Summary
The differentiation of CD4+ helper T cell subsets with diverse effector functions is accompanied by changes in metabolism required to meet their bioenergetic demands. We find follicular B helper T (Tfh) cells exhibited less proliferation, glycolysis, and mitochondrial respiration, accompanied by reduced mTOR kinase activity compared to T helper 1 (Th1) cells in response to acute viral infection. IL-2-mediated activation of the Akt kinase and mTORc1 signaling was both necessary and sufficient to shift differentiation away from Tfh cells, instead promoting that of Th1 cells. These findings were not the result of generalized signaling attenuation in Tfh cells, as they retained the ability to flux calcium and activate NFAT transcription factor-dependent cytokine production. These data identify the interleukin-2 (IL-2)-mTORc1 axis as a critical orchestrator of the reciprocal balance between Tfh and Th1 cell fates and their respective metabolic activities following acute viral infection.
Graphical Abstract

Introduction
The differentiation of functionally distinct CD4+ T helper (Th) cell subsets from naïve precursors occurs through the concerted actions of cognate peptide:major histocompatibility complex class II (MHCII) molecular interactions, co-stimulation, and polarizing cytokine signals. Follicular B helper T (Tfh) cells are distinguished from other Th cells by their selective role in initiating and orchestrating germinal center (GC) responses, with promotion of immunoglobulin affinity maturation and development of memory B and long-lived plasma cells (Crotty, 2011).
Differential cytokine signaling regulates Tfh versus Th1 cell differentiation. Interleukin-6 (IL-6) and autocrine IL-21 signaling via the STAT3 transcription factor potentiates Bcl6 up-regulation and differentiation of Tfh cells (Eto et al., 2011; Karnowski et al., 2012; Linterman et al., 2010; Nurieva et al., 2008; Ray et al., 2014), whereas IL-2 activation of STAT5 suppresses STAT3 binding to the Bcl6 locus, and promotes the expression of the transcription factor PR domain zinc finger protein 1 (B lymphocyte-induced maturation protein-1, Blimp-1), necessary for Th1 cell differentiation (Johnston et al., 2012; Oestreich et al., 2012). The expression of Bcl6 and Blimp-1 are mutually exclusive, with overexpression of either sufficient to drive the differentiation of Tfh or Th1 cells, respectively, at the expense of the other (Johnston et al., 2009). Tfh cells accordingly have reduced interleukin-2 receptor α chain (IL-2Rα, CD25) expression and p-STAT5 signaling, and as a result, reduced Blimp-1 synthesis, enabling their Bcl6-dependent differentiation (Choi et al., 2013; Ray et al., 2014).
While IL-2 induction of Blimp-1 through p-STAT5 is important for the Th1 cell differentiation, this cytokine also signals via phosphatidylinositol-3-OH kinase (PI(3)K), the serine-threonine kinase Akt, and the nutrient sensor and metabolic regulator mTOR (Powell et al., 2012). CD28 also is an inducer of PI3K, as well as IL-2 production, during T cell priming (Harada et al., 2003). Thus, T cell co-stimulation and IL-2 jointly feed into the PI3K pathway, enabling effector T cells to activate mTOR, with the latter promoting cellular growth, nutrient uptake, protein synthesis, and clonal expansion (Brennan et al., 1997; Sinclair et al., 2013). Th1, Th2, and Th17 cells depend on mTOR signaling to varying degrees to guide their expression of lineage-defining transcription factors -- T-bet, GATA3, and RORγt, respectively -- and to carry out their specialized effector functions (Delgoffe et al., 2009; Powell et al., 2012). In contrast, the differentiation of regulatory T (Treg) and memory CD8+ T cells is fostered by attenuated mTOR activity (Delgoffe et al., 2009; Michalek et al., 2011) and a reliance on fatty acid oxidation (O’Sullivan et al., 2014; Pearce et al., 2009; van der Windt et al., 2012).
Because Tfh cell differentiation requires reduced IL-2 and STAT5 signaling, these cells are likely to exhibit reduced mTOR activity (Johnston et al., 2012). This idea finds support in the observation that the expression of T-bet and granzymes, which are dependent on IL-2 and mTOR signaling in CD4+ and CD8+ T cells (Delgoffe et al., 2009; Rao et al., 2010), is reduced in Tfh cells. Additionally, Bcl6 has recently been reported to downregulate genes associated with glycolysis, with T-bet conversely inhibiting Bcl6-mediated repression of genes involved in its regulation (Oestreich et al., 2014).
Herein, we have used an acute viral model in order to better understand the role of IL-2 and mTOR signaling in Tfh cell development and function. We found Tfh cells are less proliferative and have less glycolysis and mitochondrial oxidation than Th1 cells, results stemming from a paucity in IL-2 signaling and activation of mTOR through PI3K and Akt. While Akt and mTOR signaling in response to IL-2 was essential to promote Blimp-1 and T-bet expression and differentiation of the Th1 cell lineage, this occurred at the expense of Tfh cells. Despite the reduction in these signaling cascades, we found that calcium influx in Tfh cells, nuclear translocation of the transcription factor nuclear factor of activated T cells (NFAT), and NFAT-mediated production of the canonical Tfh cell cytokine IL-21 remained intact, demonstrating that there is a selective defect in Akt and mTOR signaling in these cells. These findings unveil a linear pathway of IL-2→Akt-mTOR signaling in regulating the reciprocal identity and metabolism of Tfh and Th1 cells following acute viral infection.
