Abstract
Blinding ocular herpetic disease in humans is due to herpes simplex virus type 1 (HSV-1) reactivations from latency, rather than to primary acute infection. The cellular and molecular mechanisms that control the HSV-1 latency-reactivation cycle remain to be fully elucidated. The aim of this study was to determine if reactivation of the HSV-1 latency associated transcript (LAT) deletion mutant (dLAT2903) was impaired in this model, as it is in the rabbit model of induced and spontaneous reactivation and in the explant TG induced reactivation model in mice. The eyes of mice latently infected with wild type HSV-1 strain McKrae (LAT(+) virus) or dLAT2903 (LAT(−) virus) were irradiated with UV-B and reactivation was determined. We found that compared to LAT(−) virus, LAT(+) virus reactivated at a higher rate as determined by shedding of virus in tears on days 3 to 7 after UV-B treatment. Thus, the UV-B induced reactivation model of HSV-1 appears to be a useful small animal model for studying the mechanisms involved in how LAT enhances the HSV-1 reactivation phenotype. The utility of the model for investigating the immune evasion mechanisms regulating the HSV-1 latency/reactivation cycle and for testing the protective efficacy of candidate therapeutic vaccines and drugs are discussed.
Keywords: HSV-1, LAT, UV-B, recurrent herpetic disease, eye, animal model, immunology, virology
INTRODUCTION
Following primary infection at the eye, herpes simplex virus (HSV-1) replicates, travels up innervating neuronal axons to the trigeminal ganglia (TG) where it establishes a life-long latent infection in sensory neurons. Herpes simplex virus (HSV-1) stromal keratitis (HSK) is a leading cause of corneal blindness in the United States (Smith et al, 1980). HSK is an immuno-pathological process that is triggered by sporadic reactivation of HSV-1 from a latent state in sensory neurons, transport down neuronal axons, and shedding in tears (Chentoufi and Benmohamed, 2012; Meyers, 1975; Stuart et al, 2004). This leads to recurrent bouts of inflammation and progressive corneal scarring (Nesburn, 1983). However, the viral/host factors involved, and the underlying innate and adaptive immune mechanisms and their kinetics, remain to be fully elucidated. Major symptoms of recurrent herpes disease in the eye include clouding of the cornea and neovascularization, both of which can impair vision and lead to loss of sight. Over 400,000 people in the United States have a history of recurrent herpetic ocular disease (Farooq and Shukla, 2012; Xu et al, 2002). Estimates are that up to 90% (Farooq and Shukla, 2012; Kaufman et al, 2005; Xu et al, 2006) of adults in the United States harbor latent HSV-1, making them susceptible to developing HSK (Kumaraguru et al, 1999; Rowe et al, 2013).
The HSV-1 latency associated transcript gene (LAT) is expressed during latency (Rock et al, 1987; Spivack and Fraser, 1987; Stevens et al, 1987; Stroop et al, 1984) and is the only viral gene that is consistently detected as being abundantly transcribed at this time (Rock et al, 1987). The remaining 80 plus viral genes are shut down during latency. In mice, small amounts of mRNA from some of these genes are sometimes detected during latency. This is likely a result of sporadic abortive reactivations in single neurons, sometimes called “molecular reactivation” (Feldman et al, 2002; Margolis et al, 2007). Experiments in mice and rabbits have shown that LAT plays a critical role in enhancing the reactivation phenotype (Hill et al, 1990; Leib et al, 1989; Perng et al, 1994; Trousdale et al, 1991). This appears to be a function of LAT’s anti-apoptosis activity (Inman et al, 2001; Perng et al, 2000), since wild type levels of reactivation can be restored to a LAT(−) virus by inserting one of several different alternative anti-apoptosis genes in place of LAT (Jin et al, 2008; Jin et al, 2007; Jin et al, 2005; Perng et al, 2002). LAT may also contribute to latency/reactivation via numerous immune evasion mechanisms, such as its ability to directly or indirectly delay or interfere with interferon production (Peng et al, 2005), protect against CD8+ T-cell killing by blocking granzyme B induced apoptosis (Jiang et al, 2011), promote exhaustion of CD8+ T-cells (Allen et al, 2011; Chentoufi et al, 2011), and increase HVEM expression (Allen et al, 2014) (herpes virus entry mediator, a member of the tumor necrosis family) which can act as a switch to decrease T cell function.
Mouse studies of HSV-1 reactivation from latency have been limited by the fact that spontaneous reactivation of HSV-1 accompanied by return of the reactivated virus to the eye, either does not occur, or occurs at a rate too low for study (Feldman et al, 2002; Gebhardt and Halford, 2005). Thus, most HSV-1 reactivation studies in mice have been done ex vivo using the trigeminal ganglia (TG) explant induced reactivation model (Deshmane et al, 1993; Devi-Rao et al, 1994; Leib et al, 1989; Perng et al, 2001; Spivack et al, 1995). This is an ex vivo system in which reactivation is induced by sacrificing the mouse, removing the TGs, cutting them into small pieces, culturing the pieces in tissue culture media, and monitoring the media for the appearance of reactivated infectious virus. Whether the ex vivo TG explant induced model of HSV-1 reactivation reliably mirrors the in vivo situation in humans is unclear. HSV-1 reactivation in mice can also be induced by other methods including hyperthermia, sodium butyrate, cyclophosphamide plus dexamethasone, cadmium, cellophane, xylene, retinoic acid, iontophoresis of epinephrine, dimethyl sulfoxide, and physical restraint (Blyth et al, 1980; Cook et al, 1991; Halford et al, 1996; Harbour et al, 1981; Higaki et al, 2003; Higaki et al, 2002; Hill et al, 1982; Himmelein et al, 2011; Neumann et al, 2007; Sawtell and Thompson, 1992; Toma et al, 2008; Zlotnik et al, 1970). A potential drawback of these models is that there are no reports of recurrent eye disease being induced. Thus these models may not be optimal for the study of therapeutic interventions aimed at preventing recurrent HSK.
