Abstract
The α2δ auxiliary subunits of voltage-gated Ca2+ channels (VGCCs) are important modulators of VGCC function. Gabapentin interacts with α2δ1 and α2δ2 subunits and is reported to reduce Ca2+ channel current amplitude (ICa). This study aimed to determine the effects of gabapentin on VGCCs in retinal ganglion cells (RGCs). Whole cell patch clamp was used to record ICa in isolated RGCs, and calcium imaging was used to measure Ca2+ transients from RGCs in situ. Immunohistochemistry was used to detect the presence of α2δ1-containing VGCCs in isolated RGCs in the absence and presence of gabapentin pretreatment. Acute administration of gabapentin reduced ICa and Ca2+ transients compared to control conditions. In isolated RGCs, pretreatment with gabapentin (4–18 h) reduced ICa, and cell surface α2δ1 staining was reduced compared to nonpretreated cells. Acute administration of gabapentin to isolated RGCs that had been pretreated further reduced ICa. These results show that gabapentin has both short-term and long-term mechanisms to reduce ICa in isolated RGCs. Some Ca2+ channel blockers have been shown to protect RGCs in retinal trauma suggesting that modulation of VGCCs by gabapentin may prevent the deleterious effects of elevated Ca2+ levels in RGCs in trauma and disease.
Keywords: α2δ Ca channel subunit, Alpha2delta subunit, Cacna2d1, Retinal ganglion cell, Gabapentin
Introduction
Gabapentin has been approved for the treatment of many clinical conditions involving the central nervous system since its initial FDA approval almost 20 years ago. Initially, gabapentin was designed to mimic the actions of GABA in the central nervous system; however, it has become clear that gabapentin has distinct actions unrelated to those of GABA (Rock et al., 1993). Numerous studies investigating the actions of gabapentin have not provided a precise mechanism of action. Early studies in cultured neurons were unable to detect any action of gabapentin on common central nervous system receptors or ion channels (Rock et al., 1993), but subsequent studies have shown that gabapentin interacts with the α2δ auxiliary subunits of voltage-gated Ca2+ channels (VGCCs; Gee et al., 1996). However, the degree to which gabapentin affects the function of VGCCs appears to differ between experimental models (Stefani et al., 1998; Martin et al., 2002; Sutton et al., 2002; Li et al., 2006; Hendrich et al., 2008). The aim of the present study was to determine whether acute and chronic application of gabapentin modulates VGCC function in retinal ganglion cells (RGCs).
The primary function of VGCCs, to allow calcium into cells, is achieved by the pore-forming α1 subunit of the channel. For the high-voltage activated (HVA) class of Ca2+ channels, there are seven isoforms of α1 that determine the physiological and pharmacological characteristics of the VGCC subtype (reviewed by Dolphin, 2009a,b). However, it has become clear that the auxiliary subunits of these channels also play important roles in VGCC function and modulation (Birnbaumer et al., 1998; Walker and De Waard, 1998). HVA VGCCs typically associate with two auxiliary subunits; a β-subunit (subtypes 1–4) interacts with the α1 subunit on the intracellular face of the plasma membrane, whereas an α2δ subunit (subtypes 1–4) associates with the α1 subunit on the extracellular face of the membrane (reviewed by Dolphin, 2009a,b). Interestingly, there appears to be little selectivity in the association between α1 subunit isoforms and auxiliary subunit subtypes (Schlick et al., 2010). Intracellular β auxiliary subunits of VGCCs have been shown to be involved in second messenger signaling pathways [i.e., phosphorylation by protein kinase A (PKA); Gerhardstein et al., 1999], modulation of channel gating (reviewed by Buraei & Yang, 2010), and trafficking VGCCs to the cellular membrane (Mich & Horne, 2008). Similarly, extracellular α2δ auxiliary subunits are known to modulate VGCC trafficking to and from the cell surface (Hendrich et al., 2008). In addition, α2δ1 and α2δ2 subunits are known to contain gabapentin binding sites (Gee et al., 1996). In the present study, we sought to determine whether RGCs express α2δ1 subunits and whether VGCC function could be modulated by the α2δ ligand gabapentin.