Results
Tfh cells are less proliferative than Th1 cells following viral challenge
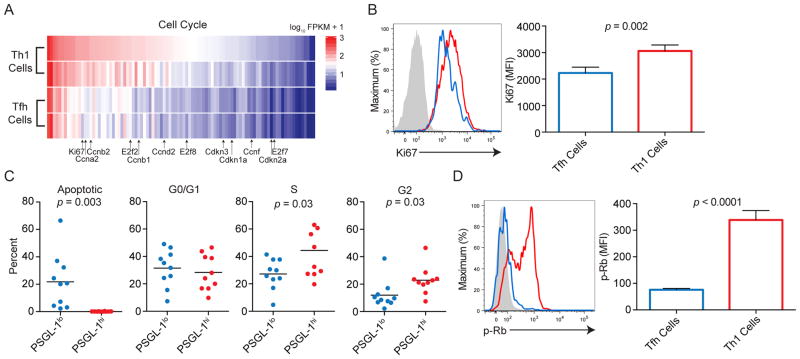
We utilized RNA-seq data (Ray et al., 2014) to better understand the signaling and metabolic pathways that control the differentiation of Tfh cells (sorted on previously defined markers CD4+ CD44+ PSGL-1lo Ly6clo CXCR5hi), with comparison to terminal effector Th1 cells (CD4+ CD44+ Ly6chi PSGL-1hi) at 8 days post-lymphocytic choriomeningitis virus (LCMV) infection (dpi) (Marshall et al., 2011; Ray et al., 2014). To parse patterns in the global gene expression programs of Tfh versus Th1 cells, we used the DAVID gene ontology website (http://david.abcc.ncifcrf.gov, Table S1). Among the top differentially expressed gene sets were those involved in cell cycle regulation, including cyclins, cyclin dependent kinases, and elongation factors (Figures 1A and S1A and Table S1), consistent with a previous report (Choi et al., 2013). To confirm whether cell cycle-affiliated protein expression was reduced in viral-specific Tfh cells compared to Th1 cells, we transferred 2 x 104 LCMV GP66 SMARTA TCR transgenic (Stg+) CD4+ T cells into congenically marked wild type recipients and infected them with the Armstrong strain of LCMV (Oxenius et al., 1998). As expression of Ly6c and PSGL-1 distinguishes Tfh from Th1 cells, and correlates with the respective cell subsets (Hale et al., 2013; Marshall et al., 2011), we used them as markers unless otherwise noted in the figure legend. Staining for the proliferation marker Ki67 was reduced in Stg+ Tfh cells compared to Stg+ Th1 cells at 8 dpi (Figure 1B), consistent with the gene expression data.
Figure 1. Tfh cells are less proliferative than Th1 cells.
(A) Cell cycle gene signature of differentially expressed transcripts in Tfh and Th1 cells. Genes selected according to DAVID analysis. Transcripts of cyclins, cyclin dependent kinases, and elongase factors are listed. (B) Ki67 staining at 8 dpi of LCMV. (C) Percentages of Stg+ CD4+ CD44+ PSGL-1lo and Stg+ CD4+ CD44+ PSGL-1hi cells that were apoptotic or at different cell cycle stages, as determined by propidium iodide (PI) staining. (D) p-Rb staining in Tfh (Stg+ CD4+ CD44+ Ly6clo PSGL-1lo) and Th1 cells (Stg+ CD4+ CD44+ Ly6chi PSGL-1hi) (Marshall et al., 2011; Ray et al., 2014). Blue and red lines represent gating on Tfh and Th1 cells, respectively, with filled grey histograms denoting naïve CD4+ T cells; (B, D). Results are representative of 2 independent experiments with at least 4 animals per experiment (B, D), with data from two independent experiments combined in (C). + SEM; (B, D). Student’s unpaired t-test; (B–D). Please see Figure S1 and Table S1.
To determine the stage of the cell cycle in which Tfh cells were arrested, we used propidium iodide staining to distinguish apoptosis and the G0/G1, S, and G2 phases of the cell cycle (Staron et al., 2011). While Tfh cells were mainly apoptotic, or in the G/0G1 phases at day 5 dpi, Th1 cells were enriched at the S and G2 phases (Figures 1C and S1B). Tfh cells also were more robustly stained with caspase and Annexin V compared to Th1 cells at 8 dpi (Figures S1C and D). Analysis of Tfh cells, designated as CXCR5hi, ICOShi, and/or PD-1hi, replicated these findings (Figures S1E and F; data not shown). Moreover, phosphorylated retinoblastoma tumor suppressor protein (p-Rb), which allows E2F-driven G1- to S-phase cell cycle progression (Sherr, Science, 1996), was significantly reduced in Stg+ Tfh compared to Stg+ Th1 cells at 5 dpi (Figure 1D).
Features of anabolic metabolism are reduced in Tfh cells compared to Th1 cells
Progression through the cell cycle is accompanied by the up-regulation of aerobic glycolytic metabolism that allows for optimal cell growth and division (Pearce and Pearce, 2013). Since Tfh cells had reduced proliferation compared to Th1 cells (Figure 1B), we asked if their metabolic function, particularly their ability to utilize glycolysis and oxidative phosphorylation, was comparatively impaired. The consumption and utilization of glucose in the presence of oxygen, or aerobic glycolysis, is upregulated in proliferative effector T cell subsets (Fox et al., 2005; Jones and Thompson, 2007). Furthermore, aerobic glycolysis as part of anabolic metabolism is differentially regulated among effector, regulatory, and memory T cell subsets that have different immunological functions and bioenergetic demands (Dang et al., 2011; Michalek et al., 2011; Pearce et al., 2009; Shi et al., 2011). We analyzed the mitochondrial mass and function of Tfh cells and Th1 cells during their development (5 dpi). While these subsets were not different in mitochondrial mass as assessed by Mitotracker Green labeling, there was a slight reduction in the mitochondrial membrane potential of Tfh cells, determined by Mitotracker Deep Red (DR) (Figures S2A and B). The ratio of mitochondrial mass to potential in Tfh cells indicated significantly reduced mitochondrial activity compared to their Th1 cell counterparts (Figure 2A). While their baseline oxygen consumption rate (OCR) was similar to that of Th1 cells, uncoupling the mitochondrial proton gradient to allow for maximal respiratory respiration using FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone), revealed a significant reduction in the maximal respiratory capacity of Tfh cells (Figure 2B, right). The fluorescent glucose analog 2-NBDG is used to assess cellular glucose uptake, with 2-NBDGlo CD8 T cells expressing more Bcl6 than 2-NBDGhi cells (Sukumar et al., 2013). Consistent with these studies, Tfh cells had reduced 2-NBDG staining (Figure 2C). And, consistent with this latter finding, Tfh cells displayed reduced extracellular acidification rates (ECAR), a measure of glycolysis, at baseline as well as upon blockade of mitochondrial ATP synthesis by oligomycin, a gauge of glycolytic flux (Figure 2D). To ensure that this phenomenon was not due to apoptotic Tfh cells, we baseline corrected the data at measurement 3 (Figure S2C). With this correction, we found that Tfh cell maximal respiratory capacity was more similar to that of Th1 cells compared to the non-baseline corrected condition, while their ability to acidify their extracellular environment remained substantially reduced. Thus, while Tfh cells had overall reduced mitochondrial and glucose metabolism, they were relatively more oxidative than glycolytic compared to Th1 cells, as demonstrated by an increase in their OCR/ECAR baseline ratio (Figure 2E). As Bcl6 competes with T-bet to regulate the glycolytic gene program of T cells (Oestreich et al., 2014), we overexpressed Bcl6 in vitro, finding that this transcription factor was able to suppress CD4+ T cell ECAR, recapitulating the reduction in glucose metabolism and higher reliance on oxidative metabolism that we observed in Tfh compared to Th1 cells (Figures 2F–H). In sum, Tfh cells are less glycolytic, and compared to Th1 cells, rely more heavily on mitochondrial function than glycolysis.