Fortunately, an in vivo HSV-1 reactivation model in mice in which significant recurrent corneal disease is induced has been developed (Laycock et al, 1991). In this model, mice are ocularly infected with HSV-1 to establish cohorts of latently infected mice. The eyes of the latently infected mice are subsequently irradiated with UV-B light. This induces reactivation of the virus and its return to the eye, as determined by the detection of infectious HSV-1 in the tears of approximately 50% of eyes between days 3 and 7 post UV-B exposure. Recurrent herpetic eye disease also occurs at high levels in this model. The use of this UV-B model appears to be mostly limited to the lab that developed this model and investigators from that lab (Keadle et al, 2008; Keadle et al, 1997; Keadle et al, 2002a; Keadle et al, 2002b; Keadle et al, 2005; Keadle et al, 2002c; Keadle et al, 2001; Keadle et al, 2000; Laycock et al, 1991; Morris et al, 2012a; Stuart and Keadle, 2012a; Stuart et al, 2008; Walker et al, 1998). A recent video (Morris et al, 2012a) demonstrating the UV-B model convinced us to attempt the model in our lab. We were particularly interested in determining if wild type (LAT(+)) virus would reactivate at a higher rate than LAT(−) virus following UV-B induced reactivation, as is the case for spontaneous and in vivo induced reactivation in rabbits (Hill et al, 1990; Perng et al, 1994) and for TG explant induced reactivation (Leib et al, 1989) and heat stress induced reactivation (Sawtell and Thompson, 1992) in mice.
We report here that: (i) We were successful in performing the UV-B model of HSV-1 induced reactivation in latently infected mice and the results were consistent with those previously reported (Laycock et al, 1991; Morris et al, 2012a); and (ii) Compared to LAT(−) virus (dLAT2903), wild type (LAT(+)) HSV-1 reactivated at a higher rate in the UV-B mouse model, as determined by shedding of virus in tears on days 3 to 7 after UV-B treatment. The usefulness of the UV-B light mouse model in investigating the possible LAT-mediate immune evasion mechanisms that regulate HSV-1 latency/reactivation and in testing the efficacy of candidate therapeutic vaccines and drugs are discussed.
MATERIALS AND METHODS
Cell lines
Rabbit skin (RS) cells were maintained in Eagle minimal essential medium (MEM) with 2 mM L-glutamine, 0.1 mM nonessential amino acids, 1mM sodium pyruvate, 10% fetal bovine serum (Promega Scientific), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Sigma, St. Louis, MO).
Viruses
All viruses were triple plaque purified and passaged only two or three times in rabbit skin (RS) cells prior to use. LAT(+) wild-type (wt.) HSV-1 strain McKrae is the parental virus for the LAT(−) mutant dLAT2903. Both viruses have been previously described (Perng et al, 1994).
Mice
Eight- to 10-week-old female C57BL/6 mice (Jackson Labs) were used in all studies. All animal studies conform to the UC Irvine IACUC guidelines and the guidelines of the US National Institute of Health.
Infection of mice
Ocular infection of mice was performed as described by (Morris et al, 2012a) using 1×106 pfu of either LAT(+) McKrae or LAT(−) dLAT2903 per eye. Briefly, mice were anesthetized and corneas were scarified (i.e., the epithelium was lightly scratched) in a crosshatched pattern of 4 to 5 vertical and 4 to 5 horizontal scratches using a 25-gauge needle. Each mouse received an i.p. injection of 0.5 ml of pooled serum containing HSV-1 neutralizing antibodies with a 50% plaque reduction neutralization titer of approximately 1:128. The serum we used differed from that previously used (Morris et al, 2012a) as we used pooled serum from rabbits latently infected with HSV-1 instead of pooled human serum. In the pilot study (experiment 1 in Fig. 2 below) only the right eyes of mice were infected, consistent with the description of the UV-B mouse model (Morris et al, 2012a). In experiment 2 in Figs. 2 and 3 below and in the experiment shown in Fig. 4 below, both eyes were infected.
Figure 2. UV-B induced reactivation of virus from mice latently infected with HSV-1 strain McKrae.
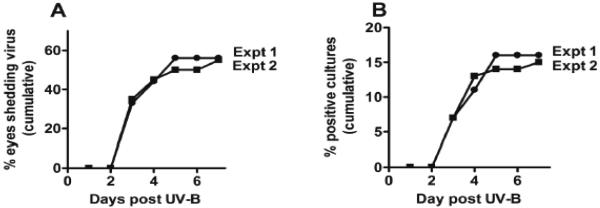
Mice were infected and ~30 days later when latency was well established, virus was induced by UV-B irradiation of eyes as described in Materials & Methods and (Morris et al, 2012a). Panel A: The cumulative percent of eyes that shed UV-B induced reactivated virus within one week of UV-B irradiation. Panel B: The cumulative percent of virus positive cultures (daily eye swabs plated on indicator cells). Expt. 1: Results of the right eyes of 9 mice. Expt. 2: Results of both eyes from 10 mice (20 eyes).
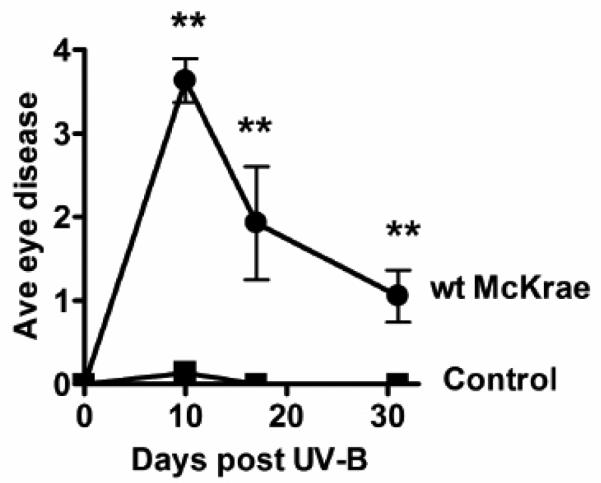
Figure 3. Recurrent eye disease following UV-B irradiation.
The eyes from the experiment 1 shown in figure 2 were monitored for eye disease (clouding on a scale of 0 to 4 (Morris et al, 2012a)) on days 10, 17, and 31 post UV-B irradiation. Groups: wt McKrae, 15 eyes; Control, naïve age matched mice receiving the same UV-B irradiation treatment, 20 eyes. “**” Indicates highly significant differences on that day with a P value <0.001 as determined by a two sided Fisher exact test.
Figure 4. UV-B induced reactivation of LAT(+) versus LAT(−) virus.
