Abstract
The precise role of Wnt/β-catenin signaling during prostatic development and tumorigenesis is unclear. Axin2 is a direct transcriptional target of β-catenin. Recent studies have shown that Axin2-expressing cells have stem/progenitor cell properties in a variety of mouse tissues. Here, we genetically labeled Axin2-expressing cells at various time points and tracked their cellular behavior at different developmental and mature stages. We found that prostatic Axin2-expressing cells mainly express luminal epithelial cell markers and are able to expand luminal cell lineages during prostatic development and maturation. They can also survive androgen withdrawal and regenerate prostatic luminal epithelial cells following androgen replacement. Deletion of β-catenin or expression of stabilized β-catenin in these Axin2-expressing cells results in abnormal development and oncogenic transformation, respectively. Our study uncovers a critical role of Wnt/β-catenin-responsive cells in prostatic development and regeneration, and that dysregulation of Wnt/β-catenin signaling in these cells contributes to prostatic developmental defects and tumorigenesis.
Keywords: Wnt Signaling, β-catenin, Axin2, Prostatic progenitor cells
INTRODUCTION
The prostate gland is an accessory reproductive endocrine-target organ in males, which contributes secretion to seminal fluid [1, 2]. Mouse prostatic development initiates at embryonic day 17.5 (E17.5) from the urogenital sinus (UGS) upon rising levels of testicular androgens [3]. Thus, it has been speculated that certain UGS cells carry out prostatic stem cell properties to commit to prostatic cell fate during embryonic prostatic development. After birth, the unbranched buds and ducts in the ventral, dorsal, and lateral prostatic lobes grow peripherally to give rise to lobe-specific ductal branching patterns [2–4]. Morphogenesis of the entire prostatic complex in mice is completed between postnatal days 15 and 30 (P15–30). Continued growth and maturation occurs during puberty (P25–40) when circulating androgens levels rise [1, 2, 5]. It is believed that prostatic stem/progenitor cells play a decisive role in controlling prostatic development and maturation. The consequent observation that prostatic regeneration occurs after repeated cycles of androgen deprivation and replacement further confirms the existence of prostatic stem cells [3, 4, 6]. Recent studies have demonstrated that basal and luminal cells are independently sustained in adult mice [7–10]. In contrast, prostatic postnatal development is governed by unipotent basal and luminal progenitors, as well as basal multipotent stem cells that differentiate into all three epithelial cell lineages, including luminal, basal and neuroendocrine cells [8]. Although many paracrine signaling pathways, including Wnt/β-catenin, are implicated in the embryonic prostatic stem cell niche [11, 12], detailed mechanisms of prostatic stem/progenitor cell regulation during prostatic development and regeneration are still largely unknown.
Wnt growth factors activate different intracellular targets through either the "canonical" or the "non-canonical" pathways [13]. The canonical signaling pathways are primarily mediated through β-catenin [14] and play a critical role in development, morphogenesis, and organogenesis [15–17]. The Wnt/β-catenin signaling pathway is required in the development of the prostate at the perinatal stage [18–21]. Deletion of the β-catenin gene (Ctnnb1) in the mouse prostate during embryonic stages results in significantly decreased prostatic budding and abrogates prostatic development [20]. Axin2 expression is upregulated by activated Wnt signaling [22], and Axin2 is also involved in the negative feedback loop of the Wnt signaling pathway [23]. Recent studies have shown that Axin2-expressing cells have stem/progenitor cell properties in a variety of mouse tissues [24–27]. In this study, we investigate the significance of Wnt/β-catenin-responsive cells throughout prostatic development and regeneration. Using Axin2CreERT2 and other related mouse models, we genetically labeled Axin2-expressing cells at various time points and tracked their cellular behavior at different developmental and mature stages. Our study uncovers the novel role of Wnt/β-catenin-responsive cells in prostatic development and regeneration, and that dysregulation of Wnt/β-catenin signaling in these cells contributes to prostatic developmental defects and tumorigenesis.
MATERIALS AND METHODS
Animals
Axin2CreERT2 (Jackson Laboratories; stock 18867) [27] and Ctnnb1(ex2-6)fl (Jackson Laboratories; stock 4152) mice [28] were obtained from Dr. Roel Nusse. RosamTmG (Jackson Laboratories; stock 7676) mice [29] were provided by Dr. Liqun Luo. Ctnnb1(ex3)fl mice{Harada, 1999 #41} were obtained from Dr. Makoto M. Taketo. All animal procedures were based on animal care guidelines approved by Stanford University’s Administrative Panel on Laboratory Care (APLAC).
Mouse procedures
Castration of adult male mice was performed as described previously{Sugimura, 1986 #10}. Briefly, both testicles and epididymis were removed through a scrotal approach from anesthetized mice. The distal end of the spermatic cord was ligated with silk thread. For androgen supplement, testosterone pellets (12.5 mg, Innovative Research of America) were placed in castrated mice subcutaneously. For tamoxifen induction, mice received a single intraperitoneal injection of 4 mg/25 g body weight tamoxifen (Sigma) suspended in corn oil. This corresponds to a total does of 1 mg tamoxifen for prepubescent mice (injected at P14) and 4 mg of tamoxifen for adult mice (injected at P60). To label Axin2-expressing cells in embryos, pregnant mothers received a single intraperitoneal injection at E16.5, totaling 1 mg/25 g body weight. BrdU (80 mg/kg; Sigma) was administered by intraperitoneal injection daily for 3 consecutive days during prostate development to label proliferating cells.
Histology and immunostaining
Prostatic tissues were fixed in 10% neutral-buffered formalin and processed into paraffin, and 5 µm serial sections were cut and processed from xylene to water through a decreasing ethanol gradient. For histology, Hematoxylin-eosin staining was performed using standard protocols. For immunohistochemistry, slides were treated by boiling in 0.01 M Citrate buffer (pH 6.0) for antigen retrieval, incubated in 0.3% H2O2 for 15 min, blocked in 5% normal goat serum for 30 min, and incubated with primary antibodies diluted in 1% normal goat serum at 4°C overnight. Slides were incubated with biotinylated secondary antibodies for 1 hour then with horseradish peroxidase streptavidin (SA-5004, Vector Laboratories) for 30 min, and visualized by DAB kit (SK-4100, Vector Laboratories). Slides were counterstained with 5% (w/v) Harris Hematoxylin, subsequently mounted with Permount Mounting Medium (SP15–500, Fisher Scientific).
For immunofluorescence staining and detection of mTmG signals, mouse tissues were fixed in 4% PFA at 4°C overnight, cryoprotected in 30% sucrose at 4°C overnight, and embedded in OCT (Tissue-Tek). 5 µm sections were obtained using a Leica cryostat, and slides were washed three times with PBS. For detection of mTmG signals, slides were directly mounted with VECTASHIELD Mounting Medium with DAPI (H-1200, Vector Laboratories). For immunofluorescence staining, slides were treated for antigen retrieval, blocked in 5% normal goat serum for 30 min, and incubated with primary antibodies diluted in 1% normal goat serum at 4°C overnight. Slides were then incubated with fluorescence secondary antibodies for 1 hour, and then mounted with VECTASHIELD Mounting Medium with DAPI.
