Abstract
Hemophilic arthropathy is a debilitating condition that can develop as a consequence of frequent joint bleeding despite adequate clotting factor replacement. The mechanisms leading to repeated spontaneous bleeding are unknown. We investigated synovial, vascular, stromal and cartilage changes in response to a single induced hemarthrosis in the FVIII-deficient mouse. We found soft tissue hyperproliferation with marked induction of neoangiogenesis and evolving abnormal vascular architecture. While soft tissue changes were rapidly reversible, abnormal vascularity persisted for months and, surprisingly, was also seen in uninjured joints. Vascular changes in FVIII-deficient mice involved pronounced remodeling with expression of α-Smooth Muscle Actin (SMA), Endoglin (CD105) and vascular endothelial growth factor, as well as alterations of joint perfusion as determined by in vivo imaging. Vascular architecture changes and pronounced expression of α-SMA appeared unique to hemophilia, as these were not found in joint tissue obtained from mouse models of rheumatoid arthritis (RA) and osteoarthritis (OA) and from patients with the same conditions. Evidence that vascular changes in hemophilia were significantly associated with bleeding and joint deterioration was obtained prospectively by dynamic in vivo imaging with musculoskeletal ultrasound and power Doppler of 156 joints (elbows, knees and ankles) in a cohort of 26 patients with hemophilia at baseline and during painful episodes. These observations support the hypothesis that vascular remodeling contributes significantly to bleed propagation and development of hemophilic arthropathy. Based on these findings, the development of molecular targets for angiogenesis inhibition may be considered in this disease.
Keywords: Hemophilia, arthropathy, neoangiogenesis, vascular remodeling, hemarthrosis
Introduction
Factor (F)VIII (Hemophilia A) or FIX (Hemophilia B) manifests with spontaneous joint bleeding in childhood [1] that results in “target joints”, defined as joints with several consecutive bleeds within a 6 month period [2]. Target joints often progress to hemophilic arthropathy [2, 3] characterized by joint deformities, synovial hypertrophy, and cartilage and bone destruction. Prophylactic clotting factor replacement can reduce joint bleeding and the development of hemophilic arthropathy markedly, but cannot entirely prevent it [3–6]. Therefore, hemophilic arthropathy is an important disabling comorbidity in adult patients, whereby the timing and intensity of prophylaxis in childhood appears to influence disease burden later in life [5, 7]. While early start and intensity as well as adherence and continued access to clotting factor all weigh in favor of joint health in hemophilia, there is evidence that once a target joint has formed prophylaxis cannot halt progressive range of motion deficits [3]. Also, despite access to prophylaxis in childhood, ~30–50% of young adults with hemophilia living in industrialized countries suffer from hemophilic arthropathy [8–10], which has become an important focus of management outside of clotting factor replacement [11]. Since clotting factor replacement alone is only partially effective for the prevention and treatment of hemophilic arthropathy, additional treatment strategies are desirable and their development could be enabled by a better understanding of the pathobiology of disease progression. However, information about such pathobiological processes is limited since hemophilia patients died at a young age last century due to virally contaminated clotting factor preparations [12], and in this context joint disease was of lesser importance.
The pathology of hemophilic arthropathy is unique in that iron toxicity is implicated and thought to underlie synovial hyperplasia and cartilage deterioration. Although hemophilic arthropathy may resemble osteoarthritis (OA) or rheumatoid arthritis (RA), and some molecular changes may have common denominators such as inflammation or cartilage destruction [13], hemophilic arthropathy does not fit either category and remains poorly understood. On a molecular level, tissue proliferation seems to be instigated by synovial apoptosis resistance through expression of the proto-oncogene C-MYC [14] or the p53 binding protein MDM2 [15], local hypoxia with production of proinflammatory cytokines, and upregulation of hypoxia inducible factor-1α [16, 17]. Also, recruitment of circulating endothelial progenitor cells [18] and neoangiogenesis with overexpression of vascular endothelial growth factor (VEGF) are thought to contribute [17, 18]. However, the reasons for continued progression of joint disease remain unknown, and may be associated with perpetuated clinical but also subclinical bleeding irrespective of clotting factor replacement [11, 19]. Based on clinical observations of abnormal vascularity and perfusion patterns in joints of hemophilia patients [11, 18], we hypothesized that these vascular changes may play a major role for perpetuated joint bleeding and thereby contribute to the progression of arthropathy.
Towards that end, we investigated the time course of synovial, vascular, stromal and cartilage changes in response to a single induced hemarthrosis in a mouse model of hemophilia, compared vascular architecture changes to mouse models of RA and OA, and explored the extent to which these findings may relate to the development of hemophilic arthropathy in patients.
We found that induced hemarthrosis in the FVIII-deficient mouse leads to soft tissue hyperproliferation with marked induction of neoangiogenesis. The surprising findings were reversibility of soft tissue changes, while vascular remodeling continued for months and also occurred in uninjured joints. Such vascular changes were not present in RA or OA. Live imaging studies in hemophilia recapitulated findings in the mouse and demonstrated a significant association of abnormal vascularity with subclinical joint bleeding and joint deterioration. These observations support the hypothesis that vascular remodeling facilitates re-bleeding and is a major contributor to the development of hemophilic arthropathy.
Methods
Mouse models
Animal experiments were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute (TSRI) and performed with skeletally mature mice (12–16 weeks old). Right knee intra-articular bleeding in FVIII-deficient BalbC mice was induced by subpatellar puncture [20]. Bleeding was determined by hematoma formation [20] and decreases in hematocrit. Spun hematocrits were determined as previously described [21]. Mice were anesthetized with Isoflurane for all procedures, and Buprenorphine (0.1 mg/kg) was administered intraperitoneally 1–2 times daily as needed after hematoma formation for pain control until resolution of immobility. Collagen-induced arthritis (CIA) and surgical OA were induced as described in DBA2J and C57BL/6J mice [22, 23]. Joints were processed and stained with antibodies as described [23].
Incapacitation by avoidance of right hind leg use after subpatellar puncture was assessed using the Incapacitance Meter (Bioseb; Vitrolles Cedex, France). Unilateral weight bearing differences (gm) were recorded and averaged over at least 40 intervals (15 sec for each interval). Values were expressed as ratio of left/right leg weight bearing capacity. The method assesses natural adjustment to the degree of pain by adapting weight distribution on both rear paws [24].
Human tissue
The harvesting of human tissues at the time of total knee replacement surgery was approved by the Scripps Human Subjects Committee and the University of California (UCSD) Human Research Protection Program (HRPP).
Evaluation of cartilage and soft tissue changes
Synovial, stromal, cartilage and vascular changes were studied by histopathology after Safranin-O-Green staining. Semiquantitative scoring was performed using Valentino-, Krenn- and Glasson-scoring systems [25–27]. Vascularity was determined as total vessel count per joint encompassing all synovial and stromal tissue between femoral and tibial growth plates. Individual vessel diameters were determined by ruler at 40-fold magnification. For elliptical vessels the larger diameter was recorded unless it exceeded the smaller diameter by > 2.5-fold. Determination of vessel diameter is depicted in Supplemental Figure 1. Chondrocyte counts were performed within the inner 50% of the tibial plateau. Percentage of Proliferating Cell Nuclear Antigen (PCNA)-positive cells of the total cell number was determined in ‘hot spot’ areas at 20-fold magnification [28].
Quantification of gene expression
Total RNA was isolated from synovia of the right (injured) and left (control) knees using the Qiagen RNAeasy kit (Qiagen, Hilden, Germany). cDNA was prepared from 1 µg RNA samples using iscript cDNA synthesis kit and 2 µL were amplified by RT-PCR reactions with 1× SYBR green supermix on a CFX96 real time system (all from Bio-Rad, Hercules, CA). Relative expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method [29].
