Abstract
DNA damage and DNA damage response (DDR) in neurulation stage embryos under maternal diabetes conditions are not well understood. The purpose of this study was to investigate whether maternal diabetes and high glucose in vitro induce DNA damage and DDR in the developing embryo through oxidative stress. In vivo experiments were conducted by mating superoxide dismutase 1 (SOD1) transgenic male mice with wild-type (WT) female mice with or without diabetes. Embryonic day 8.75 (E8.75) embryos were tested for the DNA damage markers, phosphorylated histone H2A.X (p-H2A.X) and DDR signaling intermediates, including phosphorylated checkpoint 1 (p-Chk1), phosphorylated checkpoint 2 (p-Chk2), and p53. Levels of the same DNA damage markers and DDR signaling intermediates were also determined in the mouse C17.2 neural stem cell line. Maternal diabetes and high glucose in vitro significantly increased the levels of p-H2A.X. Levels of p-Chk1, p-Chk2, and p53, were elevated under both maternal diabetic and high glucose conditions. SOD1 overexpression blocked maternal diabetes-induced DNA damage and DDR in vivo. Tempol, a SOD1 mimetic, diminished high glucose-induced DNA damage and DDR in vitro. In conclusion, maternal diabetes and high glucose in vitro induce DNA damage and activates DDR through oxidative stress, which may contribute to the pathogenesis of diabetes-associated embryopathy.
Keywords: Diabetic embryopathy, embryo, DNA damage, DNA damage response, maternal diabetes, high glucose
1. Introduction
Maternal diabetes-associated birth defects occur in 6–10% of live births, representing a significant maternal-fetal health problem[1–3]. Neural tube defects and congenital heart defects are the most common types of birth defects induced by maternal diabetes[1–3]. Previous studies have demonstrated that embryos exhibit high levels of oxidative stress under maternal diabetic or high glucose conditions[1–7]. Reactive oxygen species of oxidative stress adversely modify protein, lipid, and DNA that lead to cellular dysfunction and thereby contribute to the pathogenesis of diabetes-associated complications[8, 9]. Studies have shown that both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) induce DNA damage[10]. However, the role of maternal diabetes on embryonic DNA damage remains unclear.
Oxidative stress is the vital cause for DNA damage[11]. Oxidative stress may induce about 10,000 DNA alterations per cell per day[12]. In order to maintain genetic stability in the event of DNA damage, The DNA damage response (DDR) and the DNA repair pathway are activated for recognition of DNA damage lesions and promoting DNA damage repair[11, 12]. DDR is comprised of two main pathways: the Ataxia-telan-giectasia mutated (ATM)-Checkpoint 2 (ATM-Chk2) and ATR-Rad3-related (ATR)-Checkpoint 1 (Chk1) (ATR-Chk1)[11]. ATM-Chk2 dependent DDR is activated primarily in response to double stranded DNA breaks (DSBs)[11]. Activated ATM possesses kinase activity, which phosphorylates multiple substrates including Chk2, p53, and histone H2A.X[12]. The ATR-Chk1 pathway is activated by UV, IR, methyl methanesulfonate (MMS) and mitomycin (MMC) in response to stalled DNA replication forks and DNA damage and causes phosphorylation of Chk1 at serine 345[11]. The phosphorylation of Chk1 enhances its kinase activity and phosphorylates its downstream substrates and facilitates cell cycle arrest and DNA damage repair[11]. Both ATM-Chk2 and ATR-Chk1 pathway can be triggered by oxidative stress[11].
Because maternal diabetes mainly affects the neural stem cells in the developing embryo, the present study aims to determine whether maternal diabetes or high glucose in vitro induces DNA damage and DDR in mouse embryos and neural stem cells, respectively. We found that maternal diabetes and high glucose in vitro induced DNA damage by increasing phosphorylation of H2A.X, activating the DDR signaling pathway and its downstream effector p53. Furthermore, oxidative stress was responsible for DNA damage and DDR activation. Thus, DNA damage and DDR activation may play critical roles in high glucose-induced apoptosis and maternal diabetes-induced embryopathy.