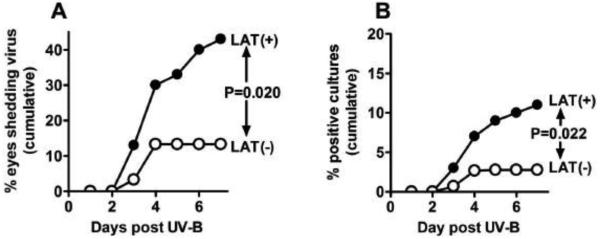
Fifteen mice/group (30 eyes) were infected, subjected to UV-B irradiation 30 days p.i., and induced reactivation analyzed as described in the legend to Figure 2. P values were determined by 2-sided Fisher exact test.
UV-B irradiation of mouse eyes, monitoring shedding of reactivated HSV-1 in tears, and monitoring recurrent eye disease
UV-B irradiation was done exactly as described (Morris et al, 2012a), including using the same UV-B light source, except that in some experiments both eyes were used. Briefly, anesthetized mice were placed on a TM20 Chromato-Vu transilluminator (UVP, San Gabriel, CA), which emits UV-B at a peak wavelength of 302 nm. Each mouse was positioned on a piece of cardboard containing a hole that is the same size as the mouse’s eye. This allowed just the eye to be irradiated by the UV-B source. Each eye was irradiated with 250 mJ of UV-B light cm2 (approximately 1 minute exposure on the transilluminator). On days 3, 4, 5, 6, and 7 post UV-B irradiation, eyes were swabbed and tear films plated on RS cells for detection of reactivated virus as we previously described for monitoring lytic virus replication in eyes of rabbits and mice and spontaneously reactivated virus in eyes of rabbits (Jin et al, 2005; Perng et al, 1994). Recurrent eye disease was monitored at various times post UV-B irradiation as described (Morris et al, 2012a).
RESULTS
Inducing reactivation of HSV-1 latency by UV-B irradiation as judged by detection of infectious virus in tears of mice
We first did a small pilot study using 9 infected and 2 uninfected eyes following the published procedure (Morris et al, 2012a) as closely as possible. An outline of the time course of the UV-B induced reactivation experiments in this report is illustrated in Fig. 1. As described in Materials & Methods, nine C57BL/6 female mice were anesthetized. The right cornea of each mouse was lightly scarified and infected with 1×106 pfu/eye of the McKrae strain of HSV-1 as eye drops. Just prior to infection each mouse received an i.p. injection of 0.5 ml of immune serum containing HSV-1 neutralizing antibody to partially protect against acute eye disease and death. Thirty days post infection the nine infected eyes were irradiated with 250 mJoules of UV-B (302 nm). The right eyes of 2 naïve age- and sex-matched control mice were subjected to the same UV-B irradiation. The only known variance from the published procedure was that we used anti-HSV-1 pooled rabbit immune serum instead of pooled human immune serum to help protect the infected mice against the primary HSV-1 infection. Following UV-B irradiation, tear swabs were collected daily for 7 days and plated on indicator cells (RS cells) to detect the presence of infectious virus that had reactivated in the corresponding TG and returned to the eye. Fig. 2A (Expt. 1) shows the cumulative percent of eyes that had at least one virus positive tear culture in this study. Infectious virus indicative of latent virus that reactivated in the corresponding TG and returned to the eye was detected in approximately 55% of the eyes in this experiment. These results are similar to those previously reported (Laycock et al, 1991; Morris et al, 2012a) and show that UV-B irradiation induces reactivation of HSV-1 that can be detected by shedding of virus in tears.
Figure 1. Schematic representation of the time line used in the UV-B model.
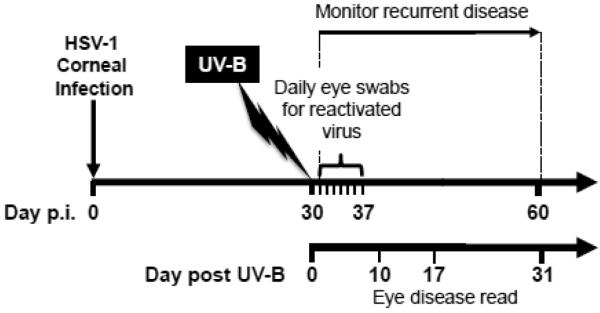
Mice were infected with HSV-1 as described in Materials & Methods closely adhering to the published model as shown in a video (Morris et al, 2012a). Tears were collected on the indicated days (days 0-7). Eye disease was scored on the indicated days (days 10, 17, 31).
We also determined the cumulative number of virus positive tear film cultures (Fig. 2B; Expt. 1). This takes into account multiple days on which an eye shed virus, and is one of the standard analyses we perform when analyzing spontaneous reactivation in rabbit eyes (Perng et al, 1994). Since in this, and subsequent experiments, we never detected virus in any mouse eye prior to day 3 post UV-B treatment or after day 7 post UV-B treatment, the total number of cultures used for these calculations excluded days 0, 1, and 2 and days 8 and later. The calculation was done as follows: [(number of virus positive tear film cultures on that day + the number of positive cultures on all preceding days) / (total number of tear film cultures evaluated from day 3 to day 7)] × 100%. Thus, the denominator was 9 eyes × 5 swabs (days 3, 4, 5, 6, 7) or 45.
To confirm the results in the pilot study, and to determine if using both eyes rather than just one eye per mouse would produce similar results, both eyes from 10 mice (20 eyes/group) were either infected with wt McKrae or mock infected (naïve mice). All other manipulations were identical to those in the pilot study. The HSV-1 reactivation results are shown in Fig. 2A and 2B (Expt. 2). Both the cumulative percent of eyes shedding reactivated virus and the cumulative percent of virus positive cultures were virtually identical to those in the smaller pilot study. These results confirmed that we were able to perform the mouse UV-B induced reactivation model, as judged by induction of shedding virus in mouse tears.
Inducing recurrent eye disease using UV-B
In addition to inducing shedding of virus in tears, the UV-B model is also reported to induce recurrent herpetic disease as judged by stromal clouding (Laycock et al, 1991; Morris et al, 2012). To confirm that we were also able to reproduce this result, eye disease was determined in the eyes of the mice in experiment 2 before and after UV-B irradiation. One day prior to UV-B, all eyes in both groups were scored on a scale of 0 to 4 by examination under a dissecting microscope, as previously described (Morris et al, 2012). Five eyes had significant disease prior to UV-B irradiation. This was a result of the primary ocular infection. These eyes were removed from the study because the existing disease would make it impossible to score disease induced by UV-B reactivation of HSV-1. All 20 eyes in the control group were normal prior to UV-B. Following UV-B irradiation, the amount of disease in individual eyes was evaluated and scored on the days indicated in Fig. 3, by an individual who was masked as to which group the eye was from. Between days 10 to 31 post UV-B irradiation the average eye disease score was significantly higher in the HSV-1 infected group compared to the uninfected control group. These results were also similar to those previously reported (Morris et al, 2012a), indicating that the UV-B model was working as expected.