Synovial perfusion studies
Joint tissue perfusion was studied using high resolution musculoskeletal ultrasound (MSKUS) with the GE Logiq e model in mice [30] (Supplemental Figure 2) and the GE Logiq S8 model in patients (General Electrics, Fairfield, CT, USA) with grey scale (B-mode) and Power Doppler (PD), using transducer frequencies of 8–16 MHz and standardized protocols [31–33]. Patients with severe or moderate hemophilia were aged ≥ 21 years and had at least one target joint. Joints were examined at baseline and during acute painful episodes. Pain was self-assessed by visual analog scale (0 no pain; 10 worst pain). Radiographic and clinical joint status was determined by Pettersson Scores [34] and Hemophilia Joint Health Scores (HJHS) [35]. In mice, quantification of PD signal was performed using the Sigma Scan Pro Program. In patients, PD signal was scored semiquantitatively [36] in 3 different anatomical locations in each joint, and added to a total score (min=0; max=9). Scoring locations: Elbow: humero-radial joint axial, longitudinal and olecranon fossa; Knee: medial and lateral recesses and medial menisceal area; Ankle: tibio-talar joint axial, longitudinal and lateral sinus tarsi (Supplemental Figure 3). Prospective data acquisition was approved by the UCSD HRPP. Written informed consent was obtained from all patients.
Statistics
Two-tailed Student T-, Mann-Whitney-, Kruskal Wallis- or Wilcoxon signed rank tests were used where appropriate. Correlations between joint score measures and PD signal were evaluated with Spearman rank correlation. P-values < 0.05 were considered significant for all analyses. Logistic regression was used to examine the association between joint bleed status and PD score, Pettersson Score and HJHS. Colinearity among the three predictors was evaluated using methods described [37, 38]. Statistical analyses were performed using SAS Version 9.4 (Cary, NC) or GraphPad Prism V6 (San Diego, CA).
Results
Synovial, stromal and cartilage changes and hind leg incapacitation following induced hemarthrosis in FVIII-deficient mice
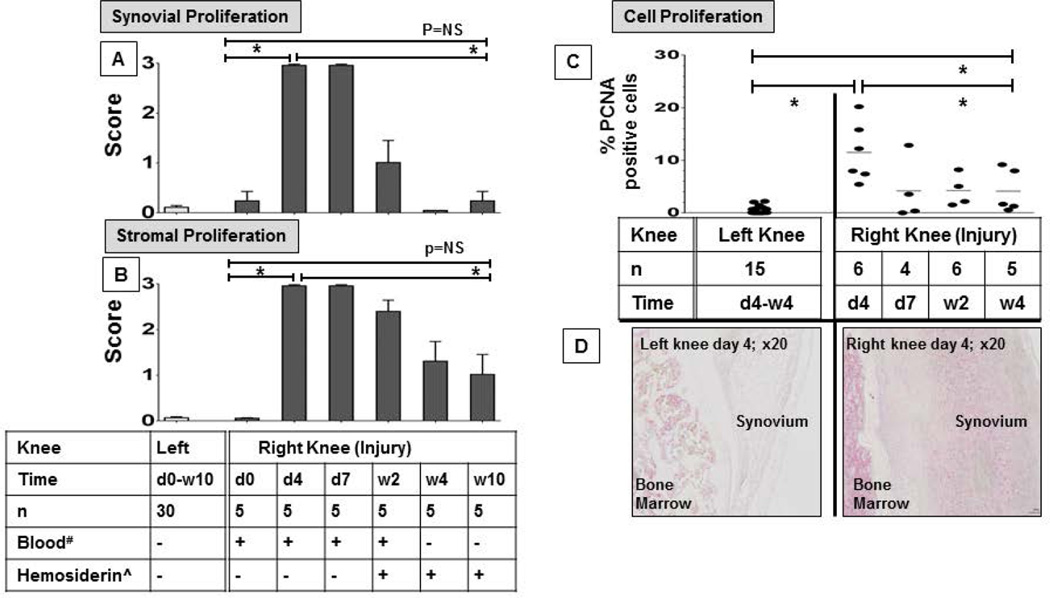
Subpatellar puncture of the right knee in FVIII-deficient mice resulted in joint bleeding and caused a significant decrease of the hematocrit from 47% (SEM 0.4) to 31% (SEM 2.0) two days post-injury (n ≥25 per group; p=0.0001) (Supplemental Figure 4). Joints were scored for synovial, stromal and cartilage changes 4 and 7 days and 2, 4 and 10 weeks post-injury (n=5 per group) and compared to baseline as well as to the uninjured control knee. Scoring examples are depicted in Supplemental Figure 5. Joint bleeding resulted in pronounced synovial (Figure 1A) and stromal (Figure 1B) hyperplasia in the injured knee, without significant changes in the uninjured knee. At 4 and 7 days post-injury, maximal scores (mean score = 3 out of 3) for synovial and stromal changes were present, but these changes were reversible. By week 4, synovial cellularity had returned to baseline (mean score = 0.2 of 3; p<0.0001) and stromal appearance had improved significantly to near baseline (mean score = 1.3 of 3; p=0.002). There was no further improvement in stromal appearance detectable at 10 weeks (Figure 1A/B; Supplemental Figure 6).
Figure 1. Time course of soft tissue proliferation following induced hemarthrosis in FVIII-deficient mice.
The right knee of FVIII-deficient mice was subjected to subpatellar puncture. Tissue changes were recorded over a time period of 10 weeks, and compared to baseline and to changes in the left, uninjured knee. Time points for the uninjured knee were combined since no changes occurred. A) Synovial and B) strornal changes were studied by histopathology using semi-quantitative scoring according to Valentino and Krenn after safranin-0-Green staining. Tissue proliferation was graded from 0 to 3, where 0 represents no, and 3 the highest grade of proliferation. *Erythrocytes in the joint space or ^Hemosiderin detected by histology. C) Cell proliferation was determined by immunohistochemistry staining for PCNA and expressed as percentage of PCNA+ cells among total cell number. D) Representative PCNA stains of the right and left knee on day 4. Horizontal lines represent the mean, and error bars represent standard error of the mean. Mann Whitney test (A/B) or Kruskal Wallis with Dunns multiple comparison test (C) was to determine statistical significance; * denotes statistical significance (p≤0.002). d, Day. w, Weeks. NS, Not Significant. PCNA, Proliferating Cell Nuclear Antigen.
To determine to what extent synovial and stromal hyperplasia were due to resident cell proliferation versus cell recruitment after injury, immunostaining for PCNA, a marker of cell proliferation was performed. Percentages of PCNA+ cells among total cells (proliferation index) were determined in ‘hot spot’ areas of hyperplastic tissue in the injured knee during a 4 week time course (n=4–6 mice per group; time points (d)ay 4, d7, (w)eek 2 and w4), and the results were compared to the uninjured control knee (Figure 1C). The cell proliferation index in the uninjured control knees was low and unchanged over time (mean 0.6 %; n=15; SEM 0.2); therefore, all values were combined and defined as “baseline”. In the injured knees, the cell proliferation index was highest at d4 (mean 11.5%; p< 0.0001 compared to baseline), declined markedly thereafter (~ mean= 4.2% during d7- w4; p-values of d7- w4 vs d4 = 0.03–0.08), but remained significantly higher compared to baseline 4 weeks post injury (p=0.004; baseline vs w4) (Figure 1C/D). These findings demonstrate continued low-grade proliferation 4 weeks post injury, consistent with the mild but persistent stromal changes.