2. Materials and Methods
2.1. Animals and reagents
Wild-type (WT) C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). SOD1-Tg mice in the C57BL/6J background were revived from frozen embryos by the Jackson Laboratory (Stock number: 002298). Streptozotocin (STZ) from Sigma (St. Louis, MO) was dissolved in sterile 0.1 M citrate buffer (pH4.5). The procedures for animal use were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
2.2. Mouse models of diabetic embryopathy
The mouse model of diabetic embryopathy has been described previously[13]. Briefly, ten-week old WT female mice were intravenously injected daily with 75 mg/kg STZ over two days to induce diabetes. Using STZ to induce diabetes is not a complicating factor because STZ is cleared from the bloodstream rapidly (STZ serum half-life, 15 minutes), and pregnancy is not established until one-to-two weeks after STZ injections. Diabetes was defined as a 12 hours fasting blood glucose level of ≥ 14 mM. To generate SOD1 embryos, we crossed SOD1-Tg male mice with nondiabetic or diabetic WT female mice. Male and female mice were paired at 3:00 P.M., and pregnancy was established by the presence of the vaginal plug next morning, and noon of that day was designated as day 0.5 (E0.5). WT female mice were treated with vehicle injections as nondiabetic controls. On E8.75 (at 6:00 P.M.), mice were euthanized and conceptuses were dissected out of the uteri, embryos with the yolk sacs were removed from the deciduas and then yolk sacs were removed from the embryos. The embryos were used for analyses.
2.3. Cell culture
C17.2 mouse neural stem cells, originally obtained from ECACC (European Collection of Cell Culture), were maintained in DMEM (5 mM glucose) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin at 37° in a humidified atmosphere of 5% CO2. The C17.2 cells are newborn mouse cerebellar progenitor cells transformed with retroviral v-myc.
2.4. Western blotting analysis
Equal amounts of protein from embryos or cells were resolved by the SDS-PAGE gel electrophoresis and transferred onto Immunobilon-P membranes (Millipore, Billerica, MA). Membranes were incubated in 5% nonfat milk for 45 minutes and then were incubated for 18 hours at 4°C with the following primary antibodies at dilutions of 1:1000 in 5% nonfat milk: p-Chk1, Chk1, p-Chk2, Chk2, p53, p-H2A.X, H2A.X and SOD1(Cell Signaling Technology). Membranes were then exposed to goat anti-rabbit or anti-mouse secondary antibodies. To confirm that equivalent amounts of protein were loaded among samples, membranes were stripped and probed with a mouse antibody against β-actin (Abcam). Signals were detected using the SuperSignal West Femto Maximum Sensitivity Substrate kit (Thermo Scientific). All experiments were repeated three times with the use of independently prepared tissue lysates.
2.5. Statistics
Data are presented as means ± S.E.M. Three embryos from three separate dams were used for the in vivo studies and cell cultures experiments were repeated three times. One-way ANOVA was performed using the SigmaStat 3.5 software, a Tukey test was used to estimate the significance. Statistical significance was accepted when P < 0.05.
3. Results
3.1. Maternal diabetes and high glucose induce phosphorylation of H2A.X
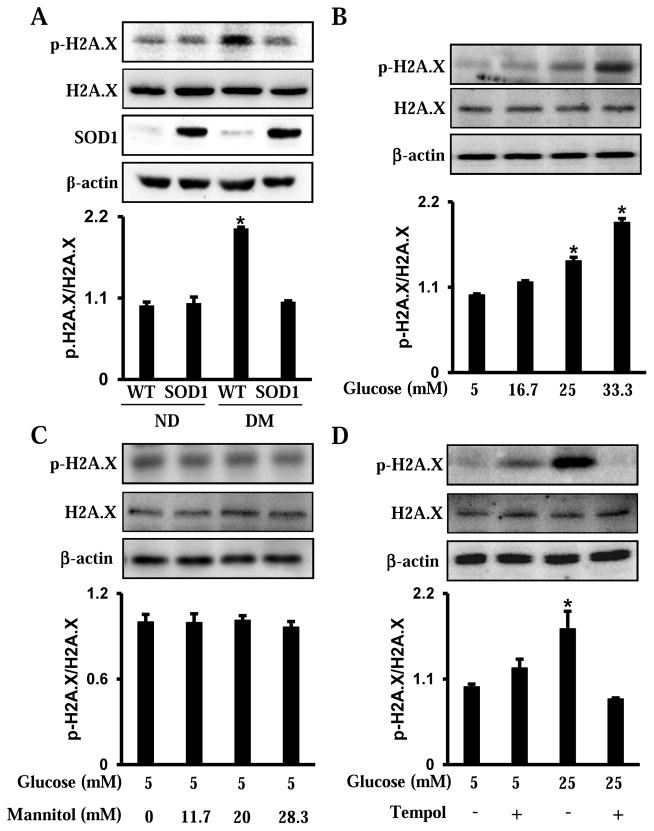
The histone variant H2A.X is extensively phosphorylated at Serine 139, which is referred to γ-H2A.X, by the ATM and ATR kinase at DNA break sites[12]. Therefore, phosphorylation of H2A.X represents one of the earliest DNA damage-induced markers, and is essential for the sustained recruitment of various checkpoint and DNA repair proteins to the DNA damage sites[12]. Maternal diabetes significantly increased p-H2A.X levels in developing embryos (Fig. 1A). The C17.2 mouse neural stem cell line was used to determine whether high glucose in vitro induces phosphorylation of H2A.X. The C17.2 cells were cultured either under normal glucose (5 mM glucose) or high glucose (16.7, 25 and 33.3 mM glucose) conditions. High glucose increased the levels of p-H2A.X in a dose-dependent manner (Fig. 1B). Moreover, mannitol was used as an osmotic control of high glucose. High mannitol concentrations had no effect on the levels of p-H2A.X (Fig. 1C). Thus, our data indicate that maternal diabetes or high glucose triggers DNA damage in vivo and in vitro, respectively.