Previous studies have shown that VGCCs and particularly α2δ1 subunit expression at the cell membrane are increased after nerve injury (Bauer et al., 2009). This increase in α2δ1 has been associated with increased pain signal transmission in the spinal cord (Li et al., 2004). Transgenic mice overexpressing α2δ1 show behaviors similar to those seen in neuropathic pain, even in the absence of nerve injury, and increased expression of α2δ1 results in increased ICa (Li et al., 2006). It is well established that excessive levels of intracellular calcium led to cellular dysfunction and even death. In the retina, studies have shown that blocking VGCCs on RGCs reduces cell loss after optic nerve injury (Osborne et al., 2004). However, nonselective inhibition of VGCCs using traditional calcium channel blockers may not be the best therapeutic strategy since calcium is required for many intracellular processes and its levels are normally tightly regulated (Osborne et al., 2004). In addition, in the setting of injury where VGCCs are upregulated, simply blocking the channel may be insufficient to prevent a pathologic rise in intracellular calcium levels. For this reason, strategies to modulate VGCCs after neuronal injury have been of particular interest. Indeed, studies using animal models of neuropathic pain have demonstrated that administration of gabapentin prevents the upregulation of α2δ1-containing VGCCs after nerve injury (Bauer et al., 2009). The objective of the present study was to determine whether gabapentin influenced α2δ1 membrane expression and VGCC function in RGCs. The results of this study demonstrate that α2δ1 can be modulated by gabapentin in isolated RGCs and whole retina both on acute and chronic time scales, raising the possibility that gabapentin administration may provide neuroprotection to RGCs in the setting of optic nerve damage.
Materials and Methods
Purified RGC culture
Experimental protocols were approved by the Dalhousie University Committee on Laboratory Animals and performed in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals (CCAC, Ottawa, ON: Vol. 1, second edition 1993; Vol. 2, 1984).
The two-step panning procedure to purify RGCs has been previously described (Barres et al., 1988; Hartwick et al., 2004). Briefly, litters of Long-Evans rats (Charles River, Montreal, QU) were sacrificed at postnatal day 7 by overexposure to isoflurane and by decapitation. Dissected eyecups were immersed in Hibernate-A (BrainBits, Springfield, IL) with 2% B27 supplements and 10 μg/ml gentamicin. Retinas were removed and treated as described in Hartwick et al. (2004). Purified ganglion cells were plated onto poly-d-lysine/laminin-coated Biocoat glass coverslips (12-mm round; BD Biosciences, Bedford, MA) in 4-well tissue culture plates at a density of 2.5 × 104 cells per well. Cultures were maintained at 37°C in a humidified 5% CO2–air atmosphere. Patch clamp recordings were made on the second day following cell dissociation and panning.
Immunohistochemical procedures
Coverslips containing purified ganglion cells were fixed in 4% paraformaldehyde in 0.01-M PBS [Cedarlane Laboratories, Burlington, ON; 5 min, room temperature (RT)]. RGCs were washed with PBS and treated with normal goat serum blocking solution (Sigma Aldrich, Oakville, ON; 20% in PBS, 30 min, RT). Coverslips were incubated for 18–24 h (4°C) with α2δ1 Ca2+ channel subunit primary antibody (affinity purified rabbit polyclonal, EPFPSAVTIKSWVDK, extracellular epitope; 1:200 in PBS; Alomone Laboratories, Jerusalem, Israel), then washed in PBS. Samples were incubated with Alexa Fluor 546 goat antirabbit antibody (Life Technologies Corp., Burlington, ON) for 2 h (RT), washed with PBS and mounted on microscope slides using VectaShield (Vector Laboratories, Burlington, ON). Cells were not permeabilized with Triton-X (omitted from all immunohistochemical buffers) to limit antibody staining to the cell surface. To determine the selectivity of the α2δ1 antibody, preadsorption control samples were performed in parallel using the supplied control peptide (Alomone Labs). The control peptide was incubated with the α2δ1 antibody (2:1 by weight, 4°C, overnight) prior to adding the suspension to the isolated RGCs. In addition, secondary antibody controls (omission of primary antibody) were performed to ensure selectivity of the Alexa Fluor 546 goat antirabbit for the α2δ1 antibody.
Confocal imaging
All immunohistochemistry samples were imaged using a Nikon Eclipse C1 confocal microscope (60×, 1.40 N.A., Plan Fluor oil immersion objective; Nikon Canada Inc., Toronto, ON). For each experiment, the gain was adjusted using a positive control sample (α2δ1 antibody on cells without any drug treatment) and maintained at the same level for all drug-treated samples. For negative control samples (preadsorption and secondary antibody controls), the gain was increased to ensure the absence of staining. In these samples, cells were visualized under bright field to ensure that there were cells in the field of view being imaged. Z-stacks were acquired using the smallest pinhole setting and 0.25 μm optical sections (total thickness of isolated RGCs was approximately 15 μm).
Offline analysis of all confocal images was performed using ImageJ (NIH, Baltimore, MD) and ClampFit (Molecular Devices, Sunnyvale, CA) software. All optical sections were flattened into single z-stacked image using the maximum fluorescence intensities of each section (MAX setting in ImageJ). The brightness and contrast of z-stacked images were optimized using ImageJ; however, no filters or background subtractions were applied to any image. Quantification of cellular staining in the absence and presence of gabapentin was estimated by determining the area under the curve (AUC) of the fluorescence intensity within a 16-μm2 (average cell soma size in these experiments) box centered on the cell body in the z-stacked image (ImageJ, ClampFit). Background fluorescence was subtracted from each sample by determining the AUC of a 16-μm2 box adjacent to the cell of interest.