Figure 2. Tfh cells have reduced metabolic function compared to Th1 cells.
(A) Mitotracker Deep Red (DR)/Mitotacker Green ratio of Tfh and Th1 cells at day 5 dpi. (B) Baseline OCR for Tfh and Th1 cells (left); Seahorse® traces for oxygen consumption rate (OCR) of Tfh and Th1 cells at 8 dpi, with measurements taken at baseline (calculated as the average of all three baseline data points) and after treatment with oligomycin, FCCP, etomoxir, and rotenone (right). (C) 2-NBDG uptake by Tfh and Th1 cells at 5 dpi. (D) Baseline ECAR (left); Seahorse® traces for extracellular acidification rate (ECAR) of Tfh and Th1 cells at 8 dpi (right). (E) Ratio of OCR to ECAR for Tfh and Th1 cells at baseline. (F–H) OCR, ECAR, and OCR/ECAR ratios of Stg cells following retroviral overexpression of Bcl6, compared to control vector (MIGR1). Blue and red lines represent gating on Tfh and Th1 cells, respectively, with the filled grey histogram (C) denoting naïve CD4+ T cells; (B–D). Results are representative of 2–3 independent experiments with 3–5 animals per experiment; (A–H). Student’s unpaired t-test; (A), (B, left panel), (C), (D, left panel), and (E–H). +SEM; (A–H). Two-Way Anova with multiple comparisons comparing means at each time point; (B) and (D), right panels. * p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001. Please see Figure S2.
Tfh cells compared to Th1 cells also have reduced Akt and mTOR signaling
The reduced glycolytic metabolism of Tfh compared to Th1 cells suggested that there might be differences in their integration of signaling pathways. Our RNA-seq data indicated that Tfh cells had reduced expression of cell cycle, growth, and metabolism signaling cascades, including members of the Ras and phosphoinositide 3-kinase (PI3K) families (Figures 3A and S1A, and Table S2). Integrated Pathway Analysis (IPA) using the Ingenuity platform (http://www.ingenuity.com) revealed a pattern of gene expression in Tfh cells compared to Th1 cells consistent with inhibition of PI3K and Akt signaling (i.e., reduced Pik3c2b and Pik3cg), and of factors that can facilitate anabolic metabolism (i.e., reduced Rps6ka1 and Rps6ka5) (Figures 3A and Table S3). Although generally regulated post-translationally, there is evidence for transcriptional regulation of these pathways. For example, PI3K is positively regulated at the transcriptional level via TNFα and NFκB signaling in ovarian cancer (Yang et al., 2008), consistent with the finding that Th1 cells reside in the proinflammatory splenic red pulp, while Tfh cells localize in white pulp B cell follicles (Marshall et al., 2011). In line with these data, we found p-Akt308, p-Akt473, the ribosomal protein S6 (p-S6), and p-FoxO1 and 3a to be reduced in Tfh cells (Figures 3C–F). Tfh cells were also substantially smaller than Th1 cells over the course of their development (Figure 3G), with reduced expression of amino acid receptor CD98 (Figure 3H). Thus, Tfh cells displayed a reduction in activity of Akt and the nutrient sensor mTOR compared to Th1 cells (Figure 3B).
Figure 3. Tfh cells have reduced signaling via metabolic pathways compared to Th1 cells.
(A) Signature of differentially expressed signaling-pathway genes in Tfh and Th1 cells. Genes associated with the Ras and PI3K pathways are highlighted. (B) Th1 cell PI3K signaling pathway (left); components of the Th1 cell pathways that could be inhibited to recapitulate the Tfh cell signaling signature according to IPA (right). Relative size of the Th1 versus Tfh cell is representative of size determined by flow cytometry (see (G)). (C–D) p-Akt308 and p-Akt473 staining, respectively, in Tfh and Th1 cells at 5 dpi. (E) p-S6 staining of Tfh and Th1 cells at 5 dpi. (F) p-FoxO1 and 3a staining of Tfh and Th1 cells at 5 dpi. (G) p-S6 by FSC-A mean fluorescence intensity (MFI) in Tfh and Th1 cells at 5 dpi (left); FSC-A at 3, 5 and 8 dpi of Tfh and Th1 cells (right). (H) CD98 expression of Tfh and Th1 cells at 5 dpi. Blue and red lines represent gating on Tfh and Th1 cells, respectively, with the filled grey histograms denoting naïve CD4+ T cells; (C–H). Results are representative of two independent experiments with 5 animals per experiment; (C–H). +SEM; (C–H). Student’s unpaired t-test; (C–F, H). Two-Way Anova with multiple comparisons comparing means at each time point (G). ** p ≤ 0.01, **** p ≤ 0.0001. Please see Table S2 and Table S3.