Antibodies
The following primary antibodies were used: anti-GFP (rabbit, 1:1,000, A11122, Molecular Probes), anti-GFP (mouse, 1:300, 2955, Cell Signaling), anti-K5 (rabbit, 1:3,000, PRB-160P, Covance), anti-K8 (mouse, 1:2,500, MMS-162P, Covance), anti-p63 (mouse, 1:200, sc-8431, Santa Cruz), anti-β-catenin (rabbit, 1:500, sc-7199, Santa Cruz), anti-β-catenin (mouse, 1:500, 610154, BD Transduction Laboratories), anti Ki67 (mouse, 1:1,000, NCL-ki67, Novacastra), anti-E-cadherin (mouse, 1:300, c20820, BD Transduction Laboratories), anti-androgen receptor (rabbit, 1:500, sc-816, Santa Cruz), anti-synaptophysin (rabbit, 1:500, 18-0130, Invitrogen), anti-BrdU (mouse, 1:200, 5292, Cell Signaling), and anti-Nkx3.1 (rabbit, 1:3,000, provided by Dr. Cory Abate-Shen, Columbia University, New York). The biotinylated anti-rabbit or anti-mouse secondary antibody (BA-1000 or BA-9200, Vector Laboratories), or anti-rabbit or anti-mouse conjugated to AlexaFluor488 or to AlexaFluor594 (Molecular Probes) secondary antibody was used for were used for immunohistochemistry or immunofluorescence staining, respectively.
Microscope image acquisition
Images of H&E and immunohistochemistry were acquired on an Axio Lab. A1 microscope using 10× and 40× Zeiss A-Plan objectives with a Canon EOS 1000D camera and using Axiovision software (Carl Zeiss). Images of immunofluorescence staining and mTmG signals and were acquired on an Nikon ECLIPSE E800 epi-fluorescence microscope using 20× and 40× Nikon Plan Fluor objectives with an QImaging RETIGA EXi camera and using QCapture software (QImaging). Cell numbers were counted manually using 40× photomicrographs as described in Supplementary Tables S1–S7. Statistical analyses were performed using 2-tailed Student’s t test or 2-way ANOVA.
RESULTS
Wnt/β-catenin-responsive cells in the embryonic UGE contribute to the luminal cell lineage
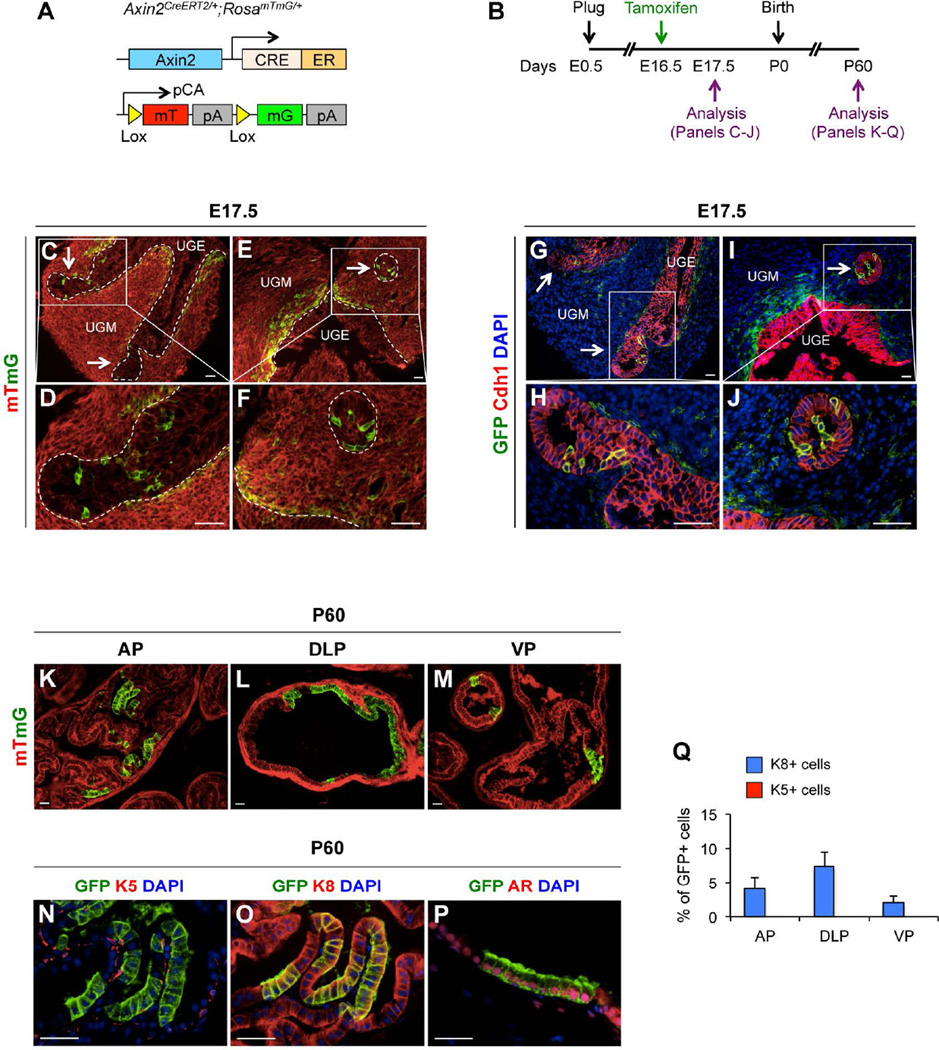
Wnt signaling is critical for cell fate commitment and determination in a variety of tissues and organs during embryonic development [15–17]. Expression of Axin2 has been observed in the urogenital sinus epithelium (UGE) [20]. In Axin2CreERT2/+;RosamTmG/+ mice [27] (Fig. 1A), tamoxifen (TM) induced Cre activity results in a permanent genetic mark in the form of a switch from membrane-bound tdTomato (mT) to membrane-bound green fluorescent protein (mGFP) expression through recombination of the floxed reporter loci in targeted cells. These cells can pass the genetic marker, mGFP expression, onto their offspring, thereby allowing the developmental fate of the Wnt/β-catenin-responsive lineage to be traced. Using this mouse model, we first examined the distribution of Wnt/β-catenin-responsive cells at embryonic stages and to trace the developmental fate of the mGFP-labeled cells (Fig. 1B). Mouse prostatic development initiates at E17.5 from UGS [3], and thus we administered TM to pregnant females at E16.5 and analyzed the male UGS of E17.5 embryos (Fig. 1B). Analysis of dissected urogenital tissues showed that Axin2-expressing cells are located within the urogenital sinus mesenchyme (UGM) (Fig. 1C, 1E) surrounding UGE, and in newly formed epithelial buds (arrows, Fig. 1C–1F). Using immunofluorescence approaches, we assessed the cellular properties of embryonic Wnt/β-catenin-responsive cells and found the expression of E-cadherin (Fig. 1G–1J), p63 (Supporting Information Fig. S1B–S1E), Nkx3.1 (Supporting Information Fig. S1F, S1G), K5 (Supporting Information Fig. S1H, S1I), and K8 (Supporting Information Fig. S1J, S1K) overlaid with mGFP staining in Axin2-expressing cells within the epithelial budding tips. To determine whether Wnt/β-catenin-responsive cells in the embryonic UGS contribute to prostate gland development, we continued to analyze the male offspring once these mice had reached adulthood (Fig. 1B). At postnatal day 60 (P60), clusters of mGFP-positive epithelial cells were observed in all three different prostatic lobes (Fig. 1K–1M). These mGFP-positive cells are limited to within luminal cell layers (Fig. 1K–1M), and co-expressed luminal cell markers, K8 (Fig. 1O) and androgen receptor (AR) (Fig. 1P), but not basal or neuroendocrine cell markers, such as K5 (Fig. 1N), p63 (Supporting Information Fig. S1L), and synaptophysin (Supporting Information Fig. S1M). Quantifying mGFP-positive cells among total K8-positive luminal cells in different prostatic lobes showed 4.2% ± 1.6% in anterior prostate (AP), 7.3% ± 2.1% in dorsolateral prostate (DLP), and 2.1% ± 1.0% in ventral prostate (VP), respectively (Fig. 1Q; Supporting Information Table S1). Notably, we rarely observed mGFP-positive cells in stromal areas of the mouse prostate at P60 (Supporting Information Fig. S1N, S1O). These data demonstrate the existence and cellular identity of Wnt/β-catenin-responsive cells in mouse embryonic UGS, and their ability to give rise to prostatic luminal cells during postnatal development.
