Abstract
Background
Neurodevelopmental disabilities persist in survivors of neonatal hypoxic-ischemic encephalopathy (HIE) despite treatment with therapeutic hypothermia. Cerebrovascular autoregulation, the mechanism that maintains cerebral perfusion during changes in blood pressure, may influence outcomes. Our objective was to describe the relationship between acute autoregulatory vasoreactivity during treatment and neurodevelopmental outcomes at 2 years of age.
Methods
In a pilot study of 28 neonates with HIE, we measured cerebral autoregulatory vasoreactivity with the hemoglobin volume index (HVx) during therapeutic hypothermia, rewarming, and the first 6 h of normothermia. The HVx, which is derived from near-infrared spectroscopy, was used to identify the individual optimal mean arterial blood pressure (MAPOPT) at which autoregulatory vasoreactivity is greatest. Cognitive and motor neurodevelopmental evaluations were completed in 19 children at 21–32 months of age. MAPOPT, blood pressure in relation to MAPOPT, blood pressure below gestational age + 5 (ga + 5), and regional cerebral oximetry (rSO2) were compared to the neurodevelopmental outcomes.
Results
Nineteen children who had HIE and were treated with therapeutic hypothermia performed in the average range on cognitive and motor evaluations at 21–32 months of age, although the mean performance was lower than that of published normative samples. Children with impairments at the 2-year evaluation had higher MAPOPT values, spent more time with blood pressure below MAPOPT, and had greater blood pressure deviation below MAPOPT during rewarming in the neonatal period than those without impairments. Greater blood pressure deviation above MAPOPT during rewarming was associated with less disability and higher cognitive scores. No association was observed between rSO2 or blood pressure below ga + 5 and neurodevelopmental outcomes.
Conclusion
In this pilot cohort, motor and cognitive impairments at 21–32 months of age were associated with greater blood pressure deviation below MAPOPT during rewarming following therapeutic hypothermia, but not with rSO2 or blood pressure below ga + 5. This suggests that identifying individual neonates’ MAPOPT is superior to using hemodynamic goals based on gestational age or rSO2 in the acute management of neonatal HIE.
Electronic supplementary material
The online version of this article (doi:10.1186/s12883-015-0464-4) contains supplementary material, which is available to authorized users.
Keywords: Autoregulation, NIRS, Hypoxic-Ischemic Encephalopathy, Therapeutic Hypothermia, Neurodevelopmental Outcomes
Background
Neonatal hypoxic-ischemic encephalopathy (HIE) affects approximately 3 in 1000 births and is the most common cause of perinatal brain injury in full-term neonates [1, 2]. Long-term severe sequelae of neonatal HIE include intellectual disability and cerebral palsy. In children who received therapeutic hypothermia for HIE, the incidence of cerebral palsy is approximately 17 % and the incidence of IQ < 70 is 27 % [3]. Based on these incidence rates, in the United States, the financial burden of HIE-induced intellectual disabilities exceeds $3.4 billion per year, and the costs of HIE-induced cerebral palsy exceed $1.9 billion per year [3–5]. Multicenter, randomized controlled trials of therapeutic hypothermia for neonatal HIE demonstrate incomplete neuroprotection. In the Total Body Hypothermia for Neonatal Encephalopathy Trial, 55 % of HIE survivors who received hypothermia had persistent neurologic abnormalities at age 6–7 years, including 21 % with cerebral palsy and 22 % with moderate or severe disabilities [6]. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network trial of therapeutic hypothermia in HIE found that 35 % of survivors who received hypothermia had moderate or severe disabilities at 6–7 years of age [3]. Therefore, additional modifiable factors and potential adjuvant therapies to hypothermia must be identified to improve neurologic outcomes.
Dysregulated cerebral blood flow may be a key component in secondary neurologic injury in HIE [7]. Cerebrovascular autoregulation maintains relatively constant cerebral blood flow across changes in perfusion pressure. This physiologic mechanism functions within a specific range of blood pressure, and the mean arterial blood pressure (MAP) with optimal autoregulatory function is termed the optimal MAP (MAPOPT). The hemoglobin volume index (HVx) monitors autoregulatory vasoreactivity by correlating changes in arterial blood pressure to changes in relative total tissue hemoglobin (rTHb), a surrogate measure of cerebral blood volume obtained by near-infrared spectroscopy (NIRS). HVx is based on the premise that autoregulatory vasodilation and vasoconstriction induce changes in cerebral blood volume that are proportional to changes in rTHb [8]. HVx can identify MAPOPT in neonates with HIE [9, 10]. We previously reported that blood pressure deviation below MAPOPT during rewarming is associated with greater brain injury on MRI in pilot studies of autoregulation during HIE [9, 10]. However, whether blood pressure autoregulation during therapeutic hypothermia and rewarming in the neonatal period is associated with later neurodevelopmental outcomes remains unknown. Neuroprotective blood pressure ranges for HIE are poorly defined, and many clinicians use regional cerebral oximetry (rSO2) or maintain blood pressures at gestational age in weeks +5 mmHg (ga + 5) to help guide hemodynamic goals in neonates [11].
The goal of this observational pilot study was to describe the relationship between blood pressure autoregulation during therapeutic hypothermia for treatment of neonatal HIE and cognitive and motor neurodevelopmental outcomes at approximately 2 years of age. We hypothesized that 1) greater blood pressure deviation below MAPOPT would be associated with neurodevelopmental disabilities; 2) the rSO2 would not be associated with disability; and 3) greater time spent with blood pressure below the ga + 5 would not be associated with disability. We tested each of these hypotheses by comparing autoregulation measurements made during hypothermia, rewarming, and the first 6 h of normothermia to neurodevelopmental outcomes of children 2 years later.
Methods
This study was approved by the Johns Hopkins University (JHU, Baltimore, MD) Institutional Review Board. Written, informed consent for HVx monitoring was obtained from the neonates’ parents upon admission to the JHU neonatal intensive care unit (NICU) and again before the 2-year neurodevelopmental follow-up evaluations, which took place at the Kennedy Krieger Institute (KKI, Baltimore, MD). All neonates who were admitted to the NICU between September 2010 and October 2012 were screened for study eligibility, which was based on the diagnosis of HIE according to criteria used by the NICHD Neonatal Research Network’s clinical trial of hypothermia in neonatal HIE [12]. Briefly, these infants were diagnosed with moderate to severe HIE based on clinical exam and blood gas from the umbilical cord or first hour of life with pH <7.15 or a base deficit >10 mmol/L. If a blood gas measurement was not available, 10-min Apgar score <5 or assisted ventilation for ≥10 min after birth, an acute perinatal event, and moderate to severe encephalopathy were used to diagnose HIE. Additional eligibility criteria for this pilot study included gestational age ≥35 weeks, birth weight ≥1800 g, initiation of whole-body cooling within 6 h of birth, presence of an arterial blood pressure cannula, and a parent who spoke English as the primary language. Neonates who did not have an arterial blood pressure cannula, who had a coagulopathy with active bleeding, or who had congenital anomalies or other diagnoses that could make cooling unsafe were not eligible for the study. Moreover, children who were involved in the foster care system at the time of neurodevelopmental follow-up were ineligible for the study. Seventeen of the children in the current study were part of the cohort in which we previously reported an association between blood pressure autoregulatory vasoreactivity measured by HVx and brain injuries on MRI [9].