Electrophysiological recordings
In all recordings, isolated ganglion cells were perfused with extracellular bathing solution containing (mM): 137 NaCl, 5 KCl, 1 MgCl , 2.5 CaCl , 22.2 glucose, and 5 HEPES, adjusted to pH 7.4 with NaOH. To isolate Ca2+ channel currents (ICa) in the absence and presence of gabapentin, the extracellular solution immediately surrounding the cell of interest was changed using a rapid perfusion inlet (VC8-S, ALA Scientific, Albany, NY) containing (in mM) 110 choline chloride, 5 KCl, 1 MgCl2 7 BaCl2, 15 TEACl, 0.14-aminopyridine, 20 glucose, 10 HEPES, and 1 μM tetrodotoxin, adjusted to pH 7.4 with CsOH. Bath applied gabapentin (acute treatment) was diluted in this barium choline extracellular solution and administered to RGCs using the ALA perfusion system (1–2 ml/min, 21–25°C).
Patch electrodes with 5–10 MΩ tip resistance were pulled from fire polished borosilicate glass capillary tubes using a micropipette puller (Sutter Instruments, Novato, CA). The intracellular pipette solution contained (in mM) 140 CsCl, 0.8 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES, adjusted to pH 7.2 with CsOH. The bath reference electrode consisted of an agar bridge with an AgCl wire. Cell voltage was clamped with an Axopatch-1D amplifier (Molecular Devices) using whole-cell capacitance and series resistance compensation. Voltage command protocols and current signal acquisition were controlled by pClamp 10 software (Molecular Devices). All protocols were initiated from a holding potential of −60 mV. The current signal was filtered at 0.5 kHz (Ithaco 4302 Dual 24dB/octave filter, Ithaca, NY) and digitized at 1 kHz with Digidata 1440 (Molecular Devices). Currents were digitally leak and capacitance transient subtracted using ClampFit (Molecular Devices) and SigmaPlot software (Systat Software Inc, San Jose, CA). All measurements made in the presence of bath applied gabapentin were taken after recordings had reached steady-state (drug exposure for 1–2 min).
Calcium imaging
Calcium imaging experiments were performed in accordance with the guidelines for the welfare of experimental animals issued by the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals (2002) and the University of California-Los Angeles Animal Research Committee. Sprague-Dawley rats (3–5 weeks of age) were deeply anesthetized, decapitated, and the eyes enucleated. The lens and the anterior portion of the eye were removed and the calcium indicator fluo-4 pentapotassium salt (0.5 μl of 40 mM stock in H2O) was injected into the optic nerve stump ~1 mm posterior to the globe. The eyecup was placed in saline solution and bubbled with 95% O2/5% CO2 for 1 h at room temperature in the dark. The retina was then isolated from the eyecup, divided into quadrants, and one quadrant mounted on a glass slide using a harp slice grid (ALA Scientific, HSG 5DD). Solutions were delivered via an 8-channel superfusion system (ALA Scientific) and contained (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, 25 NaHCO3, and 10 glucose (bubbled with 95% O2/5% CO2). Cells were depolarized with paired pulses of buffer containing elevated [K+]o (60 mM for 33 s per pulse; NaCl reduced to 68 mM, all other components remained the same). Fluo-4 confocal fluorescence images were acquired at ~5 s intervals with a Zeiss LSM 5 Pa laser scanning microscope using a water-immersion Axoplan 40× (NA 0.8) objective. Excitation was provided by an argon laser (488 nm), whereas emissions were collected using a 505-nm long-pass (LP) filter. Fluorescence intensity values were acquired by placing regions of interest (ROIs) on RGC somata and axon bundles. The ratio of fluo-4 fluorescence intensity change (Ca2+ transient amplitude) between the first pulse (always recorded in normal buffer) and the second K+ pulse measured in either normal buffer or in the presence of gabapentin (10 μM for 5 min prior to and during the second K+ pulse) was reported. This method was used to reduce the effects of fluo-4 loading variability between experiments to allow comparison of Ca2+ transients between unpaired groups.
Drugs and chemicals
All chemicals and reagents, unless otherwise noted, were obtained from Sigma Aldrich. Gabapentin was purchased from Ascent Scientific (Princeton, NJ). Tetrodotoxin citrate (TTX) was purchased from Alomone Labs. Fluo-4 pentapotassium salt was purchased from Invitrogen (Carlsbad, CA). All drugs and reagents were prepared in double distilled water as stock solutions (stored at −20°C) and warmed to room temperature or prepared fresh immediately before performing experiments. Drug pretreatments were performed by administering drugs directly to the cell culture dishes during incubation (37°C).
