Abstract
Activation of the aryl hydrocarbon receptor (AhR) transcriptionally induces phase I (cytochrome P450 (CYP) 1A1) and phase II (NAD(P)H quinone oxidoreductase 1 (NQO1) detoxifying enzymes. The effects of the classical and nonclassical AhR ligands on phase I and II enzymes are well studied in human hepatocytes. Additionally, we observed that the proton pump inhibitor, omeprazole (OM), transcriptionally induces CYP1A1 in the human adenocarcinoma cell line, H441 cells via AhR. Whether OM activates AhR and induces the phase II enzyme, NAD(P)H quinone oxidoreductase 1 (NQO1), in fetal primary human pulmonary microvascular endothelial cells (HPMEC) is unknown. Therefore, we tested the hypothesis that OM will induce NQO1 in HPMEC via the AhR. The concentrations of OM used in our experiments did not result in cytotoxicity. OM activated AhR as evident by increased CYP1A1 mRNA expression. However, contrary to our hypothesis, OM increased NQO1 mRNA and protein via an AhR-independent mechanism as AhR knockdown failed to abrogate OM-mediated increase in NQO1 expression. Interestingly, OM activated Nrf2 as evident by increased phosphoNrf2 (S40) expression in OM-treated compared to vehicle-treated cells. Furthermore, Nrf2 knockdown abrogated OM-mediated increase in NQO1 expression. In conclusion, we provide evidence that OM induces NQO1 via AhR-independent, but Nrf2-dependent mechanisms.
Keywords: Omeprazole, Aryl hydrocarbon Receptor, Fetal Human Pulmonary Microvascular Endothelial Cells, Cytochrome P450 1A1, NAD(P)H Quinone Oxidoreductase 1, nuclear factor erythroid 2–related factor 2
Introduction
The aryl hydrocarbon receptor (AhR) is a member of basic - helix – loop – helix / PER – ARNT – SIM family of transcriptional regulators [1–3]. In humans, the AhR is highly expressed in the lungs, thymus, kidney and liver [4]. The AhR is predominantly cytosolic, localized in a core complex comprising two molecules of 90-kDa heat shock protein and a single molecule of the co-chaperone hepatitis X-associated protein-2 [5, 6]. AhR activation results in a conformational change of the cytosolic AhR complex that exposes a nuclear localization sequence(s), resulting in translocation of this complex into the nucleus [7, 8]. In the nucleus, AhR dissociates from the core complex, dimerizes with the AhR nuclear translocator, and initiates transcription of many phase I and phase II enzymes such as cytochrome P450 (CYP) 1A1, glutathione S-transferase-α(GST-α), and NAD(P)H quinone reductase-1 (NQO1), by its interaction with the xenobiotic responsive elements present in the promoter region of these genes [9–12]. AhR is of particular interest to toxicologists and extensive research has been conducted on its role in the bioactivation of polycyclic and aromatic hydrocarbons leading to carcinogenesis [13]. However, the creation of knockout and transgenic mice has provided mechanistic insights into the potential role(s) that AhR might play in normal physiological homeostasis [14–17].
Several structurally diverse compounds activate AhR. The protypical or classical AhR ligands are characteristically planar, aromatic, and hydrophilic molecules that includes polycyclic aromatic hydrocarbons such as benzo [α] pyrene and halogenated aromatic hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin [18]. Additionally, several nonclassical synthetic compounds such omeprazole, lansoprazole, thiabendazole, and primaquine can activate AhR-dependent gene expression indirectly. Although these compounds are not AhR ligands by themselves, they are thought to activate AhR-dependent gene expression, either via metabolic conversion into a ligand or by their ability to affect a cellular pathway that results in AhR activation [19–23].