Clinical care in the NICU
All clinical care was determined by the treating clinicians and by NICU protocol. Neonates received whole-body hypothermia with a cooling blanket (Mul-T-Blanket Hyper/Hypothermia Blanket and Mul-T-Pad Temperature Therapy Pad; Gaymar Medi-Therm III, Gaymar Industries, Orchard Park, NY) to a goal rectal temperature of 33.5 ± 0.5 °C for 72 h. They were rewarmed over 6 h (goal 0.5 °C/h) to normothermia (36.5 °C). The clinicians determined the hemodynamic goals, decided when to implement vasoactive or inotropic medications, and selected the sedation regimens. When vasoactive medications were needed, dopamine was initiated followed by dobutamine, epinephrine, or milrinone infusions as necessary. Morphine, fentanyl, or hydromorphone boluses and infusions were used for sedation as necessary. Full montage electroencephalograms (EEGs) were conducted during hypothermia and after rewarming in addition to continuous amplitude-integrated EEG monitoring (Brainz BRM3 Monitor or CFM Olympic Brainz Monitor, Natus Medical Inc., San Carlos, CA) during hypothermia, rewarming, and the first 6 h of normothermia. Phenobarbital was administered to treat electrographic or clinical seizures; thereafter, levetiracetam, fosphenytoin, or topiramate was used for persistent seizures. Clinicians could view the rSO2, as measured by the NIRS, and blood pressure, as measured by continuous cardio-respiratory monitors, but they were blinded to HVx. Respiratory support parameters, including nasal cannula, high flow nasal cannula, or ventilator support with endotracheal tube were recorded during the rewarming period. Clinical histories and clinical variables were obtained by chart reviews.
Autoregulation monitoring
Adhesive, neonatal cerebral oximetry probes were placed bilaterally on the neonates’ foreheads and connected to an INVOS 5100 NIRS machine (INVOS; Covidien, Boulder, CO) according to manufacturer guidelines. We synchronously sampled the NIRS signals and arterial blood pressure from the patient hemodynamic monitor at 100 Hz and processed the data with ICM+ software (Cambridge Enterprises, Cambridge, UK) using a bedside computer. The ICM+ software calculated HVx using a continuous, moving correlation coefficient between MAP and the rTHb (a surrogate measure of cerebral blood volume obtained by NIRS) after filtering out high-frequency waves from pulse and respiration [8, 13]. Each calculation of HVx incorporated consecutive, paired, 10-s averaged values from 300-s duration, thereby utilizing 30 data points for each HVx calculation. HVx is a continuous variable that ranges from –1 to +1. Negative or near-zero HVx represents functional vasoreactivity (and therefore intact autoregulation) because MAP and rTHb either negatively correlate or are not correlated. When blood pressure decreases and vasoreactivity becomes impaired, HVx becomes positive and approaches +1 because MAP and rTHb positively correlate. We manually removed artifacts in the NIRS and MAP signals (e.g., arterial line flushes), and we excluded data that comprised <1 % of the recording period as an additional measure to remove artifacts.
The right and left HVx values were averaged and sorted into 5-mmHg bins of MAP to generate bar graphs. (None of the neonates had unilateral intracranial lesions on follow-up MRI.) We identified the MAPOPT in each time period (hypothermia, rewarming, first 6 h of normothermia) as the bin that had the most negative HVx when the bar graph exhibited an overall trend of increasing HVx values as MAP deviated from this nadir (Fig. 1a and b). When a nadir in HVx could not be identified, the neonate was coded as having an unidentifiable MAPOPT (Fig. 1c and d). These values were identified by an investigator who was blinded to the neurodevelopmental outcome (JKL).
Fig. 1.
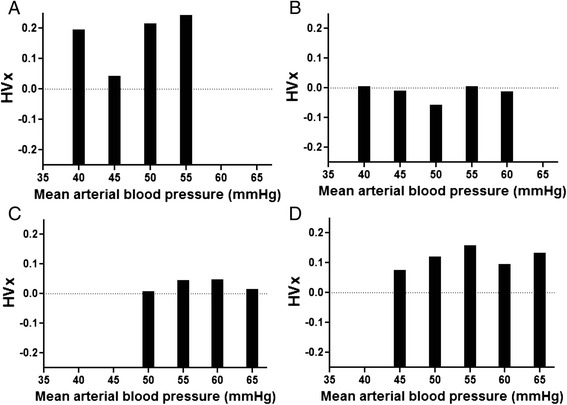
Representative hemoglobin volume index (HVx) bar graphs from individual neonates illustrate identification of the optimal mean arterial blood pressure (MAPOPT) at the nadir of HVx. MAPOPT values were 45 mmHg for patient 1 (a) and 50 mmHg for patient 2 (b). Patients 5 (c) and 25 (d) did not have a nadir in HVx and were therefore coded as having an unidentifiable MAPOPT
Blood pressure data were analyzed by three methods within each of the three time periods. First, we calculated the amount of time the neonate spent with blood pressure below, at, or above MAPOPT and analyzed this as a percentage of the autoregulation monitoring period. Second, we determined the maximal blood pressure deviation below or above MAPOPT [9, 10]. Third, we calculated the area under the curve (AUC) to combine the extent of blood pressure deviation below MAPOPT and the amount of time spent with blood pressure below MAPOPT. We analyzed time as the absolute duration of autoregulation monitoring to determine the AUC. The AUC (min•mmHg/h) was calculated as time (minutes) spent with blood pressure below MAPOPT and blood pressure deviation (mmHg) below MAPOPT, and then normalized for the duration of monitoring (hours) [10]. In addition, we calculated the percentage of time that neonates spent with blood pressure below the ga + 5 in each period. Finally, we analyzed the rSO2 using the mean between right and left cerebral hemispheres.
