Abstract
Background
The purpose of this study was to evaluate predictors of early distant brain failure (DBF) and salvage whole brain radiotherapy (WBRT) after treatment with stereotactic radiosurgery (SRS) for brain metastases and create a clinically relevant risk score in order to stratify patients’ risk of these events.
Methods
We reviewed records of 270 patients with brain metastases treated with SRS between 2003-2012. Pre-treatment patient and tumor characteristics were analyzed by univariate and multivariable analyses. Cumulative incidence (CI) of first DBF and salvage WBRT were calculated. Significant factors were used to create a score for stratifying early (6-month) DBF risk.
Results
No prior WBRT, total lesion volume <1.3 cm3, primary breast cancer or malignant melanoma histology, and multiple metastases (≥2) were found to be significant predictors for early DBF. Each factor was ascribed one point due to similar hazard ratios. Scores of 0-1, 2, and 3-4 were considered low, intermediate, and high risk, respectively. This correlated with 6-month CI of DBF of 16.6%, 28.8%, and 54.4%, respectively (p<0.001). For patients without prior WBRT, the 6-month CI of salvage WBRT by 6-months was 2%, 17.7%, and 25.7%, respectively (p<0.001).
Conclusion
Early DBF after SRS requiring salvage WBRT remains a significant clinical problem. Patient stratification for early DBF can better inform the decision for initial treatment strategy for brain metastases. The provided risk score may help predict for early DBF and subsequent salvage WBRT if initial SRS is used. External validation is needed prior to clinical implementation.
Keywords: Brain metastases, Stereotactic Radiosurgery, Salvage Whole Brain Radiation Therapy, Distant Brain Failure, Predictive Modeling
Introduction
Brain metastases are a common cause of morbidity and mortality in the adult cancer population, occurring in up to 30% of patients.1 Traditionally, whole brain radiation therapy (WBRT) has been the predominant treatment of choice for multiple lesions and the predominant adjuvant therapy after surgical resection for a limited numbers of metastases. Use of WBRT can provide rapid attenuation of neurologic symptoms and reduce the risk of neurologic death.2 However, WBRT is associated with increased risk of significant neurocognitive side effects, such as memory loss, as early as 4 months post-treatment.3
The increasing use of stereotactic radiosurgery (SRS) either alone or combination with WBRT is supported by several randomized trials.4-6 Due to the consistent lack of survival benefit associated with the addition of WBRT, as well as its detrimental effects on quality of life and neurocognitive function3, 7, there has been a treatment paradigm shift in favor of initial treatment with SRS alone. This strategy requires close surveillance and is associated with significant increased risk of distant brain failure (DBF). While most DBF events can be effectively salvaged with additional SRS8, 9, WBRT is used as salvage in cases of multiple, large volume, or extensive distant brain failures.
The ideal candidate for initial SRS would not only have a limited number of brain metastases, low volume extracranial disease, and good performance status, but would also have a low risk of early DBF that would necessitate salvage WBRT. Patients that require short interval salvage WBRT are less likely to experience the neurocognitive benefit of SRS alone, resulting in two successive modalities of cranial radiotherapy. Alternatively, patients at low-risk for DBF have a higher likelihood of being controlled with SRS alone, and would be expected to maintain a higher quality of life by prolonging the time to WBRT.7 In order to maximize the benefits of each modality, it is necessary to improve stratification of patients based on risk of early DBF. The purpose of this study was to develop a quantitative risk stratification score to predict the risk of short interval (6-month) DBF and salvage WBRT use using pretreatment clinical patient and tumor characteristics for patients with brain metastases treated with SRS alone.