Omeprazole (OM), a benzimidazole derivative, is a proton pump inhibitor that inhibits gastric acid secretion both in humans [24] and in animals [25, 26]. It has been widely used in the management of gastric acid disorders in humans [24]. Studies have shown that omeprazole activates AhR in human and rat hepatocytes [27, 28]. Recently, we observed that OM increases AhR-regulated CYP1A1 expression in the human adenocarcinoma cell line, H441, [29]. Whether OM can modulate the AhR-regulated phase II enzyme, NQO1, in fetal primary human microvascular endothelial cells (HPMEC) is unknown. Thus, the goals of this study were to investigate the effects of OM on AhR-mediated expression of NQO1 in HPMEC. Specifically, we chose HPMEC for our experiments because they express AhR [30] and they are used to study mechanisms of diseases such as bronchopulmonary dysplasia (BPD) where AhR might play a significant role [31, 32]. Using these cells, we tested the hypothesis that OM will increase NQO1 expression in wild type HPMEC, but not in AhR-deficient HPMEC.
Materials and Methods
Cell culture and treatment
HPMEC, the primary microvascular endothelial cells derived from the lungs of human fetus were obtained from ScienCell research laboratories (San Diego, CA; 3000). HPMEC were grown in 95% air and 5% CO2 at 37°C in specific endothelial cell medium according to the manufacturer’s protocol. Cells were treated with either 0.01% v/v dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, MO; 276855) or 100 μM omeprazole (OM) (Sigma Aldrich St. Louis, MO; O104) for up to 48 h.
Cell viability assay
Cell viability was determined by a colorimetric assay based on the ability of viable cells to reduce the tetrazolium salt, MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), to formazan. HPMEC were seeded onto 96-well microplates and treated with DMSO or OM at varying concentrations (0.5 – 100 μM) for up to 48 h. The cell viability was then assessed by MTT reduction assays as outlined in the MTT Assay protocol (American Type Culture Collection, Manassas, VA).
Determination of Functional Activation of AhR
It is widely established that functional activation of AhR results in its translocation into the nucleus, which results in transcriptional activation of the phase I enzyme, CYP1A1. Therefore, we determined the functional activation of AhR by analyzing the expression of CYP1A1 mRNA levels.
Real-time RT-PCR assays
Cells were grown on six-well plates to 60–70% confluence, after which they were treated with DMSO or OM. At 24 or 48 h of exposure, total RNA was isolated and reverse transcribed to cDNA as mentioned before [29]. Real-time quantitative RT-PCR analysis was performed with 7900HT Real-Time PCR System using iTaq Universal SYBR Green Supermix (Biorad, Hercules, CA; 1725121). The sequences of the primer pairs were hAhR: 5′-CACCGATGGGAAATGATACTATCC-3′ and 5′-GGTGACCTCCAGCAAATGAGTT-3′; hCYP1A1: 5′-TGGATGAGAACGCCAATGTC-3′ and 5′-TGGGTTGACCCATAGCTTCT-3′; hNQO1: 5′-ACGCCC-GAATTCAAATCCT-3′ and 5′-CCTGCCTGGAAGTTTAGGTCAA-3′; hNrf2: 5′-AAA CCA GTG GAT CTG CCA AC-3′ and 5′-GAC CGG GAA TAT CAG GAA CA-3′; hβ-actin: 5′-TGACGTGGACATCCGCAAAG-3′ and 5′-CTGGAAGGTGGACAGCGAGG-3′. β-actin was used as the reference gene.
Western Blot Assays
Whole-cell protein extracts from the cells treated with DMSO or OM 100 for up to 48 h were obtained by using nuclear extraction kit (Active Motif, Carlsbad, CA; 40010) [29]. β-actin was used as a reference protein. The protein extracts were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4°C with the following primary antibodies: anti-AhR antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-5579, dilution 1:500), anti-CYP1A1 antibody (gift from P.E. Thomas, Rutgers University, Piscataway, NJ, dilution 1:500), anti-NQO1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-16464, dilution 1:500), anti-β-actin antibody (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-47778, dilution 1:1000), anti-Nrf2 (Santa Cruz Biotechnologies, Santa Cruz, CA; sc-722, dilution 1:500) and anti-phosphoNrf2 (S40) antibodies (Abcam; ab76026, Cambridge, MA; dilution 1:1000). The immuno-reactive bands were detected by chemiluminescence methods.