Figure 1. Axin2-expressing cells in the embryonic UGE contribute to the luminal cell lineage.
(A): A scheme of mT or mGFP protein expression in Axin2-expressing cells of Axin2CreERT2/+;RosamTmG/+ mice. (B): A scheme of experimental designs in labeling and analyzing Axin2-expressing cells using Axin2CreERT2/+;RosamTmG/+ mice during embryonic (E) and postnatal (P) stages. (C–F): E17.5 UGS showing mT (red) and mGFP (green) labeling. Arrows indicate newly formed epithelial buds. (G–J): Immunofluorescence staining of mGFP (green) and E-cadherin (Cdh1) (red) in UGS at E17.5. Arrows indicate the newly formed epithelial buds. (K–M): mT and mGFP staining in different prostatic lopes of Axin2CreERT2/+;RosamTmG/+ mice at P60. AP, anterior prostate; DLP, dorsolateral prostate; VP, ventral prostate. (N–P): Co-immunofluorescence staining of mGFP (green) with K5, K8, or AR as well as DAPI in the prostate of Axin2CreERT2/+;RosamTmG/+ mice at P60. Scale bars, 20 µm. (Q): Percentages of K5 + or K8+ and mGFP+ cells in different prostatic lopes at P60.
Prepubescent Wnt/β-catenin-responsive cells contribute to the luminal lineage expansion
Although the growth of mouse prostatic buds initiates at E17.5 [2], substantial elongation, branching, and patterning of prostatic ducts continues after birth. Because the branching and patterning of prostatic lobes is completed about three weeks after birth [2], we administered TM to Axin2CreERT2/+;RosamTmG/+ mice at P14 and analyzed them at P19 to assess Wnt/β-catenin responsive cells in the prepubescent prostates (Fig. 2A). At P19, we observed that most Axin2-expressing cells were K8-positive luminal epithelial cells in different prostatic lobes (Fig. 2B–2F). Quantifying these luminal mGFP-positive cells showed 7.9% ± 2.7% in AP, 5.8% ± 1.6% in DLP, and 5.0% ± 1.7% in VP, respectively (Fig. 2Q; Supporting Information Table S2). Additionally, we also observed a few K5-positive basal epithelial Axin2-expressing cells, although they are very limited and less than mGFP-positive luminal cells: 0.9% ± 0.6% in AP, 0.8% ± 0.4% in DLP, and 0.8% ± 0.5% in VP (Fig. 2R; Supporting Information Fig. S2D, Table S2). We did not observe synaptophysin, a neuroendocrine cell marker, positive Axin2-expressing cells (Supporting Information Fig. S2C).
Figure 2. Axin2-expressing cells in the prepubescent prostate gland contribute to luminal lineage expansion.
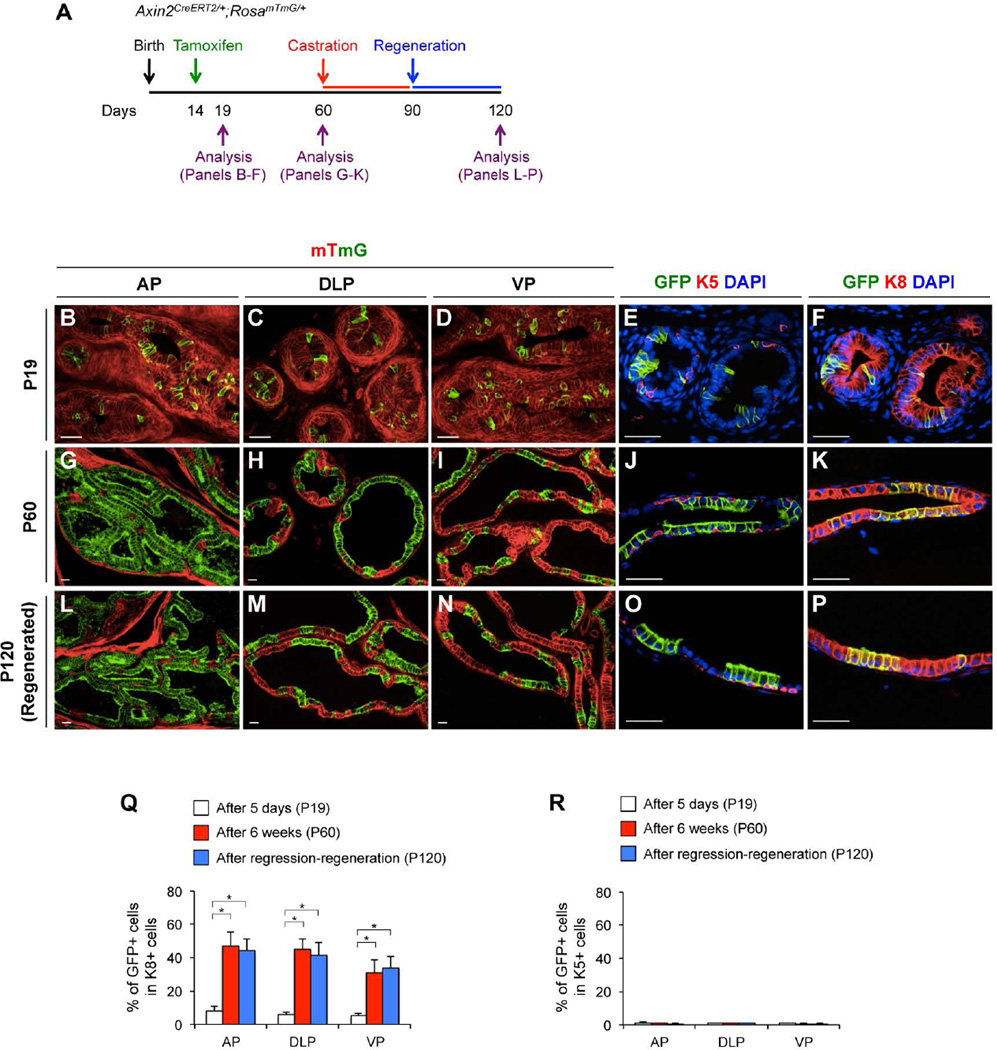
(A): A scheme of experimental designs to label and analyze prepubescent Axin2-expressing cells in the prostate of Axin2CreERT2/+;RosamTmG/+ mice. Expression of mT (red) and mGFP (green) protein in different prostatic lopes of Axin2CreERT2/+;RosamTmG/+ mice at P19 (B–D), P60 (G–I), and P120 (L–N). Co-immunofluorescence staining of mGFP (green) and K5 (red) or K8, as well as DAPI was preformed in the prostate of Axin2CreERT2/+;RosamTmG/+ mice. Representative images in DLP at P19 (E–F), P60 (J–K), and P120 (O–P) were shown. Percentages of mGFP+ cells in K8+ (Q) and in K5+ cells (R) at P19 (white bars), P60 (red bars), or P120, after a round of regression/regeneration (blue bars) in different prostatic lopes. Error bars indicate standard deviation. *, P < 0.01 by ANOVA. Scale bars, 20 µm.
