mutants generate a variant host response culminating in dissemination and lethal infection [47]. If this were the case, then co-inoculation with RB50 would provide T6SS-mediated immune modulation that would affect the fate of RB50ΔclpV. Alternatively, if systemic survival is intrinsic to the bacteria harboring the mutation and not mediated by indirect effects on the host, then the T6SS-dependent immune response generated by co-infection would not alter RB50ΔclpV systemic infection. To distinguish between these possibilities, Rag1-/- mice were inoculated with RB50 alone, RB50ΔclpV alone, or co-inoculated with RB50 and RB50ΔclpV. Similar numbers of RB50 and RB50ΔclpV were recovered from respiratory tract organs (lungs and nasal cavities) of Rag1-/- mice by day 21 p.i., regardless of whether they were inoculated separately or co-inoculated (Fig 4A). However, only RB50ΔclpV was recovered from systemic organs (p<0.05), and the numbers of RB50ΔclpV recovered from co-inoculated mice were similar to RB50ΔclpV inoculated alone (Fig 4B). Therefore, a functional T6SS provided by RB50 in the co-inoculation did not hinder the ability of RB50ΔclpV to reach and remain in systemic organs by day 21 p.i., and conversely the altered immune response with RB50ΔclpV infection still did not enable systemic survival of RB50 in the co-infected mice (Fig 4B). Loss of clpV only affects the bacteria with the mutation, suggesting that the T6SS affects direct interactions between bacteria and immune cells recruited to the site of infection. Additionally, numbers of RB50 and RB50ΔclpV were efficiently lowered in systemic organs when injected directly into the bloodstream (S3 Fig), once again suggesting that the intracellular niche may be utilized to protect B. bronchiseptica and enable trafficking to systemic organs.
Fig 4. Loss of clpV is required for systemic recovery.
Groups of Rag1-/- mice were either infected singly with RB50 (blue circles, n = 3) or RB50ΔclpV (red circles, n = 4) or co-infected with RB50 and RB50ΔclpV (red squares and blue squares, n = 4), and (A) respiratory tract bacterial numbers or (B) systemic organ bacterial numbers were enumerated on day 21 post-inoculation. This experiment was performed twice with similar results and a representative dataset is shown. ND signifies not detected. Grey line indicates the limit of detection.
Deletion of clpV increases B. bronchiseptica intracellular survival in vitro
Based on the results above, we hypothesized that B. bronchiseptica manipulates Antigen Presenting Cells (APCs) to house and traffic bacteria to systemic organs during infection, and that long-term survival in those immune cells is achieved through the loss of the T6SS. To determine whether loss of the T6SS affects intracellular survival, a gentamicin protection assay was performed to estimate intracellular survival of RB50 and RB50ΔclpV in RAW264.7 macrophages [48]. After a 1 hour treatment with gentamicin, similar numbers of RB50 and RB50ΔclpV were recovered from RAW264.7 macrophages (Fig 5A) suggesting that phagocytosis and early intracellular survival of B. bronchiseptica is T6SS-independent. However, after 24 hours numbers of intracellular RB50ΔclpV (~1x105 CFU/mL) were approximately 1000-fold higher than that of RB50 (~1x102 CFU/mL) (p<0.05) and remained over 1000-fold higher at 48 hours (p<0.05)(Fig 5A). Similarly, infection of RAW264.7 macrophages with a human isolate naturally lacking its T6SS (D445) yielded similarly high levels of recovered intracellular bacteria by 24 hours (S4 fig). Thus, loss of a functional T6SS increased in vitro intracellular survival of B. bronchiseptica in RAW264.7 macrophages. A cytotoxicity assay [29] was performed to analyze whether lower intracellular RB50 recovery by 24 hours correlates with higher cell death. Intracellular RB50 and RB50ΔclpV caused similar levels of cell death within 1 hour (Fig 5B), but by 24 hours intracellular RB50 caused higher levels of cell death than RB50ΔclpV (p<0.05)(Fig 5B). A trypan blue exclusion cell viability assay [49] confirmed these results (data not shown). Therefore, B. bronchiseptica can survive intracellularly in RAW264.7 macrophages, but the T6SS inhibits long-term intracellular survival by killing its eukaryotic host cell. These data suggest that APCs may act as vehicles for the transport of B. bronchiseptica to systemic organs during in vivo infection and may enable persistence of RB50ΔclpV in systemic organs of Rag1-/- mice.