Small interfering RNA (siRNA) transfections
HPMEC were seeded in fibronectin-coated 6-well plates at 60–70% confluence 24 h before transfection. Transfections were then performed with either 50 or 100 nM control siRNA (Dharmacon, Lafayette, CO; d-001810) or 50 nM AhR specific siRNA (Dharmacon, Lafayette, CO; L-004990) or 100 nM Nrf2 specific siRNA (Dharmacon, Lafayette, CO; L-003755) using LipofectamineRNAiMAX (Life Technologies, Grand Island, NY; 13778030). After 24 h of transfection, the cells were treated with DMSO or OM for up to 48 h. siRNA mediated knockdown of AhR or Nrf2 was validated by determining the expression of AhR and Nrf2 mRNA and protein by real time RT PCR analysis and western blotting, respectively. Additionally, the cells were harvested at the indicated time points to determine the expression of NQO1 mRNA and protein.
Analyses of data
The results were analyzed by GraphPad Prism 5 software. At least three separate experiments were performed for each measurement, and the data are expressed as means ± SEM. The effects of OM, AhR or Nrf2 gene and their associated interactions for the outcome variables were assessed using ANOVA techniques. Multiple comparison testing by the posthoc Bonferroni test was performed if statistical significance of either variable or interaction was noted by ANOVA. A p value of <0.05 was considered significant.
Results and Discussion
In this study, we investigated the effects of OM on the expression of NQO1 enzyme in wild type (WT), AhR- and Nrf2-deficient HPMEC in vitro. The present study demonstrates that OM induces NQO1 expression in HPMEC via mechanism(s) entailing Nrf2 activation. In human fetal lung-derived WT and AhR-deficient HPMEC in vitro, OM transcriptionally induced NQO1 expression when compared to controls, whereas, in Nrf2-deficient HPMEC, the lack of OM-mediated induction of NQO1 enzyme correlated with the deficiency of a functional Nrf2 gene.
The AhR is a versatile transcription factor that has important physiological functions in addition to its widely established role in the induction of a battery of genes involved in the metabolism of xenobiotics. Studies from our laboratory and others have reported that AhR may be a crucial regulator of oxidant stress and inflammation through the induction of several detoxifying enzymes or via “cross-talk” with other signal transduction pathways. Several in vitro studies suggest that OM activates AhR in human and rat hepatocytes [27, 28, 33, 34] and the mechanistic role of AhR in the induction of CYP1A enzymes by OM in vitro has been extensively studied [29, 35, 36]. However, whether OM induces the phase II enzyme, NQO1, via AhR is unknown. Therefore, we conducted experiments with OM in primary human fetal lung-derived HPMEC in vitro, both in the presence and absence of a functional AhR, to delineate the precise role of AhR in OM-mediated induction of NQO1 enzyme.
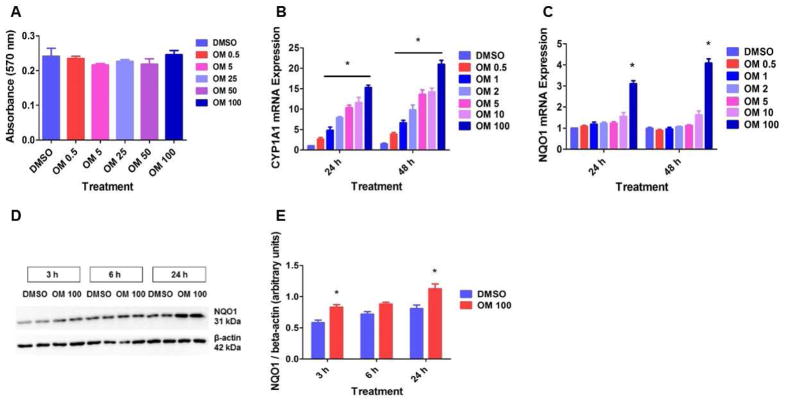
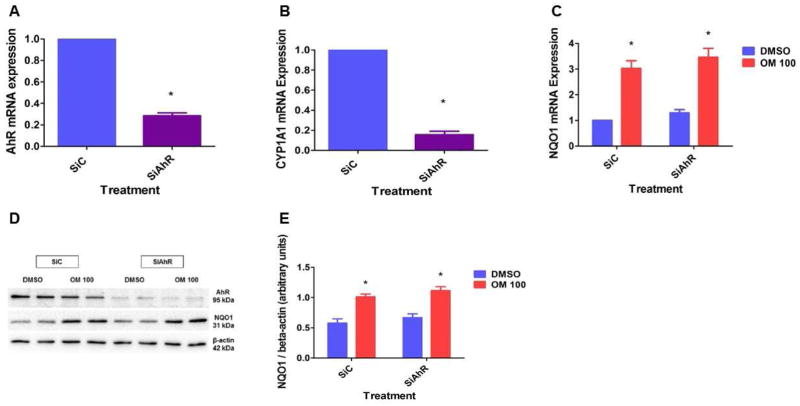