Data analysis
All data are reported as the means ± s.e.m. Data analysis was performed using Clampfit 10 (Molecular Devices), ImageJ (NIH) and Prism 4 (GraphPad Inc., La Jolla, CA) software. Graphing and statistical analyses were performed using SigmaPlot 11.0 (Systat Software Inc). Specific statistical tests used are noted in the figure legend in the appropriate Results sections. P values of less than 0.05 were considered statistically significant. All pair-wise multiple comparison procedures were performed in SigmaPlot, using the Holm–Sidak method. Figures containing confocal images were prepared using CorelDraw X4 (Corel Corporation, Ottawa, ON) graphics software.
Results
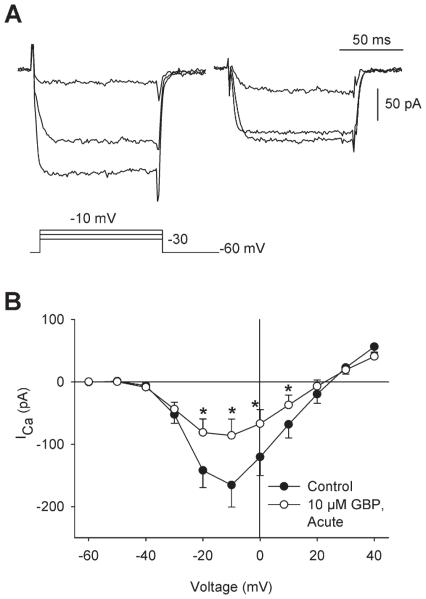
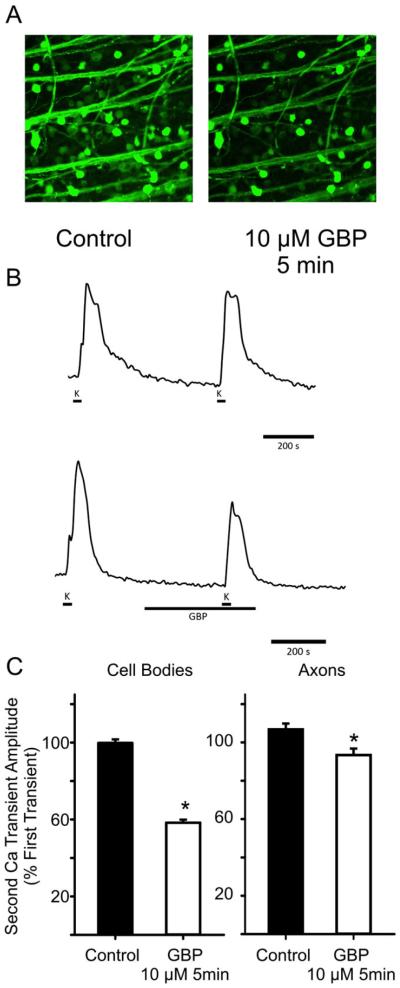
The aim of the present study was to determine the effects of gabapentin administration on Ca2+ currents (ICa) in isolated RGCs and Ca2+ transients in intact retina. First, gabapentin (10 μM) was bath applied to isolated RGCs for 2 min. Using whole cell patch clamp techniques, ICa was recorded continuously before and during this acute administration of gabapentin. Fig. 1A shows an example of a patch clamp recording of ICa from an isolated RGC before (left) and after (right) administration of gabapentin (10 μM) for 2 min. This example shows that gabapentin reduced ICa at all three voltage steps shown. Mean data showed that gabapentin significantly reduced ICa between −20 and +10 mV compared to paired control recordings (Fig. 1B). The I–V relationships show use-dependence of gabapentin, with greater block at more depolarized potentials, as is often found in drug actions on ion channels due to the higher probability of drug interaction with the open channels. Similarly, in intact retina, acute administration of gabapentin (10 μM, 5 min) caused a reduction in Ca2+ transient amplitudes in both RGC somata and axon bundles (Fig. 2). Fig. 2A shows examples of fluo-4 fluorescence during membrane depolarization (60 mM K+) in the absence (left) and presence of gabapentin (right, 10 μM GBP administered 5 min before and during membrane depolarization). Ca2+ transients measured from these fluorescence images showed that intracellular Ca2+ levels were increased to a lesser extent in the presence of gabapentin (Fig. 2B, bottom) compared to control conditions (Fig. 2B, top) in RGC cell bodies (similar results in axon bundles, data not shown). Fig. 2C (left) shows mean data demonstrating that Ca2+ transient amplitudes in RGC somata, measured as a percentage of the first depolarization-induced transient, were significantly smaller in retinas treated with gabapentin (10 μM, 5 min prior to and during second depolarization) compared to those not exposed to the drug. Similarly, mean data from ROIs measured along RGC axon bundles (Fig. 2C, right) showed significant reductions in Ca2+ transients in response to gabapentin administration compared to control retinas. These results demonstrate a rapid effect of gabapentin on intracellular Ca2+ levels in RGCs and their axons.