UV-B irradiation induces more virus reactivation (shedding) in mice latently infected with wild type LAT(+) virus compared to the LAT(−) virus dLAT2903
It is well established that in rabbits the HSV-1 LAT plays an important role in both the in vivo spontaneous and induced reactivation phenotypes. Similarly LAT plays an important role in induced reactivation of HSV-1 in the mouse TG explant induced model and in the mouse heat stressed model. However, some LAT mutants do not behave similarly in the in vivo rabbit model and the ex vivo mouse TG explant model (Loutsch et al, 1999; Maggioncalda et al, 1994). It was therefore of interest to determine if LAT plays a significant role in the in vivo UV-B reactivation model in mice.
We therefore infected both eyes of 15 mice per group with either wild type McKrae (LAT(+) virus) or dLAT2903 (LAT(−) virus), which was derived from McKrae. All procedures were as described above. Following UV-B irradiation, virus reactivation (shedding in tears) was determined and analyzed as in Figure 1 (Table 1, Fig. 4A and 4B). Reactivation of LAT(+) virus was significantly greater than LAT(−) virus by both analyses. The results for the percent of virus positive cultures (induced reactivation) seen from day 3 to 7 after UV-B irradiation are reminiscent of the percent of virus positive tear cultures seen in rabbits from day 31 to 56 post infection (spontaneous reactivation) (Perng et al, 1994). Thus, the UV-B induced reactivation mouse model readily distinguishes between LAT(+) and LAT(−) viruses and provides an additional model for determining the reactivation phenotype of HSV-1 mutants.
Table 1.
UV-B induced reactivation in LAT(+) versus LAT(−) micea.
| Virus | No. eyes shedding reactivated virus/no. eyes (%) |
No. reactivated virus positive cultures/no. cultures (%) |
|---|---|---|
| LAT(+) (wt McKrae) | 13/30 (43%) | 16/180 (9%) |
| LAT(−) (dLAT2903) | 4/30 (13%) | 5/180 (3%) |
| P value (Fisher exact) | P=0.02 | P=0.022 |
Mice were ocularly infected as described in Materials & Methods and the legend to figure 4 with LAT(+) (wild type McKrae) virus (the parental virus for dLAT2903) or LAT(−) (dLAT2903) virus. Reactivation was induced by UV-B irradiation as described in Materials & Methods.
DISCUSSION
Previous studies have used the UV-B mouse model of induced HSV-1 reactivation to investigate the protective efficacy of vaccines and drugs (e.g., acyclovir), and to determined immune mechanisms that protect from or promote recurrent HSK (Keadle et al, 2008; Keadle et al, 1997; Keadle et al, 2002a; Keadle et al, 2002b; Keadle et al, 2005; Keadle et al, 2002c; Keadle et al, 2001; Keadle et al, 2000; Laycock et al, 1991; Morris et al, 2012a; Stuart and Keadle, 2012a; Stuart et al, 2008; Walker et al, 1998). To our knowledge the effect of the HSV-1 LAT gene has not previously been investigated in the UV-B model.
LAT is both an indicator of HSV-1 latency and a critical gene in the latency/reactivation life cycle of HSV-1 (Hill et al, 1990; Leib et al, 1989; Perng et al, 1994; Rock et al, 1987; Spivack and Fraser, 1987; Stevens et al, 1987; Trousdale et al, 1991). Unlike other HSV-1 genes whose expression is tightly regulated in a cascade fashion of gene expression (i.e., α, β, and γ genes), LAT is expressed at all times. The LAT promoter functions in the absence of any other HSV-1 gene (Zwaagstra et al, 1989; Zwaagstra et al, 1990) and LAT can be detected in tissue culture almost immediately after infection (Spivack and Fraser, 1988a; Spivack and Fraser, 1988b). LAT expression remains on and LAT RNA accumulates such that at late times after tissue culture infection LAT is abundant. During latency in animal models and in humans, LAT is the only viral gene that is consistently and abundantly detected (Deatly et al, 1987; Rock et al, 1987; Stevens et al, 1987). A low level of lytic cycle gene expression that is sometimes detected in ganglia during latent infection of mice (Feldman et al, 2002) is likely due to a small number of neurons undergoing abortive reactivation. Detection of lytic cycle gene expression in rabbits and in humans is likely due to viral reactivation in a small subset of neurons since spontaneous reactivation occurs in rabbits and humans.
LAT has anti-apoptosis activity (Perng et al, 2000) and can also act as an immune evasion gene (Allen et al, 2011; Allen et al, 2014; Chentoufi et al, 2012; Chentoufi et al, 2011; Jiang et al, 2011; Peng et al, 2005). Both of these LAT functions likely play a role in how LAT enhances the reactivation phenotype. Mouse models that require either the induction of reactivation or the detection of reactivation to take place ex vivo cannot fully take into account effects of LAT’s immune evasion activity on HSV-1 reactivation and recurrent disease. The rabbit model of HSV-1 latency/reactivation appears to more closely resemble the human situation, since both spontaneous reactivation and induced reactivation take place completely in vivo. Unfortunately, because of limited availability of rabbit immunological reagents, and knock-in and knock-out rabbits, the ability to study the underlying cellular and molecular immune mechanisms in rabbits does not approach the robustness of such studies in mice. Thus, a fully in vivo reactivation model in the mouse in which the influence of LAT on the reactivation phenotype can be investigated, would be a valuable asset for deciphering the cellular and molecular immune mechanisms that regulate HSV-1 latency/reactivation.