The concentration of OM used in this study was comparable to those used in previous studies [37, 38]. More importantly, we included the blood concentrations that are observed in humans following OM therapy [39]. Initially, we tested the cytotoxicity of OM in HPMEC by MTT assay. OM in concentrations up to 100 μM did not affect the viability of HPMEC (Fig. 1A). To determine the effects of OM on the functional activation of AhR, we initially determined the expression of the prototypical marker of AhR activation, CYP1A1, in HPMEC treated with the vehicle, DMSO, or OM. Real-time RT-PCR analyses indicated that OM increases CYP1A1 mRNA levels in a dose-dependent manner when compared to DMSO-treated cells (Fig. 1B). Next, we determined the effects of OM on the expression of the AhR-regulated phase II enzyme, NQO1. OM increased NQO1 mRNA (Fig. 1C) and protein levels (Figs. 1D and E). Interestingly, when compared to its effects on CYP1A1 expression, OM induced NQO1 expression only at a higher concentration of 100 μM, which suggests that OM has a differential concentration specific effect on phase I and II enzymes. To ascertain whether the AhR is a crucial regulator of OM-mediated increase in NQO1 expression in fetal HPMEC, we performed AhR siRNA transfection experiments to knockdown AhR. AhR siRNA significantly decreased AhR (Fig. 2A) and CYP1A1 (Fig. 2B) mRNA and AhR protein (Fig. 2D) expression. Surprisingly, AhR deficiency failed to abrogate OM-mediated increase in NQO1 mRNA (Fig. 2C) and protein (Figs. 2D and E) expression. These findings thus disprove our hypothesis that OM induces NQO1 expression via AhR-mediated mechanisms.
Figure 1. OM-treated HPMEC display increased CYP1A1 and NQO1 expression.
HPMEC were treated with DMSO or OM for up to 48 h, following which: cell viability was assessed by MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) reduction activities (A); RNA was extracted for CYP1A1 (B) and NQO1 (C) mRNA expression; and whole protein was extracted for immunoblotting using anti-NQO1 or β-actin antibodies (D). Densitometric analyses wherein NQO1 band intensities were quantified and normalized to β-actin (E). Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=3). Two-way ANOVA showed an effect of OM and time of exposure for CYP1A1 and NQO1 expression in this figure. *, p < 0.05 vs. DMSO-treated cells.
Figure 2. OM increases NQO1 expression via an AhR-independent mechanism.
HPMEC were transfected with either 50 nM control (SiC) or AhR (SiAhR) siRNA. Twenty-four hours after transfection, RNA and whole-cell protein was extracted to determine AhR (A) and CYP1A1 (B) mRNA and AhR protein (D) expression, respectively. Additionally, 24 h after transfection, the cells were treated with DMSO or OM for up to 48 h, following which RNA and whole-cell protein was extracted to determine NQO1 mRNA (C) and protein (D) expression, respectively. NQO1 band intensities were quantified and normalized to β-actin (E). Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=3). Two-way ANOVA showed an effect of OM, but not the AhR gene for the dependent variable, NQO1, in this figure. Significant differences between DMSO and OM-treated cells, and between control and AhR siRNA-transfected cells are indicated by *.