NFAT signaling is intact in Tfh cells
We next examined signaling pathways downstream of the TCR, observing that Tfh cells had reduced NFAT-affiliated gene transcription (Figure S3A and Table S4). Activation of this family of transcriptional cofactors with AP-1 and NF-κB, among others, by calcium influx in response to TCR and costimulatory signals upregulates the expression of genes involved in T cell priming, such as Il2 and Cd25 (Hermann-Kleiter and Baier, 2010; Pollizzi and Powell, 2014). Although the expression of many NFAT-dependent gene transcripts was reduced in Tfh cells, the Tfh transcriptional profile contained a small subset that were up-regulated (e.g., Egr2, Egr3, and Rnf128) by this transcription factor in the absence of cofactors (Figure 4A) (Macián et al., 2002; Wherry, 2011). This transcriptional profile is consistent with the activity of NFAT in the absence of that of AP-1, in agreement with the finding that Tfh cells have reduced AP-1 transcriptional activity due to Bcl6-AP-1 protein-protein interactions (Hatzi et al., 2015). Therefore, we asked if Tfh cells maintained the ability to flux calcium upon stimulation, finding that they did so as well, if not more robustly, than Th1 cells at 8 dpi (Figure 4B). Using Amnis Imagestream® analysis, we also observed an increase in the nuclear:cytoplasmic ratio of Nfatc1 at baseline and upon stimulation in Tfh cells compared to Th1 cells (Figures 4C–E and S3B). While cyclosporine A (CsA) blocked nuclear translocation of Nfatc1 in CD4+ T cells, treatment with rapamycin (RAPA) had little effect (Figures S3C and D). To this end, we examined the ability of Stg+ Tfh cells to produce IL-21 and IFN-γ ex vivo 8 dpi in response to LCMV GP66 stimulation in the presence of CsA or RAPA. As we had limited antibody-conjugated fluorophore options for staining PSGL-1, we gated Tfh cells as Stg+ CD4+ CD44+ Ly6clo CXCR5hi and Th1 cells as Stg+ CD4+ CD44+ Ly6chi CXCR5lo. Although IL-21 and IFN-γ production was essentially ablated by CsA treatment, RAPA had little effect (Figures 4F and G), consistent with the observation that NFAT is required for IL-21 signaling in vitro (Kim et al., 2005). We also asked if Tfh cells require glucose or oxidative phosphorylation to promote cytokine responses using the glucose analog 2-DG and oligomycin, finding that both bioenergetic pathways were necessary (Figure S3E). These data indicate that while mTOR activity is inactive in Tfh compared to Th1 cells, calcium signaling with NFAT nuclear translocation is operational and required for Tfh cell cytokine production. In addition, both oxidative phosphorylation and glucose uptake are required for Tfh cell cytokine production, despite mTOR dispensability, in contrast to Treg cells where both mTOR and glucose are dispensable (Delgoffe et al., 2009; Michalek et al., 2011).
Figure 4. Calcium and NFAT signaling promote Tfh cell function.
(A) NFAT gene signature in Tfh and Th1 cells at 8 dpi. Highlighted transcripts are associated with NFAT signaling independent of cofactors. (B) Calcium influx as assessed by the ratio of Fura-4 to Fura-Red in Tfh (black line) and Th1 (grey line) cells. (C) Amnis Imagestream® survey of nuclear NFAT in PSGL-1lo and PSGL-1hi cells as determined by the similarity dilate algorithm (Volk et al., 2014), with an increase associated with nuclear NFAT. (D) Graph of similarity dilate in PSGL-1lo (solid bars) and PSGL-1hi (grey bars) cells in unstimulated conditions, or following stimulation with ionomycin with or without cyclosporine A (CsA) treatment. (E) Amnis Imagestream® images of ionomycin-treated Tfh cells and Th1 cells. (F) IFN-γ and IL-21 staining of Ly6clo CXCR5hi Tfh cells with or without CsA or rapamycin (RAPA) treatment. (G) Results from (F) based on cytokine amounts expressed in untreated samples (% of maximum). DP = Tfh cells staining for both IL-21 and IFN-γ; SP = Tfh cells staining for IFN-γ alone. Results are representative of two independent experiments with 3–5 animals per experiment; (B–G). +SEM; (D and G). One-Way Anova with multiple comparisons; (D and G). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Please see Figure S3, Figure S4 and Table S4.
Signaling via PI3K, Akt, and mTOR promotes Th1 cell differentiation at the expense of that of Tfh cells
Bcl6 controls the differentiation of Tfh cells, while T-bet and Blimp-1 regulate Th1 cell fate (Johnston et al., 2009; Oestreich et al., 2011). However, how these transcription factors affect metabolic properties in T cells is unclear. Our RNA-seq results confirmed that transcripts of transcription factors associated with Tfh versus Th1 cell differentiation segregated with their respective populations (Figure 5A and Table S5). For example, Prdm1 (Blimp-1) expression was markedly elevated in Th1 cells compared to Tfh cells, with the latter having relatively higher expression of Bcl6. Accordingly, we used a Blimp-1 YFP reporter to segregate these subsets early during their differentiation following LCMV infection (Rutishauser et al., 2009), asking if the reduction in Tfh versus Th1 cell Akt and mTOR activities (Figure 3) was concordant with the expression of transcription factors associated with Tfh and Th1 cell fates. As expected, Blimp-1 YFP+ CD4+ T cells were enriched in cells of the Th1, but not Tfh cell, phenotype (Figure 5B). Virtually all Blimp-1 YFP+ cells were blastogenic, as evidenced by increased FSC-A, and displayed increased surface expression of the PI3K-dependent amino acid receptor, CD98 (Figures 5C and D). p-S6 was also enriched in the Blimp-1 YFP+ population in vitro, and dependent on PI3K, Akt, and mTOR signaling as determined using the inhibitors LY294002 (LY), AktI, and RAPA, respectively (Figure 5E) (Staron et al., 2014). Furthermore, treatment with LY and RAPA reduced the ECAR of in vitro activated T cells (Figure S4A), in line with a role for mTOR in inducing glycolysis in Blimp-1 YFP+ cells.
Figure 5. Signaling via PI3K, Akt, and mTOR promotes Th1 cell differentiation at the expense of that of Tfh cells.
(A). Transcripts of transcription factors that are upregulated or downregulated in Tfh and Th1 cells. (B) Blimp-1 YFP+ and Blimp-1 YFP− cells gated on Ly6clo PSGL-1lo Tfh cells and Ly6clo PSGL-1lo Th1 cells (left). Percentages of Tfh cells and Th1 cells that make up the Blimp-1 YFPlo and YFPhi gates at 8 dpi (right). (C and D) FSC-A and CD98 expression in Blimp-1 YFPlo and YFPhi cells at 5 dpi. (E) p-S6 expression compared to Blimp-1 YFP expression for CD4+ T cells cultured in vitro with 1.0 μM CD3, 1.0 μM CD28, and 10 nM IL-2 (left). p-S6 expression in the same culture conditions, without or with inhibitors to PI3K (LY294002, LY, 25 μM), Akt (AktI, 1 μM), or mTOR (RAPA, 100 nM). (F) Blimp-1 YFP expression in CD4+ T cells following stimulation with varying concentrations of CD3 and CD28 (both each equal in concentration from 0 – 1.0 μM) without or with added IL-2 (0–100 ng/mL) (left). Blimp-1 YFP expression in CD4+ T cells using the culture conditions as in (E) (right). (G) Bcl6 expression in CD4+ T cells cultured in vitro with 1.0 μM CD3, 1.0 μM CD28, and 10 ng/mL IL-2, without or with addition of inhibitors to PI3K, Akt, or mTOR, as in (E). Filled grey histogram (G) denotes naïve CD4+ T cells. Representative of two independent experiments with 5 animals per experiment; (B–D). Representative of two independent experiments; (E–G). +SEM; (B–E). Two-Way Anova with multiple comparisons; (B). Student’s unpaired t-test; (C, D). **** p ≤ 0.0001. Please see Table S5.