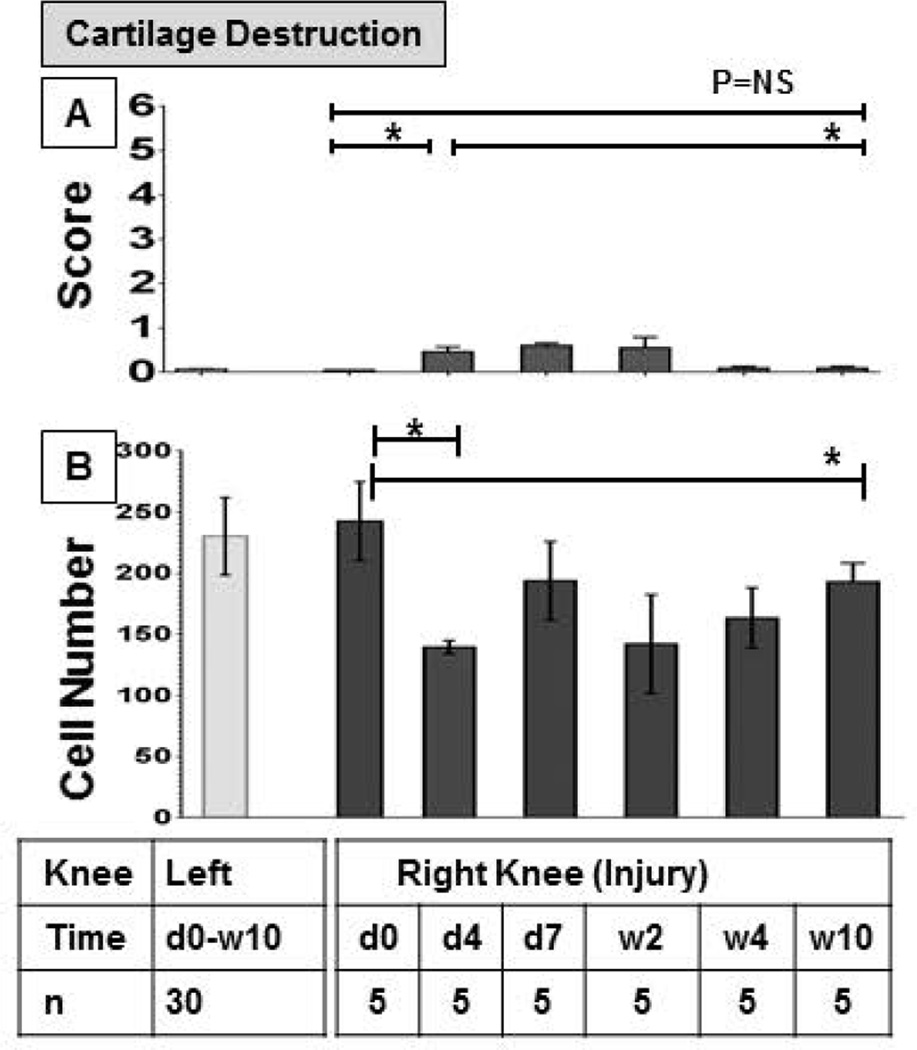
Cartilage changes occurred in parallel with soft tissue changes and were mild. Changes consisted mostly of glycosaminoglycan (GAG) loss with only few mice demonstrating higher degrees of cartilage injury such as fibrillation or superficial erosions (Figure 2A). Compared to baseline, mean scores were higher (mean score = 0.5 of 6) 4 days, 7 days and 2 weeks post- injury (p=0.007) and reverted to baseline thereafter. To appreciate more subtle changes in cellularity, we also determined chondrocyte counts over the 10 week period (Figure 2B). Mean chondrocyte counts in the punctured knee had decreased by almost 50% from 242 to 139 at day 4 (p=0.0004). Recovery was only partial. At 10 weeks the mean chondrocyte count was still significantly lower than at baseline (193; p=0.008). Mean chondrocyte counts in the control knee were comparable to baseline values of the injured knee, and remained unchanged over the entire time (231; SEM 5.5; n=33) (Figure 2B).
Figure 2. Time course of cartilage changes following induced hemarthrosis in FVIII-deficient mice.
The right knee of FVIII-deficient mice was subjected to subpatellar puncture. A) Cartilage damage was studied by histopathology using semi-quantitative Glasson scoring after Safranin-0-Green staining and graded on a scale from 0 to 6(0 = no abnormalities; 6= findings of most destruction). B) Chondrocytes were quantified manually under the microscope at 20-fold magnification; all cells within the inner 50% of the tibial plateau were counted after Safranin-0-Green staining. Changes in the right knee were studied over 10 week period and compared to baseline and changes in the left knee. Time points for the left knee were combined since no changes occurred. Errors bars represent standard error of the mean. Statistical analysis was performed with Mann Whitney (A) or Kruskal Wallis with Dunns multiple comparison tests (B); * denotes statiscal significance (p≤0.05). d, Day. w, Weeks. NS, Not significant.
Erythrocytes were present only for 2 weeks post-injury. Hemosiderin became detectable in soft tissue surrounding all injured joints at 2 weeks post-injury, and some hemosiderin deposits were still detectable at 10 weeks (Figure 1A/B).
Weight-bearing capacity of the injured leg was studied at baseline and 2 days and 10 weeks post-injury (n=5–7 mice per group). At baseline, weight was equally distributed between right and left hind legs. Severe impairment of weight-bearing of the injured leg was noted 2 days after injury. Weight-bearing of the uninjured leg exceeded weight-bearing of the injured right leg by 3.3-fold (p<0.0001). In keeping with reversible soft tissue changes, weight-bearing returned to (near) baseline at week 10 (1.6-fold increase of weight-bearing on the injured vs the uninjured hind leg; p=0.08 compared to baseline) (Supplemental Figure 7).
Time course of vascularity changes and VEGF expression following induced hemarthrosis
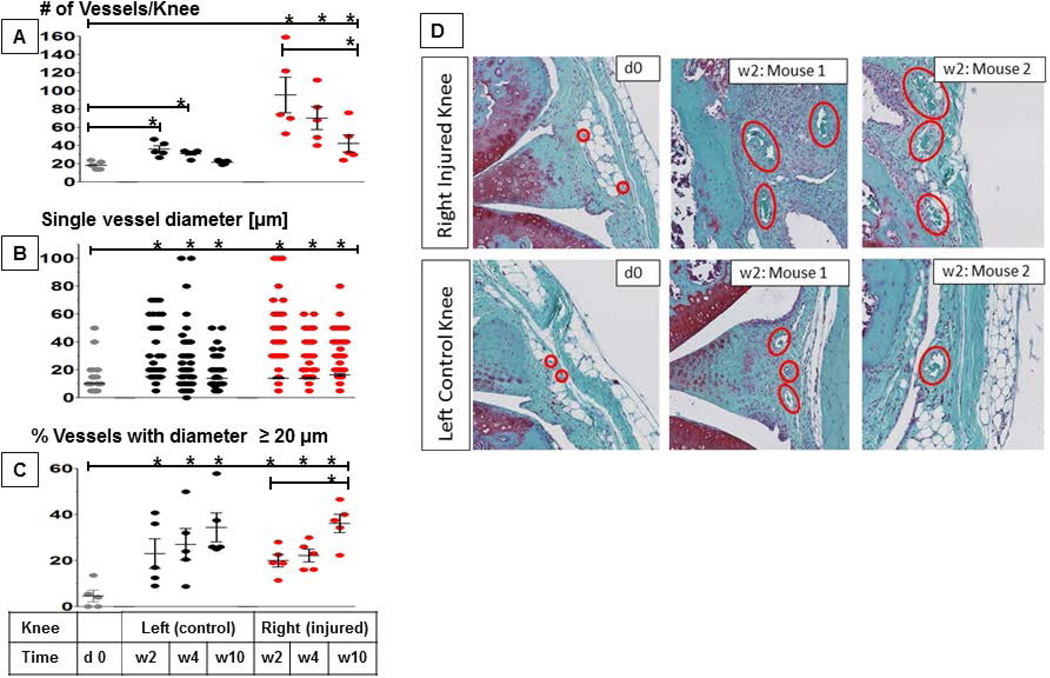
To study vascular changes associated with hemarthrosis in FVIII-deficient mice, the number and size of vessels were determined at baseline and in both knees at 2, 4 and 10 weeks after right knee injury (Figure 3). Vascularity at d4 and d7 could not be determined due to the presence of blood which prevented distinction between intra- and extravascular localization of erythrocytes. At baseline, the mean number and diameter of vessels in knee joints were 18.4 and 10 µm, respectively (Figure 3A/B). Two weeks after injury, the mean number and diameter of vessels in the injured knee increased significantly to 95.6 and 14.1 µm (p≤0.002). Similarly, the percentage of vessels with a diameter ≥ 20 µm, which is considered the upper limit of normal vessel size in synovial tissue,[39] rose from 4.6% at baseline to 20% (p≤0.003) (Figure 3C). While the mean number of vessels gradually decreased thereafter (Figure 3A), mean vessel diameter and the percentage of vessels with a diameter ≥ 20 µm did not, and further increased to 16.5 µm and 36.1% at w10 (p<0.02 compared to baseline) (Figure 3B/C). Surprisingly, similar, albeit less dramatic, changes in vascularity were also observed in the uninjured control knee (Figure 3D).