Neurodevelopmental evaluation
When the children were 21–32 months of age, they were evaluated for neurodevelopmental function in a single visit at KKI during a routinely scheduled clinical visit or a one-time research visit. Clinical visits were part of routine and regularly scheduled care in the KKI NICU follow-up clinic and included a neurologic exam, administration of the Capute Scales [14, 15] completed by or under the supervision of a developmental pediatrician or neonatologist, and a motor evaluation by a physical therapist. The Capute Scales are designed to assess language and visual–motor streams of development in children with a cognitive age ≤36 months. At research visits, the children participated in a battery that included the Mullen Early Scales of Development and the Gross Motor Function Measure (GMFM) administered by a neuropsychologist or a neurodevelopmental pediatrician. The Mullen is a comprehensive standardized measure of visual perception, language, and motor skill acquisition in children from birth to 68 months of age [16]. The GMFM is a detailed and quantitative measure of gross motor development that is frequently used to evaluate motor skill acquisition in individuals with cerebral palsy [17]. Neurodevelopmental outcomes were classified as impaired based on a Mullen Early Learning Composite Standard Score or Capute Full Scale Developmental Quotient <85 and a Gross Motor Function Classification (GMFC) of II-V based on GMFM performance or clinical neurologic and motor exam [18, 19]. This classification translates functionally to below average cognitive ability and the ability to walk with limitations. In contrast, neurodevelopmental outcomes were classified as unimpaired based on a Mullen Early Learning Composite Standard Score or Capute Full Scale Developmental Quotient ≥ 85 and a GMFC of I based on GMFM performance or clinical neurologic and motor exam. This classification translates functionally to average cognitive ability and the ability to walk without limitations. Investigators who conducted or supervised the neurodevelopmental examinations (VJB, GG, EC) were blinded to the blood pressure and autoregulation data.
Statistical analysis
Data were analyzed with SigmaPlot (v11.0, Systat Software Inc., Chicago, IL) and SAS v9.2 (SAS Institute Inc., Cary, NC). Graphs were generated with GraphPad Prism (v5.03, GraphPad Software Inc., La Jolla, CA). We present the data as means with standard deviations (SD) or medians with interquartile ranges (IQR) when appropriate. Differences were considered significant at p < 0.05. Neurodevelopmental outcomes were dichotomized into impaired or unimpaired, and the Mullen Early Learning Composite scores were analyzed as a continuous variable. MAPOPT values and the percentage of time spent with blood pressure below MAPOPT during each period (hypothermia, rewarming, and the first 6 h of normothermia) were compared by using Wilcoxon signed rank tests. Blood pressure, MAPOPT, and rSO2 data with respect to neurodevelopmental outcomes were tested separately within each time period. MAPOPT; the percentage of time spent with blood pressure below, at, or above MAPOPT; the maximal blood pressure deviation below or above MAPOPT; AUC; the percentage of time spent with blood pressure below ga + 5; and rSO2 were compared between children with and without impairments by using Mann Whitney rank sum tests. MAPOPT, blood pressure data in relation to MAPOPT and ga + 5, and rSO2 were compared to Mullen scores by using Spearman correlations. Seizure activity and the receipt of a vasopressor (dopamine, dobutamine, or epinephrine) were compared between children with and without impairments by using Fisher exact tests.
Results
Twenty-eight neonates with HIE received therapeutic hypothermia and had HVx monitoring. Nineteen of those children participated in neurodevelopmental follow-up examinations at 21–32 months of age. Therefore, data are presented for 19 children (10 girls, 9 boys). Their mean gestational age was 38.9 weeks (n = 19; SD = 1.5). During the autoregulation monitoring period, 11 (58 %) neonates had clinical or electrographic seizures that were treated with phenobarbital. Four of these neonates received additional antiepileptic therapy, including levetiracetam, fosphenytoin, lorazepam, or topiramate for persistent seizures. Thirteen (68 %) neonates received vasopressors during HVx monitoring, including 13 with dopamine, four with dobutamine, and one with epinephrine. Morphine infusions were administered to four neonates, and a hydromorphone infusion was given to one neonate. Fourteen neonates were intubated for synchronized intermitted mandatory ventilation (13) or high frequency jet ventilation (1). Seven neonates received nasal continuous positive airway pressure or high-flow nasal cannula respiratory support. During the rewarming period, four intubated neonates had adjustments to their ventilator respiratory rate (range of increase in respiratory rate: 5–14 breaths/min) or peak inspiratory pressure (range of change in peak inspiratory pressure: 1–6 cmH2O). One neonate had inhaled nitric oxide initiated during rewarming. Nine neonates had adjustments made in the inhaled oxygen concentration delivered through nasal cannula (2), high flow nasal cannula (1), or endotracheal tube (6; range of change in inhaled oxygen concentration: 5–55 %). No patients received extracorporeal membrane oxygenation (ECMO). Clinical data upon admission to the NICU and physiologic and laboratory data are presented in Tables 1 and 2.
Table 1.
Clinical characteristics of neonates with hypoxic-ischemic encephalopathy upon admission to the neonatal intensive care unit
| Parameter | N | Median (IQR) |
|---|---|---|
| Apgar at 1 min | 19 | 1 (1, 2) |
| Apgar at 5 min | 19 | 3 (2, 5) |
| Apgar at 10 min | 19 | 5 (3, 6) |
| Cord blood pH | 15 | 7.00 (6.91, 7.05) |
| Cord blood base deficit | 15 | −11 (–10,–15) |
| Arterial pH within 1 h of life | 19 | 7.07 (6.94, 7.24) |
| Base deficit within 1 h of life | 19 | −17 (–13,–22) |
IQR interquartile range
Table 2.
Physiologic and laboratory data during autoregulation monitoring
| Parameter | Hypothermia (n = 19) | Rewarming (n = 17) | Normothermia (n = 16) |
|---|---|---|---|
| Temperature (°C) | 33.5 (0.5) | 35.1 (0.9) | 36.8 (0.3) |
| Heart rate (bpm) | 110 (17) | 117 (17) | 133 (18) |
| pHa | 7.38 (0.05) | 7.36 (0.07) | 7.36 (0.06) |
| PaO2 a | 130 (72) | 100 (42) | 121 (50) |
| PaCO2 a | 43 (8) | 50 (10) | 48 (6) |
| Hemoglobin (g/dL) | 15.7 (2.1) | 14.0 (0.5) | 13.5 (0.7) |
All values are presented as mean (SD)
Bpm beats per minute
aArterial blood gas
Neurodevelopmental outcomes
Nineteen children had neurodevelopmental outcomes evaluated at a median age of 25 months (range, 21–32 months). Fifteen children were evaluated in research visits. Because one of those participants did not complete the GMFM, the GMFC level was determined by using the Mullen Gross Motor performance and clinical judgment. Four children had clinical evaluations. Children who participated in the full research battery had a mean performance on the Mullen Early Learning Composite within the normal range (n = 15; mean = 88.87; SD = 18.52). GMFM scores (n = 14; mean = 84.23; SD = 22.57) were similar to those of 2–4-year-old children with cerebral palsy who can walk without assistance (n = 25; mean = 81.2; SD = 13.5) [18]. The children who had clinical visits also had average mean performance (n = 4; mean = 86.00; SD = 51.61). Overall, 11 (58 %) children had typical performance or mild delays in neurodevelopment based on cognitive performance in the average range and the ability to walk without limitations. These 11 children were coded as having an unimpaired neurodevelopmental outcome for the analysis. Eight (42 %) children had more significant delays based on below-average cognitive performance or the inability to walk without limitations. These eight children were coded as having an impaired neurodevelopmental outcome for the analysis. Seizures or the receipt of a vasopressor were not associated with having an impaired neurodevelopmental outcome (p = 1.000 for seizures; p = 1.000 for vasopressors).