Figure 1.
Maternal diabetes and high glucose in vitro induce H2A.X phosphorylation. A. Phosphorylation of H2A.X in E8.75 embryos from nondiabetic or diabetic dams, in the presence or absence of SOD1 overexpression. B. Phosphorylation of H2A.X in neural stem cells treated with glucose (5, 16.7, 25, 33.3 mM) for 48 hours. C. Phosphorylation of H2A.X in neural stem cells treated with normal glucose (5 mM) plus mannitol (0, 11.7, 20, 18.3 mM) for 48 hours. D. Phosphorylation of H2A.X in neural stem cells treated with normal (5 mM) or high glucose (25 mM) for 48 hours, in the presence or absence of Tempol (100 μM). Within each panel, the membrane was probed first for the phosphorylated protein and sequentially stripped for probing of the total protein content and β-actin. The quantification of the blot is shown in the bar graph. Experiments were repeated three times (n = 3). Values are the means ± SEM from three separate experiments. * indicates significant differences (P < 0.05) compared to the other three groups (A, D) or the normal glucose (5 mM) groups (B).
3.2. Inhibiting oxidative stress blocks maternal diabetes- and high glucose-induced DNA damage
Our previous studies have demonstrated that oxidative stress mediates the adverse effects of maternal diabetes, and the antioxidant enzyme, SOD1 overexpression in SOD1 Tg mice blocks maternal diabetes-induced oxidative stress in the developing embryo [14–16]. To test whether oxidative stress is involved in maternal diabetes-induced DNA damage, wild-type (WT) and SOD1 overexpressing embryos from nondiabetic and diabetic dams were used to determine the levels of p-H2A.X. SOD1 overexpression suppressed the increase of p-H2A.X levels by maternal diabetes (Fig. 1A). Furthermore, the SOD1 mimetic Tempol abrogated high glucose-induced H2A.X phosphorylation in vitro (Fig. 1D). These findings suggest that mitigating oxidative stress abrogates maternal diabetes- and high glucose-induced DNA damage.
3.3. Maternal diabetes induces Chk1 and Chk2 phosphorylation through oxidative stress
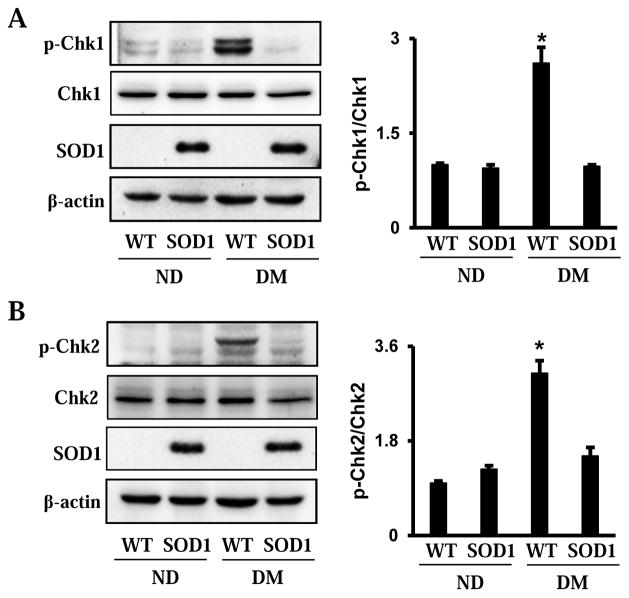
In order to reveal the involvement of Chk1 and Chk2 in the maternal diabetes-induced DDR in diabetic embryopathy, the phosphorylation levels of Chk1 and Chk2 in the developing embryo were determined. Maternal diabetes significantly induced the phosphorylation of Chk1 and Chk2, and this effect was blocked by overexpression of SOD1 (Fig. 2A and B).