Figure 3. Vascular changes following induced hemarthrosis in FVIII-deficient mice.
The right knees of FVIII-deficient mice were subjected to subpatellar puncture. Vascularity changes were assessed histologically following Safranin-0-Green staining at 40-fold magnification. Findings were recorded at 2, 4 and 10 weeks post-injury and compared to baseline as well as to the uninjured left knee (n=5–6 per group). Error bars represent standard of the mean. A) Total vessel count per joint in synovial and stromal tissue between fernoral and tibial growth plates. B) Individual diameters of all vessels in each joint. C) Percentage of vessels in each joint with a diameter ≥ 20 µm. D) Representative examples stained with Safranin-O, depicting the increase of vessel diameter in the right and also left knee 2 weeks after injury compared to baseline in 6 different mice. Statistical significance was determined by Kruskal Wallis test with Dunns multiple comparison test. * indicates statistical significance (p ≤ 0.05). Error bars represent the SEM.
Since VEGF is a known mediator of neoangiogenesis, and has been shown previously to be expressed in human hemophilic synovium [18], its differential gene expression by RT-PCR in synovial tissue of both injured and uninjured knees was investigated at baseline, and at 7 and 17 days after induced hemarthrosis. Compared to baseline, VEGF expression (isoform A) in the injured knee increased significantly by ~2.5 fold on day 7, and ~15-fold on day 17 (p<0.0001). Again, surprisingly, similar findings were present in the contralateral control knee, with ~10-fold increase of VEGF expression on day 17 (p<0.0001) (Supplemental Figure 8). These findings explain the unexpected neovascularization observed in the contralateral control knee and generate the hypothesis that systemic mediators may regulate local VEGF expression.
Murine and human vascular perfusion changes in response to joint bleeding
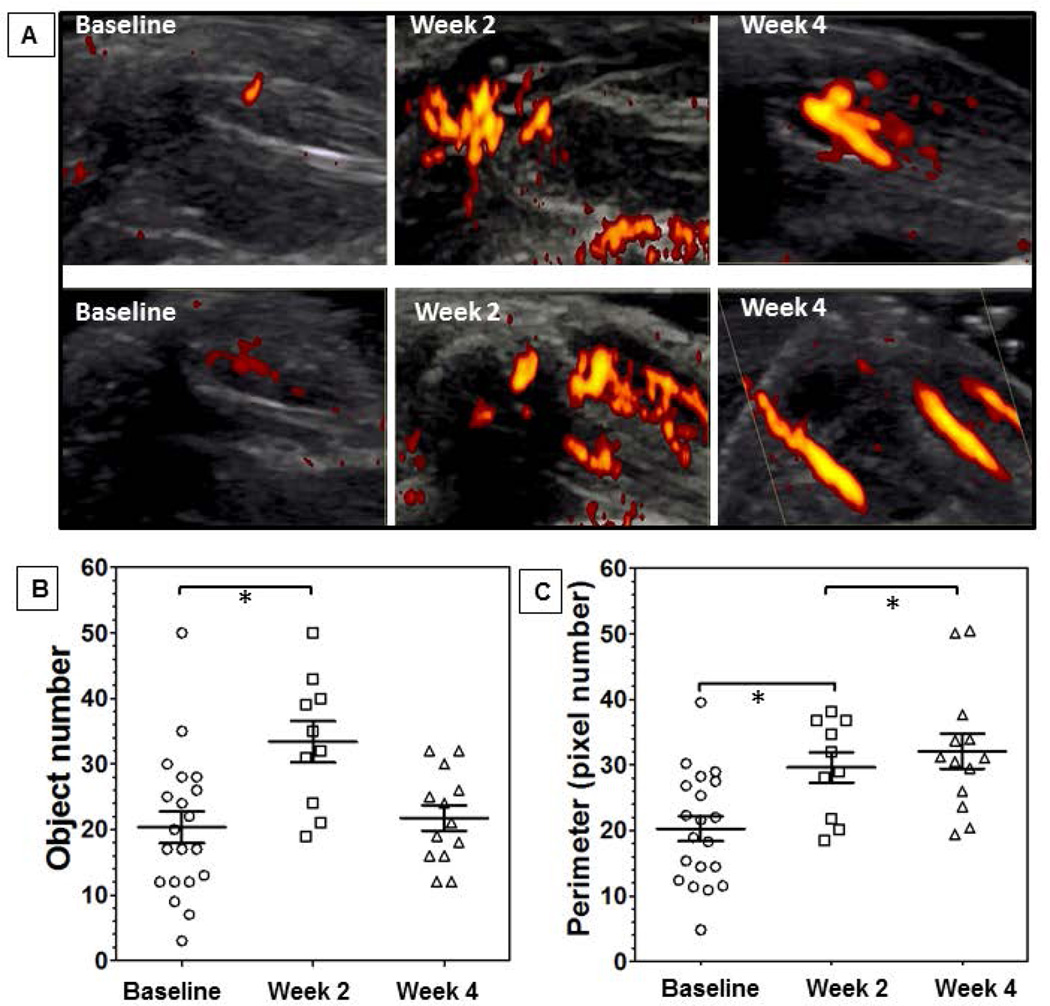
To study vascularity in response to bleeding in vivo, we examined the injured knees in FVIII-deficient mice as well as the joints of patients with hemophilia at baseline and during painful episodes using MSKUS and PD. In FVIII-deficient mice (n=10 per group), perfusion was quantified at baseline and 2 and 4 weeks post injury. Marked neoangiogenesis was present in most mice at 2 and 4 weeks. Vascular appearance changed, in that vessels at 2 weeks were smaller, more numerous and appeared sprouting, whereas at 4 weeks shapes appeared elongated and confluent (Figure 4A). Objective PD quantification revealed a significant increase in vessel number (~ 2-fold; p ≤ 0.01) and perimeter (combined vascular circumferences) (~ 1.5-fold; p ≤ 0.01) at w2 (Figure 4B/C). At week 4, the vascular perimeter remained increased while vessel enumeration had declined significantly, similar to baseline. These findings mirror the visual impression of confluence of smaller into larger vascular structures over time, are consistent with our histological observations (Figure 3), and indicate pronounced neovascularization with continued vascular remodeling in response to joint bleeding. A full set of PD images is provided in Supplemental Figure 9.
Figure 4. Vascular architecture and joint tissue perfusion changes following induced hemarthrosis in FVIII-deficient mice.
The right knees of FVIII-deficient mice were subjected to subpatellar puncture. Vascularity was studied using high resolution musculoskeletal ultrasound and power Doppler. A) Representative examples of 6 different mouse joints examined at baseline, and after 2 and 4 weeks post injury. Quantification of vascularity by B) vessel count (object number) and C) changes in vascular circumference (object perimeter) n=10 mice per group. Statistical significance was determined by Kruskal Wallis with Dunns multiple comparisons test. *denotes statistical significance ≤ 0.05. Error bars represent SEM.