Autoregulatory vasoreactivity
All 19 neonates with measured neurodevelopmental outcomes were monitored during therapeutic hypothermia. HVx monitoring was terminated before rewarming in one neonate because of technical complications with monitoring and in a second who was transferred to the pediatric ICU for potential ECMO (ECMO was not initiated). One neonate did not receive HVx monitoring during normothermia because the arterial blood pressure cannula was removed. MAPOPT was identified in 15/19 (79 %) neonates during hypothermia, 17/17 (100 %) during rewarming, and 14/16 (88 %) during the first 6 h of normothermia. HVx was monitored for a median of 30.5 h (IQR, 22.4–46.5) during hypothermia, 6.5 h (IQR, 5–8) during rewarming, and 6 h (IQR, 6–6) during normothermia. MAPOPT ranged from 35 to 65 mmHg, with the majority of MAPOPT values between 45 and 55 mmHg. Values for MAPOPT were similar during hypothermia, rewarming, and the first 6 h of normothermia (p = 0.831 for hypothermia vs. rewarming; p = 0.313 for hypothermia vs. normothermia; and p = 0.685 for rewarming vs. normothermia; Fig. 2).
Fig. 2.
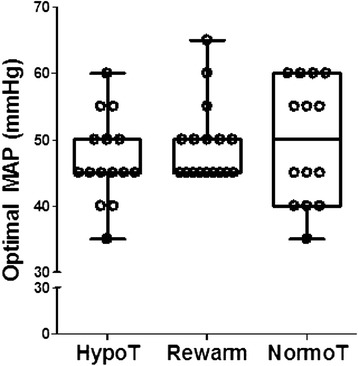
Optimal mean arterial blood pressure (MAP) values were similar during hypothermia (hypoT; n = 15), rewarming (rewarm; n = 17), and the first 6 h of normothermia (normoT; n = 14). p = 0.831 for hypothermia vs. rewarming; p = 0.313 for hypothermia vs. normothermia; p = 0.685 for rewarming vs. normothermia by Wilcoxon signed rank tests. Box plots with whiskers (5th–95th percentiles) are shown. Each circle represents one neonate
The MAP ranged from 30 to 70 mmHg but remained between 40 and 60 mmHg most of the time (Fig. 3). The percentage of time that neonates spent with blood pressure below MAPOPT was similar between time periods. More specifically, neonates spent a median of 6 % (IQR, 1–25) of the hypothermia period and 41 % (IQR, 8–59) of the rewarming period with blood pressure below MAPOPT (p = 0.119). Neonates spent a median of 31 % (IQR, 0–87 %) of the normothermia period with blood pressure below MAPOPT (p = 0.083 for hypothermia vs. normothermia; p = 0.903 for rewarming vs. normothermia).
Fig. 3.
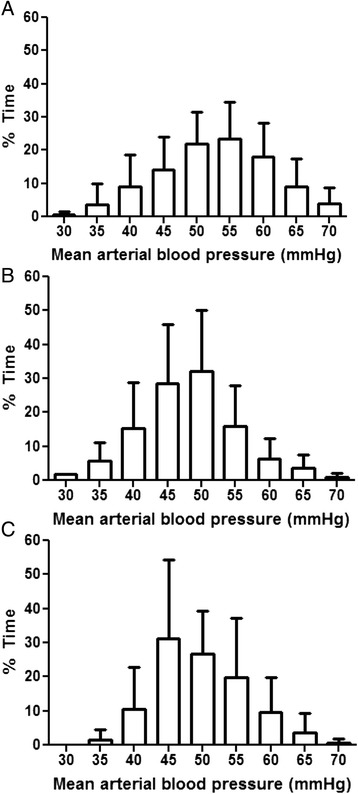
The percentages of time during hypothermia (n = 19; a), rewarming (n = 17; b), and normothermia (n = 16; c) that neonates spent at each level of mean arterial blood pressure. Data are shown as means with SDs
Values for MAPOPT during rewarming were significantly higher among children who developed impairments (n = 8) than in those who were unimpaired (n = 9; p = 0.023; Fig. 4a). MAPOPT values were similar between children with impairments (n = 5) and those without impairments during hypothermia (n = 10; p = 0.949) and during normothermia (n = 7 children with impairments; n = 7 children without impairments; p = 0.383).
Fig. 4.
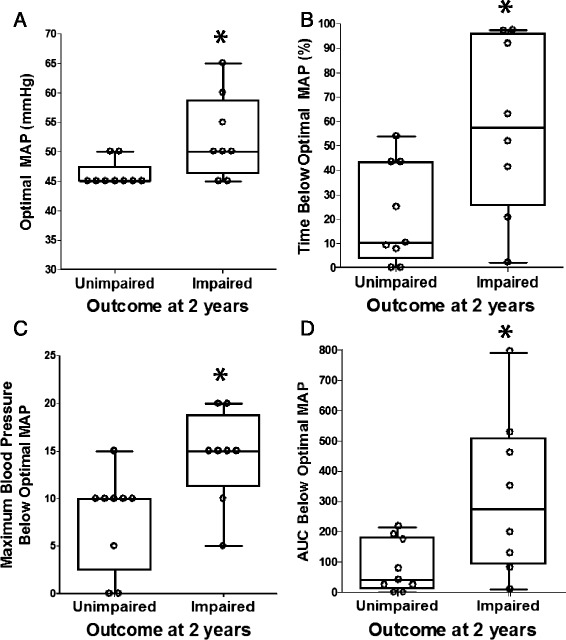
Optimal mean arterial blood pressure (MAP) and blood pressure below optimal MAP during the neonatal rewarming period in relation to neurodevelopmental outcome at approximately 2 years of age. In comparison to children without impairments (n = 9; unimpaired), children who developed impairments (n = 8; impaired) had higher optimal MAP values (*p = 0.023; a), spent a greater percentage of time with blood pressure below optimal MAP (*p = 0.048; b), had greater maximal blood pressure deviation below optimal MAP (*p = 0.019; c), and had greater area under the curve (AUC) below optimal MAP (*p = 0.039; d) during rewarming. Data were analyzed by Mann Whitney rank sum tests. Box plots with whiskers (5th–95th percentiles) are shown. Each circle represents one child
Neurodevelopmental outcome of children at approximately 2 years was associated with a longer duration of blood pressure below the individual neonate's MAPOPT during the neonatal rewarming period. Children who developed impairments (n = 8) spent a greater percentage of time with blood pressure below MAPOPT than did children without impairments (n = 9; p = 0.048; Fig. 4b). Additionally, children with impairments (n = 8) had greater maximal blood pressure deviation below MAPOPT (p = 0.019; Fig. 4c) and greater AUC below MAPOPT (p = 0.039; Fig. 4d) during rewarming than did those without impairments (n = 9). No associations were identified between impairment at 2 years and the percentage of time spent with blood pressure below MAPOPT, maximal blood pressure deviation below MAPOPT, or AUC during hypothermia and normothermia (p > 0.10 for all comparisons; Additional file 1: Table S1).