Fig. 1.
Acute administration of gabapentin reduced ICa in isolated rat RGCs. (A) Examples of ICa recorded from an individual RGC under voltage-clamp conditions in the absence (left) and presence of GBP (right; 10 μM, 2 min). Corresponding voltage command protocol shown below current traces. (B) Mean current–voltage (I–V) relationships showed that a brief administration of 10 μM GBP (2 min; hollow symbols) significantly reduced ICa compared to control [filled symbols; n = 7; *P < 0.05, two-way repeated-measure (RM) ANOVA vs. control, paired data].
Fig. 2.
Application of gabapentin reduced the amplitude of Ca2+ transients in adult rat RGC bodies and axon bundles. (A) Examples of fluo-4 fluorescence images showing intracellular Ca2+ transients produced in response to membrane depolarization (evoked with 60 mM K+) in retinal wholemounts in the absence (left) and presence of GBP (10 μM, 5 min; right). (B) Examples of time course measurements of intracellular Ca2+ levels in RGC cell bodies in the absence (top) and presence of gabapentin (bottom). Solid lines shown under traces indicate application of high K+ and gabapentin solutions. Similar time courses were generated for axon bundles in the absence and presence of gabapentin. (C) The ratio of the second Ca2+ transient to the first was used to measure mean responses in the absence and presence of gabapentin. Mean data showed that GBP (10 μM 5 min prior to and during membrane depolarization; hollow bars) reduced Ca2+ transient amplitudes compared to control (filled bars) in both cell somata (left: Control: 99.8 ± 1.8%; n = 239 cells, 10 experiments; GBP: 58.3 ± 1.6%; n = 313 cells, 9 experiments; *P < 0.05, one-way ANOVA, unpaired data) and axon bundles (right: Control: 106.8 ± 3.0%, n = 40 axon bundles, 7 experiments; GBP: 93.4 ± 3.4 %, n = 43 axon bundles, 5 experiments; *P < 0.05, one-way ANOVA, unpaired data).
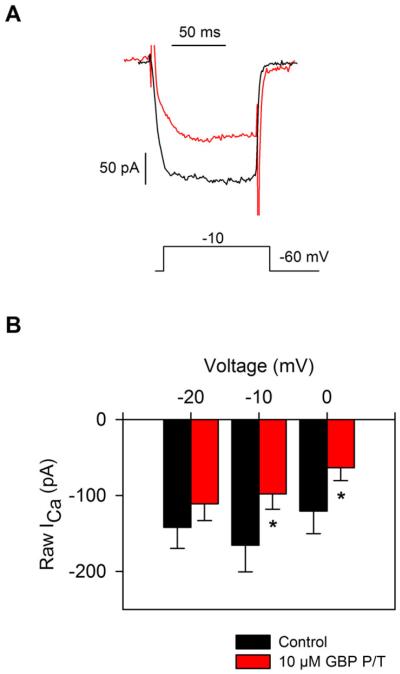
Next, to determine the effects of gabapentin over longer time periods (i.e., chronic administration), gabapentin was added to the isolated RGC culture medium (10 μM) during the incubation period before electrophysiological experiments were performed. Experiments were performed either 4 or 18 h after gabapentin administration. Data collected from these two gabapentin pretreatment time points were not significantly different; therefore, these data were combined into a single group. Fig. 3A compares ICa recordings from two different isolated RGCs in the absence of gabapentin pretreatment (black trace) and a different cell that had been pretreated with gabapentin (red trace, 10 μM GBP, 4 h). These recordings show that pretreatment of RGCs with gabapentin caused a reduction in ICa. Mean data showed that ICa was significantly reduced in isolated RGCs that had been pretreated with gabapentin compared to the control group of cells that had not been pretreated (Fig. 3B, unpaired data).
Fig. 3.
Pretreatment of RGCs with gabapentin reduced ICa in isolated rat RGCs. (A) Representative voltage clamp recordings of ICa from two isolated RGCs in the absence (black) and presence of GBP pretreatment (10 μM, 4 h; red). (B) Mean data show that ICa recorded from RGCs after an extended exposure to GBP (10 μM, 4–18 h; red bars) was significantly smaller compared to cells that had not been pretreated with GBP (black bars; n = 7–9; *P < 0.05, two-way RM ANOVA, unpaired data).