Figure 2.
Maternal diabetes induces Chk1 and Chk2 phosphorylation in embryos. A. Phosphorylation of Chk1 in E8.75 embryos from nondiabetic or diabetic dams, in the presence or absence of overexpression of SOD1. B. Phosphorylation of Chk2 in E8.75 embryos from nondiabetic or diabetic dams, in the presence or absence of overexpression of SOD1. Within each panel, the membrane was probed first for the phosphorylated protein and sequentially stripped for probing total protein and β-actin. The quantification of blot was showed in the bar graph. Experiments were repeated three times (n = 3). Values are the means ± SEM from three separate experiments. * indicates significant differences (P < 0.05) compared with other three groups.
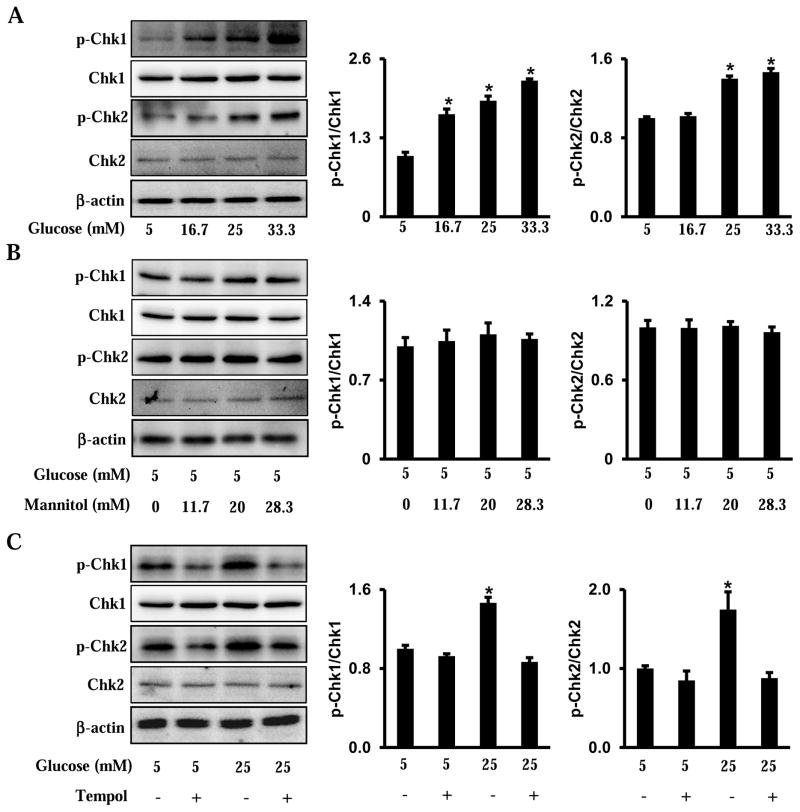
The phosphorylation of Chk1 and Chk2 was validated through in vitro experiments. High glucose increased the phosphorylation of Chk1 and Chk2 in a dose-dependent manner (Fig. 3A). Mannitol had no effect on the phosphorylation of Chk1 and Chk2 in cultured cells (Fig. 3B). Furthermore, Tempol abolished high glucose-induced phosphorylation of Chk1 and Chk2 (Fig. 3C).
Figure 3.