Methods
Patient Selection
Institutional review board approval was obtained for this study. We reviewed the medical records of consecutive patients treated with single fraction linear accelerator (LINAC)-based framed SRS alone between January 2003 and June 2012. Additional eligibility criteria included age at least 18 years, pathologic diagnosis of cancer, radiographic evidence of at least one brain metastasis if an intact lesion, primary SRS or post-operative SRS to the resection cavity, and at least 4 months of imaging follow-up. Patients were excluded if they had classically radiosensitive histologies (e.g. lymphoma, germ cell tumors), had previous cranial SRS, or were planned for combination WBRT and SRS therapy for the current brain lesion(s). Prior WBRT was allowed in order to determine the effect of previous WBRT on subsequent DBF risk after initial DBF. This left 270 patients with 480 individual lesions eligible for analysis.
Radiation Therapy
Details on SRS planning and delivery have been previously published.10 In brief, patients were treated with single-fraction LINAC-based framed SRS and planned using either Brainscan or iPlan treatment-planning software (Brainlab, Feldkirchen, Germany). All patients underwent a high-resolution treatment-planning MRI scan with and without contrast before CT simulation. The treatment-planning MRI scan was registered to the CT simulation using Brainlab software. The gross target volume (GTV) was defined as the entire enhancing lesion or resection cavity, including any residual enhancing disease. The GTV was then expanded from 0 to 2 mm in all directions to create a planning target volume (PTV). Typical expansions were 1 mm to PTV for intact lesions and 1 mm clinical target volume (CTV) and 1 mm PTV expansion (for a total of 2 mm) for resection cavities. Prescribed dose varied according to lesion size and was consistent with the findings from Radiation Therapy Oncology Group (RTOG) protocol 90-05.11 Targets up to 20 mm in diameter were typically dosed to 21 Gy, 21-30 mm to 18 Gy, and 31-40 mm to 15 Gy.
Follow Up and Assessment of Progression
Follow-up with MRI was at 1 month after treatment, then every 3 months thereafter, unless clinically indicated at an earlier time point. DBF was defined as the occurrence of at least one new enhancing intracranial metastatic lesion occurring outside of the 80% isodose line of the previously treated lesion(s). Cases where imaging was equivocal between local tumor progression and radiation necrosis were adjudicated using consensus from the multidisciplinary brain tumor board. When necessary, advanced techniques including MRI SPECT, MRI perfusion, brain positron emission tomography (PET), and/or surgical resection were used to differentiate radiographic changes. Patients with a limited number of distant brain failure lesions or who had received previous WBRT were first considered for salvage with local therapy (generally SRS). Salvage WBRT was used for patients with diffuse or significant number of brain metastases not amenable to local therapy. This decision was left to the discretion of the treating physician and patient.
Statistical Analysis
Primary outcomes for this analysis included (1) time to first DBF from the first SRS treatment session and (2) time to salvage WBRT from first SRS session for patients without previous WBRT. DBF within 6 months of first SRS was deemed an early event based on clinical experience prior to analysis and was the main time point of interest. Pre-treatment patient and tumor characteristics were analyzed in relation to these outcomes in order to stratify risk as low, intermediate, and high and to generate a clinically usable score for risk stratification. Secondary outcomes included overall survival (OS), which was the time to death from any cause following first SRS session.
Statistical analysis was conducted using SAS Version 9.3 and R Version 2.15.3. Competing risk analysis was conducted and cumulative incidence was estimated for DBF and WBRT. For both, death without the event of interest was considered a competing risk. For analysis of DBF, if patients did not experience DBF or death, they were censored at the time of last brain imaging or salvage WBRT. For analysis of WBRT, only patients without previous WBRT were included and patients without WBRT or death were censored at the time of last brain imaging. OS rates were estimated using the Kaplan-Meier method.
In order to determine which factors should be incorporated into a DBF risk score, a multivariable competing risk analysis was conducted using a proportional subdistribution hazards’ regression model as described by Fine and Gray.12, 13 The following variables were considered: gender, primary cancer, ECOG performance status, active systemic disease, extracranial metastatic sites, previous WBRT, number of lesions treated, age at treatment, and total volume of lesions (cm3). Continuous variables were categorized using quintiles and all categorical variables were dummy coded. Best subset selection was used to choose the final set of variables. The number of parameters to include in the final model was determined based on tenfold cross validation.14 To measure model performance, a cause-specific concordance index (c-index) for competing risk models was used, measured at the time point of interest, 6 months.15 The smallest number of parameters with a cross-validated performance estimate within one standard error of the best model was chosen.14 Once the number of parameters to include in the model was chosen, best subset selection was run on the full sample.