To explore whether the above genetically labeled Wnt/β-catenin-responsive cells have stem/progenitor properties in prostatic growth and maturation during puberty, we traced prepubescent Axin2-expressing cells by tamoxifen administration to Axin2CreERT2/+;RosamTmG/+ mice at P14 and analyzed the contribution of mGFP-labeled cells to the adult prostatic epithelia (P60) (Fig. 2A). Although less than 8% of the mGFP-positive luminal cells existed in prostatic lobes at P19 (Fig. 2Q), more than 46.5% ± 8.3%, 44.6% ± 6.0% and 30.8% ± 7.6% of mGFP-positive luminal cells appear in AP, DLP, and VP, respectively, at P60 (Fig. 2G–2K, 2Q; Supporting Information Table S2). In contrast, there is no significant difference in the numbers of K5-positive basal mGFP-positive cells between P19 and P60 (Fig. 2R; Supporting Information Fig. S2G, Table S2). Immunofluorescence analyses showed that those expanded mGFP-positive luminal cells express AR (Supporting Information Fig. S2H) and Nkx3.1 (Supporting Information Fig. S2I), but not p63 (Supporting Information Fig. S2E) or synaptophysin (Supporting Information Fig. S2F). Results from these tracing experiments demonstrate that the prepubescent Wnt/β-catenin-responsive cells have unipotent luminal progenitor properties and are able to expand the luminal cell population throughout puberty.
To determine the fate of prepubescent Wnt/β-catenin-responsive cells in adult prostates during tissue turnover, we administered TM to Axin2CreERT2/+;RosamTmG/+ mice at P14 then induced extensive prostatic epithelial turnover at P60 by a classic prostatic regression-regeneration assay (Fig. 2A). After a round of prostatic regression-regeneration, we observed that mGFP-positive cells remain in the luminal layers (Fig. 2L–2P), but not in basal layers (Fig. 2O; Supporting Information Fig. S2L) or in neuroendocrine cells (Supporting Information Fig. S2K). The percentage of mGFP-positive luminal cells in the regenerated prostates (P120) was similar to the percentage of mGFP-positive luminal cells in the intact prostates (P60) (Fig. 2Q; Supporting Information Table S2). We also measured the proliferative rates of both mGFP-positive and negative luminal cells during the chase period, and observed more BrdU-labeled cells in mGFP-positive than negative cells (Supporting Information Fig. S2N–S2R). Our observation suggests that Wnt/β-catenin-responsive cells in the prepubescent prostate gland act as unipotent luminal progenitors and enable epithelial proliferation during prostatic epithelial regeneration.
β-catenin is required in prepubescent Wnt/β-catenin-responsive cells for expanding the luminal lineages
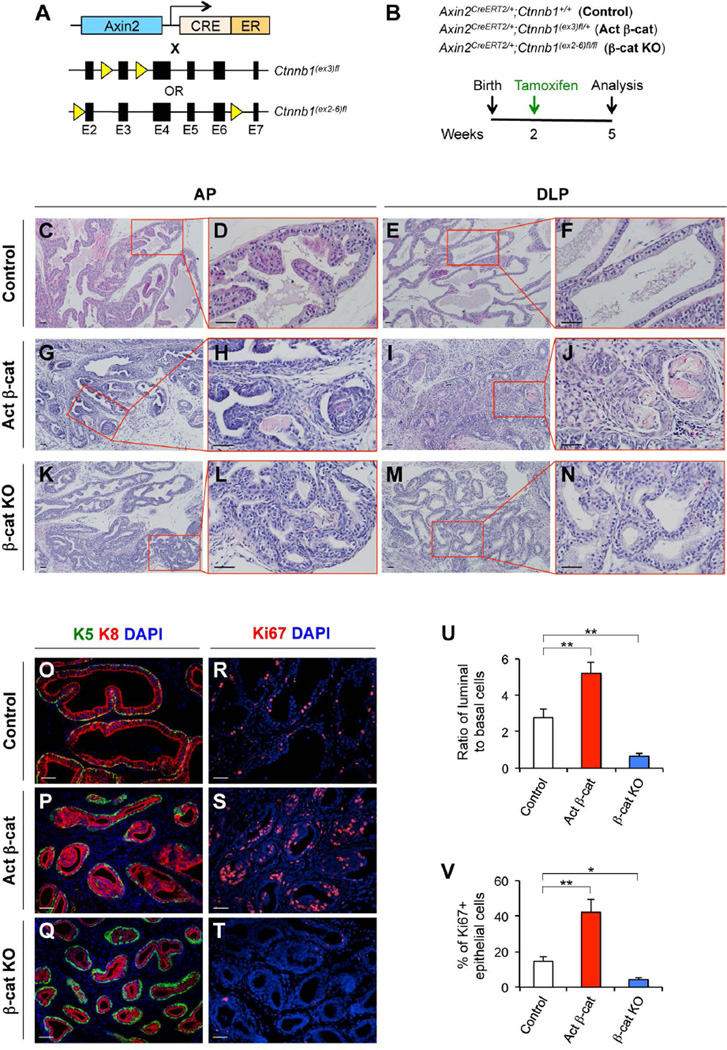
We next examined the biological significance of Wnt/β-catenin signaling in prepubescent Axin2-expressing cells to expand the luminal lineages during puberty. We utilized both Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl (β-cat KO) and Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Activated β-cat) mice, in which either Ctnnb1 is deleted or a stabilized form of the β-catenin is expressed in Axin2-expressing cells conditionally after TM administration, respectively (Fig. 3A). These mice and controls were administered TM at P14 and then analyzed at P35, just after puberty (Fig. 3B). Interestingly, we observed reduced prostatic gland size, decreased lumen secretion, and abnormal branching morphogenesis in both Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Fig. 3G–3J) and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice (Fig. 3K–3N). In addition, there appears to be a decreased cytoplasmic/nuclear ratio in prostatic luminal cells of Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Fig. 3G–3J) and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice (Fig. 3K–3N) compared with luminal cells of Axin2CreERT2/+;Ctnnb1+/+ controls (Fig. 3C–3F). Intriguingly, prostatic intraepithelial neoplasia (PIN) lesions with atypical cells and intraluminal keratin were observed in Axin2CreERT2/+;Ctnnb1(ex3)fl/+ mice with activated β-cat (Fig. 3H, 3J). In PIN lesions of Axin2CreERT2/+;Ctnnb1(ex3)fl/+ mice, the expression of stabilized β-catenin protein was detected in luminal cells that also express K8 (Supporting Information Fig. S3H) and AR (Supporting Information Fig. S3G), but not in basal cells that express K5 (Supporting Information Fig. S3E, S3F). Interestingly, we observed a high luminal to basal cell ratio of 5.2:1 in Axin2CreERT2/+;Ctnnb1(ex3)fl/+ prostates and a low luminal to basal cell ratio of 0.7:1 in Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl prostates, whereas the ratio of luminal to basal cells in Axin2CreERT2/+;Ctnnb1+/+ prostates was retained at 2.8:1 (Fig. 3O–3Q, 3U; Supporting Information Table S3). To assess whether abnormal expression of β-catenin in the prepubescent Wnt/β-catenin-responsive cells affects epithelial proliferation, we analyzed Ki67, a marker of proliferating cells, positive epithelial cells in those mouse prostates. We observed a significant increase, 42.2% ± 7.3%, and a decrease, 4.3% ± 1.3%, in Ki67 immunostaining of prostatic epithelial cells in Axin2CreERT2/+;Ctnnb1(ex3)fl/+ with activated β-cat, and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice (β-cat -KO), respectively, in comparison to controls, 14.6% ± 3.0%, in Axin2CreERT2/+;Ctnnb1+/+ mice (Fig. 3R–3T, 3V; Supporting Information Table S4). These results demonstrate a crucial role of Wnt/β-catenin signaling in prostatic epithelial cell proliferation during puberty as well as regulating the basal/luminal cell ratio, implicating that the dysregulation of Wnt/β-catenin signaling in prepubescent Axin2-expresseing cells may directly contribute to prostatic ductal defects and abnormal epithelial differentiation.