High glucose induces Chk1 and Chk2 phosphorylation in neural stem cells. A. Phosphorylation of Chk1 and Chk2 in cultured cells treated with glucose (5, 16.7, 25, 33.3 mM) for 48 hours. B. Phosphorylation of Chk1 and Chk2 in cultured cells treated with normal glucose (5 mM) plus mannitol (0, 11.7, 20, 18.3 mM) for 48 hours. C. Phosphorylation of Chk1 and Chk2 in cultured cells treated with normal (5 mM) or high (25 mM) glucose for 48 hours, in the presence or absence of Tempol (100 μM). Within each panel, the membrane was probed first for the phosphorylated protein and sequentially stripped for probing total protein and β-actin. The quantification of blot was showed in the bar graph. Experiments were repeated three times (n = 3). Values are the means ± SEM from three separate experiments. * indicates significant differences (P < 0.05) compared with other three groups (C) or the normal glucose (5 mM) groups (A).
3.4. Maternal diabetes and high glucose induce p53 accumulation via oxidative stress
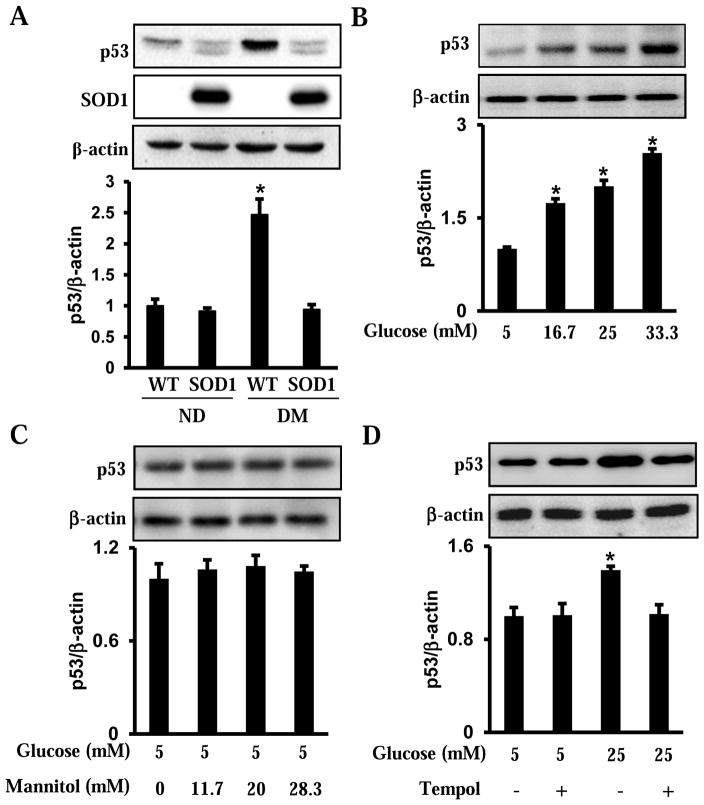
The phosphorylation of p53 is the result of Chk1 and Chk2 phsophorylation. The stabilized p53 due to phsophorylation subsequently accumulates in cells, and, thus, promoted its transcription factor activity that triggers cell cycle arrest or cell death[17]. Indeed, consistent with Chk1 and Ckh2 phosphorylation, p53 protein levels were significantly increased in embryos exposed to maternal diabetes compared with embryos from nondiabetic dams (Fig. 4A). Overexpression of SOD1 in embryos reduced the level of p53 to that in embryos from nondiabetic dams (Fig. 4A). Similarly, high glucose induced the increase of p53 in the C17.2 mouse neural stem cells. This finding was not seen in its osmotic control, mannitol (Fig. 4B and C). Moreover, Tempol suppressed high glucose-induced increase of p53 (Fig. 4D).
Figure 4.
Maternal diabetes and high glucose in vitro induce p53 accumulation. A. Levels of p53 in E8.75 embryos from nondiabetic or diabetic dams, in the presence or absence of SOD1 overexpression. B. Levels of p53 in neural stem cells treated with glucose (5, 16.7, 25, 33.3 mM) for 48 hours. C. Levels of p53 in neural stem cells treated with normal glucose (5 mM) plus mannitol (0, 11.7, 20, 18.3 mM) for 48 hours. D. Levels of p53 in neural stem cells treated with normal (5 mM) or high glucose (25 mM) for 48 hours, in the presence or absence of Tempol (100 μM). Within each panel, the membrane was probed first for the p53 protein and sequentially stripped to probe for β-actin. The quantification of the blot is shown in the bar graphs. Experiments were repeated three times (n = 3). Values are the means ± SEM from three separate experiments. * indicates significant differences (P < 0.05) compared to the other three groups (A, D) or the normal glucose (5 mM) groups (B).