To create DBF risk scores, parameter estimates from the resulting model were divided by the natural log of 2. This way, each one point increase in the risk score can be interpreted as a 2-fold increase in the risk of DBF. To simplify the scoring system, points were rounded to the nearest integer since this did not make a substantial difference in the results. To assess the association of the DBF risk groups with the outcomes, both cumulative incidence functions with Gray's test and univariate proportional subdistribution hazards’ regression models were used. The bootstrap cross-validation c-index was reported at 6 months.
Results
Patient and Tumor Characteristics
Two hundred and seventy patients with a total of 480 brain metastases were eligible for analysis, with a median imaging follow-up period of 26.2 months (range 4-112 months) for patients without DBF, WBRT, or death. Non-small cell lung cancer (37%), breast cancer (23%), and malignant melanoma (21%) were the most common primary histologies. Eighty-five patients (31%) had received previous WBRT, with median dose of 35 Gy (range 24-45 Gy).
All patients received single fraction LINAC-based SRS. Most patients (59%) had a single brain metastasis, with 61 patients (22%) treated for 2, and 49 patients (18%) treated for ≥3 lesions. See Table 1 for additional patient and treatment characteristics.
Table 1.
Patient and Tumor Characteristics
| N=270 | % | ||
|---|---|---|---|
| Gender | Male | 127 | 47 |
| Female | 143 | 53 | |
| Primary Cancer | Non-small cell lung cancer | 99 | 36.7 |
| Breast cancer | 63 | 23.3 | |
| Melanoma | 56 | 20.7 | |
| Other | 52 | 19.3 | |
| ECOG PS | 0 | 137 | 50.9 |
| 1 | 109 | 40.5 | |
| 2 or 3 | 23 | 8.6 | |
| Missing | 1 | - | |
| Active Systemic Disease | No | 140 | 52 |
| Yes | 129 | 48 | |
| Missing | 1 | - | |
| Extracranial Metastatic Sites | No | 133 | 49.4 |
| Yes | 136 | 50.6 | |
| Missing | 1 | - | |
| Previous WBRT | No | 185 | 68.5 |
| Yes | 85 | 31.5 | |
| CTX During SRS (<30 Days) | No | 213 | 79.2 |
| Yes | 56 | 20.8 | |
| Missing | 1 | - | |
| Intact and Resected Metastases | Intact | 406 | 85.5 |
| Resected | 86 | 14.5 | |
| Number of Metastases Treated at First Session | 1 | 160 | 59.3 |
| 2 | 61 | 22.6 | |
| ≥3 | 49 | 18.1 | |
| Median if ≥3 (Range) | 4 (3-7) | ||
| Age (years) | 18-44 | 53 | 19.6 |
| 45-54 | 68 | 25.2 | |
| 55-59 | 48 | 17.8 | |
| ≥60 | 101 | 37.4 | |
| Median (Range) | 56 (18-87) | - | |
| Total Volume from First Session (cm^3) | 0-<0.3 | 45 | 17.6 |
| 0.3-<1.3 | 56 | 21.9 | |
| 1.3-<3.3 | 54 | 21.1 | |
| 3.3-<7.6 | 50 | 19.5 | |
| ≥7.6 | 51 | 19.9 | |
| Mean (SD) | 4.02 (4.76) | ||
| Median (Range) | 2.21 (0.032-22.3) | ||
| Number of Metastases Treated at First Session | Mean (SD) | 1.78 (1.27) | |
| Median (Range) | 1 (1-7) | ||