Figure 3. Prepubescent Axin2-expressing cells require β-catenin to expand luminal cell lineages.
(A): A scheme of conditional expression of stabilized β-catenin or deletion of β-catenin in compound mouse models. (B): Experimental strategy for analyzing Axin2CreERT2/+;Ctnnb1+/+ (control), Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Activated β-cat), and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl (β-cat KO) mice. (C–N): H&E staining of AP and DLP isolated from the above mice. (O–T): Co-immunofluorescence staining of prostate tissues isolated from the control, Activated β-cat, and β-cat KO mice. (U): Ratio of luminal (K8+) versus basal (K5+) cells in control, Activated β-cat, and β-cat KO mice. (V): Percentages of Ki67 positive epithelial cells in the prostate of the above different mice. Error bars indicate standard deviation. *, P < 0.05; **, P < 0.01 by Student’s t tests. Scale bars, 50 µm.
Castration-resistant Wnt/β-catenin-responsive cells are unipotent luminal progenitors that enable prostatic epithelial regeneration
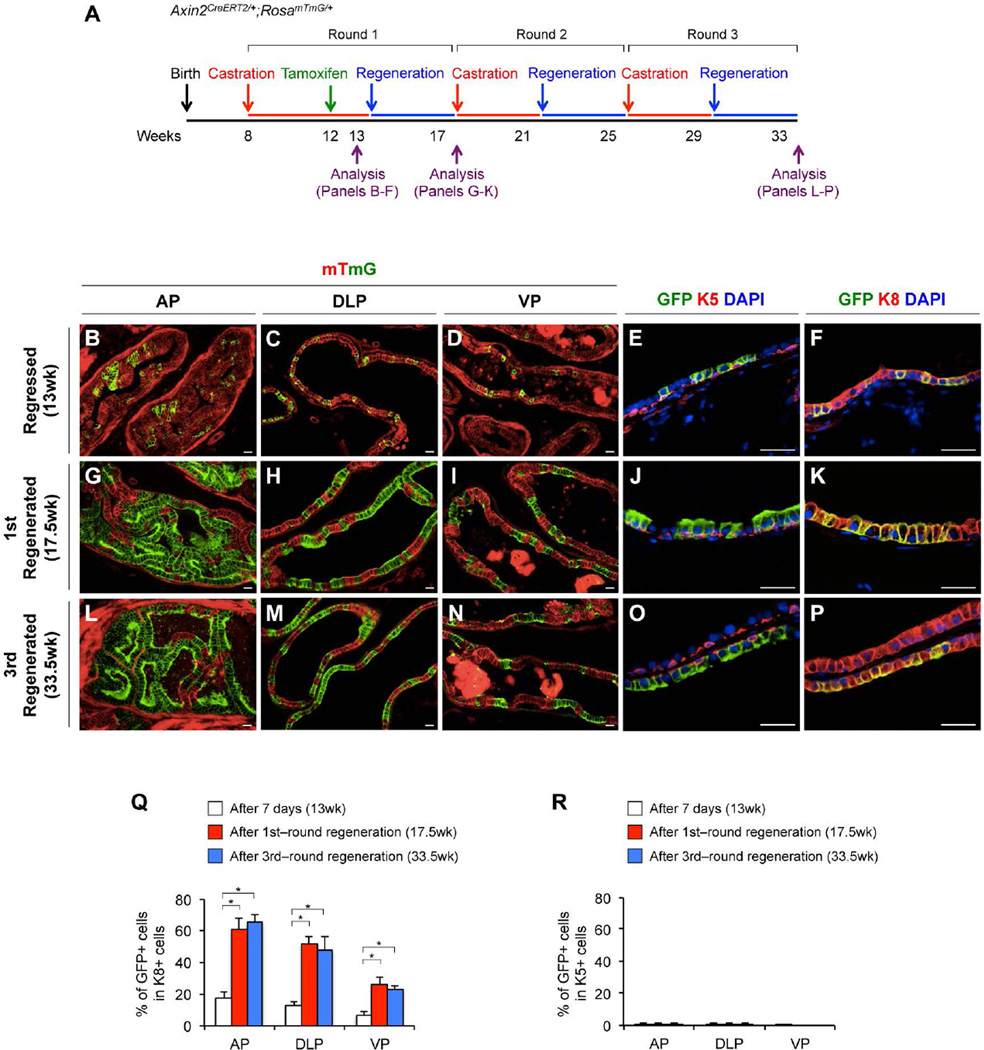
Castration-resistant Nkx3.1-expressing cells (CARNs) retain prostatic luminal cell properties and the ability to generate all three different prostatic epithelial lineages [30]. A recent study showing that adult murine luminal and basal cells are independently sustained during prostatic regression-regeneration further implies the presence of castration-resistant unipotent luminal progenitors [7]. Here, we examined the existence and identity of castration-resistant Wnt/β-catenin-responsive cells in the mouse prostate. Axin2CreERT2/+;RosamTmG/+ mice were castrated at 8 weeks of age, administered TM at 12 weeks of age, and then analyzed at 13 weeks of age (Fig. 4A). Histologically, we observed regressed glands in all prostatic lobes of castrated mice (Fig. 4B–4D). Markedly atrophic glandular/ductal profiles appear within a relatively prominent concentric peri-glandular/−ductular fibromuscular stroma (data not shown), which we interpret as hormone-withdrawal atrophy of these glands. We observed that the majority of genetically labeled Axin2-expressing cells after 5-week-castration reside in luminal layers and were K8 immunoreactive (Fig. 4B–4F). Quantifying these K8-positive mGFP-positive castration-resistant cells in different prostatic lobes showed 17.2% ± 3.7% in AP, 12.5% ± 2.5% in DLP, and 6.4% ± 2.7% in VP, respectively (Fig. 4Q; Supporting Information Table S5). We also detected a small portion of K5-positive Axin2-expressing cells in basal cell layers of different prostatic lobes, which are 1.1% ± 0.6% (AP), 0.9% ± 0.5% (DLP) and 0.1% ± 0.1% (VP), respectively (Supporting Information Fig. S4G, Table S5). Interestingly, using the Nkx3.1-specific antibody, only a few castration-resistant Axin2-expressing epithelial cells were also Nkx3.1-positive (Supporting Information Fig. S4B– S4D).
Figure 4. Axin2-expressing cells in the regressed prostate gland show unipotent luminal progenitor properties.
(A): A scheme for the multiple round repression-regeneration assays. (B–P): mT (red) and mGFP (green) staining and co-immunofluorescence analyses in different prostatic lopes at different time points (see Fig. 4A). Percentages of mGFP+ cells in K8+ (Q) and in K5+ cells (R) at 7 days after tamoxifen injection (white bars), after the 1st round of regression-regeneration (red bars), or after the 3rd round of regression/regeneration (blue bars) in AP, DLP and VP. Error bars indicate standard deviation. *, P < 0.01 by ANOVA. Scale bars, 20 µm.