TCR and cytokine signaling precedes the up-regulation of transcription factors required for T cell subset differentiation. In CD4+ T cells, IL-2 induces Blimp-1 expression at the expense of that of Bcl6 (Oestreich et al., 2012). Using graded doses of TCR and IL-2, we confirmed that Blimp-1 YFP was increased by TCR signaling and IL-2, with the latter effectively lowering the threshold of such expression (Figure 5F, left). While Blimp-1 YFP expression was almost entirely PI3K-, Akt-, and mTOR-dependent, as determined by inhibition of their respective signaling (Figure 5F, right), Bcl6 expression was largely insensitive to these interventions, with the exception of the PI3K inhibitor, LY294002 (Figure 5G). These inhibitors were specific to the IL-2-mediated activation of PI3K, as noted by a loss of p-S6 expression (Figure S4B, right), and intact p-STAT5 expression (Figure S4B, left). These data suggest Bcl6 expression is independent of Akt and mTOR signals, whereas cells expressing Blimp-1 are enriched in mTOR activity, with Akt and mTOR signals necessary to induce expression of the latter transcription factor.
mTOR alters the balance of Tfh versus Th1 cell differentiation
mTOR is necessary for Th1 subset differentiation (Delgoffe et al., 2009); however, its role in Tfh cell development is unclear. To this end, we retrovirally transduced constructs containing an mTOR small-hairpin RNA (shRNA) or control vector into CD4+ Stg+ T cells (Araki et al., 2009), followed by their transfer into wild type recipients and assessment of Tfh and Th1 cell percentages at 8 dpi with LCMV. To validate the efficacy of this vector, we confirmed that its transduction led to a reduction in cell size and CD98 expression (Rao et al., 2010) (Figures 6A and B). We next queried the effect of mTOR silencing on Tfh and Th1 cell development in vivo. Compared to cells bearing the control vector, percentages and numbers of Th1 cells were significantly reduced following mTOR silencing (Figures 6C and S5B, respectively), with a reduction in T-bet expression (Figure 6D). The effects on Tfh cells were more variable, however, with an increase in their percentage (Figure 6C) albeit without a significant change in their numbers (Figure S5B) and a minimal reduction in Bcl6 expression in transduced cells (Figure 6E).
Figure 6. mTOR alters the balance of Tfh versus Th1 cell differentiation.
(A and B) FSC-A and CD98 expression of total Stg+ T cells, transduced with an mTOR small-hairpin RNA (shRNA) or an empty control vector, 8 dpi following LCMV infection. (C) Percentages of Ly6chi PSGL-1hi Th1 and Ly6clo PSGL-1lo Tfh cells, as gated on CD4+ CD44hi Stg+ cells. (D, E) T-bet and Bcl6 expression in total Stg+ transduced T cells. (F) Percentages of Ly6chi PSGL-1hi Th1 and Ly6clo PSGL-1lo Tfh cells, as gated on CD4+ CD44hi Stg+ cells. (G) T-bet expression in total Stg+ transduced T cells. (H) MFI c-Myc following stimulation of Stg+ cells with GP66 in the presence or absence of rapamycin (RAPA). (I) c-Myc expression in Tfh and Th1 cells at 8 dpi. (J) Control or constitutively active (CA)-c-Myc transduced Stg+ T cells gated on Tfh and Th1 cells. Black and red lines for A, B, D, and E represent gating on control and mTOR shRNA transduced Stg+ T cells, respectively, with dashed lines denoting naïve CD4+ T cells. Black, blue, and red lines for G represent gating on control, Raptor, and Rictor shRNA transduced Stg+ T cells, respectively, with filled grey histograms denoting naïve CD4+ T cells. Results are representative of 2–4 independent experiments with 4–5 animals per condition per experiment; (A–J). +SEM; (A, B, D, E, G–I). Student’s unpaired t-test; (A–E, I, and J). One-Way Anova with multiple comparisons; (F–H). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Please see Figure S5.
We next assessed the ability of mTOR-deficient T cells to drive GC B cell formation. As OT-II TCR transgenic animals are unable to promote GC responses upon LCMV challenge (Marshall et al., 2015) (Figure S5C), we used this system to adoptively transfer transduced Stg+ T cells to promote B cell responses. We transferred 7,000-sorted control or mTOR shRNA transduced Stg+ T cells into these mice, the latter were equally capable in promoting GC B cell differentiation compared to animals receiving control-transduced cells (Figures S5D and E). We also found, through testing one of the cohorts, that recipients of either condition were equally capable in helping B cells promote pathogen specific antibody responses compared to infected transgenic mice without receipt of transferred cells (Figure S5F), consistent with the dispensability of mTOR signaling in Tfh cell function.
Because mTORc1 and mTORc2 have been shown to promote different T cell subsets, with the requirement of mTORc1 for Th1 and Th17 cells (Delgoffe et al., 2009), and mTORc2 for Th2 cells (Lee et al., 2010), we used shRNA vectors targeted against the Raptor (mTORc1) and Rictor (mTORc2) complexes to determine the roles of mTORc1 and mTORc2 in Th1 versus Tfh cell development. Raptor silencing, similar to that of total mTOR, induced Tfh cell differentiation at the expense of that of Th1 cells with reduction in T-bet expression (Figures 6F and G, and S5G). However, Rictor silencing had minimal effects on Tfh cell development, while promoting that of Th1 cells, albeit without a significant effect on T-bet expression. These findings suggest that mTORc2 promotes Tfh cell differentiation, a role that is dispensable in the absence of both complexes.
Given the finding that mTOR directs the balance of Tfh versus Th1 cell differentiation toward the latter, we wondered if c-Myc, which operates downstream of mTOR signaling to facilitate a glycolytic program in T cells (Wang et al., 2011), could deter Tfh cell differentiation. We confirmed that TCR-dependent c-Myc expression was dependent on mTOR in vitro (Figure 6H). Consistent with this finding, c-Myc expression was significantly increased in Th1 cells compared to Tfh cells (Figure 6I), and ectopic expression of a constitutively active c-Myc was sufficient to promote Th1 cell differentiation and markers associated with anabolic metabolism compared to that of Tfh cells (Figure 6J) (Wang and Green, 2012). Thus, mTORc1 and c-Myc signaling are important promoters of Th1 cell differentiation over that of Tfh cells.