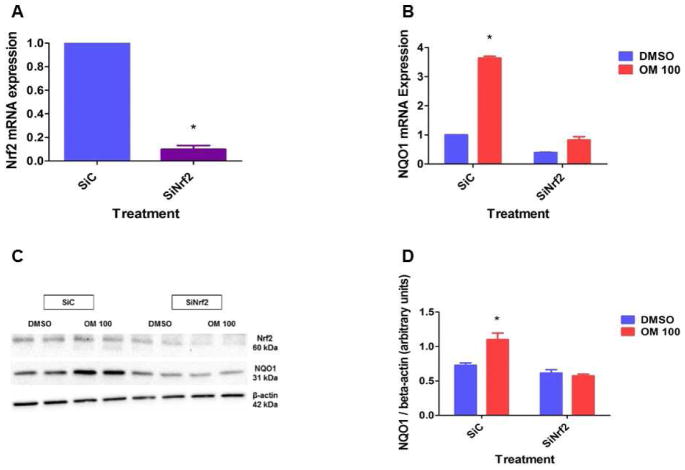
Nuclear factor erythroid 2–related factor 2 (Nrf2), which regulates the antioxidant response element (ARE)-driven gene battery is a major transcription factor that modulates NQO1 expression [40]. Hence, we finally conducted experiments to determine whether OM induces NQO1 via Nrf2. Interestingly, we observed that OM-treated cells had increased phosphoNrf2 (S40) expression compared to vehicle-treated cells (Figs. 3B, C, and E). This finding suggests that OM activates Nrf2 signaling since phosphorylation of Nrf2 at S40 leads to dissociation of Nrf2 from its inhibitor Kelch-like ECH-associated protein 1, which in turn results in the translocation of Nrf2 into the nucleus where it activates ARE-mediated gene expression [41]. OM has been similarly shown to increase nuclear Nrf2 accumulation in mice [42]. Although Mahmoud-Awny et.al. [43] observed that OM increases Nrf2 mRNA levels in rats with an ischemic/reperfusion insult, OM had no effect on the Nrf2 mRNA levels (Fig. 3A) in our study. These discrepant findings may be attributed to the differences in species, cell type, and the nature of underlying insult. Identical Nrf2 mRNA levels (Fig. 3A) between vehicle- and OM-treated cells suggest that OM activates Nrf2 pathway via unknown posttranscriptional mechanisms in HPMEC. To determine whether Nrf2 is a critical regulator of NQO1 expression in OM-treated cells, we knocked down Nrf2 by transfecting the cells with Nrf2 siRNA. Nrf2 siRNA significantly decreased Nrf2 and NQO1 mRNA (Figs. 4A and B) and protein (Figs. 4C and D) expression, which indicates that Nrf2 regulates the constitutive expression of NQO1 in HPMEC. Furthermore, OM failed to increase NQO1 mRNA (Fig. 4B) and protein (Figs. 4C and D) expression in Nrf2-deficient cells, supporting the hypothesis that OM-mediated induction of the NQO1 enzyme occurs via Nrf2-dependent mechanisms. Although a previous study has shown that OM increases hepatic NQO1 mRNA levels in rats [44], the mechanism of NQO1 induction was not investigated. To the best of our knowledge, this is the first study to demonstrate that Nrf2 is directly involved in OM-mediated induction of NQO1 in HPMEC in vitro. The mechanisms of OM-mediated activation of Nrf2 are unknown and deserve further investigations.
Figure 3. OM-treated HPMEC display increased phosphoNrf2(S40) protein expression.
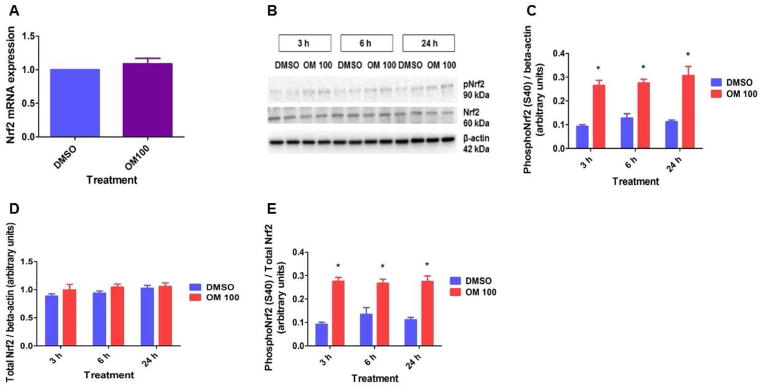
HPMEC were treated with DMSO or OM for up to 24 h, following which, following which RNA and whole-cell protein was extracted for real-time RT-PCR analyses of Nrf2 mRNA (A) and Nrf2 (B and D) and phosphoNrf2(S40) (pNrf2) (B, C and E) protein expression, respectively. Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=3). Two-way ANOVA showed an effect of OM but not of time for the dependent variable, phosphoNrf2 (S40), in this figure. Significant differences between DMSO and OM-treated cells are indicated by *, p < 0.05.