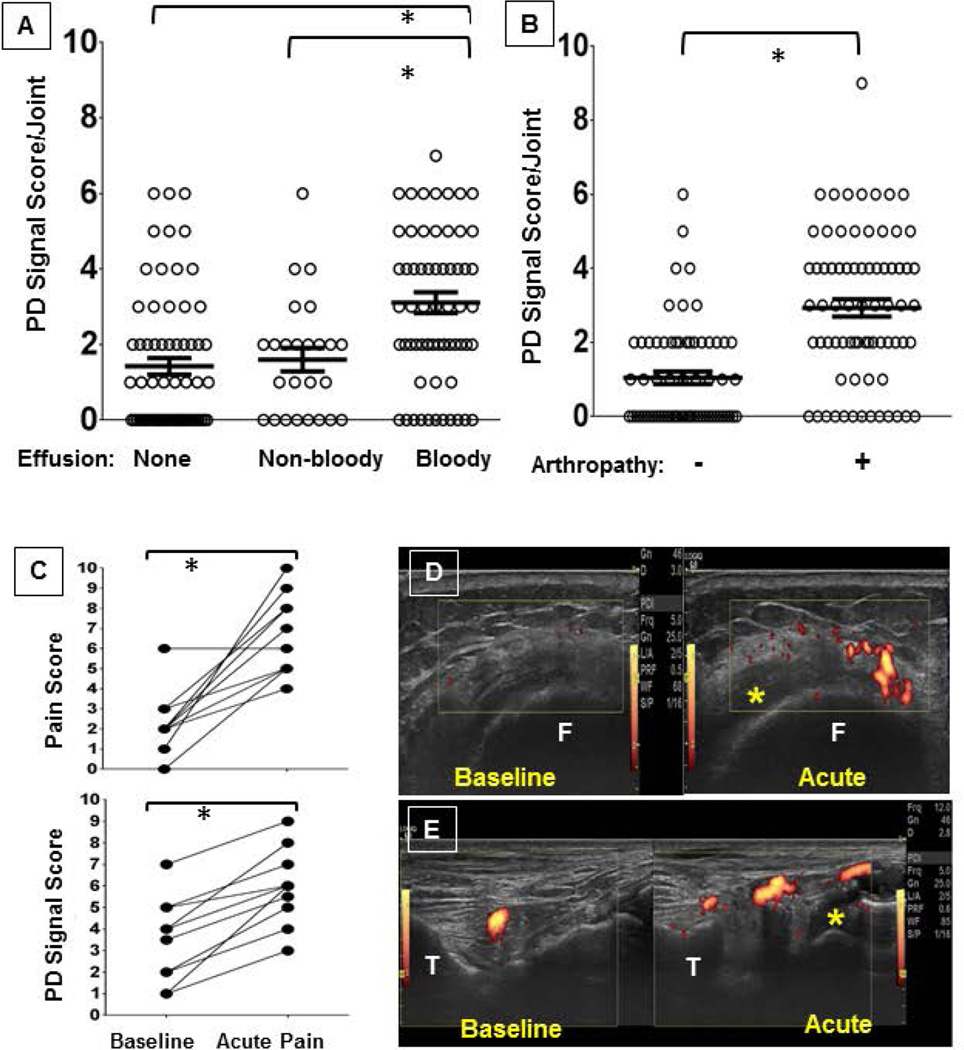
To study to what extent vascular changes, joint bleeding and degree of arthropathy are inter-related, joint bleed status [40, 41], PD signal, and radiographic and clinical joint scores were determined in 156 joints (both ankles, elbows and knees) of 26 adult patients with hemophilia (Hemophilia A, n=21; Hemophilia B, n=5) at baseline. Mean age was 41 years (SEM 3). All patients with severe, and one with moderate, hemophilia (n=21) were on long-term prophylactic clotting factor replacement therapy. Eighty three joints (53%) were arthropathic with a Pettersson score ≥ 1. The mean number of arthropathic joints per patient was 3 (SEM 0.4). Effusions were present in 58% of joints (90/156), of which 71% were bloody (64/90). Although a positive PD signal was found more frequently in joints with a bloody effusion than in joints without (83% vs. 55%; p< 0.01), almost every patient (22 of 26) had a positive PD signal in at least one joint without a bloody effusion. However, the mean PD signal score was significantly higher in those joints with bloody effusions (3.1) compared to joints with non-bloody effusions or no effusion (1.6 and 1.4, respectively; p-values < 0.01) (Figure 5A). The PD score was also significantly higher in arthropathic joints compared to non-arthropathic joints (3.0 vs. 1.0; p<0.0001) (Figure 5B), as well as in joints with an abnormal HJHS compared to normal joints (2.6 vs 1.4; p=0.001). In turn, bloody effusions were significantly more prevalent in arthropathic joints compared to non-arthropathic joints (45/83, 54% vs 19/73, 27%; p≤0.01) and, of concern, more than half of the bloody effusions in non-arthropathic joints (11/19) were found in joints that were already clinically compromised (HJHS ≥1 [maximum worst score 20]). Importantly, joint bleed status was independently associated with PD signal score (odds ratio [OR] = 1.45 [95% CI: 1.13, 1.86, p = 0.0035]) and with Pettersson score (OR = 1.21 [95%CI: 1.03, 1.43, p = 0.0185]) after adjusting for Hemophilia Joint Health Score (Supplemental Table 1).
Figure 5. joint tissue perfusion in human hemophilic joints in relation to hemarthrosis or arthropathy at baseline and during painful episodes.
Elbows, knees and ankles (n=156) of 26 hemophilia patients were examined by high resoultion musculoskeletal ultrasound for the presence or absence of poer Doppler (PD) signal ar baseline with or without A) bloody effusions, B) arthropathy, C) when experiencing pain. Statistical significance was performed with Mann Whitney tests (A/B) and Wilcoxon signed rank test (C). *p-values of ≤ 0.05 denote statistical significance. Error bars represent SEM. Representative vases depicting the D) medial recess of the right knee in transverse axis and E) right talotibiar joint in longitudinal axis. * indicates compressible bloody effusion. Images are depicted as captured during painful edisodes with direct comparison to baseline at identical scan settings; for improved sequential depiction figure D was cut and the baseline scan was moved to the left. PD, Power Doppler. F, Femur. T, Tibia.
During the first 6 months after baseline examination, 10 patients presented with acute pain in arthropathic joints. In all acutely painful joints, positive PD signals had been present at baseline, and bloody effusions pre-existed in 5. At the time of painful presentation, all 10 joints were diagnosed with bloody effusions, and 3 of 5 preexisting complex effusions had noticeably increased in volume. Mean pain and PD signal scores had increased significantly to 6.7 (SEM 0.6) and 6.0 (SEM 0.6) from 2.3 (SEM 0.5) and 3.5 (SEM 0.6) at baseline, respectively (Figure 5C). The acute vascularity changes in association with hemarthroses are shown for 2 of the cases in Figure 5D/E, capturing knee and ankle hemarthroses in a 67 year old patient with severe Hemophilia B and a 36 year old patient with severe Hemophilia A.
Mesenchymal progenitor cell and fibroblast response to hemarthrosis, osteoarthritis and rheumatoid arthritis
To determine to what extent vascular remodeling rather than acute vessel dilation was involved in the observed changes of vessel size associated with hemarthrosis, tissue sections from knees of FVIII-deficient mice were examined 2 weeks after induced hemarthrosis for their expression of α-SMA and CD105. α-SMA is the most widely used marker for myofibroblasts, which are mesenchymal-type progenitor cells displaying features of contractile smooth muscle cells, and which play a substantial role in vascular remodeling [42, 43]. Strong α-SMA expression is present in remodeled arteries in vascular disorders such as pulmonary hypertension [44]. CD105 (endoglin) is a mesenchymal progenitor cell marker involved in neoangiogenesis and in maintenance of vascular integrity in response to injury [45]. CD105 is also an early mesenchymal stem cell marker, present on cells isolated from murine and human synovium that have tissue repair and chondrogenic repair capacity [46, 47]. CD105 and α-SMA are both expressed on human and murine cells, which was important when comparing findings across murine and human samples.
To determine if signs of vascular remodeling also could be found in other arthritic conditions and were relevant in human disease, CD105 and α-SMA expression analyses were performed on synovial tissues of knees from mice with OA or RA, and on synovial tissue from patients with hemophilia, OA or RA (n=2–5 per group).