IL-2 signals via mTOR to regulate Th1 versus Tfh cell differentiation
Given the role of mTOR signaling in promoting Th1 cell differentiation over that of Tfh cells, we next investigated its upstream driver. IL-2 was a good candidate, given its signaling via STAT5 and mTOR that promotes the Th1 cell fate (Brennan et al., 1997; Johnston et al., 2012), and given our finding that Blimp-1hi Th1 cells were enriched in mTOR activity, with IL-2 induction of Blimp-1 dependent on the latter, along with PI3K and Akt signaling (Figures 5F–G). While Blimp-1 YFP+ cells, as expected, were CD25hi following LCMV challenge (Figures 7A and S6A), we also detected expression of CD25 in Blimp-1 YFPlo cells in vitro, suggesting that its up-regulation can precede that of Blimp-1 (Figure S6B, left). Accordingly, we observed that CD25hi Blimp-1 YFPlo cells, as well as their CD25hi YFPhi counterparts, had increased p-S6 (Figure S6B, right), finding a linear relationship between its phosphorylation and increasing concentrations of IL-2 (Figure 7B). Increasing doses of IL-2 also up-regulated CD25 expression (Figure S6C), yet, suppression of Akt and mTOR signaling by AktI and RAPA, respectively, while inhibiting p-S6 expression (Figure 7B), did not greatly alter that of CD25 (Figure S6C), suggesting that p-S6 was not directly responsible for its expression.
Figure 7. mTOR signaling is downstream of that of CD25.
(A) CD25 expression in Stg+ Blimp-1 YFPhi and YFPlo cells at 5 dpi. (B) p-S6 expression in wild type CD4+ T cells following stimulation with a range of αCD3 and CD28 concentrations (0–1 μg/mL) cultured without or with a range of IL-2 amounts (0–100 ng/mL). LY294002, AktI, and RAPA, inhibiting signaling via PI3K, Akt, and mTOR, respectively, were used at concentrations as in Figure 5, and tested in conditions with 1 μg/mL αCD3 and 1 μg/mL CD28 and 10 ng/mL IL-2. (C) p-S6 staining in Stg+ CD4+ CD44+ cells at 5 dpi. (D) Single- and double-transduced Stg+ cells gated on Ly6clo PSGL-1lo Tfh and Ly6chi PSGL-1hi Th1 cells and normalized to their control vectors (LMP, MIGR1-Thy1.1, LMP and MIGR1-Thy1.1; dotted line). (E) T-bet and Bcl6 expression in single- and double-transduced cells normalized to the mean of their control vectors (dotted line). Blue and red lines in (C) represent gating on Tfh and Th1 cells, respectively, with the filled histogram denoting naïve CD4+ T cells. Results are representative of 2 independent experiments with 5 animals per condition per experiment; (A–E). Student’s unpaired t-test (A and C); one-way Anova with multiple comparisons (D, E). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001. Please see Figure S6.
STAT5 signaling promotes up-regulation of CD25, which not only feeds-forward to enhance p-STAT5 activity, but also promotes augmented PI3K and Ras signaling (Brennan et al., 1997). This indicated that IL-2 could promote Th1 versus Tfh cell development through regulating mTOR activity. To test this idea, we silenced CD25 expression on Stg+ cells using an shRNA, which markedly reduced p-S6 and p-Rb staining in total Stg+ cells in vivo at 5 dpi (Figures 7C and S6D). We then used the CD25 shRNA vector without, or in, combination with a vector expressing constitutively active myristolated-Akt (myr-Akt) (Aguilar et al., 2007). As previously reported, silencing of CD25 reduced the development of Th1 cells and T-bet expression at 5 dpi, while promoting that Tfh cells (Figures 7D and S6E–F) and Bcl6 (Figure 7E) (Johnston et al., 2012). Conversely, overexpression of myr-Akt promoted Th1 cell differentiation and T-bet upregulation at the expense of Tfh cells and Bcl6 expression (Figures 7D and E, and S6E–F). In double-transduced cells, myr-Akt restored Th1 cells to wild type amounts when CD25 signaling was suppressed, suggesting that Akt activity could circumvent the need for IL-2 signaling during such differentiation (Figures 7D and S6E–F). Similarly, T-bet and Bcl6 were restored by myr-Akt (Figure 7E). Thus, IL-2 appears to represent a central rheostat, signaling via PI3K, Akt, and mTOR to regulate Tfh and Th1 cell differentiation in vivo.
Discussion
In tune with their differing functions and migratory patterns, we found that Tfh cells utilized distinct signaling pathways and differed in their metabolic requirements compared to Th1 cells. The former were less proliferative, and displayed an inability to maximally engage aerobic glycolysis, likely due to reduced mTOR activity in these cells, compared to Th1 cells. In line with these data, IL-2 activation of Akt and mTOR was necessary for induction of the Th1 cell transcription factor Blimp-1, but not the Tfh cell transcription factor Bcl6. Furthermore, the preferential differentiation of Tfh cell differentiation that occurred in the context of CD25 silencing was reversed by hyper-activation of Akt. Therefore, IL-2 is likely to be epistatic to mTOR activity for commitment to the Th1 cell lineage, functioning as a critical rheostat in the regulation of the reciprocal differentiation and metabolism of Th1 versus Tfh cells. Yet, despite reductions in PI3K, Akt, and mTOR, Tfh cells retained the ability to flux calcium, and demonstrated enhanced basal and activation-induced nuclear translocation of the transcription factor NFATc1. NFAT activity, but not signaling via mTOR, was functionally required downstream of the TCR for production of the canonical Tfh cytokine, IL-21.
Nutrient uptake is also a major contributor to T cell subset differentiation and function. Th1, Th17, and Th2 cells all require mTOR and glucose for their development, whereas Treg cells neither require mTOR nor glucose, instead utilizing exogenous fatty acids (Delgoffe et al., 2009; Michalek et al., 2011). Our findings that Tfh cells have reduced mitochondrial function and glucose uptake, as well as reduced maximal respiratory capacity and extracellular acidification compared to Th1 cells, are indicative of a relatively quiescent metabolic state. Because Tfh cells are less reliant on high amounts of glucose and mTOR activity for their function, these cells share some of the same molecular and metabolic underpinnings as Treg cells. Indeed, their reduced IL-2-driven glycolysis program, negatively regulated by Bcl6 (Oestreich et al., 2014), has metabolic consequences for these cells in vivo. Despite their reduced metabolic capacity, however, we showed that Tfh cells require both glucose and mitochondrial ATPase for cytokine secretion, suggesting that they uptake glucose as well utilize oxidative phosphorylation to maintain function.