PS = Performance Status, WBRT = Whole Brain Radiation Therapy, CTX = Chemotherapy, SRS = Stereotactic Radiosurgery, SD = Standard Deviation
Recurrence and salvage WBRT use
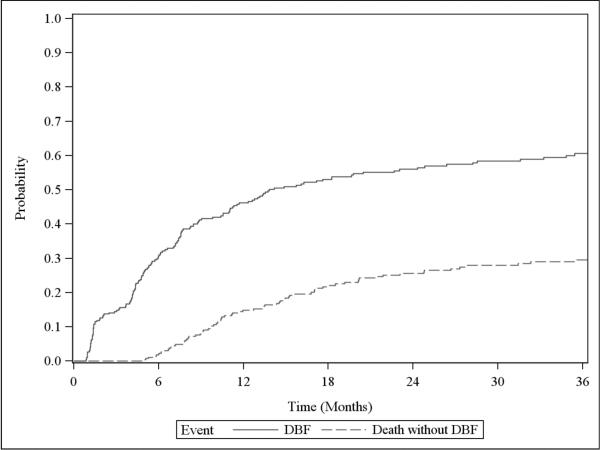
The 6 and 12-month cumulative incidence of DBF in all 270 patients was 30.6% (95% confidence interval [CI] 25.2–36.2%) and 46.2% (95% CI 40.1-52.1%), respectively (Figure 1). DBF occurred in 164 patients. Ninety-one (55%) were salvaged with SRS, 42 (26%) with WBRT, 21 (13%) received no therapy, 8 (5%) with surgery, and 2 (1%) with fractionated partial brain radiation.
Figure 1.
Cumulative incidence of distant brain failure for all patients
The 6 and 12-month cumulative incidence of salvage WBRT in the 185 patients without previous WBRT was 15.3% (95% CI 10.5–20.9%) and 24.2% (95% CI 18.2–30.6%), respectively (Supplemental Figure 1). Six and 12-month cumulative incidence of DBF in this cohort was 33.9% (95% CI 27.1-40.8%) and 48.6% (95% CI 41-55.6%), respectively.
In the multivariable analysis of prognostic factors, the following parameters were found to be significant predictors of increased risk of DBF: no previous WBRT (p=0.025), total volume of treated lesions <0.3 cm3 (p=0.007) and 0.3-1.3cm3 (p=0.034), total number of lesions treated ≥2 (p=0.012), and breast cancer (p=<0.001) or malignant melanoma (p=0.015) histology (Table 2).
Table 2.
Multivariate Competing Risk Analysis of Distant Brain Failure
| Distant Brain Failure |
|
|---|---|
| Covariate | Hazard Ratio |
| Previous WBRT: No | 1.59 (1.06-2.38) |
| Total Volume from First Session (cm^3): 0-<0.3 | 1.79 (1.17-2.75) |
| Total Volume from First Session (cm^3): 0.3-<1.3 | 1.53 (1.03-2.28) |
| Total Volume from First Session (cm^3): >1.3 | Reference |
| Primary Cancer: Breast cancer | 1.85 (1.28-2.67) |
| Primary Cancer: Melanoma | 1.77 (1.12-2.79) |
| Primary Cancer: Non-breast cancer or melanoma | Reference |
| Number of Metastases Treated at First Session: 2 | 1.68 (1.12-2.52) |
| Number of Metastases Treated at First Session: >=3 | 1.88 (1.15-3.08) |
| Number of Metastases Treated at First Session: 1 | Reference |
WBRT = Whole Brain Radiation Therapy, HR = Hazard Ratio
To calculate the DBF risk score, one point is added for each of the following risk factors: no previous WBRT, total lesion volume for initial SRS <1.3 cm3, primary breast cancer or malignant melanoma, and multiple lesions (≥2) treated with SRS. The score was calculated for the 256 patients (95%) with complete data available. The resulting scores ranged from 0-4. Both the lowest and highest scores occurred infrequently (<5.1%). Consequently, scores of 0 and 1 were grouped together into the low risk group. A score of 2 was considered intermediate risk, and 3-4 was considered high risk (Table 3). Ninety-one patients (36%) were low risk, 98 (38%) were intermediate risk, and 67 (26%) were high risk.
Table 3.