We then tracked the fate of the castration-resistant Wnt/β-catenin-responsive cells in Axin2CreERT2/+;RosamTmG/+ mice through androgen-mediated prostatic regeneration (Fig. 4A). The percentage of mGFP-positive luminal cells in different prostatic lobes is more than 3 fold higher in the regenerated prostates (at week 17.5) than in the regressed prostates (at week 13) (Fig. 4G–4K, 4Q; Supporting Information Table S5). In contrast, there was no significant expansion of mGFP-positive cells in the basal layers (Fig. 4R; Supporting Information Fig. S4H, S4J, Table S5). To assess the long-term self-renewal capacity of castration-resistant Wnt/β-catenin-responsive cells, we subjected Axin2CreERT2/+;RosamTmG/+ mice to three-rounds of regression-regeneration (Fig. 4A). Quantifying total mGFP-positive cells in the regenerated prostatic lobes at week 33.5 showed that mGFP-positive cells preserved the number of their descendants in the luminal layers after multiple rounds of tissue regeneration (Fig. 4L–4Q; Supporting Information Table S5). Thus, we conclude that Wnt/β-catenin-responsive cells in the regressed prostates possess unipotent luminal progenitor properties that show long-term proliferative potential during multiple rounds of tissue regeneration.
Wnt/β-catenin signaling is required for castration-resistant Axin2-expressing cells to expand the luminal lineages
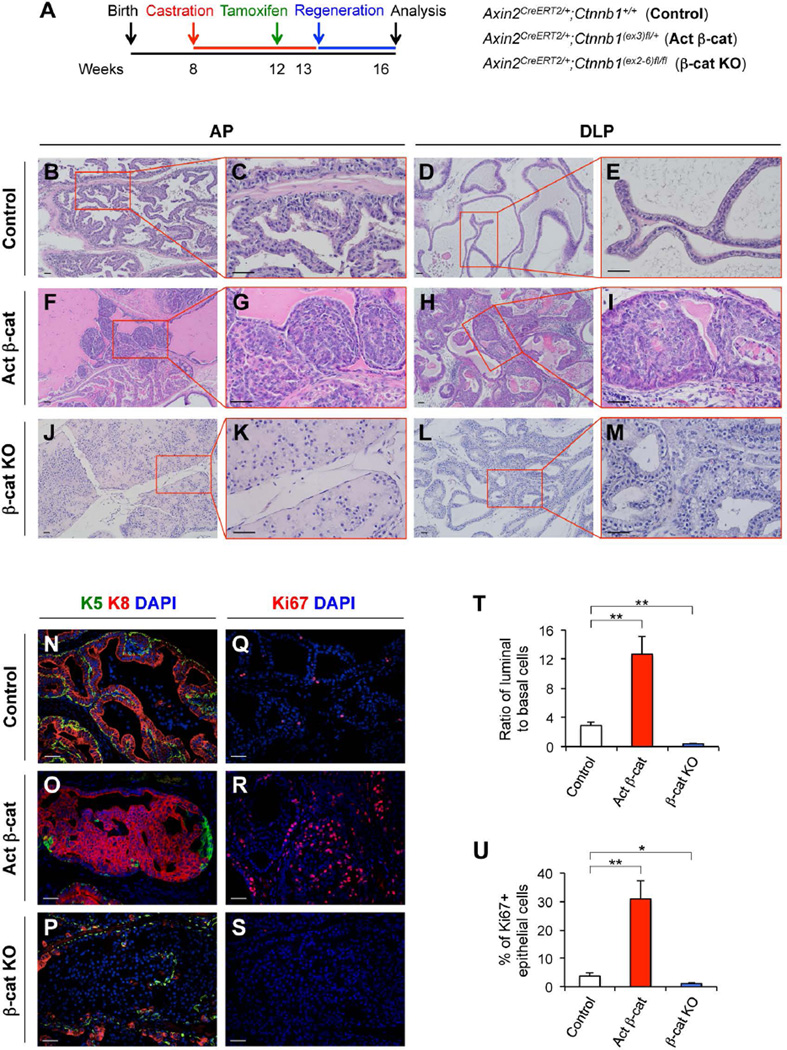
Next, we directly examined the significance of Wnt/β-catenin signaling in castration-resistant Wnt/β-catenin-responsive cells’ capability to expand the luminal lineages during androgen-mediated prostatic regeneration. Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Activated β-cat) and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl (β-cat KO) mice as well as controls, Axin2CreERT2/+;Ctnnb1+/+, were castrated at 8 weeks of age, administered TM at 12 weeks of age, supplemented with testosterone pellets at 13.5 weeks of age, and analyzed at 16.5 weeks of age (Fig. 5A). Histologically, we did not observe any abnormalities in the prostates of Axin2CreERT2/+;Ctnnb1+/+ mice (Fig. 5B–5E). However, there were pathological changes typical of intracystic adenocarcinoma (carcinoma in situ) in all prostatic lobes of Axin2CreERT2/+;Ctnnb1(ex3)fl/+ mice (Fig. 5F–5I). Tumor cells in the adenocarcinoma lesions expressed stabilized β-catenin, K8, AR and Ki67, but not K5 or p63 (Fig. S5B–5H). In Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice, we observed a lack of luminal cell enfolding and reduced branching morphogenesis in AP and DLP (Fig. 5J–5M; Supporting Information Fig. S5I, S5J). Immunohistochemical analyses confirmed the loss of β-catenin expression in “luminal-like epithelial cells”, which reside in prostatic luminal layers (Supporting Information Fig. S5M), but are not immunoreactive to K8, K5, or p63 antibodies (Supporting Information Fig. S5K, S5L); however, the expression of AR and E-cadherin was observed in some of these cells (Supporting Information Fig. S5N, S5O). To further assess the biological role of β-catenin in prostatic epithelium regeneration, we analyzed the ratio of luminal and basal cells in the prostates of the above mice using specific K8 and K5 antibodies. The ratio of prostatic luminal to basal epithelial cells in Axin2CreERT2/+;Ctnnb1+/+ control mice was maintained at 2.9:1 (Fig. 5N, 5T). However, a significant increase of luminal to basal cell ratio, approximately 12.7:1, was observed in the prostate of Axin2CreERT2/+;Ctnnb1(ex3)fl/+ mice (Fig. 5O, 5T), and a decrease of luminal to basal cell ratio, 0.4:1, appeared in the prostate of Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice (Fig. 5P, 5T; Supporting Information Fig. S5K, Table S6). This suggests a critical role of Wnt/β-catenin signaling in luminal lineage expansion during prostatic regeneration. To further assess the effect of Wnt/β-catenin signaling in the castration-resistant Axin2-expressing cells, we analyzed the epithelial proliferation in regenerated prostates by measuring Ki67-positive epithelial cells. There are 3.8% ± 1.1% of Ki67-positive epithelial cells in the prostate of Axin2CreERT2/+;Ctnnb1+/+ mice, whereas 31.0% ± 6.4% and 1.1% ± 0.5% in the prostates of Axin2CreERT2/+;Ctnnb1(ex3)fl/+ and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl mice, respectively (Fig. 5Q–5S, 5U; Supporting Information Fig. S5F, Table S7). In summary, our results demonstrate the decisive role of Wnt/β-catenin signaling in castration-resistant Axin2-expressing cell-mediated epithelial cell regeneration, and the dysregulation of this signaling pathway resulting in abnormal prostatic development and tumorigenesis.