4. Discussion
This study provides evidence that maternal diabetes induces DNA damage and DDR during the neurulation stage of developing embryos. The DNA damage marker p-H2A.X was increased in the developing embryo by maternal diabetes and this elevation was suppressed by overexpression of SOD1. In addition, the data indicate that the DDR pathways are activated by maternal diabetes manifested by increased phosphorylation of Chk1, p-Chk2, and p53 accumulation. Overexpression of SOD1 in embryos diminished DDR activation. The occurrence of DNA damage and DDR in the developing embryo by high glucose of diabetes was recapitulated through in vitro studies with mouse neural stem cells. High glucose induced DNA damage and DDR in neural stem cells, and these effects were abrogated by the application of the SOD1 mimetic Tempol. These findings indicate that maternal diabetes induces DNA damage and DDR in the developing embryo, and these events may ultimately contribute to maternal diabetes-associated birth defects, particularly neural tube defects.
Human studies have shown that diabetes-induced DNA damage occurs in a variety of cells and tissues, including lymphocytes[18, 19], mononuclear cells[20], leukocytes[21], erythrocytes[22], islet cells[23], and visceral adipose tissue[24]. Animal studies have shown that diabetes increases the levels of the oxidative DNA damage marker 8-oxo-dG in the rat renal cortex[25, 26], testis[27], and corpus cavernosum[28]. However, the extent of DNA damage in diabetes-associated embryos remains unclear. Rats with severe diabetes show higher levels of DNA damage in leukocytes of the offspring[29, 30]. Other groups have shown that the mutation frequency of the neutral target gene lacI in fetuses is increased under maternal diabetic environments and high glucose conditions in vitro[31, 32]. The increase in fetal DNA mutations can be attributed to high glucose-mediated DNA damage[31, 32]. Our study provides direct evidence that maternal diabetes induces DNA damage during the neurulation stage of embryos by establishing the presence of increased p-H2A.X and DDR activation. Furthermore, we ascertained that maternal diabetes-induced oxidative stress contributed to the DNA damage by diminishing the adverse effects in embryos and neural stem cells with the implementation of SOD1 and Tempol, respectively. These findings are consistent with those of previous studies in other systems[33]. For example, thalidomide is a teratogenic compound and thalidomide-induced oxidative stress increases the DNA damage in rabbit embryos[33]. Collectively, our findings further support the oxidative stress hypothesis in the induction of diabetic embryopathy[1, 2].
Maternal diabetes-induced DNA damage may involve multiple types of DNA breaks or lesions. This is further supported by the fact that some groups have observed single stranded DNA breaks (SSBs) in peripheral blood lymphocytes from human samples of type 1 and type 2 diabetes mellitus[18–20, 22]. The occurrence of DSBs by identifying a well-validated DSB marker, p-H2A.X, in peripheral blood lymphocytes from a group of type 1 diabetes mellitus adolescent patients[34]. These results are consistent with our findings because we also observed an increase of p-H2A.X in mouse embryos from diabetic mothers.
The DDR pathways are activated by oxidative stress and they are used to detect DNA lesions, signal their presence, and arrest cell cycle progression to promote DNA damage repair[11]. Human studies have shown that DNA repair capacity is inhibited in lymphocytes by oxidative stress induced by type 1 and type 2 diabetes mellitus[18, 19, 22]. Diabetes-induced oxidative DNA damage is associated with the down-regulation of 8-oxoG-DNA glycosylase (OGG1), a DNA repair enzyme[25]. Collectively, we propose that maternal diabetes-induced DNA damage results from a decrease in DNA repair capacity. In future studies, we may aim to investigate the mechanisms underlying maternal diabetes-induced DNA repair dysfunction in embryos.
The DDR pathways are activated for DNA repair purposes[11]. However, when DNA damage exceeds the cellular capacity to repair or the repair pathways are suppressed, the accumulation of DNA damage will overwhelm cells and result in cell death or apoptosis[29]. The present study shows that when there is maternal diabetes-induced DNA damage, p53 accumulates in embryos. p53 is a transcription factor that induces cellular growth arrest or apoptosis[17]. Our previous studies have demonstrated that neural stem cell apoptosis in the developing neuroepithelium is the causal event for maternal diabetes-induced neural tube defects[1, 2]. We have elucidated the roles of oxidative stress, ER stress, and their molecular mediator in embryonic apoptosis[1, 2, 4, 6, 7, 35, 36]. DNA damage and the accumulation of p53 may be other important factors in maternal diabetes-induced embryonic apoptosis.