Risk Score predicting Early (6-month) Distant Brain Failure
| Points | Characteristic |
|---|---|
| 1 | No previous WBRT |
| 1 | Total volume of disease <1.3cm3 |
| 1 | Primary Breast or Melanoma |
| 1 | ≥2 brain metastases |
| Points | Risk Group | 6-month DBF | HR (95% CI) and p-value | 6-month Salvage WBRT | HR (95% CI) and p-value |
|---|---|---|---|---|---|
| ≥3 points | High Risk | 54.4% | 3.04 (2.01 – 4.61), p<0.001 | 25.7% | 4.15 (2.03 – 8.48), p<0.001 |
| 2 points | Intermediate Risk | 28.8% | 1.64 (1.11 – 2.44), p=0.013 | 17.7% | 2.28 (1.08 – 4.81), p=0.03 |
| 0-1 points | Low Risk | 16.6% | Reference | 2% | Reference |
DBF = Distant Brain Failure, WBRT = Whole Brain Radiation Therapy, HR = Hazard Ratio, CI = Confidence Interval
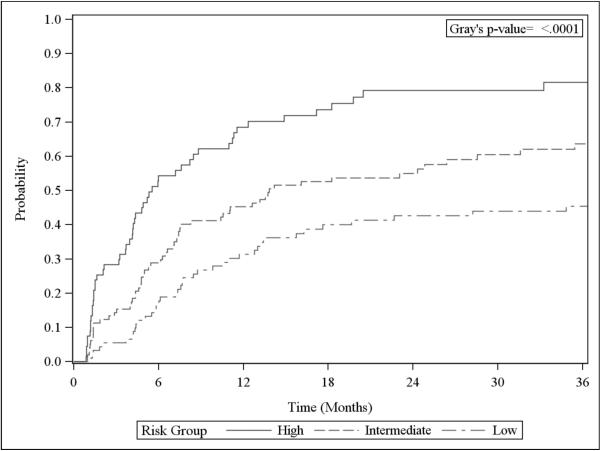
There were significant univariate associations between risk group and DBF. The 6-month cumulative incidence of DBF for the low, intermediate, and high-risk groups was 16.6% (95% CI 9.8–25.1%), 28.8% (95% CI 20.2–38.1%), and 54.4% (95% CI 41.4–65.6%), respectively (p<0.001, Figure 2). The hazard ratios (HR) for the high and intermediate risk groups versus the low risk group were 3.04 (95% CI 2.01–4.61, p<0.001) and 1.64 (95% CI 1.11–2.44, p=0.013), respectively. The c-index of this model was 0.657 (Supplemental Table 1).
Figure 2.
Cumulative incidence of distant brain failure by risk groups
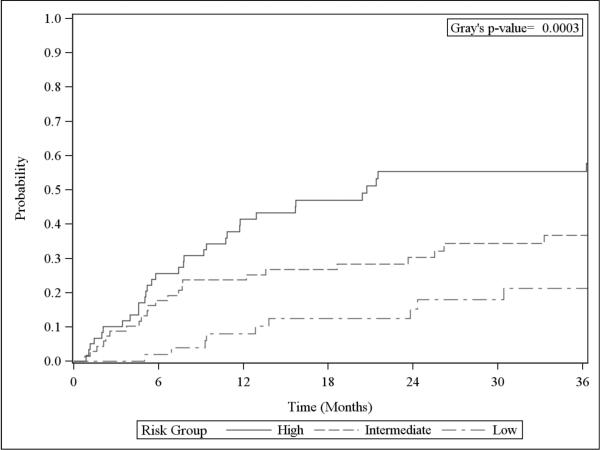
These risk groups were assessed in relation to the endpoint of salvage WBRT use in the 179 patients without previous WBRT and the necessary data available to calculate the score. Fifty-two patients (29%) were low risk, 68 (38%) were intermediate risk, and 59 (33%) were high risk. The 6-month cumulative incidence of salvage WBRT for the low, intermediate, and high-risk groups was 2% (95% CI 0.2– 9.2%), 17.7% (95% CI 9.7–27.7%), and 25.7% (95% CI 15.2–37.4%), respectively (p<0.001, Figure 3). The hazard of salvage WBRT for the high-risk group was 4 times the hazard for the low risk group (HR 4.15, 95% CI 2.03–8.48, p<0.001) and the hazard for the intermediate risk group was twice that of the low risk group (HR 2.28, 95% CI 1.08–4.81, p=0.03). The c-index of this model, measured at 6 months was 0.673 (Supplemental Table 2).