Figure 4. OM increases NQO1 expression in HPMEC via Nrf2-dependent mechanisms.
HPMEC were transfected with either 100 nM control (SiC) or Nrf2 (SiNrf2) siRNA. Twenty-four to forty-eight hours after transfection, RNA and whole-cell protein was extracted to determine Nrf2 mRNA (A) and protein (C) expression, respectively. Additionally, 24 h after transfection, the cells were treated with DMSO or OM for up to 48 h, following which RNA and whole-cell protein was extracted to determine NQO1 mRNA (B) and protein (C) expression, respectively. NQO1 band intensities were quantified and normalized to β-actin (D). Data are representative of at least three independent experiments. Values are presented as means ± SEM (n=3). Two-way ANOVA showed an effect of OM and Nrf2 gene and an interaction between them for the dependent variable, NQO1, in this figure. Significant differences between DMSO and OM-treated cells, and between control and Nrf2 siRNA-transfected cells are indicated by *.
In summary, we provide evidence that OM induces pulmonary NQO1 enzyme in vitro via AhR-independent, but Nrf2-dependent mechanisms. Our results suggest that OM can be used to investigate Nrf2 biology in the lung, which can lead to the discovery of novel therapies in the prevention and treatment of oxidative stress-induced disorders like BPD in premature infants, and acute respiratory distress syndrome, chronic obstructive pulmonary disease, and malignancies in adults.
Highlights.
We investigated whether omeprazole induces NQO1 in human fetal lung cells.
Omeprazole induces the phase II enzyme, NQO1, in human fetal lung cells.
AhR deficiency fails to abrogate omeprazole-mediated induction of NQO1.
Omeprazole increases phosphoNrf2 (S40) protein expression in human fetal lung cells.
Nrf2 knockdown abrogates the induction of NQO1 by omeprazole in human lung cells.
Acknowledgments
This work was supported by grants from National Institutes of Health HD-073323 to B.S. and [ES-009132, HL-112516, HL-087174, and ES-019689] to B.M., and American Heart Association BGIA20190008 to B.S.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARE
antioxidant response element
- BPD
bronchopulmonary dysplasia
- CYP
cytochrome P450
- DMSO
dimethylsulfoxide
- HPMEC
human pulmonary microvascular endothelial cells
- MTT
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2–related factor 2
- OM
omeprazole
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sogawa K, Fujii-Kuriyama Y. Ah receptor, a novel ligand-activated transcription factor. J Biochem. 1997;122:1075–1079. doi: 10.1093/oxfordjournals.jbchem.a021864. [DOI] [PubMed] [Google Scholar]
- 3.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci. 2005;94:1169–1186. doi: 10.1002/jps.20324. [DOI] [PubMed] [Google Scholar]
- 5.Denis M, Cuthill S, Wikstrom AC, Poellinger L, Gustafsson JA. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988;155:801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- 6.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 7.Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Molecular pharmacology. 1994;45:428–438. [PubMed] [Google Scholar]
- 8.Hord NG, Perdew GH. Physicochemical and immunocytochemical analysis of the aryl hydrocarbon receptor nuclear translocator: characterization of two monoclonal antibodies to the aryl hydrocarbon receptor nuclear translocator. Molecular pharmacology. 1994;46:618–626. [PubMed] [Google Scholar]
- 9.Emi Y, Ikushiro S, Iyanagi T. Xenobiotic responsive element-mediated transcriptional activation in the UDP-glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996;271:3952–3958. doi: 10.1074/jbc.271.7.3952. [DOI] [PubMed] [Google Scholar]