Fig 5. Deletion of clpV increases intracellular survival in vitro.
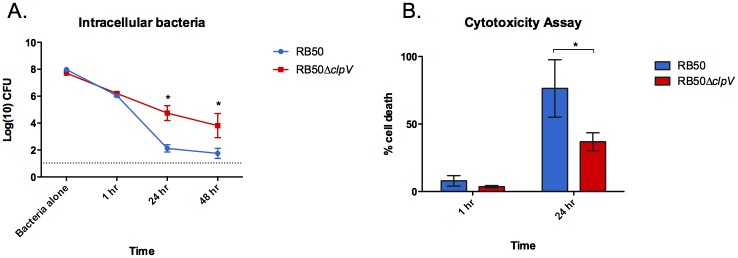
(A) RAW264.7 macrophages were infected with RB50 (blue, n = 4) or RB50ΔclpV (red, n = 4) at an MOI of 100 and bacterial invasion and intracellular survival was determined at 1, 24, and 48 hour after addition of gentamicin. The experiment was conducted five times with similar results and a representative dataset is shown. (B) The cytotoxicity of RAW264.7 macrophages infected with RB50 (blue) or RB50ΔclpV (red) at an MOI of 100 was determined 1 hour and 24 hours after gentamicin application. The average percent cytotoxicity of four wells in three different experiments was measured by (LDH release from a well / LDH release from positive control well) x 100 ±SE is shown. * denotes p value <0.05. Grey line indicates limit of detection.
The T6SS and adaptive immune components limit in vivo intracellular survival
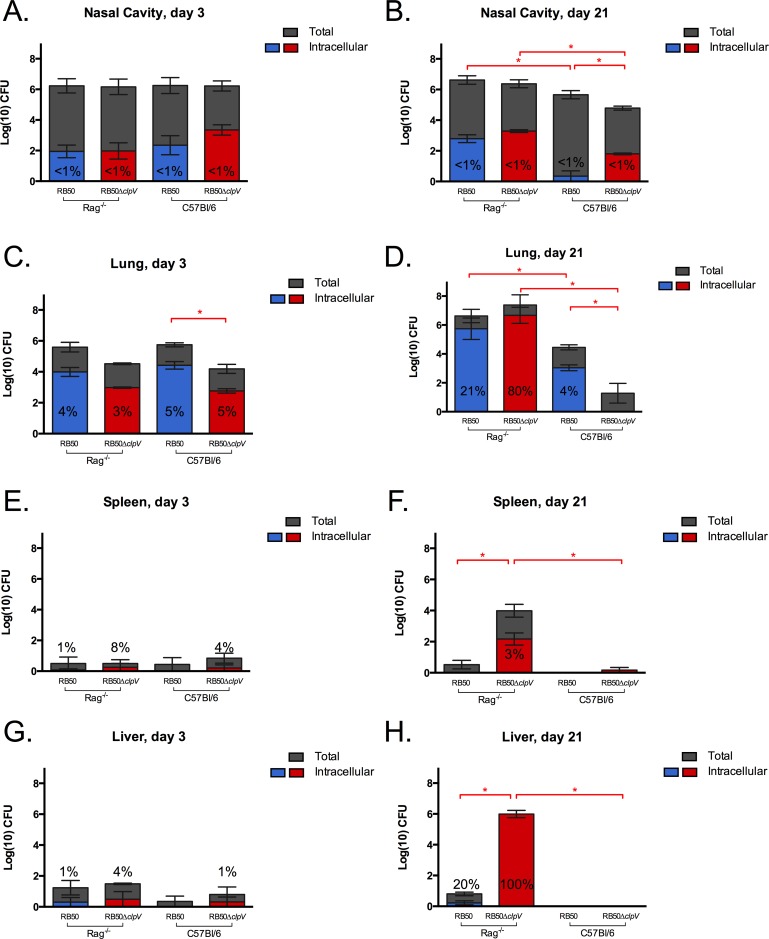
To investigate whether the T6SS affects intracellular survival in vivo, we gentamicin treated homogenized organs recovered from mice inoculated with RB50 or RB50ΔclpV and then enumerated surviving (intracellular) bacteria [19]. Less than 1% of RB50 and RB50ΔclpV recovered from the nasal cavities of both C57Bl/6 and Rag1-/- mice on days 3 and 21 p.i. survived intracellularly (Fig 6A and 6B). However, approximately 4% of RB50 in the lungs of C57Bl/6 mice on days 3 and 21 p.i. were gentamicin resistant (Fig 6C and 6D), suggesting that a proportion of B. bronchiseptica survives intracellularly in the lungs during infection and that there may be organ-specific differences in the proportion of bacteria that are intracellular. A similar proportion of RB50 (5%) was intracellular in lungs of Rag1-/- mice on day 3 p.i., but those numbers increased to 21% by day 21 p.i. (Fig 6C and 6D). Intracellular B. bronchiseptica was therefore observed in both wild-type and immunodeficient mice but increased over time only in the latter, indicating that adaptive immune components control numbers of intracellular B. bronchiseptica in the lungs.
Fig 6. Deletion of clpV increases intracellular survival in vivo.