Most adults carry latent HSV-1, shed reactivated virus frequently, but remain asymptomatic having no recurrent disease (Kumar et al, 2009; Schacker et al, 1998; Wald et al, 1995). In contrast, a small proportion of individuals are symptomatic and have frequent recurrent disease (Kumar et al, 2009; Schacker et al, 1998; Wald et al, 1995). Cross talk between the immune system and the virus orchestrate HSV latency/reactivation (Allen et al, 2011; Allen et al, 2014; Chentoufi et al, 2012; Chentoufi et al, 2011; Ghiasi et al, 1992; Jiang et al, 2011; Peng et al, 2005). Although most herpetic disease is due to viral reactivation, rather than to primary acute infection (Nesburn, 1983; Nesburn et al, 1998a; Nesburn et al, 1998b), the vast majority of experimental models investigating the immune mechanisms that orchestrate recurrent herpes disease have used the mouse model of primary acute infection, instead of more relevant models of recurrent disease (Morris et al, 2012b; Stuart and Keadle, 2012a; Webre et al, 2012). The extrapolation to human recurrent disease is uncertain, because the protective and pathological mechanisms that operate during primary herpetic disease are, in most part, different from those that operate during recurrent herpetic disease (Morris et al, 2012a; Stuart and Keadle, 2012a; West et al, 2014). The UV-B model of HSV-1 induced reactivation is more likely to immunologically and pathologically mimic human recurrent ocular herpetic disease (Morris et al, 2012a; Stuart and Keadle, 2012a; Webre et al, 2012).
In addition to explant cultivation of TG and UV-B irradiation of eyes, other methods have also been used to induce reactivation of HSV-1 in mice. These include hyperthermia, sodium butyrate, cyclophosphamide plus dexamethasone, cadmium, cellophane, xylene, retinoic acid, iontophoresis of epinephrine, dimethyl sulfoxide, and physical restraint (Blyth et al, 1980; Cook et al, 1991; Halford et al, 1996; Harbour et al, 1981; Higaki et al, 2003; Higaki et al, 2002; Hill et al, 1982; Himmelein et al, 2011; Neumann et al, 2007; Sawtell and Thompson, 1992; Toma et al, 2008; Zlotnik et al, 1970). In these models, HSV-1 reactivation was assessed either by the presence of infectious virus in the ganglia in vitro or shedding of virus in tears. Shimeld (Shimeld et al, 1989; Shimeld et al, 1990) compared immunosuppressive drugs (cyclophosphamide plus dexamethasone) to UV-B irradiation. Both induced HSV-1 reactivation judged by shedding of virus in tears, but only UV-B induced recurrent eye disease. To our knowledge, only UV-B has been reported to induce significant recurrent HSK, making it the most useful mouse model for investigating the cellular and immunological mechanisms involved in recurrent herpetic eye disease. The UV-B method used in the present study was developed by Shimeld et al. (Shimeld et al, 1989; Shimeld et al, 1990; Shimeld et al, 1996a; Shimeld et al, 1995; Shimeld et al, 1996b; Shimeld et al, 1997) and adopted and modified by (Laycock et al, 1994; Laycock et al, 1991; Laycock et al, 1993; Pepose et al, 1992; Stuart and Keadle, 2012b) and (Morris et al, 2012a). The present study extends the UV-B model by showing that LAT(+) (wt HSV-1) has a higher frequency of reactivation in this model than does LAT(−) HSV-1.
It should be noted that the UV-B induced reactivation model is not limited to C57BL/6 mice. With the NIH strain of mice UV-B induced reactivation is 70–90% as determined by detection of infectious virus in tear films (Keadle et al, 1997; Laycock et al, 1991; Stuart et al, 2004). UV-B successfully induced HSV-1 reactivation in latently infected HLA transgenic mice made on either a C57BL/6 or a BALB/c genetic background (not shown).
In the studies reported here, the recently described detailed procedures for the UV-B mouse model of HSV-1 reactivation (Morris et al, 2012a) were followed as closely as possible. We found that UV-B irradiation of the eyes of latently infected mice induced reactivation of the LAT(+) virus more efficiently than it induced reactivation of the LAT(−) virus, as judged by detection of infectious virus in tears between days 3 to 7 post UV-B. The ability of LAT to enhance the reactivation phenotype in the in vivo UV-B induced reactivation model was similar to the ability of LAT to enhance reactivation in other mouse models and in rabbits. In addition, the sensitivity of the UV-B mouse model to distinguish between LAT(+) and LAT(−) viruses was highly reminiscent of the rabbit model. Specifically, the percent of reactivated virus positive cultures between days 3 and 7 post UV-B irradiation was 9% for LAT(+) virus and 3% for LAT(−) virus. This decreased reactivation with LAT(−) virus to approximately 33% that of LAT(+) virus is similar to what is seen for spontaneous reactivation in latently infected rabbits (Perng et al, 1994). In our lab, this decreased spontaneous reactivation rate of LAT(−) viruses in the rabbit model is very consistent, while in the mouse TG explant model the difference between LAT(+) and LAT(−) viruses is sometimes not as clear cut. Published results for at least one LAT mutant also suggest that the in vivo rabbit model may be more sensitive in detecting HSV-1 mutational effects on the reactivation phenotype than ex vivo mouse models (Loutsch et al, 1999; Maggioncalda et al, 1996). For these and other reasons discussed above, we feel that this fully in vivo mouse UV-B induced reactivation model of HSV-1 should be a highly useful addition to deciphering the mechanism(s) by which LAT enhances the reactivation phenotype and for investigating the cellular and molecular immune mechanisms involved in HSV-1 reactivation in general.
Every year approximately 20,000 individuals in the United States develop symptoms of recurrent, painful and potentially blinding ocular herpetic disease due to sporadic spontaneous reactivation of HSV-1 from latently infected sensory neurons of the TG (Kumar et al, 2009; Schacker et al, 1998; Wald et al, 1995). To date, there is no licensed therapeutic vaccine that can effectively stop or reduce HSV-1 reactivation from latency. Current long term anti-viral drug therapies (e.g. Acyclovir and derivatives) reduce recurrent ocular disease in symptomatic individuals by only ~40%, and do not eliminate virus reactivation (HEDS, 1998). Identifying the mechanisms that lead to HSV-1 reactivation from latently infected sensory neurons, the root of recurrent disease, would help in developing more effective immunotherapies to prevent or reduce viral shedding in tears and, hence, reduce recurrent herpetic disease and blindness (reviewed in (Kuo et al, 2014). The protective efficacy of candidate therapeutic vaccines and drugs against ocular herpes must first be pre-clinically tested in reliable animal models of recurrent ocular herpetic disease. We have found that the UV-B in vivo model of HSV-1 induced reactivation also works in our “humanized” HLA-A*0201 transgenic mouse model of ocular herpes (not shown). This will allow us in future studies to test the therapeutic efficacy of candidate vaccines based on human HLA-A*0201-restricted epitopes (Khan et al, 2015).