While STAT5-induced Blimp-1 expression is critical for regulating the balance between Th1 and Tfh cell fates, our data indicated that Blimp-1 expression is dependent on Akt and mTOR signaling. One potential explanation for the requirement of STAT5 in the induction of Blimp-1 is its requirement in the upregulation of the high-affinity IL-2 receptor alpha chain, CD25, which stabilizes IL-2 and IL-2R interactions, thereby potentiating IL-2 signaling (Smith, 1988; Villarino et al., 2007; Waldmann et al., 2001). Indeed, CD25 expression was virtually absent in the CD4+ T cells lacking STAT5 (Johnston et al., 2012). In addition to activating STAT5, IL-2 activates both Ras and PI3K. The IL-2Rβ cytoplasmic tail induces activation of all of these pathways; upon IL-2 ligation, IL-2Rβ phosphorylates STAT5 at IL-2Rβ510 and PI3K at IL-2Rβ392, while it stimulates Ras signaling via IL-2Rβ338 phosphorylation of Shc (Brennan et al., 1997). Therefore, STAT5-induced CD25 up-regulation likely serves not only to positively feedback on STAT5 activity, but also to enhance IL-2-induced PI3K and Ras signaling. Our findings show that ectopic expression of a constitutively active Akt (myr-Akt) restored Th1 cell differentiation in the absence of high-affinity IL-2 signaling via CD25. These data argue that IL-2 activation of Akt and mTOR signaling is critical in orchestrating the reciprocal differentiation of Th1 and Tfh lineages, and demonstrate that IL-2 conducts two signaling arms that are important for T cell differentiation, via STAT5 and PI3K (Figure S6G, red Th1 cell). In line with this hypothesis, Treg cells, which expand in the absence of mTOR (Delgoffe et al., 2009), and which suppressor function is defunct with ablation of the phosphatase PTEN that suppresses PI(3)K function (Huynh et al., 2015; Shrestha et al., 2015), require IL-2Rβ activation of STAT5 for maintenance of FoxP3 expression (Burchill et al., 2007; Yao et al., 2007). Therefore, Treg cells have intact IL-2 signaling via STAT5, while their signaling via PI3K is attenuated by PTEN (Figure S6G, green Treg cell). We found that IL-2 signaling in Tfh cells, unlike Treg and Th1 cells, was impaired in both pathways, which explains their reduction in CD25 expression and upregulation of Bcl6 via STAT3 binding, as well as their reduction in mTOR signaling and anabolic metabolism (Figure S6G, blue Tfh cell).
How do Tfh cells suppress CD25 signaling, and subsequently that of Akt and mTOR, during their differentiation? Expression of CD25 and the canonical Tfh cell marker CXCR5 is mutually exclusive early following LCMV Armstrong and Listeria monocytogenes infections (Johnston et al., 2012; Pepper et al., 2011). Tfh cells also are unaffected by CD25 ablation, unlike Th1 cells, suggesting that this cytokine is not important for Tfh cell development. It remains to be tested whether TCR:MHCII-peptide dwell-time interactions (Tubo et al., 2013) or ICOS signaling can directly suppress CD25 upregulation in Tfh cells, thus protecting them from high-affinity IL-2 signaling and subsequent Akt and mTOR activation. However, we have shown that STAT3 protects against the effects of STAT5 regulation at the Bcl6 locus. It may do the same at the CD25 locus, as we found that STAT3-deficient Tfh cells have an increase in CD25 expression (Ray et al., 2014). These mutant Tfh cells, which have characteristics of Th1 cells, also have patterns of gene expression associated with enhancement of c-Myc, NFκB, and E2f1 cellular pathways, according to IPA analysis (Ray et al., 2014). Thus, competing cytokine signals appear to shield Tfh cells from IL-2 signaling, aiding their differentiation.
The study of T cell subset signaling and metabolism has implications for creating therapeutics for infectious and autoimmune diseases. Targeting the mTOR pathway in T cells would preferentially promote Tfh cell differentiation while suppressing Th1 cell differentiation, as we have shown. However, global treatment with metabolic inhibitors, such as rapamycin, is likely to affect other cell types that are essential for Tfh cell formation; i.e., dendritic cells or GC B cells. Thus, while targeting metabolic pathways may be useful in design of vaccination strategies or therapy of autoimmunity, the effects of these interventions on differential regulation of CD4+ T cell fate need careful consideration.
Experimental Procedures
Mice and LCMV infection
Mice were housed in specific pathogen-free conditions at the Yale School of Medicine (New Haven, CT). C57BL/6 (B6) mice were purchased from the National Cancer Institute (Bethesda, MD), with SMARTA Tg (Tg(TcrLCMV)1Aox) from Hans Hengartner at the University of Zürich in Switzerland, Blimp-1 YFP animals (B6.Cg-Tg(Prdm1-EYFP)1Mnz/J) from Michel Nussenzweig at New York University, and OTII Tg mice from Jackson Laboratories. All were used at 6–8 wk of age. The Institutional Animal Care and Use Committee of Yale University approved all procedures involving mice. They were infected with LCMV Armstrong by i.p. injection of 2 x 105 PFU per mouse.
PI3K, Akt, mTOR, NFAT, Glucose, and Oxidative Phosphorylation Inhibitor in vitro Treatments
Inhibitors for PI3K (LY294002, 25 μM), AKT (AktI, 1 μM), mTOR (RAPA, 100 nM), and NFAT (CsA, 1 μM) were used to treat Blimp-1 YFP cells for 2 days with varying doses of IL-2 (0 – 100 ng/mL) and TCR signaling (αCD3 and αCD28 at matching concentrations from 0 – 1 μM). For cytokine staining, RAPA was used at 200 nM, CsA at 1 μM, 2-DG at 50 μM, and Oligomycin (ATPase inhibitor, 0.5 mM), added 15 minutes before stimulation of cells with GP61–80 peptide.
Seahorse Extracellular Flux Assay
Seahorse analysis experiments were performed as previously described (Henao-Mejia et al., 2013) and discussed in brief in the Supplemental Experimental Procedures.
Calcium Flux Assay
D8 Stg CD4+ T cells were stained for markers of Tfh (CD4+ Thy1.1+ Ly6clo PSGL-1lo) or Th1 (CD4+ Thy1.1+ Ly6chi PSGL-1hi) cells on ice for 20 mins and then these cells were loaded with a mixture of Fura-4AM (1μg/ml) and Fura-Red (2μg/ml) (Life Technologies) at 37°C for 10 mins. After washing twice with PBS, cell pellets were suspended with RPMI containing 10% FBS. Intracellular calcium was analyzed by flow cytometry for 30 seconds to acquire baseline levels followed by cell stimulation with ionomycin. Calcium influx was determined as the ratio between Fura-Red and Fura-4AM fluorescence intensity.