Better neurodevelopmental outcome was associated with greater time spent with blood pressure above MAPOPT and greater blood pressure deviation above MAPOPT during rewarming. Children who developed impairments (n = 8) spent a smaller percentage of the rewarming period with blood pressure above MAPOPT (p = 0.039; Fig. 5a) and had less maximal blood pressure deviation above MAPOPT (p = 0.021; Fig. 5b) than did children without impairments (n = 9). This association was not present for hypothermia or normothermia (p > 0.10 for all comparisons; Additional file 1: Table S1). Moreover, disability was not associated with the percentage of time spent with blood pressure at MAPOPT in any time period (p > 0.10 for all comparisons; Additional file 1: Table S1).
Fig. 5.

Blood pressure above the optimal mean arterial blood pressure (MAP) during the neonatal rewarming period in relation to neurodevelopmental outcome at approximately 2 years of age. When compared to children without impairments (n = 9; unimpaired), those who developed impairments (n = 8; impaired) spent a lower percentage of the rewarming period with blood pressure above optimal MAP (*p = 0.039; a) and had less maximal blood pressure deviation above optimal MAP (*p = 0.021; b) during rewarming. Data were analyzed by Mann Whitney rank sum tests. Box plots with whiskers (5th–95th percentiles) are shown. Each circle represents one child
The Mullen score at the 2-year evaluation also correlated with blood pressure in relation to MAPOPT during the neonatal rewarming period. A higher Mullen score correlated with a greater percentage of time spent in the rewarming period with blood pressure above MAPOPT (n = 13; r = 0.560, p = 0.044; Fig. 6a) and a greater maximal blood pressure deviation above MAPOPT during rewarming (n = 13; r = 0.585; p = 0.035; Fig. 6b). Similarly, maximal blood pressure deviation below MAPOPT during rewarming and the Mullen score were negatively correlated (n = 13; r = –0.563; p = 0.044; Fig. 6c). The proportion of the rewarming period spent with blood pressure below MAPOPT did not correlate with the Mullen score (n = 13; r = –0.465 p = 0.102). No correlations were identified between the Mullen score and duration of time with blood pressure above or below MAPOPT or blood pressure deviation from MAPOPT during hypothermia or normothermia (p > 0.10 for all comparisons; Additional file 2: Table S2). The Mullen score also did not correlate with the percentage of time spent at MAPOPT or with the AUC below MAPOPT in any time period (p > 0.05 for all comparisons; Additional file 2: Table S2).
Fig. 6.
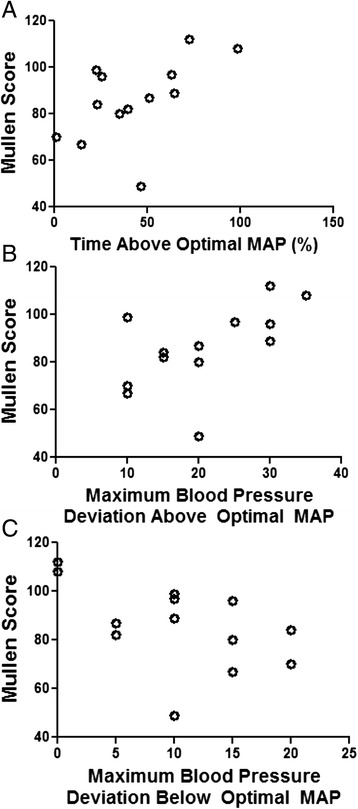
Blood pressure in relation to the optimal mean arterial blood pressure (MAP) during the neonatal rewarming period and the Mullen score at approximately 2 years of age. Higher Mullen scores correlated with a greater percentage of the rewarming period spent with blood pressure above optimal MAP (n = 13; r = 0.560, p = 0.044; a) and greater maximal blood pressure deviation above optimal MAP (n = 13; r = 0.585; p = 0.035; b). Lower Mullen scores correlated with greater maximal blood pressure deviation below optimal MAP (n = 13; r = –0.563; p = 0.044; c). Data were analyzed by Spearman correlations. Each circle represents one child
Cerebral oximetry and blood pressure in relation to gestational age
When all children were analyzed (including those with an unidentifiable MAPOPT), the mean rSO2 in any period (hypothermia, rewarming, or normothermia) was not associated with future impairment or Mullen score (p > 0.10 for all comparisons; Additional file 1: Tables S1 and Additional file 2: Table S2). The percentages of time during the hypothermia, rewarming, and normothermia periods that neonates spent with blood pressure below ga + 5 also were not associated with future impairment or Mullen score (p > 0.10 for all comparisons; Additional file 1: Table S1 and Additional file 2: Table S2). Moreover, neonates spent little time with blood pressure below their gestational age (Fig. 3).
Discussion
Several findings relevant to the treatment of neonatal HIE are suggested by this observational pilot study. The range of MAP with optimized cerebrovascular autoregulatory vasoreactivity may be identified by using HVx. Further, deviation from MAPOPT during rewarming was associated with outcome. Children with impairments at approximately 2 years of age had significantly higher MAPOPT values during the neonatal rewarming period than did children without impairments. Neurodevelopmental impairment in children was associated with more time spent at blood pressure below MAPOPT, having greater maximal blood pressure deviation below MAPOPT, and having greater AUC below MAPOPT during the rewarming period. Children without impairments spent more time with blood pressure above MAPOPT and had greater blood pressure deviation above MAPOPT during rewarming than did those with impairments. Furthermore, higher Mullen scores at 2 years significantly correlated with neonates spending more time with blood pressure above MAPOPT and having greater blood pressure deviation above MAPOPT during rewarming. An association was observed only between neurodevelopmental outcome and blood pressure in relation to MAPOPT during rewarming; no association was apparent between outcome and blood pressure during hypothermia or normothermia. Finally, neither the rSO2 nor time spent with blood pressure below ga + 5 during hypothermia, rewarming, or normothermia was associated with neurodevelopmental outcome. Although a causal relationship between blood pressure autoregulation and neurodevelopmental outcomes cannot be determined in this small, observational pilot study, our findings reveal an association between better neurodevelopmental outcomes and having blood pressures that remain within or above MAPOPT during rewarming. They further suggest that identifying each individual neonate’s MAPOPT with HVx may serve as a better method than rSO2 alone or rules based on gestational age to select blood pressure goals.
Although the overall performance of the 19 children evaluated at 2 years was in the average range, the mean cognitive scores were lower than those in normative samples [3, 6], and 42 % of the children had impairments in cognitive or motor function. The large variability in performance of the children who had clinical evaluations was likely due to the small number of children in this group. Nine (32 %) children with HIE who received autoregulation monitoring in the NICU did not have neurodevelopmental outcome data available for this study. Nonetheless, the observed associations between neurodevelopmental outcomes and autoregulation in the children with available data carry important considerations for the hemodynamic management of neonatal HIE that deserve further study.