In summary, our study provides the first direct evidence that high glucose of diabetes or high glucose in vitro leads to DNA damage, DDR activation, and p53 accumulation in the neurulation stage of embryos and neural stem cells, respectively. These findings suggest that DNA damage may participate in maternal diabetes-induced apoptosis in developing embryo. These discoveries may help us understand the mechanisms that underlie maternal diabetes-induced birth defects, particularly neural tube defects.
Supplementary Material
Highlights.
Maternal diabetes induces DNA damage and its response in the developing embryos;
High glucose triggers DNA damage and its response in neural stem cells;
Oxidative stress is responsible for maternal diabetes or high glucose-induced DNA damage and its response;
Targeting DNA damage and its response may be potential therapeutic interventions in diabetes-induced neural tube defects.
Acknowledgments
This research is supported by NIH R01DK083243, R01DK101972, R01DK103024 and an American Diabetes Association Basic Science Award (1-13-BS-220).
Footnotes
Disclosure: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang P, Reece EA, Wang F, Gabbay-Benziv R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. American journal of obstetrics and gynecology. 2015;212:569–579. doi: 10.1016/j.ajog.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang F, Reece EA, Yang P. Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. American journal of obstetrics and gynecology. 2015;213:125–134. doi: 10.1016/j.ajog.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbay-Benziv R, Reece EA, Wang F, Yang P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World journal of diabetes. 2015;6:481–488. doi: 10.4239/wjd.v6.i3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide Dismutase 1 in vivo Ameliorates Maternal Diabetes-Induced Apoptosis and Heart Defects through Restoration of Impaired Wnt Signaling, Circulation. Cardiovascular genetics. 2015 doi: 10.1161/CIRCGENETICS.115.001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Reece EA, Yang P. Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. American journal of obstetrics and gynecology. 2015;212:650 e651–611. doi: 10.1016/j.ajog.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Wang F, Fu M, Wang C, Quon MJ, Yang P. Cellular Stress, Excessive Apoptosis, and the Effect of Metformin in a Mouse Model of Type 2 Diabetic Embryopathy. Diabetes. 2015;64:2526–2536. doi: 10.2337/db14-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Yang P. The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicological sciences : an official journal of the Society of Toxicology. 2015;144:186–196. doi: 10.1093/toxsci/kfu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 9.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrine reviews. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 10.Milic M, Frustaci A, Del Bufalo A, Sanchez-Alarcon J, Valencia-Quintana R, Russo P, Bonassi S. DNA damage in non-communicable diseases: A clinical and epidemiological perspective. Mutation research. 2015;776:118–127. doi: 10.1016/j.mrfmmm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Yan S, Sorrell M, Berman Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cellular and molecular life sciences : CMLS. 2014;71:3951–3967. doi: 10.1007/s00018-014-1666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Li X, Xu C, Eckert RL, Reece EA, Zielke HR, Wang F. Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Science signaling. 2013;6:ra74. doi: 10.1126/scisignal.2004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Weng H, Reece EA, Yang P. SOD1 overexpression in vivo blocks hyperglycemia-induced specific PKC isoforms: substrate activation and consequent lipid peroxidation in diabetic embryopathy. American journal of obstetrics and gynecology. 2011;205:84 e81–86. doi: 10.1016/j.ajog.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng H, Li X, Reece EA, Yang P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2012;206:448 e441–447. doi: 10.1016/j.ajog.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Reece EA, Yang P. Superoxide dismutase 1 overexpression in mice abolishes maternal diabetes-induced endoplasmic reticulum stress in diabetic embryopathy. American journal of obstetrics and gynecology. 2013;209:345 e341–347. doi: 10.1016/j.ajog.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer science. 2014;105:370–388. doi: 10.1111/cas.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blasiak J, Arabski M, Krupa R, Wozniak K, Zadrozny M, Kasznicki J, Zurawska M, Drzewoski J. DNA damage and repair in type 2 diabetes mellitus. Mutation research. 2004;554:297–304. doi: 10.1016/j.mrfmmm.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Merecz A, Markiewicz L, Sliwinska A, Kosmalski M, Kasznicki J, Drzewoski J, Majsterek I. Analysis of oxidative DNA damage and its repair in Polish patients with diabetes mellitus type 2: Role in pathogenesis of diabetic neuropathy. Advances in medical sciences. 2015;60:220–230. doi: 10.1016/j.advms.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Song F, Jia W, Yao Y, Hu Y, Lei L, Lin J, Sun X, Liu L. Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed Type 2 diabetes. Clinical science. 2007;112:599–606. doi: 10.1042/CS20060323. [DOI] [PubMed] [Google Scholar]