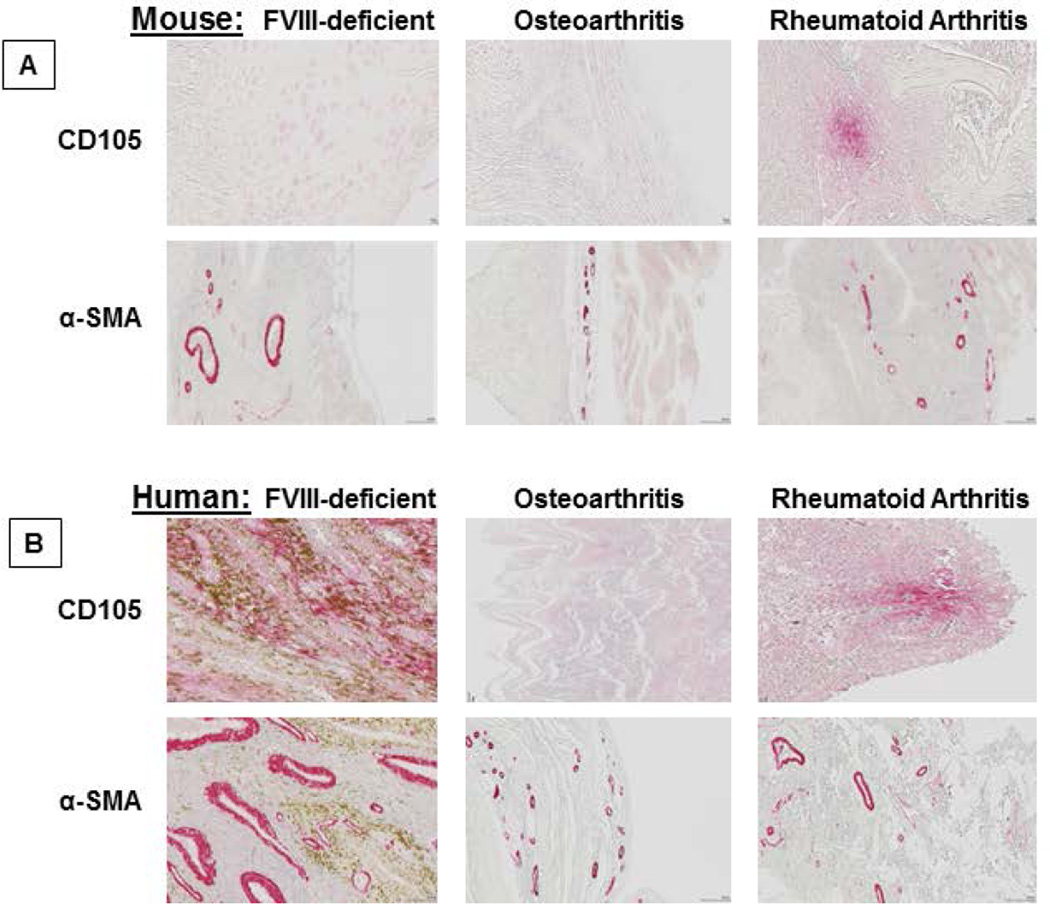
CD105 expression was more uniformly distributed in synovial tissue from FVIII-deficient mice after knee injury, rare in synovial tissue from mice with OA (few cells were present in tissue from 1 of 4 mice), but pronounced and clustered in synovial tissue from mice with RA. Representative examples are shown in Figure 6A. α-SMA expression was only found perivascularly in FVIII-deficient mice compared to mice with OA or RA, and the vessels expressing α-SMA appeared considerably larger and thicker. The marked perivascular expression of α-SMA in large distorted vessels and the evenly wide-spread tissue distribution of CD105 were unique to hemophilia and distinguished hemophilic synovium from RA and OA synovium (Figure 6A). Findings in the mouse were comparable to findings in human specimens (Figure 6B).
Figure 6. Expression of CD105 and α-SMA in murine and human specimens from patients with different types of arthritis.
Tissue sections from injured knees of FVIII-deficient of mice 2 weeks after injury, from knees of mice with osteoarthrities and rheumatoid arthrities, as well as synovial specimens from patients with the same conditions were studied for expression of CD105 and α-SMA. Representative examples are shown for A) murine and B) human specimens. Magnification was 40-fold and 20-fold for CD105 and α-SMA. α–SMA, α-Smooth Muscle Acin.
Discussion
While there is good evidence that frequent joint bleeding induces synovial hypertrophy, cartilage damage and bony changes, creating the syndrome of hemophilic arthropathy [13, 48], the causes for progression of the arthropathy, often despite prophylactic clotting factor replacement, are unclear. Here we provide evidence for uncontrolled vascular remodeling in response to bleeding in hemophilia. These results support the hypothesis that aberrant vascular remodeling in hemophilia facilitates re-bleeding or perpetuated bleeding, and may be a critical mechanism predisposing joints to develop hemophilic arthropathy. Induced joint bleeding in the FVIII-deficient mouse resulted in rapid, pronounced synovial and stromal hyperplasia with functional hind leg incapacitation, all of which were reversible 4–10 weeks after the injury. Transient hyperplasia was predominantly due to peripheral cell recruitment rather than resident cell proliferation since the proliferation index by PCNA expression was relatively low. Cartilage changes were very mild, mostly consisting of reversible GAG loss and slow recovery of cellularity, consistent with findings in other animal models [49, 50]. This suggests that a single joint bleed induced by needle puncture in the BalbC FVIII-deficient mouse predisposes to, but does not cause, severe permanent damage of soft tissues and cartilage unless repair mechanisms are challenged by repetitive bleeding [20, 51, 52]. It was surprising that vascular changes were not reversible and, moreover, occurred in the contralateral uninjured knee joint, including local VEGF expression. This indicates that joint bleeding may provoke systemic angiogenic stimuli resulting in hypervascularity in otherwise unaffected joints. While effects caused by persistent anemia are unlikely since repeated large volume blood draws in mice do not result in persistent anemia [53], we cannot exclude that acute blood loss or anemia may have altered gene expression patterns and elicited neoangiogenesis. However, our findings are consistent with observations that systemic VEGF levels are elevated in patients with hemophilia [18], RA [54, 55], and even OA [56] and that incubation of endothelial cells with serum from hemophilic patients causes vascular sprouting that is suppressed by VEGF-inhibitors [18]. Since the architecture of hemophilic vessels, encompassing large, elongated, thickened, and tortuous shapes that appeared confluent was different from the numerous small and rare vessels in RA and OA, additional mediators or angiogenesis pathways unique to hemophilia, such as local or systemic inflammation, may contribute to the process of vascular remodeling [16, 52, 57].
FVIII-deficient mice do not suffer from spontaneous joint bleeding, which limits the applicability of this model to provide insights into mechanisms of re-bleeding. We therefore explored directly the extent to which abnormal vascularity may underlie perpetuated joint bleeding and deterioration in patients with hemophilia. Subclinical joint bleeding at patients’ baseline was found in approximately 1/3rd of joints, which was comparable to previous observations [11, 40]. Excess and pronounced vascularity was more frequently present in joints with subclinical bleeding than in non-bleeding joints, whereby the odds for subclinical bleeding increased 1.45- and 1.21-fold for each point increase in PD and Pettersson score. PD signals were large, confluent and pulsatile, and have been described before as unique to hemophilic joints [40]. PD signals are established as a sensitive tool to determine abnormal microvascular flow in joints [58, 59] and are rarely detected in normal joints or OA [60, 61]. The signals are detected in active RA [55, 60], albeit weaker and more spot like [40]. These findings suggest that joint bleeding, aberrant angiogenesis and, ultimately, joint deterioration are tightly intertwined, consistent with the hypothesis that abnormal vascular structure and remodeling create a propensity for re-bleeding and contribute to joint deterioration. Moreover, detection of vascular signals in some radiographically intact but bleeding joints suggested that abnormal angiogenesis can develop in healthy joints and may precede bleeding.
More evidence that vascular changes may precede and facilitate subsequent joint bleeding came from the examination of acutely painful joints. Acute pain and more or new bleeding only occurred in joints where PD signals were present at baseline, and PD scores were significantly increased at the time of bleeding. These observations strengthen the hypothesis that dynamic vascular remodeling in hemophilia may precipitate subsequent joint bleeding despite clotting factor replacement. A prospective, long-term follow up in children would be needed to determine the exact sequence and timing of joint bleeding, mediators of angiogenesis and subsequent vascular changes.