Groups (n = 3) of Rag1-/- and C57Bl/6 mice were inoculated with 5x105 CFU RB50 or RB50ΔclpV and total bacterial numbers (grey) and intracellular RB50 (blue) and RB50ΔclpV (red) numbers were enumerated from the nasal cavity (A, B), lungs (C, D), spleen (E, F), and liver (G, H) early (day 3) and late (day 21) post-infection. This experiment was conducted twice with similar results and a representative dataset is shown. Red * denotes p value <0.05 for comparison of intracellular bacterial populations.
Fewer total RB50ΔclpV were recovered from the lungs of C57Bl/6 mice by day 3 p.i. than total RB50, but the proportion of intracellular bacteria recovered at this timepoint were similar (Fig 6C). By day 21 p.i., RB50ΔclpV numbers in C57/Bl6 lungs were substantially decreased and no intracellular bacteria were observed (Fig 6D). The T6SS is therefore required for persistence in lungs of immune-competent mice, as was shown previously [29]. In the lungs of Rag1-/- mice, however, 3% of total RB50ΔclpV were intracellular on day 3 p.i. and those numbers increased to 80% by day 21 p.i., which was substantially higher than wild type bacterial recovery in these animals (Fig 6C and 6D). Hence, loss of the T6SS enables increased recovery of intracellular B. bronchiseptica from the lungs of immunodeficient mice.
Low numbers of wild-type B. bronchiseptica were recovered from the spleens and livers of C57Bl/6 mice by day 3 p.i., none of which were intracellular in either organ (Fig 6E and 6G). Loss of clpV increased the proportion of intracellular bacteria in the spleens and livers of C57Bl/6 mice 3 days p.i. to 4% and 1%, respectively (Fig 6E and 6G). In Rag1-/- mice RB50ΔclpV proportions increased to 8% in the spleen and 4% in the liver on day 3 p.i., indicating that clpV and adaptive immune components both limit intracellular recovery from systemic organs early during infection (Fig 6E and 6G). While no RB50ΔclpV were recovered from systemic organs of wild-type mice on day 21 p.i., 3% of 104 CFU and 100% of 106 CFU were recovered intracellularly from the spleen and liver of Rag1-/- mice by day 21, respectively. This data suggests that that the T6SS prevents accumulation of intracellular B. bronchiseptica in systemic organs and that adaptive immune components are required for clearance of systemic RB50ΔclpV. Together these results indicate that in immunodeficient mice the T6SS prevents long-term intracellular survival suggesting that in an immunocompromised host, loss of the T6SS may aid in B. bronchiseptica persistence.