Cerebral NIRS is often used to monitor neonates with HIE during therapeutic hypothermia [9, 10, 20–23] because invasive neurologic monitoring is generally not feasible in such patients. The predictive value of cerebral oximetry in relation to neurologic outcomes remains unclear in HIE. For neonates with HIE who had selective head cooling, higher cerebral oximetry values during hypothermia were associated with worse outcomes, including death, cerebral palsy, or global delay [22]. In contrast, other studies report that cerebral oximetry cannot predict poor neurologic outcome at 7–10 days of life [21] or severe encephalopathy [23]. Numerous factors that affect cerebral oxygen supply and demand create variability in cerebral oximetry and make immediate interpretation of the readings difficult. These factors include the administration of sedative or anti-epileptic medications, seizures, changes in oxygen supply, hyper/hypoventilation, and fluctuations in hemoglobin levels. Moreover, the decrease in cerebral metabolic rate during therapeutic hypothermia and the subsequent increase in metabolism during rewarming are confounders. Calculating the cerebral tissue oxygen extraction may offer better correlation with brain injury than regional cerebral oximetry alone [20, 23]. Altered brain oxygen consumption that may be related to dysfunctional autoregulation [24] and regional differences in cerebral perfusion [25] have been described in preterm neonates and neonates with HIE or perinatal arterial ischemic strokes. Methods to assess autoregulation by correlating blood pressure with tissue oxygen levels or oxygen extraction measured by NIRS are being tested in neonates [26–28].
We used the autoregulation metric HVx, which incorporates measures of both oxygenated and deoxygenated hemoglobin. Therefore, HVx should be minimally affected by parameters that change tissue oxygen extraction or supply, including temperature and metabolic demand. This method may enable clinicians to develop an individualized approach for neonates with HIE by identifying and aiming for the MAPOPT at which autoregulation is most functional.
Our ability to identify MAPOPT in neonates by using HVx showed that MAPOPT values vary among individuals. Children who developed impairments had significantly higher MAPOPT during rewarming from hypothermia than did children who did not develop impairments. Intracranial hypertension raises the limits of blood pressure autoregulation [29]. It is possible that severely injured neonates are at risk of elevated intracranial pressure during rewarming [30], which could shift the blood pressure autoregulation curve to higher pressures and increase MAPOPT. Identifying MAPOPT would be particularly critical in these neonates to clarify hemodynamic goals that support autoregulation.
We also found an association between neurodevelopmental impairment and blood pressure deviation from MAPOPT during rewarming. Children with impairments spent more time with blood pressure below MAPOPT, had greater maximal blood pressure deviation below MAPOPT, and had greater AUC below MAPOPT during rewarming than did those without impairments. Greater maximal blood pressure deviation below MAPOPT correlated with a lower Mullen Early Learning Composite score. Likewise, having blood pressure that remained above MAPOPT during rewarming was associated with less impairment. Children without impairments spent a greater proportion of the rewarming period with blood pressure above MAPOPT and had greater blood pressure deviation above MAPOPT than did children with impairments. Moreover, more time with blood pressure above MAPOPT and greater maximal blood pressure deviation above MAPOPT during rewarming correlated with a higher Mullen Early Learning Composite score.
There were no associations between the 2-year neurodevelopmental outcomes and blood pressure deviation from MAPOPT during hypothermia or normothermia. The percentages of time that neonates spent with blood pressure below MAPOPT were similar in the hypothermia, rewarming, and normothermia periods. Several possibilities might explain the association between blood pressure deviation from MAPOPT during rewarming and neurodevelopmental outcomes. The inherent risk of secondary neuronal injury may be highest during rewarming. Additionally, severely injured neonates may have less stable cardiovascular regulation and diminished autoregulatory capacity during rewarming. We previously reported that spending more time and having greater blood pressure deviation below MAPOPT during rewarming were associated with greater brain injury on MRI [9, 10]. This finding might be related to an increase in MAPOPT during rewarming in severely injured neonates. Rewarming itself might adversely affect cerebral blood flow autoregulation and increase the risk of stroke [31]. Intracranial hypertension and hyperemia can occur in some brain-injured regions during rewarming [30]. Moreover, cytotoxicity from rewarming with resultant neuronal cell death may be enhanced in the post-hypoxic developing brain [32]. Other neural cells also are likely vulnerable to secondary injury after hypoxia in the developing brain. In the 24 h after rewarming, elevated serum glial fibrillary acidic protein, a biomarker of astrocyte injury, was associated with the greatest severity of clinical and MRI markers of brain injury in neonates with HIE [33].
Given the small sample size in this pilot study, we were unable to control for hemoglobin level, PaCO2, or sedation which might affect cerebral blood flow and potentially confound the interpretation of HVx. Neonates did not have any clinical changes during rewarming that would acutely change their hemoglobin level, such as blood transfusion or hemorrhage. Vasoreactivity is affected by changes in CO2 production [34], including those secondary to changing metabolic rate at different temperatures. Nonetheless, HVx is a useful metric during rewarming because it is derived from both oxygenated and deoxygenated hemoglobin and therefore should be minimally affected by shifts in the oxy-/deoxyhemoglobin balance with changing temperature and metabolic rate [9, 10, 13].
The effects of changing ventilatory support during rewarming on HVx are unclear. Changes in intrathoracic pressure can affect cerebral perfusion pressure [35] and oscillations in arterial oxygen levels from ventilator maneuvers are transmitted to the cerebral microcirculation [36]. Four intubated neonates in this study had adjustments in their ventilator respiratory rate and/or peak inspiratory pressure during rewarming. Because ventilator frequencies are filtered out before calculating HVx, [8] mechanical effects of ventilation should be minimal on HVx unless they substantially increase steady state cerebral venous blood volume. Changes in the inhaled oxygen concentration should also minimally affect HVx, which is derived from the amount of total cerebral hemoglobin and not just the oxyhemoglobin component. On the other hand, it is conceivable that the one neonate who received inhaled nitric oxide during rewarming had increased cerebral delivery of nitrite, which then could have produced cerebral vasodilation. Formal studies to examine the effects of changing ventilator support and oxygen supply on HVx measurements are warranted.
Regional SO2 and blood pressure based on ga + 5 were not associated with neurodevelopmental outcomes in this study. The “normal” MAP for a neonate is often assumed to be the neonate’s ga + 5 [11], a value that frequently serves as the goal blood pressure for critically ill neonates. Our findings in this pilot study suggest that using autoregulation monitoring to identify hemodynamic goals that support autoregulation may be superior to rSO2 or rules based on gestational age.
While the data suggest that maintaining a patient’s blood pressure near MAPOPT might improve outcome, a cause and effect relationship between blood pressure autoregulation and neurodevelopmental outcome cannot yet be determined in this observational pilot study. The risks of raising a neonate’s blood pressure must be considered. While targeting the optimal cerebral perfusion pressure to support autoregulation has not yet been explored in neonates with HIE, it is being evaluated in adult traumatic brain injury [37].