In conclusion, there are two principal findings in this report: (i) we successfully reproduced the UV-B model of HSV-1 induced reactivation; and (ii) we found that HSV-1 reactivation induced by UV-B in the mouse model was increased in the presence of LAT.
ACKNOWLEDGMENTS
This study was supported by Public Health Service NIH grants R01EY013191, 1R56AI098985, 1R56AI093133, R01EY14900, R01EY019896, R01EY024618, R01EY022365, and The Discovery Center for Eye Research. We thank Patrick Stuart for his invaluable advice in helping us to establish the UV-B model in our laboratory. We thank Dr. Nigel Fraser for reading this manuscript and providing helpful comments, and John Jacob Krochmal IV and Jairo Misael Garcia for technical assistance.
Footnotes
Conflict of interest.
All of the authors (Lbachir BenMohamed, Nelson Osorio, Ruchi Srivastava, Arif A. Khan, Jennifer L. Simpson, Steven L. Wechsler) declare that they have no conflict of interest.
REFERENCES
- Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol. 2011;85:4184–97. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Rhode-Kurnow A, Mott KR, Jiang X, Carpenter D, Rodriguez-Barbosa JI, Jones C, Wechsler SL, Ware CF, Ghiasi H. Interactions between Herpesvirus Entry Mediator (TNFRSF14) and Latency-Associated Transcript during Herpes Simplex Virus 1 Latency. J Virol. 2014;88:1961–1971. doi: 10.1128/JVI.02467-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth WA, Harbour DA, Hill TJ. Effect of immunosuppression on recurrent herpes simplex in mice. Infect Immun. 1980;29:902–907. doi: 10.1128/iai.29.3.902-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chentoufi AA, Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin Dev Immunol. 20122012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, Nesburn AB, Wechsler SL, Benmohamed L. The Herpes Simplex Virus Type 1 Latency-Associated Transcript Inhibits Phenotypic and Functional Maturation of Dendritic Cells. Viral Immunol. 2012 doi: 10.1089/vim.2011.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol. 2011;85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SD, Paveloff MJ, Doucet JJ, Cottingham AJ, Sedarati F, Hill JM. Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest Ophthalmol Vis Sci. 1991;32:1558–1561. [PubMed] [Google Scholar]
- Deatly AM, Spivack JG, Lavi E, Fraser NW. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987;84:3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Nicosia M, Valyi-Nagy T, Feldman LT, Dillner A, Fraser NW. An HSV-1 mutant lacking the LAT TATA element reactivates normally in explant cocultivation. Virology. 1993;196:868–872. doi: 10.1006/viro.1993.1548. [DOI] [PubMed] [Google Scholar]
- Devi-Rao GB, Bloom DC, Stevens JG, Wagner EK. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt BM, Halford WP. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J. 2005;2:67. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. Baculovirus-expressed glycoprotein G of herpes simplex virus type 1 partially protects vaccinated mice against lethal HSV-1 challenge. Virology. 1992;190:233–239. doi: 10.1016/0042-6822(92)91209-d. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ. Mechanisms of herpes simplex virus type 1 reactivation. J Virol. 1996;70:5051–5060. doi: 10.1128/jvi.70.8.5051-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour DA, Hill TJ, Blyth WA. Acute and recurrent herpes simplex in several strains of mice. J Gen Virol. 1981;55:31–40. doi: 10.1099/0022-1317-55-1-31. [DOI] [PubMed] [Google Scholar]
- HEDS Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- Higaki S, Gebhardt B, Lukiw W, Thompson H, Hill J. Gene expression profiling in the HSV-1 latently infected mouse trigeminal ganglia following hyperthermic stress. Curr Eye Res. 2003;26:231–8. doi: 10.1076/ceyr.26.3.231.14892. [DOI] [PubMed] [Google Scholar]
- Higaki S, Gebhardt BM, Lukiw WJ, Thompson HW, Hill JM. Effect of immunosuppression on gene expression in the HSV-1 latently infected mouse trigeminal ganglion. Invest Ophthalmol Vis Sci. 2002;43:1862–9. [PubMed] [Google Scholar]
- Hill JM, Sedarati F, Javier RT, Wagner EK, Stevens JG. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Hill TJ, Blyth WA, Harbour DA. Recurrent herpes simplex in mice; topical treatment with acyclovir cream. Antiviral Res. 1982;2:135–46. doi: 10.1016/0166-3542(82)90015-8. [DOI] [PubMed] [Google Scholar]
- Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman M, Perng G, Henderson G, Ghiasi H, Nesburn A, Wechsler S, Jones C. Region of Herpes Simplex Virus type 1 latency-associated transcript sufficient for wild type spontaneous reactivation promotes cell survival in tissue culture. J Virol. 2001;75:3636–3646. doi: 10.1128/JVI.75.8.3636-3646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, BenMohamed L, Fraser NW, Jones C, Wechsler SL. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol. 2011;85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Carpenter D, Moerdyk-Schauwecker M, Vanarsdall AL, Osorio N, Hsiang C, Jones C, Wechsler SL. Cellular FLIP can substitute for the herpes simplex virus type 1 latency-associated transcript gene to support a wild-type virus reactivation phenotype in mice. J Neurovirol. 2008;14:389–400. doi: 10.1080/13550280802216510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Perng GC, Carpenter D, Mott KR, Osorio N, Naito J, Brick DJ, Jones C, Wechsler SL. Reactivation phenotype in rabbits of a herpes simplex virus type 1 mutant containing an unrelated antiapoptosis gene in place of latency-associated transcript. J Neurovirol. 2007;13:78–84. doi: 10.1080/13550280601164333. [DOI] [PubMed] [Google Scholar]