Discussion
Many species of bacteria, including S. aureus and P. aeruginosa [50,51], utilize virulence factors to reach the bloodstream and systemic organs during infection. However, in other species bacterial factors prevent dissemination to systemic organs [52–54]. Loss of those virulence factors then causes a hypervirulent phenotype characterized by increased intracellular survival, enhanced dissemination to blood and systemic organs, and host lethality [52–54]. For example, loss of covS or lgt in Staphylococcus aureus, sciS in S. enterica, and ccpA in Streptococcus pyogenes all lead to hypervirulence and increased host lethality [47,52–54]. Although the B. bronchiseptica T6SS is required for persistence during infection of wild-type mice [29], we show that loss of this secretion system contributes to enhanced intracellular survival leading to early host death of immunodeficient mice. A large proportion of recently sequenced B. bronchiseptica strains are T6SS-negative (Fig 1), suggesting that while this secretion system plays an important role during infection [29], loss of the T6SS may also benefit B. bronchiseptica by enhancing intracellular survival. Here we showed that T6SS-deficiency is detrimental for B. bronchiseptica survival in the lungs of wild-type C57Bl/6 mice (Fig 6A and 6B). However, disruption of T6SS function is also detrimental for B. bronchiseptica in adaptive immunodeficient mice; while high numbers of RB50ΔclpV survived intracellularly at the site of infection, the spread of mutant to systemic organs via long-term survival in APCs contributed to host death, representing a “dead-end” for B. bronchiseptica (Figs 6 and 2). A similar phenotype for the T6SS has also been reported for Helicobacter hepaticus, where this secretion system plays a protective role by decreasing intracellular bacterial numbers within intestinal epithelial cells and by modulating host inflammation [55]. Since B. bronchiseptica kills its eukaryotic cellular host via a T6SS-mediated mechanism, a functional T6SS appears to be required for containment of the bacteria to the respiratory tract in immunocompromised mice and may potentially increase likelihood of transmission and ultimate success of this pathogen. In this work, we have not determined how the wild-type B. bronchiseptica are cleared from systemic organs of immunocompromised mice, and it will be interesting to elucidate the specific mechanism of clearance in future. Future work will determine whether loss of this secretion system correlates with intracellular recovery from clinical isolates of immunodeficient patients, and whether T6SS-deficient B. bronchiseptica strains are able to persist in the population because of their enhanced survival in an immunocompromised niche.
An adaptive immune response is required to contain B. bronchiseptica within the respiratory tract and to limit intracellular B. bronchiseptica numbers. Either B cells or T cells are sufficient to limit systemic T6SS-negative bacteria, though both are required for efficient clearance from the lungs (Bendor and Harvill, unpublished). However, a proportion of T6SS-sufficient B. bronchiseptica were recovered intracellularly from the lungs of wild-type mice during infection (Fig 6C and 6D). Intracellular localization shields these wild-type B. bronchiseptica from complement, antibodies, and other antimicrobials released during inflammation, potentially allowing them to evade these responses and emerge after resolution of the local inflammatory response. Future work will determine mechanisms of intracellular survival for these T6SS-sufficient B. bronchiseptica, including whether the wild-type bacteria persist within or repeatedly infect cells and whether they regulate expression of virulence factors and modulate host cell behavior in order to maintain their intracellular localization.