Given the small sample size of this pilot study, we were unable to control for potential confounders such as gender, socioeconomic status and access to therapy services that might affect neurodevelopmental outcome. There are factors in addition to hemodynamic management that may correlate with neurodevelopmental outcomes in HIE, including abnormal EEG and brain imaging [38] and non-neurologic co-morbidities. Also, the type of neonatal cerebral oximetry probe may affect the cerebral oximetry measurements in neonates [39]. Because HVx monitoring could only begin after an arterial blood pressure cannula was established during hypothermia, we analyzed the data as the percentage of the autoregulation monitoring period and normalized the AUC for the duration of monitoring to account for different durations of monitoring during the hypothermia period. Early instability in cerebral autoregulation immediately after the perinatal event was not captured in this study. We were able to monitor HVx more consistently during rewarming and the first 6 h of normothermia. HVx measures only the regional frontal cortex and cannot be used to assess other regions of the brain, including those thought to be most at risk from neonatal hypoxic ischemia [40]. Variability in regional brain oxygenation has been reported in models of neonatal HIE, including differences in oxygenation between cortical and thalamic regions [41]. Additionally, we could not validate HVx against other measures of cerebral blood flow, such as transcranial Doppler, because continuous Doppler is not feasible for 3–4 days in neonates. Nonetheless, HVx has been validated against laser-Doppler in a swine model of HIE [13], HVx correlates with intracranial pressure-derived autoregulation measures in patients [42], and HVx has been validated against transcranial Doppler in identifying MAPOPT during cardiopulmonary bypass [43].
Conclusions
In this observational pilot study of neonatal HIE and therapeutic hypothermia, we used HVx monitoring to identify associations between cerebrovascular autoregulatory vasoreactivity during the neonatal rewarming period and 2-year neurodevelopmental outcomes. MAPOPT varied among individual neonates and was higher during rewarming in children who developed impairments than in those who did not. Blood pressure deviation below MAPOPT during rewarming was associated with greater impairment and lower cognitive scores. Although future studies are warranted, our pilot data suggest that individualizing blood pressure goals based on MAPOPT, especially during the rewarming period, may be superior to rSO2 alone or blood pressure goals based on gestational age to support autoregulation and improve neurodevelopmental outcomes.
Acknowledgements
VJB was supported by NIH grant NINDS K12-NS001696 during the writing of this manuscript. JKL was supported by NIH grant K08 NS080984 during the writing of this manuscript. FJN was supported by NIH grant HD070996 during the writing of this manuscript.
Abbreviations
- HIE
Hypoxic-ischemic encephalopathy
- HVx
Hemoglobin volume index
- MAPOPT
Mean arterial blood pressure at which autoregulatory vasoreactivity is optimal
- rSO2
Regional cerebral oximetry
- NIRS
Near-infrared spectroscopy
- rTHb
Relative total tissue hemoglobin
- NICU
Neonatal intensive care unit
- EEG
Electroencephalogram
- MRI
Magnetic resonance imaging
- AUC
Area under the curve
- GMFM
Gross motor function measure
- GMFC
Gross motor function classification
- ECMO
Extracorporeal membrane oxygenation
- PaCO2
Partial pressure of carbon dioxide in blood
Additional files
Blood pressure data in relation to the optimal mean arterial blood pressure or gestational age, regional cerebral oxygen saturation, and neurodevelopmental disability. (DOCX 19 kb)
Blood pressure in relation to the optimal mean arterial blood pressure and the Mullen score. (DOC 49 kb)
Footnotes
Competing interests
JKL and FJN previously received funding from Covidien for a separate study.
Authors’ contributions
VJB participated in the design of the study, collection of the neurodevelopmental outcomes, and helped draft the manuscript. GG participated in the design of the study, collection of the neurodevelopmental outcomes, and reviewed the manuscript. EC participated in the design of the study, collection of the neurodevelopmental outcomes, and reviewed the manuscript. SC helped perform the statistical analysis and interpretation. JJ helped perform the statistical analysis and interpretation and reviewed the manuscript. CP participated in the design of the study and recruitment of participants. RK participated in the autoregulation design and analysis and reviewed the manuscript. RCV participated in the data analysis and reviewed the manuscript. MVJ participated in the design of the study, coordination of the neurodevelopmental outcomes, and reviewed the manuscript. FJN helped conceive of the study, participated in its design and reviewed the manuscript. JKL helped conceive of the study, participated in the design and autoregulation analysis, and helped to draft the manuscript. All authors read and approved of the final manuscript.
Contributor Information
Vera Joanna Burton, Email: BurtonJ@kennedykrieger.org.
Gwendolyn Gerner, Email: GernerG@kennedykrieger.org.
Elizabeth Cristofalo, Email: ecristofalo@jhmi.edu.
Shang-en Chung, Email: schung2@jhmi.edu.
Jacky M. Jennings, Email: jjennin1@jhmi.edu
Charlamaine Parkinson, Email: chenson1@jhmi.edu.
Raymond C. Koehler, Email: rkoehler@jhmi.edu
Raul Chavez-Valdez, Email: rchavez2@jhmi.edu.