- 10.Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 11.Fujisawa-Sehara A, Sogawa K, Yamane M, Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic acids research. 1987;15:4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 14.Bock KW, Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem. 2009;390:1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- 15.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:40–53. doi: 10.2183/pjab.86.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauzeau V, Carvajal-Gonzalez JM, Riolobos AS, Sevilla MA, Menacho-Marquez M, Roman AC, Abad A, Montero MJ, Fernandez-Salguero P, Bustelo XR. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem. 2011;286:2896–2909. doi: 10.1074/jbc.M110.187534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsey S, Papoutsakis ET. The Evolving Role of the Aryl Hydrocarbon Receptor (AHR) in the Normophysiology of Hematopoiesis. Stem cell reviews. 2012 doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bulletin of environmental contamination and toxicology. 1998;61:557–568. doi: 10.1007/pl00002973. [DOI] [PubMed] [Google Scholar]
- 19.Daujat M, Peryt B, Lesca P, Fourtanier G, Domergue J, Maurel P. Omeprazole, an inducer of human CYP1A1 and 1A2, is not a ligand for the Ah receptor. Biochemical and biophysical research communications. 1992;188:820–825. doi: 10.1016/0006-291x(92)91130-i. [DOI] [PubMed] [Google Scholar]
- 20.Lesca P, Peryt B, Larrieu G, Alvinerie M, Galtier P, Daujat M, Maurel P, Hoogenboom L. Evidence for the ligand-independent activation of the AH receptor. Biochemical and biophysical research communications. 1995;209:474–482. doi: 10.1006/bbrc.1995.1526. [DOI] [PubMed] [Google Scholar]
- 21.Daujat M, Charrasse S, Fabre I, Lesca P, Jounaidi Y, Larroque C, Poellinger L, Maurel P. Induction of CYP1A1 gene by benzimidazole derivatives during Caco-2 cell differentiation. Evidence for an aryl-hydrocarbon receptor-mediated mechanism. European journal of biochemistry / FEBS. 1996;237:642–652. doi: 10.1111/j.1432-1033.1996.0642p.x. [DOI] [PubMed] [Google Scholar]
- 22.Aix L, Rey-Grobellet X, Larrieu G, Lesca P, Galtier P. Thiabendazole is an inducer of cytochrome P4501A1 in cultured rabbit hepatocytes. Biochemical and biophysical research communications. 1994;202:1483–1489. doi: 10.1006/bbrc.1994.2098. [DOI] [PubMed] [Google Scholar]
- 23.Fontaine F, de Sousa G, Duchene P, Rahmani R. Cytochrome P450 Induction and Cytotoxic effects of Antimalarials in Rat Hepatocytes. Toxicology in vitro : an international journal published in association with BIBRA. 1998;12:545–549. doi: 10.1016/s0887-2333(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 24.Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 25.Larsson H, Carlsson E, Ryberg B, Fryklund J, Wallmark B. Rat parietal cell function after prolonged inhibition of gastric acid secretion. Am J Physiol. 1988;254:G33–39. doi: 10.1152/ajpgi.1988.254.1.G33. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Murakami K, Sato R, Kashimura K, Miura M, Ootsu S, Miyajima H, Nasu M, Okimoto T, Kodama M, Fujioka T. Effect of sucralfate on antibiotic therapy for Helicobacter pylori infection in mice. 2004. 2004/11/25 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol Pharmacol. 1993;43:504–508. [PubMed] [Google Scholar]
- 28.Yoshinari K, Ueda R, Kusano K, Yoshimura T, Nagata K, Yamazoe Y. Omeprazole transactivates human CYP1A1 and CYP1A2 expression through the common regulatory region containing multiple xenobiotic-responsive elements. Biochem Pharmacol. 2008;76:139–145. doi: 10.1016/j.bcp.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Shivanna B, Chu C, Welty SE, Jiang W, Wang L, Couroucli XI, Moorthy B. Omeprazole attenuates hyperoxic injury in H441 cells via the aryl hydrocarbon receptor. Free radical biology & medicine. 2011;51:1910–1917. doi: 10.1016/j.freeradbiomed.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang N, Walker MK. Crosstalk between the aryl hydrocarbon receptor and hypoxia on the constitutive expression of cytochrome P4501A1 mRNA. Cardiovascular toxicology. 