Intracellular survival of the T6SS-deficient bacteria appears to be niche specific as more intracellular bacteria were recovered in the lungs and livers than in the nasal cavities and spleens, respectively, during infection (Fig 6A–6D). This differential intracellular survival could be attributed to altered immune surveillance and response in different organs. Increased intracellular survival in the liver as compared to the spleen has also been observed with mycobacteria, where a varying immune response accounted for these differences [56]. In addition, dissimilar architecture or cellular populations of the two organs may have contributed to differences in intracellular bacterial recovery. While we used the spleen as a representative systemic organ for immune response analysis to RB50 or RB50ΔclpV infection in Rag1-/- mice (Fig 3C), it will be interesting to determine whether a different immune response in the liver contributes to the higher proportion of surviving intracellular B. bronchiseptica during infection (Fig 6F and 6H). Also, further work identifying the cell types housing Bordetella in the respiratory tract and systemic organs may shed light on the niche-specific differences in intracellular survival that were reported in this study.
All three classical bordetellae (B. bronchiseptica, B. pertussis, and B. parapertussis) have been shown to be able to survive intracellularly [5,8,11,12,57]. Of the three, B. bronchiseptica is the only species predicted to have a functional T6SS [36] while the T6SS is absent from B. pertussis and degenerated in B. parapertussis [36,39]. Additionally, O antigen has been reported to mediate B. parapertussis survival in neutrophils [12] but B. pertussis lacks the O antigen locus [39], indicating that an alternative factor is likely required for intracellular survival of B. pertussis. The T3SS appears to function similarly to the T6SS by limiting intracellular survival and systemic recovery of B. bronchiseptica [58] (Bendor and Harvill, unpublished), but is probably not functional in B. parapertussis because of pseudogenes present in the locus [39]. Lastly, higher levels of B. bronchiseptica Bvg- mutants have been recovered intracellularly in vitro than wild-type [6], but in contrast B. pertussis appears to require Bvg function for invasion and intracellular survival in macrophages in vitro [59]. Thus, there seem to be different mechanisms for intracellular survival in these three closely related species, and we have identified a system in which we can dissect their varying intracellular survival strategies. This system will be useful to investigate when and how Bordetella species utilize an intracellular niche during infection, which will provide essential information for the design of improved vaccines and therapeutics.
Supporting Information
RB50 (blue), RB50ΔclpV (red) and D445 (green) recovery from livers of Rag1-/- mice on day 7 p.i. Grey line indicates limit of detection.
(TIFF)
RB50 (blue) and RB50ΔclpV (red) recovery from livers and spleens of Rag1-/- and wild-type C57Bl/6 mice on days 3 (A) and 7 (B) p.i. * denotes p value <0.05.
(TIFF)
RB50 (blue) and RB50ΔclpV (red) recovery from lungs, livers, spleens, and kidneys of Rag1-/- mice that had been intravenously injected and dissected on days 1 (A) and 7 (B) p.i. ND—Not Detected. The grey line indicates limit of detection.
(TIFF)
Invasion and intracellular survival of RB50ΔclpV (red) and a B. bronchiseptica isolate naturally missing the T6SS (D445, green) in RAW264.7 macrophages at an MOI of 100 at 1 and 24 hours post-gentamicin application. The grey line indicates limit of detection.