- 21.Dincer Y, Akcay T, Ilkova H, Alademir Z, Ozbay G. DNA damage and antioxidant defense in peripheral leukocytes of patients with Type I diabetes mellitus. Mutation research. 2003;527:49–55. doi: 10.1016/s0027-5107(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 22.Pacal L, Varvarovska J, Rusavy Z, Lacigova S, Stetina R, Racek J, Pomahacova R, Tanhauserova V, Kankova K. Parameters of oxidative stress, DNA damage and DNA repair in type 1 and type 2 diabetes mellitus. Archives of physiology and biochemistry. 2011;117:222–230. doi: 10.3109/13813455.2010.551135. [DOI] [PubMed] [Google Scholar]
- 23.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 24.Jones DA, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Changes in markers of oxidative stress and DNA damage in human visceral adipose tissue from subjects with obesity and type 2 diabetes. Diabetes research and clinical practice. 2014;106:627–633. doi: 10.1016/j.diabres.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2′-deoxyguanosine-DNA glycosylase. Diabetes. 2008;57:2626–2636. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakimoto M, Inoguchi T, Sonta T, Yu HY, Imamura M, Etoh T, Hashimoto T, Nawata H. Accumulation of 8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes. 2002;51:1588–1595. doi: 10.2337/diabetes.51.5.1588. [DOI] [PubMed] [Google Scholar]
- 27.Kilarkaje N, Al-Hussaini H, Al-Bader MM. Diabetes-induced DNA damage and apoptosis are associated with poly (ADP ribose) polymerase 1 inhibition in the rat testis. European journal of pharmacology. 2014;737:29–40. doi: 10.1016/j.ejphar.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Kilarkaje N, Yousif MH, El-Hashim AZ, Makki B, Akhtar S, Benter IF. Role of angiotensin II and angiotensin-(1-7) in diabetes-induced oxidative DNA damage in the corpus cavernosum. Fertility and sterility. 2013;100:226–233. doi: 10.1016/j.fertnstert.2013.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Moreli JB, Santos JH, Rocha CR, Damasceno DC, Morceli G, Rudge MV, Bevilacqua E, Calderon IM. DNA damage and its cellular response in mother and fetus exposed to hyperglycemic environment. BioMed research international. 2014;2014:676758. doi: 10.1155/2014/676758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima PH, Sinzato YK, Gelaleti RB, Calderon IM, Rudge MV, Damasceno DC. Genotoxicity evaluation in severe or mild diabetic pregnancy in laboratory animals, Experimental and clinical endocrinology & diabetes : official journal. German Society of Endocrinology [and] German Diabetes Association. 2012;120:303–307. doi: 10.1055/s-0031-1299766. [DOI] [PubMed] [Google Scholar]
- 31.Lee AT, Plump A, DeSimone C, Cerami A, Bucala R. A role for DNA mutations in diabetes-associated teratogenesis in transgenic embryos. Diabetes. 1995;44:20–24. doi: 10.2337/diab.44.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Lee AT, Reis D, Eriksson UJ. Hyperglycemia-induced embryonic dysmorphogenesis correlates with genomic DNA mutation frequency in vitro and in vivo. Diabetes. 1999;48:371–376. doi: 10.2337/diabetes.48.2.371. [DOI] [PubMed] [Google Scholar]
- 33.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nature medicine. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 34.Giovannini C, Piaggi S, Federico G, Scarpato R. High levels of gamma-H2AX foci and cell membrane oxidation in adolescents with type 1 diabetes. Mutation research. 2014;770:128–135. doi: 10.1016/j.mrfmmm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Wu Y, Quon MJ, Li X, Yang P. ASK1 mediates the teratogenicity of diabetes in the developing heart by inducing ER stress and inhibiting critical factors essential for cardiac development. American journal of physiology Endocrinology and metabolism. 2015;309:E487–499. doi: 10.1152/ajpendo.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Wu Y, Gu H, Reece EA, Fang S, Gabbay-Benziv R, Aberdeen G, Yang P. Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes. 2015;64:973–988. doi: 10.2337/db14-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.