- Jin L, Perng GC, Mott KR, Osorio N, Naito J, Brick DJ, Carpenter D, Jones C, Wechsler SL. A herpes simplex virus type 1 mutant expressing a baculovirus inhibitor of apoptosis gene in place of latency-associated transcript has a wild-type reactivation phenotype in the mouse. J Virol. 2005;79 doi: 10.1128/JVI.79.19.12286-12295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Alexander DE, Leib DA, Stuart PM. Interferon gamma is not required for recurrent herpetic stromal keratitis. Virology. 2008;380:46–51. doi: 10.1016/j.virol.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Morris JL, Leib DA, Morrison LA, Pepose JS, Stuart PM. Therapeutic vaccination with vhs(−) herpes simplex virus reduces the severity of recurrent herpetic stromal keratitis in mice. J Gen Virol. 2002a;83:2361–2365. doi: 10.1099/0022-1317-83-10-2361. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morris JL, Pepose JS, Stuart PM. CD4(+) and CD8(+) cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microb Pathog. 2002b;32:255–262. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morris JL, Stuart PM. The effects of aminoguanidine on primary and recurrent ocular herpes simplex virus infection. Nitric Oxide. 2005;13:247–253. doi: 10.1016/j.niox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morrison LA, Morris JL, Pepose JS, Stuart PM. Therapeutic immunization with a virion host shutoff-defective, replication-incompetent herpes simplex virus type 1 strain limits recurrent herpetic ocular infection. J Virol. 2002c;76:3615–3625. doi: 10.1128/JVI.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Kumano Y, Pepose JS, Stuart PM. Cytokine expression in murine corneas during recurrent herpetic stromal keratitis. Ocul Immunol Inflamm. 2001;9:193–205. doi: 10.1076/ocii.9.3.193.3967. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Miller JK, Pepose JS, Stuart PM. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2000;41:96–102. [PubMed] [Google Scholar]
- Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. Phenotypic and Functional Characterization of Herpes Simplex Virus Glycoprotein B Epitope-specific Effector and Memory CD8+ T Cells from Ocular Herpes Symptomatic and Asymptomatic Individuals. J Virol: 2015 doi: 10.1128/JVI.03419-14. DOI: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Hill JM, Clement C, Varnell ED, Thompson HW, Kaufman HE. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Invest Ophthalmol Vis Sci. 2009;50:5601–5608. doi: 10.1167/iovs.09-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraguru U, Davis I, Rouse BT. Chemokines and ocular pathology caused by corneal infection with herpes simplex virus. J Neurovirol. 1999;5:42–47. doi: 10.3109/13550289909029744. [DOI] [PubMed] [Google Scholar]
- Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine. 2014;32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock KA, Brady RH, Lee SF, Osborne PA, Johnson EM, Pepose JS. The role of nerve growth factor in modulating herpes simplex virus reactivation in vivo. Graefes Arch Clin Exp Ophthalmol. 1994;232:421–425. doi: 10.1007/BF00186584. [DOI] [PubMed] [Google Scholar]
- Laycock KA, Lee SF, Brady RH, Pepose JS. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest Ophthalmol Vis Sci. 1991;32:2741–2746. [PubMed] [Google Scholar]
- Laycock KA, Lee SF, Stulting RD, Croen KD, Ostrove JM, Straus SE, Pepose JS. Herpes simplex virus type 1 transcription is not detectable in quiescent human stromal keratitis by in situ hybridization. Invest Ophthalmol Vis Sci. 1993;34:285–292. [PubMed] [Google Scholar]
- Leib DA, Bogard CL, Kosz-Vnenchak M, Hicks KA, Coen DM, Knipe DM, Schaffer PA. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutsch JM, Perng GC, Hill JM, Zheng X, Marquart ME, Block TM, Ghiasi H, Nesburn AB, Wechsler SL. Identical 371-base-pair deletion mutations in the LAT genes of herpes simplex virus type 1 McKrae and 17syn+ result in different in vivo reactivation phenotypes. J Virol. 1999;73:767–771. doi: 10.1128/jvi.73.1.767-771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioncalda J, Mehta A, Bagasra O, Fraser NW, Block TM. A herpes simplex virus type 1 mutant with a deletion immediately upstream of the LAT locus establishes latency and reactivates from latently infected mice with normal kinetics. J Neurovirol. 1996;2:268–278. doi: 10.3109/13550289609146890. [DOI] [PubMed] [Google Scholar]
- Maggioncalda J, Mehta A, Fraser NW, Block TM. Analysis of a herpes simplex virus type 1 LAT mutant with a deletion between the putative promoter and the 5' end of the 2.0-kilobase transcript. J Virol. 1994;68:7816–7824. doi: 10.1128/jvi.68.12.7816-7824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis TP, Elfman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, Voytek C. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol. 2007;81:11069–11074. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RL. Immunology of herpes simplex virus in fection. Int Ophthalmol Clin. 1975;15:37–47. doi: 10.1097/00004397-197501540-00005. [DOI] [PubMed] [Google Scholar]
- Morris J, Stuart PM, Rogge M, Potter C, Gupta N, Yin XT. Recurrent herpetic stromal keratitis in mice, a model for studying human HSK. J Vis Exp. 2012a:e4276. doi: 10.3791/4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JE, Zobell S, Yin XT, Zakeri H, Summers BC, Leib DA, Stuart PM. Mice with mutations in Fas and Fas ligand demonstrate increased herpetic stromal keratitis following corneal infection with HSV-1. J Immunol. 2012b;188:793–799. doi: 10.4049/jimmunol.1102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesburn AB. Report of the corneal disease panel: Vision research: A national plan 1983-1987. II. C.V. Mosby Co.; St. Louis: 1983. Part III. [Google Scholar]
- Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology. 1998a;252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthal Vis Sci. 1998b;39:1163–1170. [PubMed] [Google Scholar]
- Neumann DM, Bhattacharjee PS, Giordani NV, Bloom DC, Hill JM. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. J Virol. 2007;81:13248–53. doi: 10.1128/JVI.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Henderson G, Inman M, BenMohamed L, Perng GC, Wechsler SL, Jones C. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal Ganglia of acutely infected mice. J Virol. 2005;79:6162–6171. doi: 10.1128/JVI.79.10.6162-6171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose JS, Laycock KA, Miller JK, Chansue E, Lenze EJ, Gans LA, Smith ME. Reactivation of latent herpes simplex virus by excimer laser photokeratectomy. Am J Ophthalmol. 1992;114:45–50. doi: 10.1016/s0002-9394(14)77411-2. [DOI] [PubMed] [Google Scholar]