(TIFF)
Acknowledgments
We would like to acknowledge Yury Ivanov for critical review of this manuscript and all members of the Harvill lab for support and helpful discussion.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Institutes of Health Grants GM083113, AI107016, AI116186, GM113681 (to ETH, http://www.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Monack DM, Mueller A & Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2004;2: 747–765. [DOI] [PubMed] [Google Scholar]
- 2. Fortier AH, Leiby DA, Narayanan RB, Asafoadjei E, Crawford RM, Nacy CA, et al. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 1995;63: 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barry AO, Mege JL & Ghigo E. Hijacked phagosomes and leukocyte activation: an intimate relationship. J. Leukoc. Biol. 2011;89: 373–382. 10.1189/jlb.0510270 [DOI] [PubMed] [Google Scholar]
- 4. Forde CB, Shi X, Li J & Roberts M. Bordetella bronchiseptica-mediated cytotoxicity to macrophages is dependent on bvg-regulated factors, including pertactin. Infect. Immun. 1999;67: 5972–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forde CB, Parton R & Coote JG. Bioluminescence as a reporter of intracellular survival of Bordetella bronchiseptica in murine phagocytes . Infect. Immun. 1998;66: 3198–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banemann A & Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect. Immun. 1997;65: 3469–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schipper H, Krohne GF & Gross R. Epithelial cell invasion and survival of Bordetella bronchiseptica . Infect. Immun. 1994;62: 3008–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzman CA, Rohde M, Bock M. & Timmis KN. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect. Immun. 1994;62: 5528–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneider B, Gross R & Haas A. Phagosome acidification has opposite effects on intracellular survival of Bordetella pertussis and B. bronchiseptica . Infect. Immun. 2000;68: 7039–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jungnitz H, West NP, Walker MJ, Chhatwal GS & Guzmán CA. A Second Two-Component Regulatory System of Bordetella bronchiseptica Required for Bacterial Resistance to Oxidative Stress, Production of Acid Phosphatase, and In Vivo Persistence. Infect. Immun. 1998;66: 4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamberti YA, Hayes JA, Perez Vidakovics ML, Harvill ET & Rodriguez ME. Intracellular Trafficking of Bordetella pertussis in Human Macrophages. Infect. Immun. 2010;78: 907–913. 10.1128/IAI.01031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gorgojo J, Lamberti Y, Valdez H, Harvill ET & Rodríguez ME. Bordetella parapertussis survives the innate interaction with human neutrophils by impairing bactericidal trafficking inside the cell through a lipid raft-dependent mechanism mediated by the lipopolysaccharide O antigen. Infect. Immun. 2012;80: 4309–4316. 10.1128/IAI.00662-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattoo S & Cherry JD. Molecular Pathogenesis, Epidemiology, and Clinical Manifestations of Respiratory Infections Due to Bordetella pertussis and Other Bordetella Subspecies. Clin. Microbiol. Rev. 2005;18:326–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica . PLoS Pathog. 2005;1: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mills KH, Barnard A, Watkins J & Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 1993;61: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen JW, Ibsen PH, Hasløv K & Heron I. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleen of mice aerosol infected with Bordetella pertussis . Infect. Immun. 1992;60: 4563–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Müller I, Garcia-Sanz JA, Titus R, Behin R & Louis J. Analysis of the cellular parameters of the immune responses contributing to resistance and susceptibility of mice to infection with the intracellular parasite, Leishmania major . Immunol. Rev. 1989;112: 95–113. [DOI] [PubMed] [Google Scholar]
- 18. Kratz SS & Kurlander RJ. Characterization of the pattern of inflammatory cell influx and cytokine production during the murine host response to Listeria monocytogenes . J. Immunol. 1988;141: 598–606. [PubMed] [Google Scholar]
- 19. Hellwig SM, Hazenbos WL, van de Winkel JG & Mooi FR. Evidence for an intracellular niche for Bordetella pertussis in broncho-alveolar lavage cells of mice. FEMS Immunol. Med. Microbiol. 1999;26: 203–207. [DOI] [PubMed] [Google Scholar]