Michael V. Johnston, Email: Johnston@kennedykrieger.org
Frances J. Northington, Email: frances@jhmi.edu
Jennifer K. Lee, Email: jklee@jhmi.edu
References
- 1.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7(1):56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 2.Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199(6):587–95. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. NEJM. 2012;366(33):2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1–116. [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services Centers for Disease Control and Prevention. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment – United States, 2003. MMWR Morb Mortality Wkly Rep. 2004;53(03):57–9. [PubMed] [Google Scholar]
- 6.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. NEJM. 2014;371(2):140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 7.Chalak LF, Tarumi T, Zhang R. The “neurovascular unit approach” to evaluate mechanisms of dysfunctional autoregulation in asphyxiated newborns in the era of hypothermia therapy. Early Hum Dev. 2014;90:687–94. doi: 10.1016/j.earlhumdev.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40(5):1820–6. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 9.Howlett JA, Northington FJ, Gilmore MM, Tekes A, Huisman TAGM, Parkinson C, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013;74(5):525–35. doi: 10.1038/pr.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekes A, Poretti A, Scheurkogel MM, Huisman TAM, Howlett JA, Alqahtani E, et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. Am J Neuroradiol. 2015;36(1):188–93. doi: 10.3174/ajnr.A4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gretchen CB, Rayannavar AS. Cardiology. In: Engorn B FJ, editor. The Harriet Lane Handbook. 20th ed. Philadephia, PA Saunders, an imprint of Elsevier Inc; 2015. p. 127-71
- 12.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. NEJM. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 13.Larson AC, Jamrogowicz JL, Kulikowicz E, Wang B, Yang Z-J, Shaffner DH, et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J Appl Physiol. 2013;115(10):1433–42. doi: 10.1152/japplphysiol.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accardo PJ, Capute AJ. The Capute Scales: Cognitive Adaptive Test/Clinical Linguistic & Auditory Milestone Scale (Cat/Clams) Portland: Book News, Inc; 2004. [Google Scholar]
- 15.Visintainer PF, Leppert MO, Bennett A, Accardo PJ. Standardization of the Capute Scales: methods and results. J Child Neurol. 2004;19(12):967–72. doi: 10.1177/08830738040190121101. [DOI] [PubMed] [Google Scholar]
- 16.Mullen EM. Mullen Scales of Early Learning. San Antonio: Pearson Clinical Asssessments; 1995. [Google Scholar]
- 17.Russell DJ, Rosenbaum PL, Wright M, Avery LM. Gross Motor Function Measure (GMFM-66 and GMFM-88) User’s Manual 2nd Ed. London: MacKeith Press; 2013. [Google Scholar]
- 18.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 19.Beckung E, Hagberg G. Correlation between ICIDH handicap code and Gross Motor Functional Classification System in children with cerebral palsy. Dev Med Child Neurol. 2000;42:669–73. doi: 10.1017/S0012162200001237. [DOI] [PubMed] [Google Scholar]
- 20.Massaro AN, Bouyssi-Kobar M, Chang T, Vezina LG, du Plessis AJ, Limperopoulos C. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. Am J Neuroradiol. 2013;34(8):1649–55. doi: 10.3174/ajnr.A3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shellhaas RA, Thelen BJ, Bapuraj JR, Burns JW, Swenson AW, Christensen MK, et al. Limited short-term prognostic utility of cerebral NIRS during neonatal therapeutic hypothermia. Neurology. 2013;81(3):249–55. doi: 10.1212/WNL.0b013e31829bfe41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, Savini S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 2013;35(1):26–31. doi: 10.1016/j.braindev.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Wintermark P, Hansen A, Warfield SK, Dukhovny D, Soul JS. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. NeuroImage. 2014;85(1):287–93. doi: 10.1016/j.neuroimage.2013.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vis JB, Petersen ET, Alderliesten T, Groenendaal F, de Vries LS, van Bel F, et al. Non-invasive MRI measurements of venous oxygenation, oxygen extraction fraction and oxygen consumption in neonates. NeuroImage. 2014;95:185–92. doi: 10.1016/j.neuroimage.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 25.De Vis JB, Petersen ET, Kersbergen KJ, Alderliesten T, de Vries LS, van Bel F, et al. Evaluation of perinatal arterial ischemic stroke using noninvasive arterial spin labeling perfusion MRI. Pediatr Res. 2013;74(3):307–13. doi: 10.1038/pr.2013.111. [DOI] [PubMed] [Google Scholar]
- 26.Verhagen EA, Hummel LA, Bos AF, Kooi EMW. Near-infrared spectroscopy to detect absence of cerebrovascular autoregulation in preterm infants. Clin Neurophysiol. 2014;125(1):47–52. doi: 10.1016/j.clinph.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Hahn GH. Testing impact of perinatal inflammation on cerebral autoregulation in preterm neonates: evaluation of a noninvasive method. Dan Med J. 2013;60(4):B4628. [PubMed] [Google Scholar]
- 28.Baerts W, van Bel F, Thewissen L, Derks JB, Lemmers PMA. Tocolytic indomethacin: effects on neonatal haemodynamics and cerebral autoregulation in the preterm newborn. Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F419–23. doi: 10.1136/archdischild-2012-302532. [DOI] [PubMed] [Google Scholar]
- 29.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108(4):1278–83. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 30.Iida K, Kurisu K, Arita K, Ohtani M. Hyperemia prior to acute brain swelling during rewarming of patients who have been treated with moderate hypothermia for severe head injuries. J Neurosurg. 2003;98(4):793–9. doi: 10.3171/jns.2003.98.4.0793. [DOI] [PubMed] [Google Scholar]
- 31.Joshi B, Brady K, Lee JK, Easley B, Panigrahi R, Smielewski P, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–8. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Armstrong JS, Lee J-H, Bhalala U, Kulikowicz E, Zhang H et al. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2015;Jan 7:Epub ahead of time. [DOI] [PMC free article] [PubMed]
- 33.Ennen CS, Huisman TAGM, Savage WJ, Northington FJ, Jennings JM, Everett AD, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205(3):251–e1-7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock JM, Deibler AR, Whitlow CT, Tan H, Kraft RA, Burdette JH, et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am J Neuroradiol. 2009;30(2):378–85. doi: 10.3174/ajnr.A1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiehna EN, Huffmyer JL, Thiele RH, Scalzo DC, Nemergut EC. Use of the intrathoracic pressure regulator to lower intracranial pressure in patients with altered intracranial elastance: a pilot study. J Neurosurg. 2013;119(3):756–9. doi: 10.3171/2013.4.JNS122489. [DOI] [PubMed] [Google Scholar]
- 36.Klein KU, Boehme S, Hartmann EK, Szczyrba M, Heylen L, Liu T, et al. Transmission of arterial oxygen partial pressure oscillations to the cerebral microcirculation in a porcine model of acute lung injury caused by cyclic recruitment and derecruitment. Br J Anaesth. 2013;110(2):266–73. doi: 10.1093/bja/aes376. [DOI] [PubMed] [Google Scholar]
- 37.Dias C, Silva MJ, Pereira E, Monteiro E, Maia I, Barbosa S, et al. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocrit Care. 2015;23(1):92–102. doi: 10.1007/s12028-014-0103-8. [DOI] [PubMed] [Google Scholar]
- 38.Jose A, Matthai J, Paul S. Correlation of EEG, CT, and MRI Brain with Neurological Outcome at 12 Months in Term Newborns with Hypoxic Ischemic Encephalopathy. J Clin Neonatol. 2013;2(3):125–30. doi: 10.4103/2249-4847.119996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dix LML, van Bel F, Baerts W, Lemmers PMA. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res. 2013;74(5):557–63. doi: 10.1038/pr.2013.133. [DOI] [PubMed] [Google Scholar]
- 40.Ghei SK, Zan E, Nathan JE, Choudhri A, Tekes A, Huisman TAGM, et al. MR imaging of hypoxic-ischemic injury in term neonates: pearls and pitfalls. Radiographics. 2014;34(4):1047–61. doi: 10.1148/rg.344130080. [DOI] [PubMed] [Google Scholar]
- 41.Manole MD, Kochanek PM, Bayir H, Alexander H, Dezfulian C, Fink EL, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res. 2014;75(2):295–301. doi: 10.1038/pr.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, et al. Noninvasive Monitoring of Cerebrovascular Reactivity with Near Infrared Spectroscopy in Head-Injured Patients. J Neurotrauma. 2010;27(11):1951–8. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 43.Easley RB, Kibler KK, Brady KM, Joshi B, Ono M, Brown C, et al. Continuous cerebrovascular reactivity monitoring and autoregulation monitoring identify similar lower limits of autoregulation in patients undergoing cardiopulmonary bypass. Neurol Res. 2013;35(4):344–54. doi: 10.1179/1743132812Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]