Figure 5. β-catenin is required for castration-resistant Axin2-expressing cells to expand luminal lineages during prostatic regeneration.
(A): A scheme of the experimental designs for analyzing castration-resistant Wnt/β-catenin-responsive cells in Axin2CreERT2/+;Ctnnb1+/+ (control), Axin2CreERT2/+;Ctnnb1(ex3)fl/+ (Activated β-cat), and Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl (β-cat KO) mice. (B–M): H&E staining of AP and DLP tissues isolated from different mice. (N–P): Co-immunofluorescence staining of K5 (green), K8 (red), and DAPI, and Ki67 and DAPI in different mouse models. Representative images of AP were shown. (T): Ratios of luminal (K8+) versus basal (K5+) cells in prostate tissues isolated from mice received testosterone treatment for 3 weeks. (U): Percentages of Ki67+ epithelial cells in the above prostate tissues. Error bars indicate standard deviation. *, P < 0.05; **, P < 0.01 by Student’s t tests. Scale bars, 50 µm.
DISCUSSION
Mouse prostatic development initiates at embryonic days 17.5 (E17.5) from UGS upon rising levels of testicular androgens [3]. Expression of Axin2, a direct transcriptional target of β-catenin, has been observed in UGE [20]. Deletion of the Ctnnb1 gene in the mouse prostate at E15.5 results in significantly decreased prostatic budding and abrogated prostatic development [20]. However, the precise mechanisms underlying the Wnt/β-catenin signaling pathway in regulating embryonic prostatic development and organogenesis is still largely unknown. In this study, we first utilized Axin2CreERT2/+;RosamTmG/+ mice to assess the distribution of Wnt/β-catenin-responsive cells at embryonic stages. We observed that most Wnt/β-catenin-responsive cells are located within UGM as well as in newly formed epithelial budding tips at E17.5. Immunohistochemical analyses showed the expression of E-cadherin, p63, K5, K8, and Nkx3.1 in these cells. Tracking these genetically labeled cells through puberty into adulthood, we identified luminal progenitor properties of these Wnt/β-catenin-responsive cells. At P60, we observed clusters of mGFP-positive epithelial cells in all three prostatic lobes. These mGFP-positive cells are limited to luminal cell layers and are immunoreactive for K8 and the androgen receptor (AR), but not to K5, p63, or synatophysin antibodies. These data provide the first line of evidence that demonstrates the existence and cellular identity of prostatic Wnt/β-catenin-responsive cells at embryonic stages, and their ability to generate luminal lineages throughout prostatic epithelial development.
Although the growth of mouse prostatic buds initiates at E17.5, substantial elongation, growth, and patterning of prostatic ducts continues after birth [2]. Prostatic ductal branching morphogenesis starts through the interaction between prostatic epithelial buds and mesenchymal pads, which leads to the development of secondary, tertiary, and further ducts [31]. Currently, the importance of Wnt/β-catenin signaling in prostatic maturation and maintenance during puberty and adulthood is largely unclear. Therefore, we directly addressed this question in this study. Because the branching and patterning of the prostatic lobes are completed three weeks after birth [2], we genetically marked the Wnt/β-catenin responsive cells at P14 and analyzed the mice at P19. We observed that within all of the different prostatic lobes the majority of mGFP-positive cells were K8-positive luminal epithelial cells, and only a few were K5-positive basal epithelial cells. In the tracking experiments, less than 8% of the luminal cells in the prostatic lobes at P19 were mGFP-positive, while up to 50% of the luminal cells were mGFP-positive at P60 (Fig. 2; Supporting Information Fig. S2). These adult mGFP-positive cells expressed several luminal cell markers, including AR, Nkx3.1, and K8; they also appeared more proliferative in comparison with mGFP- negative cells. In contrast, we did not observe significant differences in the numbers of K5-positive mGFP-positive cells between P19 and P60. These results indicate that the prepubescent Wnt/β-catenin responsive cells have replicative properties and are able to generate the luminal cell population through puberty.
Androgen signaling plays an essential role in prostatic development and differentiation. It has been suggested that prostatic stem/progenitor cells in the adult mouse have the ability to survive androgen withdrawal and to regenerate prostatic glands when androgens are supplemented [32]. This androgen-withdrawal and supplementation cycle can be repeated several times. Although the basal epithelial cells were suggested to be responsible for prostate gland regeneration in mice, the cellular identity of the prostatic stem cell population in the adult mouse prostate is still controversial. We have shown that the prepubescent Wnt/β-catenin responsive cells are of luminal epithelial cell origin and can significantly expand the luminal epithelial cell population through puberty. In this study, we further assessed the cellular property of castration-resistant Wnt/β-catenin responsive cells. We observed that most of castration-resistant Axin2-expressing cells reside in luminal layers and are immunoreactive to K8 antibody. Using a classic prostatic regression-regeneration assay, we tested the regenerative ability of the castration-resistant Wnt/β-catenin-responsive cells. We observed a significant expansion of mGFP-positive luminal cells in different prostatic lobes of castrated Axin2CreERT2/+;RosamTmG/+ mice when they were supplemented with androgens after 4 weeks. Intriguingly, we demonstrated mGFP-labeled cells preserved the number of their descendants in the luminal layers after three-rounds of androgen withdrawal and supplementation cycles. These data suggest that the castration-resistant Wnt/β-catenin-responsive cells possess unipotent luminal progenitor properties and long-term proliferative potential during multiple rounds of tissue turnover. It has been shown that castration-resistant Nkx3.1-expressing cells (CARNs) are of luminal cell origin and retain stem cell ability being capable of generating prostatic luminal, basal and neuroendocrine cells [30]. However, we only observed a very small portion of the castration-resistant Axin2-expressing cells that are immunoresponsive to Nkx3.1 antibody. In these experiments, we also observed a small portion of castration-resistant Axin2-expressing cells being immunoreactive to K5 antibody. However, through either single or multiple androgen withdrawal and supplement cycles, we did not observe a significant increase of K5-positive mGFP-positive cells in all different prostatic lobes.
Multiple lines of evidence indicate a critical role of Wnt signaling in cell fate determination during early development [26, 33]. Conditional deletion of the β-catenin gene (Ctnnb1) in the mouse prostate during embryonic stages results in abrogated prostatic development [20]. Using Axin2CreERT2/+;Ctnnb1(ex2-6)fl/fl (β-cat KO) mice, we investigated the biological role of Wnt/β-catenin signaling in prepubescent Axin2-expressing cells. Those mice were administered TM at P14 and then analyzed at P35; where we observed reduced prostatic gland size, decreased luminal secretions, and abnormal branching morphogenesis. Using a similar approach, we also analyzed the effect of β-catenin deletion in castration-resistant Axin2-expressing cells. The lack of luminal cell enfolding and reduced branching morphogenesis was mainly observed in AP and DLP in these mice. These data is consistent with the previous finding and further supports a critical role of β-catenin in prostatic development, maturation, and regeneration.