- 20. Gueirard P, Ave P, Balazuc AM, Thiberge S, Huerre M, Milon G, et al. Bordetella bronchiseptica persists in the nasal cavities of mice and triggers early delivery of dendritic cells in the lymph nodes draining the lower and upper respiratory tract. Infect. Immun. 2003;71: 4137–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dworkin MS, Sullivan PS, Buskin SE, Harrington RD, Olliffe J, MacArthur RD, et al. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 1999;28: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 22. Coulthurst SJ. The Type VI secretion system—a widespread and versatile cell targeting system. Res. Microbiol. 2013;164: 640–654. 10.1016/j.resmic.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 23. Ma AT, McAuley S, Pukatzki S & Mekalanos JJ. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell Host Microbe. 2009;5: 234–243. 10.1016/j.chom.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cascales E. The type VI secretion toolkit. EMBO Rep. 2008;9: 735–741. 10.1038/embor.2008.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Folkesson A, Löfdahl S & Normark S. The Salmonella enterica subspecies I specific centisome 7 genomic island encodes novel protein families present in bacteria living in close contact with eukaryotic cells. Res. Microbiol. 2002;153: 537–545. [DOI] [PubMed] [Google Scholar]
- 26. De Bruin OM, Ludu JS & Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 2007;7: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suarez G, Sierra JC, Sha J, Wang S, Erova TE, Fadl AA, et al. Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 2008;44: 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christian Schlieker HZ. ClpV, a unique Hsp100/Clp member of pathogenic proteobacteria. Biol. Chem. 2005;386: 1115–27. [DOI] [PubMed] [Google Scholar]
- 29. Weyrich LS, Rolin OY, Muse SJ, Park J, Spidale N, Kennett MJ, et al. A Type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo. PloS One 2012;7: e45892 10.1371/journal.pone.0045892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trevejo RT, Barr MC, Robinson RA. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet Res. 2005;36(3):14. [DOI] [PubMed] [Google Scholar]
- 31. Cookson BT, Vandamme P, Carlson LC, Larson AM, Sheffield JV, Kersters K, et al. Bacteremia caused by a novel Bordetella species, “B. hinzii”. J Clin Microbiol. 1994. October 1;32(10):2569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bromberg K, Tannis G, Steiner P. Detection of Bordetella pertussis associated with the alveolar macrophages of children with human immunodeficiency virus infection. Infect Immun. 1991. December 1;59(12):4715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huebner ES, Christman B, Dummer S, Tang Y-W, Goodman S. Hospital-Acquired Bordetella bronchiseptica Infection following Hematopoietic Stem Cell Transplantation. J Clin Microbiol. 2006. July 1;44(7):2581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuente J, Albo C, Rodríguez A, Sopeña B & Martínez C. Bordetella bronchiseptica pneumonia in a patient with AIDS. Thorax 1994;49: 719–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meis JFGM, van Griethuijsen AJA, Muytjens HL. Bordetella bronchiseptica bronchitis in an immunosuppressed patient. Eur J Clin Microbiol Infect Dis. 1990. May 1;9(5):366–7. [DOI] [PubMed] [Google Scholar]
- 36. Park J, Zhang Y, Buboltz AM, Zhang X, Schuster SC, Ahuja U, Liu M, et al. Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genomics 2012;13: 545 10.1186/1471-2164-13-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Register KB, Ivanov YV, Harvill ET, Davison N & Foster G. Novel, host-restricted genotypes of Bordetella bronchiseptica associated with phocine respiratory tract isolates. Microbiol. 2015;161: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Register KB, Ivanov YV, Jacobs N, Meyer J, Goodfield LL, Muse SJ, et al. Draft Genome Sequences of 53 Genetically Distinct Isolates of Bordetella bronchiseptica Representing 11 Terrestrial and Aquatic Hosts. Genome Announc. 2015;3: e00152–15. 10.1128/genomeA.00152-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica . Nat. Genet. 2003;35: 32–40. [DOI] [PubMed] [Google Scholar]
- 40. Carver TJ, Rutherford KM, Berriman M, Rajandream MA, Barrell BG, Parkhill J. ACT: the Artemis Comparison Tool. Bioinforma. Oxf. Engl. 2005;21: 3422–3423. [DOI] [PubMed] [Google Scholar]