To ascertain that the dynamic nature of vascular changes was due to remodeling rather than vasodilation, we studied the expression of α-SMA- and CD105 in synovial tissue of mice with FVIII-deficiency, OA or RA, and in humans with the same conditions. We found strong expression of α-SMA in murine and human hemophilic synovial samples in the perivascular regions, delineating remarkably large, irregularly shaped vessels with thickened vessel walls. While expression of α-SMA was also present in murine and human synovial samples of OA and RA, it was much less pronounced, delineating fewer, smaller and less irregular vessels with uniformly thin walls. These findings were in agreement with the in vivo observations of distorted vascular architecture obtained by PD signals, and provide strong evidence to support distinctive vascular remodeling and angiogenic response in hemophilia compared to the other disorders.
CD105 was expressed by murine and human synovia in hemophilia, RA and OA and, as with α-SMA patterns, differed with the clinical condition. Expression was uniform in hemophilia, patchy in RA, with only rare positive cells identified in OA, suggesting different contributions of CD105+ mesenchymal progenitor cells to tissue repair in the different conditions. However, since CD105+ cells were not found localized to the perivascular areas, as were the α-SMA+ cells, their direct involvement in angiogenesis is less obvious. CD105 expression in hemophilic tissue was denser and stronger in human compared to murine tissue, possibly, because human tissue was challenged by multiple bleeding events, whereas only one bleed was induced in the mouse. It was previously shown that synovium from normal subjects is relatively deplete of CD105+ cells, suggesting that their presence contributes to tissue regeneration in hemophilia. Mesenchymal stem cells are used therapeutically to mitigate the course of inflammatory conditions [62, 63], and are being explored for treatment of OA and RA [64, 65]. Future studies should reveal their potential usefulness in hemophilic joint disease [66].
In summary, our observations in the hemophilic mouse and humans with hemophilia are consistent with the hypothesis that abnormal blood vessel formation and dynamic vascular remodeling perpetuate or facilitate bleeding in hemophilic joints that develop neoangiogenesis in response to local and/or systemic stimuli. Since subclinical bleeding was associated with vascular changes and more advanced arthropathy, whereby dynamic vascular fluctuations and more bleeding were often observed during painful events, vascular remodeling appears to be an important link to perpetuated joint bleeding and progression of disease. While perpetuation of bleeding and vascular remodeling appears to create a vicious cycle, the causal interrelations of bleeding and vascular changes are complicated and will require further study. Even aggressive prophylactic treatment practices may not be fully sufficient to prevent bleeding, angiogenesis or both once arthropathy is present. This opens new avenues to study molecular targets for angiogenesis inhibition to prevent aberrant vessel formation, potentially increasing the effectiveness of clotting factor replacement therapy.
Supplementary Material
Acknowledgements
Factor VIII-deficient mice (BALB/c background) were a generous gift of David Lillicrap, MD, (Queens University, Ontario, CNA). We thank John Larios, MPT, for the determination of HJHSs. We thank Martin Lotz, MD, for providing technical support to perform joint histology and immunohistochemistry, usage of equipment, and for synovial tissue from mice and humans with OA or RA. We thank John H. Griffin, PhD, for providing lab space, equipment and animal housing.
Funding Sources
This work was funded by grant support from Biogen for “prevalence and etiology of subclinical joint bleeding and ’joint microbleeding’ in adults with hemophilic arthropathy” (A.v.D), an Early Career Development Award from Bayer Hemophilia (A.v.D. and V.B.), a Novo Nordisk Career Development Award from the National Hemophilia Foundation (A.v.D.), by National Institutes of Health grants HL091385, CA94233, CA192658 (D.L.D.) and HL104165 (L.O.M.).
Disclosures
A.v.D. has received honoraria for participating in scientific advisory board panels, consulting and speaking engagements for Baxter Biosciences, Pfizer, Biogen, CSL-Behring, Novo Nordisk and Grifols.
Footnotes
Contributions
A.v.D. provided study concept and designed the studies, performed and interpreted joint ultrasounds, performed mouse knee injuries, contributed to interpretation of the histological results, interpreted clinical data, provided oversight and drafted the manuscript. V.B. processed joint tissues, performed immunohistochemistry, contributed to gene expression studies and interpretation of joint histology, and contributed to drafting the manuscript. M.O. processed joint tissues, performed immunohistochemistry and contributed to interpretation of joint histology. S.J. and D.L.D. performed and interpreted gene expression analyses. R.B. performed statistical analyses. T.C. coordinated the clinical study and collected clinical data. S.B., J.V.L. and M.S. provided surgical specimen. T.H. interpreted radiographs and contributed to interpretation of ultrasounds. R.M. contributed to interpretation of ultrasounds. L.O.M. contributed to study concept, data review, mouse work and manuscript drafting.
References
- 1.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–1456. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 2.Soucie JM, Cianfrini C, Janco RL, et al. Joint range-of-motion limitations among young males with hemophilia: prevalence and risk factors. Blood. 2004;103:2467–2473. doi: 10.1182/blood-2003-05-1457. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Siddiqi AE, Soucie JM, et al. The effect of secondary prophylaxis versus episodic treatment on the range of motion of target joints in patients with haemophilia. British journal of haematology. 2013;161:424–433. doi: 10.1111/bjh.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994;236:391–399. doi: 10.1111/j.1365-2796.1994.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg J, Zimmermann R, Katsarou O, et al. Controlled, cross-sectional MRI evaluation of joint status in severe haemophilia A patients treated with prophylaxis vs. on demand. Haemophilia. 2015;21:171–179. doi: 10.1111/hae.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer K, Steen Carlsson K, Petrini P, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129–1136. doi: 10.1182/blood-2012-12-470898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, van Hout BA, van der Bom JG, et al. Association between joint bleeds and Pettersson scores in severe haemophilia. Acta Radiol. 2002;43:528–532. [PubMed] [Google Scholar]
- 8.Jackson SC, Yang M, Minuk L, et al. Prophylaxis in older Canadian adults with hemophilia A: lessons and more questions. BMC Hematol. 2015;15:4. doi: 10.1186/s12878-015-0022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aznar JA, Lucía F, Abad-Franch L, et al. Haemophilia in Spain. Haemophilia. 2009;15:665–675. doi: 10.1111/j.1365-2516.2009.02001.x. [DOI] [PubMed] [Google Scholar]
- 10.Schramm W, Gringeri A, Ljung R, et al. Haemophilia care in Europe: the ESCHQoL study. Haemophilia. 2012;18:729–737. doi: 10.1111/j.1365-2516.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 11.Kidder W, Nguyen S, Larios J, et al. Point-of-care musculoskeletal ultrasound is critical for the diagnosis of hemarthroses, inflammation and soft tissue abnormalities in adult patients with painful haemophilic arthropathy. Haemophilia. 2015 doi: 10.1111/hae.12637. [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 13.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. Journal of thrombosis and haemostasis : JTH. 2010;8:1895–1902. doi: 10.1111/j.1538-7836.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 14.Wen FQ, Jabbar AA, Chen YX, et al. c-myc proto-oncogene expression in hemophilic synovitis: in vitro studies of the effects of iron and ceramide. Blood. 2002;100:912–916. doi: 10.1182/blood-2002-02-0390. [DOI] [PubMed] [Google Scholar]
- 15.Hakobyan N, Kazarian T, Jabbar AA, et al. Pathobiology of hemophilic synovitis I: overexpression of mdm2 oncogene. Blood. 2004;104:2060–2064. doi: 10.1182/blood-2003-12-4231. [DOI] [PubMed] [Google Scholar]
- 16.Roosendaal G, Vianen ME, Wenting MJ, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br. 1998;80:540–545. doi: 10.1302/0301-620x.80b3.7807. [DOI] [PubMed] [Google Scholar]
- 17.Sen D, Chapla A, Walter N, et al. Nuclear factor (NF)-kappaB and its associated pathways are major molecular regulators of blood-induced joint damage in a murine model of hemophilia. Journal of thrombosis and haemostasis : JTH. 2013;11:293–306. doi: 10.1111/jth.12101. [DOI] [PubMed] [Google Scholar]