Figure 3.
Cumulative incidence of whole brain radiation therapy by risk groups (for patients without previous whole brain radiation therapy)
Survival
The median overall survival (OS) for all patients was 17.8 months (95% CI 15.6–21.1 months), with a 1-year OS rate of 66.6%. The OS rates did not differ significantly between risk groups (p=0.84, Supplemental Figure 2). Of note, DBF event and male gender were significantly associated with worse OS in multivariate analysis, while absence of active systemic disease was protective (Supplemental Table 3).
Discussion
There is significant controversy concerning the use and timing of WBRT and/or SRS for management of brain metastases. For patients with 1-4 lesions and reasonable expectation of systemic control, therapy options include surgical resection followed by RT, WBRT plus SRS for single metastasis, SRS alone, or WBRT alone. For patients with >4 lesions, WBRT is generally preferred, however additional consideration may be made for SRS in patients with good performance status and low overall tumor volume.16
To better define the standard of care, there have been several investigations into risk stratification of DBF after SRS.17-22 Sawrie et al (2008) retrospectively investigated a cohort of 100 patients with newly diagnosed brain metastases, revealing >3 metastases, presence of extracranial metastases, and melanoma histology as significant predictors of DBF.17 One-year freedom from DBF for patients without any risk factors was 83% compared with 26% for all others. Limitations of this study include the small patient numbers, lack of a reported median imaging follow-up period, substantially higher rates of DBF than other similar published reports, and the lack of a competing risk analysis for the 1-year endpoint.
Rodrigues et al (2014) published results of 361 patients treated with LINAC-based SRS for oligometastatic disease to the brain.22 They designed a recursive partitioning analysis (RPA) that stratifies patients based on risk of DBF at 1-year. Factors including age, number of lesions, maximum gross tumor volume, and performance status were significant. Our study confirmed several of their findings, including the prognostic value of number of lesions, histology, and smaller total tumor volume. While clinically useful, this study did not relate the risk of DBF with the need for salvage WBRT.
Ayala-Peakcock et al (2014) reviewed 464 patients without prior WBRT treated with Gamma Knife.21 The study designed a nomogram predicting for DBF at 6 and 9 months and examined time to salvage WBRT. Significant predictors of DBF included progressive systemic disease, number of metastases, discovery of new metastases at time of SRS planning scan, and melanoma and squamous cell lung cancer histologies. Earlier requirement of WBRT was significantly predicted by progressive systemic disease, number of brain metastases, minimum SRS dose, and widespread metastatic disease. Our study confirmed several of these predictive factors including number of metastases and high-risk primary histologies. Lesion volume was not included in their analysis. Differences between studies include our use of cumulative incidence analysis with competing risk compared to the Kaplan-Meier method, which minimizes the risk of overestimation when there is substantial hazard for the competing risk. We expanded on time to salvage WBRT by applying our scoring system towards this endpoint and demonstrated significant and clinically relevant stratification.
Absence of prior WBRT significantly increases the risk of subsequent DBF in this study. We included patients with prior WBRT in order to determine and quantify if there was a persistent effect on subsequent DBF even after an initial DBF event. Our study shows that WBRT does have a protective effect on subsequent DBF after initial DBF. We hypothesize that WBRT may effectively control the vast majority of microscopic disease in the brain, but there may be a small resistant population that manifests as DBF without being the product of systemic reseeding of the CNS. Subsequent local therapy with SRS would then sterilize this resistant population.