- 41. Cotter PA & Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 1994;62: 3381–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buboltz AM, Nicholson TL, Weyrich LS & Harvill ET. Role of the Type III Secretion System in a Hypervirulent Lineage of Bordetella bronchiseptica . Infect. Immun. 2009;77: 3969–3977. 10.1128/IAI.01362-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kirimanjeswara GS, Mann PB & Harvill ET. Role of Antibodies in Immunity to Bordetella Infections. Infect. Immun. 2003;71: 1719–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mann PB, Kennett MJ & Harvill ET. Toll-Like Receptor 4 Is Critical to Innate Host Defense in a Murine Model of Bordetellosis. J. Infect. Dis. 2004;189: 833–836. [DOI] [PubMed] [Google Scholar]
- 45. Woolfrey BF & Moody JA. Human infections associated with Bordetella bronchiseptica . Clin. Microbiol. Rev. 1991;4: 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goodnow RA. Biology of Bordetella bronchiseptica . Microbiol. Rev. 1980;44: 722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wardenburg JB, Williams WA & Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci.2006;103: 13831–13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM & Mobley HLT. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog. 2013;9: e1003788 10.1371/journal.ppat.1003788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strober W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol.2001;Appendix 3: Appendix 3B 10.1002/0471142735.ima03bs21 [DOI] [PubMed] [Google Scholar]
- 50. Lowy FD. Staphylococcus aureus Infections. N. Engl. J. Med. 1998;339: 520–532. [DOI] [PubMed] [Google Scholar]
- 51. Koh AY, Priebe GP & Pier GB. Virulence of Pseudomonas aeruginosa in a Murine Model of Gastrointestinal Colonization and Dissemination in Neutropenia. Infect. Immun. 2005;73: 2262–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kinkel TL & McIver KS. CcpA-Mediated Repression of Streptolysin S Expression and Virulence in the Group A Streptococcus. Infect. Immun. 2008;76: 3451–3463. 10.1128/IAI.00343-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parsons DA & Heffron F. SciS, an icmF Homolog in Salmonella enterica Serovar Typhimurium, Limits Intracellular Replication and Decreases Virulence. Infect. Immun. 2005;73: 4338–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Liu G, Feng W, Zhou Y, Liu M, Wiley JA, et al. Neutrophils select hypervirulent CovRS mutants of M1T1 group A Streptococcus during subcutaneous infection of mice. Infect. Immun. 2014;82: 1579–1590. 10.1128/IAI.01458-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chow J & Mazmanian SK. A Pathobiont of the Microbiota Balances Host Colonization and Intestinal Inflammation. Cell Host Microbe 2010;7: 265–276. 10.1016/j.chom.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Curtis MM & Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126: 177–185. 10.1111/j.1365-2567.2008.03017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steed LL, Setareh M & Friedman RL. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J. Leukoc. Biol. 1991;50: 321–330. [DOI] [PubMed] [Google Scholar]
- 58. Yuk MH, Harvill ET, Cotter PA & Miller JF. Modulation of host immune responses, induction of apoptosis and inhibition of NF-κB activation by the Bordetella type III secretion system. Mol. Microbiol. 2000;35: 991–1004. [DOI] [PubMed] [Google Scholar]
- 59. Friedman RL, Nordensson K, Wilson L, Akporiaye ET & Yocum DE. Uptake and intracellular survival of Bordetella pertussis in human macrophages. Infect. Immun. 1992;60: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RB50 (blue), RB50ΔclpV (red) and D445 (green) recovery from livers of Rag1-/- mice on day 7 p.i. Grey line indicates limit of detection.
(TIFF)
RB50 (blue) and RB50ΔclpV (red) recovery from livers and spleens of Rag1-/- and wild-type C57Bl/6 mice on days 3 (A) and 7 (B) p.i. * denotes p value <0.05.
(TIFF)
RB50 (blue) and RB50ΔclpV (red) recovery from lungs, livers, spleens, and kidneys of Rag1-/- mice that had been intravenously injected and dissected on days 1 (A) and 7 (B) p.i. ND—Not Detected. The grey line indicates limit of detection.
(TIFF)
Invasion and intracellular survival of RB50ΔclpV (red) and a B. bronchiseptica isolate naturally missing the T6SS (D445, green) in RAW264.7 macrophages at an MOI of 100 at 1 and 24 hours post-gentamicin application. The grey line indicates limit of detection.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.