- 18.Acharya SS, Kaplan RN, Macdonald D, et al. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 2011;117:2484–2493. doi: 10.1182/blood-2010-05-284653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 20.Hakobyan N, Enockson C, Cole AA, et al. Experimental haemophilic arthropathy in a mouse model of a massive haemarthrosis: gross, radiological and histological changes. Haemophilia. 2008;14:804–809. doi: 10.1111/j.1365-2516.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 21.von Drygalski A, Furlan-Freguia C, Ruf W, et al. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:769–776. doi: 10.1161/ATVBAHA.112.301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevaart L, Vervoordeldonk MJ, Tak PP. Collagen-induced arthritis in mice. Methods Mol Biol. 2010;602:181–192. doi: 10.1007/978-1-60761-058-8_11. [DOI] [PubMed] [Google Scholar]
- 23.Caramés B, Taniguchi N, Otsuki S, et al. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutten K, Schiene K, Robens A, et al. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18:204–212. doi: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 25.Valentino LA, Hakobyan N. Histological changes in murine haemophilic synovitis: a quantitative grading system to assess blood-induced synovitis. Haemophilia. 2006;12:654–662. doi: 10.1111/j.1365-2516.2006.01348.x. [DOI] [PubMed] [Google Scholar]
- 26.Krenn V, Morawietz L, Häupl T, et al. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 27.Glasson SS, Chambers MG, Van Den Berg WB, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Thareja S, Zager JS, Sadhwani D, et al. Analysis of tumor mitotic rate in thin metastatic melanomas compared with thin melanomas without metastasis using both the hematoxylin and eosin and anti-phosphohistone 3 IHC stain. The American Journal of dermatopathology. 2014;36:64–67. doi: 10.1097/DAD.0b013e31829433b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Clavel G, Marchiol-Fournigault C, Renault G, et al. Ultrasound and Doppler micro-imaging in a model of rheumatoid arthritis in mice. Ann Rheum Dis. 2008;67:1765–1772. doi: 10.1136/ard.2007.083915. [DOI] [PubMed] [Google Scholar]
- 31.Martinoli C, Della Casa Alberighi O, Di Minno G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109:1170–1179. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]
- 32.Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–649. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 34.Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980:153–159. [PubMed] [Google Scholar]
- 35.Feldman BM, Funk S, Lundin B, et al. Musculoskeletal measurement tools from the International Prophylaxis Study Group (IPSG) Haemophilia. 2008;14(Suppl 3):162–169. doi: 10.1111/j.1365-2516.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 36.Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis and rheumatism. 2009;61:1194–1201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DaL S. Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- 38.Kleinbaum D, Kupper L, Nizam A, et al. Applied Regression Analysis And Other Multivariable Methods. Pacific Grove, CA: Duxbury Press/Cengage Learning; 2008. [Google Scholar]
- 39.Haywood L, Walsh DA. Vasculature of the normal and arthritic synovial joint. Histol Histopathol. 2001;16:277–284. doi: 10.14670/HH-16.277. [DOI] [PubMed] [Google Scholar]
- 40.Melchiorre D, Linari S, Innocenti M, et al. Ultrasound detects joint damage and bleeding in haemophilic arthropathy: a proposal of a score. Haemophilia. 2011;17:112–117. doi: 10.1111/j.1365-2516.2010.02380.x. [DOI] [PubMed] [Google Scholar]
- 41.Ceponis A, Wong-Sefidan I, Glass CS, et al. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia. 2013;19:790–798. doi: 10.1111/hae.12175. [DOI] [PubMed] [Google Scholar]
- 42.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 43.Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arciniegas E, Frid MG, Douglas IS, et al. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 45.Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 46.Arufe MC, De la Fuente A, Fuentes-Boquete I, et al. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145–155. doi: 10.1002/jcb.22238. [DOI] [PubMed] [Google Scholar]
- 47.Hermida-Gómez T, Fuentes-Boquete I, Gimeno-Longas MJ, et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol. 2011;38:339–349. doi: 10.3899/jrheum.100614. [DOI] [PubMed] [Google Scholar]
- 48.Lafeber FP, Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia. 2008;14(Suppl 4):3–9. doi: 10.1111/j.1365-2516.2008.01732.x. [DOI] [PubMed] [Google Scholar]
- 49.Hooiveld M, Roosendaal G, Vianen M, et al. Blood-induced joint damage: longterm effects in vitro and in vivo. J Rheumatol. 2003;30:339–344. [PubMed] [Google Scholar]
- 50.Hooiveld M, Roosendaal G, Wenting M, et al. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol. 2003;162:943–951. doi: 10.1016/S0002-9440(10)63889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakobyan N, Kazarian T, Valentino LA. Synovitis in a murine model of human factor VIII deficiency. Haemophilia. 2005;11:227–232. doi: 10.1111/j.1365-2516.2005.01080.x. [DOI] [PubMed] [Google Scholar]
- 52.Narkbunnam N, Sun J, Hu G, et al. IL-6 receptor antagonist as adjunctive therapy with clotting factor replacement to protect against bleeding-induced arthropathy in hemophilia. Journal of thrombosis and haemostasis : JTH. 2013;11:881–893. doi: 10.1111/jth.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raabe BM, Artwohl JE, Purcell JE, et al. Effects of weekly blood collection in C57BL/6 mice. J Am Assoc Lab Anim Sci. 2011;50:680–685. [PMC free article] [PubMed] [Google Scholar]
- 54.Harada M, Mitsuyama K, Yoshida H, et al. Vascular endothelial growth factor in patients with rheumatoid arthritis. Scandinavian journal of rheumatology. 1998;27:377–380. doi: 10.1080/03009749850154429. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez J, Ruiz-Esquide V, Pomes I, et al. Patients with rheumatoid arthritis in clinical remission and ultrasound-defined active synovitis exhibit higher disease activity and increased serum levels of angiogenic biomarkers. Arthritis research & therapy. 2014;16:R5. doi: 10.1186/ar4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballara S, Taylor PC, Reusch P, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis and rheumatism. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 57.Ovlisen K, Kristensen AT, Jensen AL, et al. IL-1 beta, IL-6, KC and MCP-1 are elevated in synovial fluid from haemophilic mice with experimentally induced haemarthrosis. Haemophilia. 2009;15:802–810. doi: 10.1111/j.1365-2516.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- 58.Walther M, Harms H, Krenn V, et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis and rheumatism. 2001;44:331–338. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 59.Walther M, Harms H, Krenn V, et al. Synovial tissue of the hip at power Doppler US: correlation between vascularity and power Doppler US signal. Radiology. 2002;225:225–231. doi: 10.1148/radiol.2251011272. [DOI] [PubMed] [Google Scholar]
- 60.Hall M, Doherty S, Courtney P, et al. Synovial pathology detected on ultrasound correlates with the severity of radiographic knee osteoarthritis more than with symptoms. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22:1627–1633. doi: 10.1016/j.joca.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitchen J, Kane D. Greyscale and power Doppler ultrasonographic evaluation of normal synovial joints: correlation with pro- and anti-inflammatory cytokines and angiogenic factors. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu354. [DOI] [PubMed] [Google Scholar]
- 62.Figueroa FE, Carrion F, Villanueva S, et al. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biological research. 2012;45:269–277. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 63.Griffin MD, Elliman SJ, Cahill E, et al. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells. 2013;31:2033–2041. doi: 10.1002/stem.1452. [DOI] [PubMed] [Google Scholar]
- 64.Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nature reviews Rheumatology. 2013;9:584–594. doi: 10.1038/nrrheum.2013.109. [DOI] [PubMed] [Google Scholar]
- 65.Zheng ZH, Li XY, Ding J, et al. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology (Oxford, England) 2008;47:22–30. doi: 10.1093/rheumatology/kem284. [DOI] [PubMed] [Google Scholar]
- 66.Ebihara Y, Takedani H, Ishige I, et al. Feasibility of autologous bone marrow mesenchymal stem cells cultured with autologous serum for treatment of haemophilic arthropathy. Haemophilia. 2013;19:e87–e89. doi: 10.1111/hae.12056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.