It is known from multiple phase III randomized trials that the omission of WBRT after local therapy (surgery or SRS) for brain metastases leads to higher intracranial failure rates, but without detriment in OS.3, 4, 6, 23 However, in this study, DBF event was associated with worse OS in multivariate analysis. This discrepancy is likely due to the fact that our study patient population is significantly higher risk in terms of both CNS and non-CNS progression than the phase III trial study populations. A higher proportion of patients (20%) had melanoma histology, a significant proportion of patients (18%) had ≥ 3 brain metastases treated, and patients with previous WBRT were included, meaning that a distant brain failure event in that subset was actually a second brain relapse event. All of these factors serve to increase the mortality risk of DBF versus the published trials. Although most patients did receive salvage therapy for DBF, 13% did not and this may have also contributed to the increased risk of death with DBF.
Of interest, smaller cumulative volumes of metastases predicted for a greater risk of DBF. This result reinforces a similar finding by Rodrigues et al (2014), who reported smaller GTV size was related to a greater distant brain failure risk at 1-year.22 Higher risk of DBF in patients with smaller metastases may theoretically be indicative of primaries with greater propensity to seed micro-metastases. Additionally, small lesions may have the potential to be missed by initial MRI and manifest as the early development of multiple small foci.
Initial SRS with observation compared to SRS with WBRT for patients with 1-3 brain metastases has been reported to result in improved neurocognitive outcomes and acceptable cost-effectiveness in Quality Adjusted Life Years/Expectancy (QALY/QALE) without a difference in OS.24, 25 Despite a decrease in the risk of DBF with WBRT, the associated reduction in quality of life makes this risk and subsequent cost of salvage therapies justifiable for this particular population.7 While initial WBRT has been losing popularity, it is still a useful and acceptable standard of care in the setting of multiple initial brain metastases and may be considered in a patient with high-risk of early DBF and salvage WBRT.
In addition, several recent advances have been made which significantly reduce the neurocognitive detriment of traditional WBRT, including the use of neuro-protectant drugs and hippocampal avoidance techniques.26, 27 Alternatively, the inclusion of central nervous system (CNS) active systemic therapies could be emphasized if initial SRS was used in order to reduce the risk of early DBF. Several biologic and immunologic agents have shown CNS activity in patients with brain metastases, including erlotinib in lung cancer28, lapatinib and capecitibine in HER-2 positive breast cancer29, and dabrafenib and ipilimumab in malignant melanoma.30, 31 Although initial or salvage SRS of large numbers of brain metastases is technically feasible32, whether the neurocognitive preservation benefits of SRS apply when so many lesions are treated is not yet known.
Strengths of this study include its large patient population, homogenous treatment paradigm and salvage WBRT indications, long median imaging follow-up period, and use of competing risk analyses. The primary limitation of the risk score is its unproven nature. To be fully incorporated into practice, it will require further validation in independent cohorts. Other limitations of this study include its retrospective nature, single institution experience, extended treatment period, and lack of neurocognitive and quality of life metrics. The latter two factors are critical for decision making between using local and regional brain radiotherapy and are areas of active research.
Conclusion
Early DBF after SRS requiring salvage WBRT remains a significant clinical problem. Patient stratification for early DBF can better inform the decision for initial treatment strategy for brain metastases. No prior WBRT, total lesion volume treated with SRS <1.3 cm3, primary breast cancer or malignant melanoma histology, and multiple lesions (≥2) are associated with increased risk of early (6-month) DBF.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This manuscript has not been previously published and is not under consideration in the same or substantially similar form in any other peer-reviewed media.
All authors have read and approved the manuscript. To the best of our knowledge, no conflict of interest, financial or other, exists.
This data was previously presented in abstract form at the American Society of Therapeutic Radiation Oncology (ASTRO) Annual Meeting 2014, San Francisco, California.