2007;7:282–290. doi: 10.1007/s12012-007-9007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S, Patel A, Chu C, Jiang W, Wang L, Welty SE, Moorthy B, Shivanna B. Aryl hydrocarbon receptor is necessary to protect fetal human pulmonary microvascular endothelial cells against hyperoxic injury: Mechanistic roles of antioxidant enzymes and RelB. Toxicology and applied pharmacology. 2015 doi: 10.1016/j.taap.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright CJ, Agboke F, Chen F, La P, Yang G, Dennery PA. NO inhibits hyperoxia-induced NF-kappaB activation in neonatal pulmonary microvascular endothelial cells. Pediatric research. 2010;68:484–489. doi: 10.1203/PDR.0b013e3181f917b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Backlund M, Ingelman-Sundberg M. Regulation of aryl hydrocarbon receptor signal transduction by protein tyrosine kinases. Cellular signalling. 2005;17:39–48. doi: 10.1016/j.cellsig.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Murray IA, Perdew GH. Omeprazole stimulates the induction of human insulin-like growth factor binding protein-1 through aryl hydrocarbon receptor activation. The Journal of pharmacology and experimental therapeutics. 2008;324:1102–1110. doi: 10.1124/jpet.107.132241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiizaki K, Ohsako S, Kawanishi M, Yagi T. Identification of amino acid residues in the ligand-binding domain of the aryl hydrocarbon receptor causing the species-specific response to omeprazole: possible determinants for binding putative endogenous ligands. Molecular pharmacology. 2014;85:279–289. doi: 10.1124/mol.113.088856. [DOI] [PubMed] [Google Scholar]
- 36.Backlund M, Johansson I, Mkrtchian S, Ingelman-Sundberg M. Signal transduction-mediated activation of the aryl hydrocarbon receptor in rat hepatoma H4IIE cells. The Journal of biological chemistry. 1997;272:31755–31763. doi: 10.1074/jbc.272.50.31755. [DOI] [PubMed] [Google Scholar]
- 37.Novotna A, Srovnalova A, Svecarova M, Korhonova M, Bartonkova I, Dvorak Z. Differential effects of omeprazole and lansoprazole enantiomers on aryl hydrocarbon receptor in human hepatocytes and cell lines. PloS one. 2014;9:e98711. doi: 10.1371/journal.pone.0098711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin UH, Lee SO, Pfent C, Safe S. The aryl hydrocarbon receptor ligand omeprazole inhibits breast cancer cell invasion and metastasis. BMC cancer. 2014;14:498. doi: 10.1186/1471-2407-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cederberg C, Thomson AB, Mahachai V, Westin JA, Kirdeikis P, Fisher D, Zuk L, Marriage B. Effect of intravenous and oral omeprazole on 24-hour intragastric acidity in duodenal ulcer patients. Gastroenterology. 1992;103:913–918. doi: 10.1016/0016-5085(92)90025-t. [DOI] [PubMed] [Google Scholar]
- 40.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free radical biology & medicine. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 42.Patel V, Joharapurkar A, Dhanesha N, Kshirsagar S, Detroja J, Patel K, Gandhi T, Patel K, Bahekar R, Jain M. Combination of omeprazole with GLP-1 agonist therapy improves insulin sensitivity and antioxidant activity in liver in type 1 diabetic mice. Pharmacological reports : PR. 2013;65:927–936. doi: 10.1016/s1734-1140(13)71074-0. [DOI] [PubMed] [Google Scholar]
- 43.Mahmoud-Awny M, Attia AS, Abd-Ellah MF, El-Abhar HS. Mangiferin Mitigates Gastric Ulcer in Ischemia/ Reperfused Rats: Involvement of PPAR-gamma, NF-kappaB and Nrf2/HO-1 Signaling Pathways. PloS one. 2015;10:e0132497. doi: 10.1371/journal.pone.0132497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi H, Shimamoto K, Taniai E, Ishii Y, Morita R, Suzuki K, Shibutani M, Mitsumori K. Liver tumor promoting effect of omeprazole in rats and its possible mechanism of action. The Journal of toxicological sciences. 2012;37:491–501. doi: 10.2131/jts.37.491. [DOI] [PubMed] [Google Scholar]