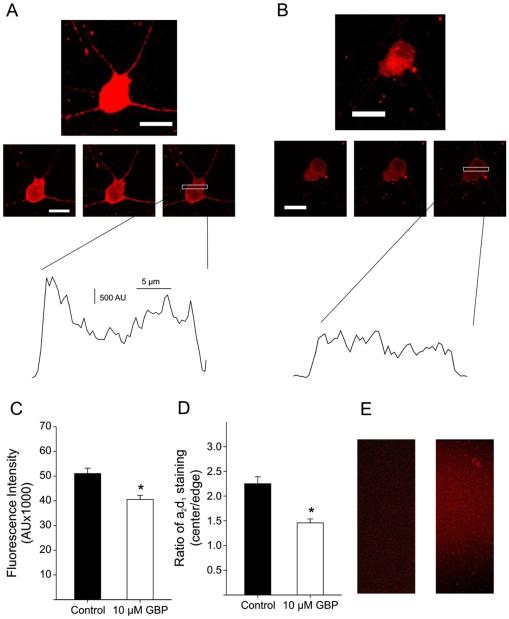
Previous studies have shown that gabapentin pretreatment reduces the presence of α2δ accessory subunit-containing calcium channels at the membrane surface of neuronal cells (Bauer et al., 2009). As gabapentin pretreatment reduced the amplitude of ICa in isolated RGCs, we aimed to determine whether gabapentin influenced the presence of α2δ1 accessory subunits on the membrane surface of RGCs. After isolation, coverslips containing isolated RGCs were pretreated with gabapentin prior to being fixed and stained with antibody against the α2δ1 calcium channel accessory subunit. To reduce staining of α2δ1 in the cytoplasm of RGCs, Triton-X (a detergent used to permeabilize cells) was excluded from all immunohistochemical solutions (Bauer et al., 2009). Fig. 4A (top) shows an example confocal z-stack image of an isolated RGC stained for α2δ1 in the absence of gabapentin pretreatment. When individual confocal sections were examined (Fig. 4A, middle), α2δ1 staining appeared to be more predominant on the cell membrane (outer edges) of the cell body, and the dendrites were also strongly labeled. Fig. 4A (bottom) shows the staining intensity of a cell plotted versus distance across the cell (cross section indicated by white box in middle panel). This plot projection shows that α2δ1 staining intensity was greater on the cell membrane compared to the cytoplasm. In parallel experiments, pretreatment of RGCs with gabapentin (10 μM, 4 h) reduced the overall staining intensity of α2δ1 in both the cell body and dendrites (z-stack, Fig. 4B, top). In addition, examination of individual confocal sections (Fig. 4B, middle) showed that α2δ1 staining did not appear to be localized to the cell membrane and was very diffuse overall. Indeed, the cross section plot profile (Fig. 4B, bottom) showed that staining was similar at the cell membrane and cytoplasm. These results suggest that gabapentin reduced the cell surface expression of α2δ1-containing Ca2+ channels. To quantify α2δ1 staining in the absence and presence of gabapentin, the fluorescence intensities of isolated RGC cell bodies were measured (Fig. 4C). The AUC for α2δ1 staining was significantly smaller in cells that had been pretreated with gabapentin compared control RGCs. In addition, measuring the ratio between the α2δ1 staining intensity at the cell membrane and cytoplasm showed that cells pretreated with gabapentin had significantly less staining at the cell membrane compared to cells that had not been treated with the drug (Fig. 4D). Secondary antibody control (Fig. 4E, left) and peptide preabsorption samples (Fig. 4E, right) showed no nonspecific labeling. These results suggest that similar to previous studies (Bauer et al., 2009), RGCs undergo gabapentin-mediated trafficking of α2δ1-containing Ca2+ channels away from the cell membrane.
Fig. 4.
Staining of α2δ1 Ca2+ channel auxiliary subunits was reduced in rat RGCs pretreated with gabapentin. (A) An example of an isolated ganglion cell stained with α2δ1 antibody in the absence of drug (60× objective; scale bars 20 μm). Top: flattened z-stack image; middle: progressive confocal sections (0.25-μm thickness, 1 μm between each image) showing greater α2δ1 staining at the outer membrane; bottom: plot projection of cross section of cell (indicated by white bar) showed higher staining intensity at the outer edge of the cell. (B) Isolated RGC stained for α2δ1 after pretreatment with gabapentin (10 μM, 4 h). Overall cellular staining was reduced, especially in the dendrites. (60× objective; scale bars 20 μm). Top: flattened z-stack image; middle: progressive confocal sections (0.25-μm thickness, 1 μm between each image) showing diffuse α2δ1 staining of the cell body and very little staining in the dendrites; bottom: plot projection of cross section of the cell (indicated by white bar) showed no difference between staining intensity at the outer edge of the cell compared to the cytoplasm. (C) Mean data showed that isolated RGCs pretreated with GBP (10 μM, 4 h; hollow bar) had significantly reduced α2δ1 staining (determined by fluorescence intensity; see Materials and methods section for detailed description) compared to cells not treated with GBP (4–9 cells per group; *P < 0.05, t-test). (D) Mean data of plot projections measured for every cell showed that cells not treated with GBP had higher ratios of α2δ1 staining at the cell membrane (outer edge) compared to the cytoplasm (cell center; 4–9 cells per group; *P < 0.05, t-test). (E) Secondary antibody control (left) and preadsorption control (right) showed no nonselective α2δ1 staining.