- Perng G, Jones C, Ciacci-Zanella H, Henderson G, Yukht A, Slanina S, Hofman F, Ghiasi H, Nesburn A, Wechsler S. Virus induced neuronal apoptosis blocked by the herpes simplex virus latency associated transcript (LAT) Science. 2000;287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng GC, Maguen B, Jin L, Mott KR, Osorio N, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Inman M, Henderson G, Jones C, Wechsler SL. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol. 2002;76:1224–1235. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng GC, Slanina SM, Ghiasi H, Nesburn AB, Wechsler SL. The effect of latency-associated transcript on the herpes simplex virus type 1 latency-reactivation phenotype is mouse strain-dependent. J Gen Virol. 2001;82:1117–1122. doi: 10.1099/0022-1317-82-5-1117. [DOI] [PubMed] [Google Scholar]
- Rock DL, Nesburn AB, Ghiasi H, Ong J, Lewis TL, Lokensgard JR, Wechsler SL. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88–101. doi: 10.1016/j.preteyeres.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NM, Thompson RL. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis. 1998;178:1616–1622. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Hill T, Blyth B, Easty D. An improved model of recurrent herpetic eye disease in mice. Curr Eye Res. 1989;8:1193–1205. doi: 10.3109/02713688909000044. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Hill TJ, Blyth WA, Easty DL. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J Gen Virol. 1990;71:397–404. doi: 10.1099/0022-1317-71-2-397. Pt 2. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Nicholls SM, Easty DL, Hill TJ. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J Gen Virol. 1996a;77:977–85. doi: 10.1099/0022-1317-77-5-977. Pt 5. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Williams NA, Easty DL, Hill TJ. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J Gen Virol. 1996b;77:2583–90. doi: 10.1099/0022-1317-77-10-2583. Pt 10. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Williams NA, Easty DL, Hill TJ. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J Gen Virol. 1997;78:3317–25. doi: 10.1099/0022-1317-78-12-3317. Pt 12. [DOI] [PubMed] [Google Scholar]
- Smith RE, McDonald HR, Nesburn AB, Minckler DS. Penetrating keratoplasty: changing indications, 1947 to 1978. Arch Ophthalmol. 1980;98:1226–1229. doi: 10.1001/archopht.1980.01020040078009. [DOI] [PubMed] [Google Scholar]
- Spivack JG, Fareed MU, Valyi-Nagy T, Nash TC, JS OK, Gesser RM, McKie EA, MacLean AR, Fraser NW, Brown SM. Replication, establishment of latent infection, expression of the latency-associated transcripts and explant reactivation of herpes simplex virus type 1 gamma 34.5 mutants in a mouse eye model. J Gen Virol. 1995;76:321–332. doi: 10.1099/0022-1317-76-2-321. [DOI] [PubMed] [Google Scholar]
- Spivack JG, Fraser NW. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987;61:3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack JG, Fraser NW. Expression of herpes simplex virus type 1 (HSV-1) latency-associated transcripts and transcripts affected by the deletion in avirulent mutant HFEM: evidence for a new class of HSV-1 genes. J Virol. 1988a;62:3281–3287. doi: 10.1128/jvi.62.9.3281-3287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack JG, Fraser NW. Expression of herpes simplex virus type 1 latency-associated transcripts in the trigeminal ganglia of mice during acute infection and reactivation of latent infection. J Virol. 1988b;62:1479–1485. doi: 10.1128/jvi.62.5.1479-1485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stroop WG, Rock DL, Fraser NW. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984;51:27–38. [PubMed] [Google Scholar]
- Stuart PM, Keadle TL. Recurrent herpetic stromal keratitis in mice: a model for studying human HSK. Clin Dev Immunol. 2012a2012:728480. doi: 10.1155/2012/728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart PM, Keadle TL. Recurrent herpetic stromal keratitis in mice: a model for studying human HSK. Clin Dev Immunol. 2012b;88:72–78. doi: 10.1155/2012/728480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart PM, Morris JE, Sidhu M, Keadle TL. CCL3 protects mice from corneal pathology during recurrent HSV-1 infection. Frontiers in Bioscience. 2008;13 doi: 10.2741/3013. [DOI] [PubMed] [Google Scholar]
- Stuart PM, Summers B, Morris JE, Morrison LA, Leib DA. CD8(+) T cells control corneal disease following ocular infection with herpes simplex virus type 1. J Gen Virol. 2004;8:2055–63. doi: 10.1099/vir.0.80049-0. [DOI] [PubMed] [Google Scholar]
- Toma HS, Murina AT, Areaux RG, Jr., Neumann DM, Bhattacharjee PS, Foster TP, Kaufman HE, Hill JM. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23:249–73. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- Trousdale MD, Steiner I, Spivack JG, Deshmane SL, Brown SM, MacLean AR, Subak-Sharpe JH, Fraser NW. In vivo and in vitro reactivation impairment of a herpes simplex virus type 1 latency-associated transcript variant in a rabbit eye model. J Virol. 1991;65:6989–6993. doi: 10.1128/jvi.65.12.6989-6993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- Walker J, Laycock KA, Pepose JS, Leib DA. Postexposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes simplex virus shedding in mice. Vaccine. 1998;16:6–8. doi: 10.1016/s0264-410x(97)00177-1. [DOI] [PubMed] [Google Scholar]
- Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Foster TP, Lukiw WJ, Thompson HW. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol. 2012;2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DM, Del Rosso CR, Yin XT, Stuart PM. CXCL1 but not IL-6 is required for recurrent herpetic stromal keratitis. J Immunol. 2014;192:1762–1767. doi: 10.4049/jimmunol.1302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J Infect Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Zlotnik I, Smith CE, Grant DP, Peacock S. The effect of immunosuppression on viral encephalitis, with special reference to cyclophosphamide. Br J Exp Pathol. 1970;51:434–9. [PMC free article] [PubMed] [Google Scholar]
- Zwaagstra J, Ghiasi H, Nesburn AB, Wechsler SL. In vitro promoter activity associated with the latency-associated transcript gene of herpes simplex virus type 1. J Gen Virol. 1989;70:2163–2169. doi: 10.1099/0022-1317-70-8-2163. [DOI] [PubMed] [Google Scholar]
- Zwaagstra JC, Ghiasi H, Slanina SM, Nesburn AB, Wheatley SC, Lillycrop K, Wood J, Latchman DS, Patel K, Wechsler SL. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64:5019–5028. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]