Retroviral Transduction and Cell Transfer
For retroviral transduction, 1μg of LMP, MIGR1-GFP, MIGR1-Thy1.1, mTOR shRNA, Myr-Akt, and/or CD25 shRNA vector with 0.5 μg of EcoHelp plasmid were used to transfect HEK293T viral packaging cells using X-tremeGENE 9 DNA transfection reagent (Roche) overnight. The media was then replaced and virus was grown for another 24 h. 6 x 106 splenocytes were activated for 24 hours using GP66 peptide stimulation and spin transduced with the supernatant of the transfection with polybrene. Post-transduction, 1 x 105 Stg+ T cells were transferred via retro-orbital injection into infected B6 recipients that were subsequently infected with LCMV Armstrong. For B cell activity analysis, Stg T cells transduced with mTOR shRNA or control vector were cultured for a period of 24 hours before sorting. These cells were then sorted on GFP positivity and transferred at 7,000 GFP+ Stg cells/mouse in to OTII mice that were subsequently infected with LCMV. These mice were sacrificed at 8 dpi to assess their B cell responses to LCMV. For nontransduced cells, 2 x 105 wild type or Blimp-1 YFP+ naïve STg+ CD4+ T cells were transferred via retro-orbital injection into wild type mice. Twelve to 24 h later, mice were infected with LCMV Armstrong by i.p. injection. At 3, 5, and 8 dpi, depending on the experiment, spleens were harvested for flow cytometry for phenotypic analysis.
Statistics
Data were analyzed as described in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. Leslie Berg for the CD25 silencing plasmid, Shane Crotty for the LMP shRNA vector, and Christophe Benoit for the Myr-Akt plasmid. We thank members of the Kaech and Craft laboratories for their suggestions and review. This work was supported by the National Science Foundation Graduate Research Fellowship Program under grant 2012099695 (J.P.R.), National Institute of Health grants F32AI094791 (H.D.M), AR40072, AR062842, AI075157, AR053495, AR063942, and the Alliance for Lupus Research.
Footnotes
Accession Numbers
The GEO accession number for the RNA-Seq data reported in this paper is GSE55596.
Author Contributions
Conceptualization, J.P.R, M.M.S, and J.C.; Methodology, J.P.R, M.M.S, J.A.S, S.M.K and J.C.; Software, J.P.R.; Validation, J.P.R., M.M.S, J.A.S., S.M.K., and J.C.; Formal Analysis, J.P.R.; Investigation, J.P.R, M.M.S, J.A.S. and J.C.; Writing – Original Draft, J.P.R, M.M.S, and J.C.; Writing – Review & Editing, J.P.R, M.M.S, J.A.S., S.M.K. and J.C.; Project Administration, J.P.R.; Funding Acquisition, S.M.K and J.C.; Resources, S.M.K and J.C.; Supervision, S.M.K and J.C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudière E, Foretz M, Viollet B, et al. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metabolism. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. The Journal of Immunology. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR Kinase Differentially RegulatesEffector and Regulatory T Cell Lineage Commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Hale JS, Youngblood B, Latner DR, Mohammed AUR, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct Memory CD4(+) T Cells with Commitment to T Follicular Helper- and T Helper 1-Cell Lineages Are Generated after Acute Viral Infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Ohgai D, Watanabe R, Okano K, Koiwai O, Tanabe K, Toma H, Altman A, Abe R. A single amino acid alteration in cytoplasmic domain determines IL-2 promoter activation by ligation of CD28 but not inducible costimulator (ICOS) J Exp Med. 2003;197:257–262. doi: 10.1084/jem.20021305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, Crotty S. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. Journal of Experimental Medicine. 2015;212:539–553. doi: 10.1084/jem.20141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nature Immunology. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 Are Reciprocal and Antagonistic Regulators of T Follicular Helper Cell Differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. Journal of Experimental Medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D’Costa K, Tarlinton DM, Kallies A, Corcoran LM. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. Journal of Experimental Medicine. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-P, Korn LL, Gamero AM, Leonard WJ. Calcium-dependent activation of interleukin-21 gene expression in T cells. J Biol Chem. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. Journal of Experimental Medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macián F, García-Cózar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM. The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife. 2015;4:e04851. doi: 10.7554/eLife.04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. The Journal of Immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T Follicular Helper Cells Is Mediated by Interleukin-21 but Independent of T Helper 1, 2, or 17 Cell Lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt GJW, Huang SCC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. Journal of Experimental Medicine. 2011;208:1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nature Immunology. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, Weinmann AS. Bcl-6 directly represses the gene program of the glycolysis pathway. Nature Immunology. 2014;15:957–964. doi: 10.1038/ni.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of Immune Responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR Kinase Determines Effector versus Memory CD8+ T Cell Fate by Regulating the Expression of Transcription Factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription Factor STAT3 and Type I Interferons Are Corepressive Insulators for Differentiationof Follicular Helper and T Helper 1 Cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1 -dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. Journal of Experimental Medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nature Immunology. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nature Immunology. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH. The Transcription Factor FoxO1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8+ T Cells during Chronic Infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117:7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Pagán AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single Naive CD4(+) T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJW, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino AV, Tato CM, Stumhofer JS, Yao Z, Cui YK, Hennighausen L, O’Shea JJ, Hunter CA. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk A, Li J, Xin J, You D, Zhang J, Liu X, Xiao Y, Breslin P, Li Z, Wei W, et al. Co-inhibition of NF-κB and JNK is synergistic in TNF-expressing human AML. Journal of Experimental Medicine. 2014;211:1093–1108. doi: 10.1084/jem.20130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic checkpoints in activated T cells. Nature Immunology. 2012;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. The Transcription Factor Myc Controls Metabolic Reprogramming upon T Lymphocyte Activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nature Immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Yang N, Huang J, Greshock J, Liang S, Barchetti A, Hasegawa K, Kim S, Giannakakis A, Li C, O’Brien-Jenkins A, et al. Transcriptional regulation of PIK3CA oncogene by NF-kappaB in ovarian cancer microenvironment. PLoS ONE. 2008;3:e1758. doi: 10.1371/journal.pone.0001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.