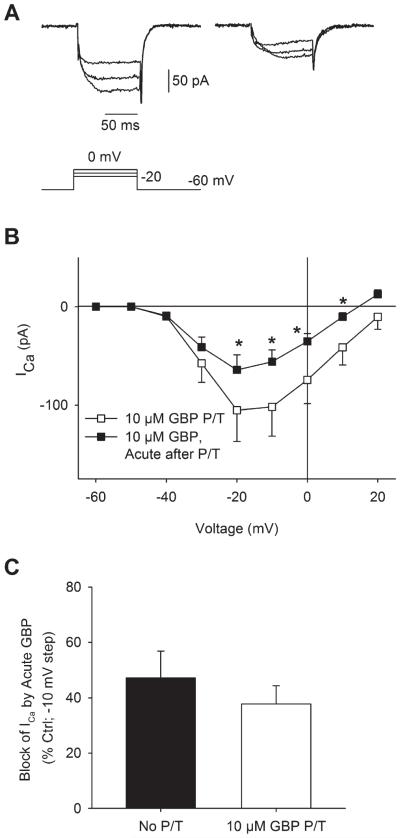
To determine whether the effects of acutely applied gabapentin on ICa in RGCs were related to channel trafficking or to another more rapid mechanism, cells that had been pretreated with gabapentin were exposed to an additional application of gabapentin during patch clamp recording. Fig. 5A shows an example of ICa recorded from an RGC that had been pretreated with gabapentin (10 μM, 18 h; left). After bath administration of gabapentin (10 μM, 2 min), ICa was further reduced (Fig. 5A; right). Mean data showed that acute gabapentin administration to RGCs that had been pretreated caused a further, significant reduction in ICa (Fig. 5B). When compared to RGCs that had not been pretreated, acute application of gabapentin showed a similar reduction in ICa in RGCs pretreated with gabapentin (Fig. 5C). These results suggest a separate more rapid mechanism of gabapentin-mediated reduction of ICa in RGCs, in addition to the α2δ1-mediated Ca2+ channel trafficking mechanism.
Fig. 5.
Acute administration of gabapentin to rat RGCs that had been pretreated further reduced ICa. (A) Examples of ICa recorded from an isolated ganglion cell pretreated with gabapentin (GBP, 10 μM, 18 h; left). Upon acute administration of gabapentin during the voltage-clamp recording (GBP, 10 μM, 2 min; right), ICa was further reduced. (B) Mean I–V relationships showed that administration of GBP to cells already pretreated with GPB significantly reduced ICa (n = 6; *P < 0.05, two-way RM ANOVA vs. pretreatment, paired data). (C) Comparison of ICa suppression by GBP in untreated and GPB-pretreated cells showed that acute application of GBP reduced ICa to a similar extent in both groups.
Discussion
The aim of the present study was to determine the effects of short duration (acute) and long duration (chronic) gabapentin administration in rat RGCs. Results showed that acutely administered gabapentin significantly reduced the amplitude of ICa in isolated RGCs and reduced Ca2+ transient amplitudes in retinal wholemounts. In addition, treating RGCs with gabapentin for prolonged periods of time reduced ICa compared to cells that had not been treated with gabapentin. Immunohistochemical experiments showed that chronic administration of gabapentin reduced the membrane expression of α2δ1 VGCC subunits in isolated RGCs. Interestingly, acute application of gabapentin to cells that had been pretreated showed further reductions in ICa. These results suggest multiple mechanisms of action of gabapentin in RGCs.
The present study is the first to show a significant reduction in ICa and Ca2+ transients in mammalian RGCs after acute administration of gabapentin. While some previous studies report no acute effect of gabapentin on calcium channels (Rock et al., 1993; Kang et al., 2002; Davies et al., 2006); several studies have documented inhibitory effects of gabapentin on VGCCs even with brief applications of the drug (Stefani et al., 1998, 2001; Martin et al., 2002; McClelland et al., 2004). There are several possibilities for the discrepancy in responsiveness of neuronal cells to gabapentin (reviewed by Uchitel et al., 2010). It is well known that gabapentin binds to α2δ auxiliary subunits of VGCCs (Gee et al., 1996) and that α2δ expression varies in different cell types (Cole et al., 2005). In addition, studies have shown that α2δ subtypes are not selectively associated with specific α1 subunit isoforms (Schlick et al., 2010). Therefore, it is plausible that different experimental neuronal preparations (and even different cell types within these preparations) will have varied expression patterns of VGCC accessory subunits; thus, respond differentially to gabapentin.