References
- 1.DeVita VTHS, Rosenberg SA. Cancer, principles and practice of oncology. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 2.Bradley KA, Mehta MP. Management of brain metastases. Semin Oncol. 2004;31:693–701. doi: 10.1053/j.seminoncol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 4.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 6.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 7.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 8.Chao ST, Barnett GH, Vogelbaum MA, et al. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer. 2008;113:2198–2204. doi: 10.1002/cncr.23821. [DOI] [PubMed] [Google Scholar]
- 9.Prabhu RS, Dhabaan A, Hall WA, et al. Clinical outcomes for a novel 6 degrees of freedom image guided localization method for frameless radiosurgery for intracranial brain metastases. J Neurooncol. 2013;113:93–99. doi: 10.1007/s11060-013-1093-7. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu R, Shu H-K, Hadjipanayis C, et al. Current Dosing Paradigm for Stereotactic Radiosurgery Alone After Surgical Resection of Brain Metastases Needs to Be Optimized for Improved Local Control. Int J Radiat Oncol Biol Phys. 2012;83:e61–e66. doi: 10.1016/j.ijrobp.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 13.Kohl M, Heinze G. %PSHREG: A SAS Macro for Proportional and Nonproportional Subdistribution Hazards Regression for Survival Analyses with Competing Risks. Section for Clinical Biometrics: Medical University of Vienna, Center for Medical Statistics, Informatics and Intelligent Systems. 2013 [Google Scholar]
- 14.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning. Springer; New York, NY: 2001. [Google Scholar]
- 15.Wolbers M, Blanche P, Koller MT, Witteman JCM, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014 doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Network NCC. [9/14/2014];Central Nervous System Cancers Version 2.2014. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- 17.Sawrie SM, Guthrie BL, Spencer SA, et al. Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys. 2008;70:181–186. doi: 10.1016/j.ijrobp.2007.05.084. [DOI] [PubMed] [Google Scholar]
- 18.Chen XJ, Xiao JP, Li XP, et al. Risk factors of distant brain failure for patients with newly diagnosed brain metastases treated with stereotactic radiotherapy alone. Radiat Oncol. 2011;6:175. doi: 10.1186/1748-717X-6-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Likhacheva A, Pinnix CC, Parikh N, et al. Validation of Recursive Partitioning Analysis and Diagnosis-Specific Graded Prognostic Assessment in patients treated initially with radiosurgery alone. J Neurosurg. 2012;117(Suppl):38–44. doi: 10.3171/2012.3.GKS1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rades D, Dziggel L, Nagy V, et al. A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother Oncol. 2013;108:123–127. doi: 10.1016/j.radonc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Ayala-Peacock DN, Peiffer AM, Lucas JT, et al. A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro Oncol. 2014;16:1283–1288. doi: 10.1093/neuonc/nou018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues G, Warner A, Zindler J, Slotman B, Lagerwaard F. A clinical nomogram and recursive partitioning analysis to determine the risk of regional failure after radiosurgery alone for brain metastases. Radiother Oncol. 2014;111:52–58. doi: 10.1016/j.radonc.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 24.Lal LS, Byfield SD, Chang EL, et al. Cost-effectiveness analysis of a randomized study comparing radiosurgery with radiosurgery and whole brain radiation therapy in patients with 1 to 3 brain metastases. Am J Clin Oncol. 2012;35:45–50. doi: 10.1097/COC.0b013e3182005a8f. [DOI] [PubMed] [Google Scholar]
- 25.Lester-Coll NH, Dosoretz AP, Yu JB. Decision analysis of stereotactic radiation surgery versus stereotactic radiation surgery and whole-brain radiation therapy for 1 to 3 brain metastases. Int J Radiat Oncol Biol Phys. 2014;89:563–568. doi: 10.1016/j.ijrobp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai H, Han B. The effectiveness of erlotinib against brain metastases in non-small cell lung cancer patients. Am J Clin Oncol. 2013;36:110–115. doi: 10.1097/COC.0b013e3182438c91. [DOI] [PubMed] [Google Scholar]
- 29.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. The lancet oncology. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 30.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. The lancet oncology. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 31.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. The lancet oncology. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.