The cellular levels of β-catenin are tightly regulated in normal cells. Mutations affecting the degradation of β-catenin can increase the cellular levels of the proteins [16]. Moreover, E-cadherin complexes with the actin cytoskeleton via catenins to maintain the functional characteristics of epithelia [34, 35]. Disruption of this complex, due primarily to loss or decreased expression E-cadherin, may also increase cellular β-catenin to promote oncogenic transformation [36, 37]. Conditional expression of stabilized β-catenin in the mouse prostate results in hyperplasia and prostatic intraepithelial neoplasia (PIN) [38, 39]. In addition to its oncogenic effect, stabilized β-catenin can also change cellular differentiation fate, which further results in either preventing prostatic maturation or inducing transdifferentiation into squamous epithelium [38–40]. Using Axin2CreERT2/+:Ctnnb1L(ex3)/+ mice, we directly assessed the role of stabilized β-catenin in Axin2-expressing cells. Activation of stabilized β-catenin expression at P14 results in PIN lesions with atypical cells and intraluminal keratin. Intriguingly, activation of stabilized β-catenin expression in castrated-resistant Axin2-expressing cells induces the formation of prostatic intracystic adenocarcinoma. Both atypical and tumor cells appear to be of luminal cell origin and with K8 and AR expression. Axin2 has been shown to be a downstream target gene of β-catenin [22], and it is also involved in regulating β-catenin degradation [23, 41]. Given that Axin2-expressing cells have stem/progenitor cell properties in a variety of mouse tissues [24–27], our findings suggest a potential role of abnormal activation of β-catenin expression in Axin2-expressing cells during the course of oncogenic transformation and tumor formation.
CONCLUSIONS
Prostatic Axin2-expressing cells express luminal epithelial cell markers and are able to expand luminal cell lineages during prostatic development and maturation. They can also survive androgen withdrawal and regenerate prostatic luminal epithelial cells following androgen replacement. Deletion of β-catenin or expression of stabilized β-catenin in these Axin2-expressing cells results in abnormal development and oncogenic transformation, respectively. Our study uncovers a critical role of Wnt/β-catenin-responsive cells in prostatic development and regeneration, and that dysregulation of Wnt/β-catenin signaling in these cells contributes to prostatic developmental defects and tumorigenesis.
Supplementary Material
SIGNIFICANCE STATEMENT.
Using Axin2CreERT2 and other mouse models, we genetically labeled Axin2-expressing cells at various time points and tracked their cellular behavior at different stages. We demonstrated for the first time that prostatic Axin2-expressing cells express luminal epithelial cell markers and are able to expand luminal cell lineages during prostatic development and maturation. These cells can also survive androgen withdrawal and regenerate luminal epithelial cells following androgen replacement. Deletion of β-catenin or expression of stabilized β-catenin in these Axin2-expressing cells results in abnormal development and oncogenic transformation.
ACKNOWLEDGEMENTS
We are grateful for Dr. Cory Abate-Shen for providing Nkx 3.1 antibody. This work was supported by the NIH grants R01CA070297, R01CA151623, U01CA166894, R21CA190021, and R01DK104941.
Footnotes
Author Contributions:
Suk Hyung Lee: Conception and design, collection and assembly of data, manuscript writing
Daniel T. Johnson: Data analysis and interpretation, manuscript writing
Richard Luong: Data analysis and interpretation, manuscript writing
Eun Jeong Yu: Collection and assembly of data
Gerald R. Cunha: Data analysis and interpretation, manuscript writing
Roel Nusse: Data analysis and interpretation
Zijie Sun: Conception and design, Data analysis and interpretation, manuscript writing
Disclosure of Potential Conflict of Interest: The authors indicate no potential conflicts of interests.
References
- 1.Staack A, Donjacour AA, Brody J, et al. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 2.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 3.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 4.Chung LW, Cunha GR. Stromal-epithelial interactions: II. Regulation of prostatic growth by embryonic urogenital sinus mesenchyme. Prostate. 1983;4:503–511. doi: 10.1002/pros.2990040509. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi N, Sugimura Y, Kawamura J, et al. Morphological and functional heterogeneity in the rat prostatic gland. Biol Reprod. 1991;45:308–321. doi: 10.1095/biolreprod45.2.308. [DOI] [PubMed] [Google Scholar]
- 6.Cunha GR, Donjacour AA, Sugimara Y. Stromal-epithelial interactions and heterogeneity of proliferative activity within the prostate. Biochem Cell Biol. 1986;64:608–614. doi: 10.1139/o86-084. [DOI] [PubMed] [Google Scholar]
- 7.Choi N, Zhang B, Zhang L, et al. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ousset M, Van Keymeulen A, Bouvencourt G, et al. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- 9.Qin J, Liu X, Laffin B, et al. The PSA(−/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 2012;10:556–569. doi: 10.1016/j.stem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Zhang B, Han SJ, et al. Targeting CreER(T2) expression to keratin 8-expressing murine simple epithelia using bacterial artificial chromosome transgenesis. Transgenic Res. 2012;21:1117–1123. doi: 10.1007/s11248-012-9598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimeault M, Mehta PP, Hauke R, et al. Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr Rev. 2008;29:234–252. doi: 10.1210/er.2007-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum R, Gupta R, Burger PE, et al. Molecular signatures of the primitive prostate stem cell niche reveal novel mesenchymal-epithelial signaling pathways. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24:881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- 14.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 15.Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- 16.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 17.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 18.Prins GS, Putz O. Molecular signaling pathways that regulate prostate gland development. Differentiation. 2008;76:641–659. doi: 10.1111/j.1432-0436.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis JC, Thomsen MK, Taketo MM, et al. beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9:e1003180. doi: 10.1371/journal.pgen.1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons BW, Hurley PJ, Huang Z, et al. Wnt signaling though beta-catenin is required for prostate lineage specification. Dev Biol. 2012;371:246–255. doi: 10.1016/j.ydbio.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ontiveros CS, Salm SN, Wilson EL. Axin2 expression identifies progenitor cells in the murine prostate. Prostate. 2008;68:1263–1272. doi: 10.1002/pros.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan D, Wiesmann M, Rohan M, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman AN, van Amerongen R, Palmer TD, et al. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/beta-catenin-responsive neural stem cells. Proc Natl Acad Sci U S A. 2013;110:7324–7329. doi: 10.1073/pnas.1305411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ardehali R, Ali SR, Inlay MA, et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc Natl Acad Sci U S A. 2013;110:3405–3410. doi: 10.1073/pnas.1220832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blauwkamp TA, Nigam S, Ardehali R, et al. Endogenous Wnt signalling in human embryonic stem cells generates an equilibrium of distinct lineage-specified progenitors. Nat Commun. 2012;3:1070. doi: 10.1038/ncomms2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Brault V, Moore R, Kutsch S, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 29.Muzumdar MD, Tasic B, Miyamichi K, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomson AA, Timms BG, Barton L, et al. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. [DOI] [PubMed] [Google Scholar]
- 32.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 33.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birchmeier W, Hulsken J, Behrens J. Adherens junction proteins in tumour progression. Cancer Surv. 1995;24:129–140. [PubMed] [Google Scholar]
- 35.Huber AH, Stewart DB, Laurents DV, et al. The cadherin cytoplasmic domain is unstructured in the absence of b-catenin: a possible mechanism for regulating cadherin turnover. J Biol Chem. 2001 doi: 10.1074/jbc.M010377200. In press. [DOI] [PubMed] [Google Scholar]
- 36.Graff JR, Herman JG, Lapidus RG, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 1995;55:5195–5199. [PubMed] [Google Scholar]
- 37.Mitchell S, Abel P, Ware M, et al. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 2000;85:932–944. doi: 10.1046/j.1464-410x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 38.Bierie B, Nozawa M, Renou JP, et al. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- 39.Yu X, Wang Y, DeGraff DJ, et al. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868–1879. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, et al. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 41.Lustig B, Jerchow B, Sachs M, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.