Studies using α2δ1 overexpression models have shown a significant reduction in ICa in response to gabapentin application (Li et al., 2006). In addition, membrane expression of α2δ1 is increased in injury or disease states (Fehrenbacher et al., 2003; Bauer et al., 2009). Thus, it has been proposed that the acute effects of gabapentin on ICa may not be detected in the absence of some kind of cellular insult (Uchitel et al., 2010). Indeed, most ex vivo cell preparations involve some kind of damage to isolate tissues and individual neurons and cell culture conditions also affect neuronal expression patterns of VGCC accessory subunits (Martin et al., 2002). The results of the present study show that isolated RGCs both expressed α2δ1 on their cell surface and showed a significant reduction in ICa in response to acute applications of gabapentin. Thus, these findings suggest that isolated RGCs may be a good model system to study the mechanism by which acute application of gabapentin reduces ICa.
The long-term (chronic) mechanism of gabapentin action involves the modulation of α2δ trafficking to the cell membrane (Bauer et al., 2009). Chronic application of gabapentin reduces ICa by decreasing the number of functional VGCCs at the cell surface (Hendrich et al., 2008; Bauer et al., 2009). Results of the present study show that chronic application of gabapentin reduced the cell surface expression of α2δ1 in isolated RGCs. These results are similar to those reported in other neuronal cell types and expression systems (Heblich et al., 2008; Hendrich et al., 2008). In addition, RGCs that had been chronically treated with gabapentin had significantly smaller ICa amplitudes than cells that had not been treated with gabapentin. These results demonstrate that reduced cell surface expression of α2δ1 after gabapentin treatment caused a parallel decrease in ICa amplitude. Interestingly, the present study is the first of our knowledge to report that cells pretreated with gabapentin show a further reduction in ICa in response to acute application of gabapentin. These results show that the chronic treatment of RGCs with gabapentin does not totally eliminate cell surface expression of α2δ1, as shown by our immunohistochemical data, leaving some α2δ subunits available on the cell surface for gabapentin binding in the acute setting. These results suggest two distinct mechanisms of gabapentin action in isolated RGCs. First, a long-term mechanism, occurring on the order of hours to days, involves gabapentin modulating the availability (cell surface expression) of viable VGCCs. This mechanism explains the efficacy of gabapentin reducing ICa in neuropathic pain models (Bauer et al., 2009). However, results of the present study suggest an additional mechanism where acute application of gabapentin is able to reduce ICa on a more rapid time scale, even after cells have been chronically treated with gabapentin. It is possible that gabapentin interacts with the channel α1 subunit directly or that it interacts with the bound α2δ subunit and through one of these actions alters channel gating. RGCs also express GABAB receptors (Koulen et al., 1998), activation of which modulates N- and L-type VGCC gating in a concentration dependent manner in RGCs of lower vertebrates (Shen & Slaughter, 1999). While binding studies show no interaction between gabapentin and GABAB receptors (Suman-Chauhan et al., 1993), there remains the possibility of GABAB receptor activation as well as other nonspecific actions of gabapentin. Further studies are required to elucidate the acute mechanism of gabapentin inhibition of ICa.
Despite gabapentin being used clinically for decades, its precise mechanism of action in different disease conditions remains unclear. Studies have determined gabapentin binding sites (Gee et al., 1996) and have started to elucidate mechanisms by which the clinical effects of gabapentin arise (Bauer et al., 2009). However, studies have demonstrated that under different conditions, gabapentin produces different effects (Stefani et al., 2001; Martin et al., 2002; Uchitel et al., 2010). In addition, other auxiliary subunits of VGCCs and other ion channels may play important roles in the actions of gabapentin (Stefani et al., 2001; Martin et al., 2002; Mich & Horne, 2008). Thus, further studies are required to determine the roles of development, environment and cellular preconditioning in VGCC subunit expression, and sensitivity to gabapentin. Understanding these factors may allow the development of a selective treatment strategy for conditions where calcium dysregulation is a predominant feature.
Acknowledgments
The authors would like to thank Dr. William Baldridge and Janette Nason for the preparation of postnatal retinal ganglion cell cultures. Funding for this work was provided by a Canadian Institutes of Health Research and Nova Scotia Health Research Foundation Regional Partnership Program grant to S.B. Part of this research and development project was conducted at the David Geffen School of Medicine at UCLA and is made possible by a contract agreement that was awarded to N.C.B. and administered by the U.S. Army Medical Research & Materiel Command (USAMRMC) and the Telemedicine & Advanced Technology Research Center (TATRC), at Fort Detrick, MD under Contract Number W81XWH-10-2-0077. Support for these studies also came from NIH EY04067, and a VA Merit Review was awarded to N.C.B. N.C.B. is a VA Career Research Scientist.
References
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2de-lta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. The Journal of Neuroscience. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel beta subunits. Journal of Bioenergetics and Biomembranes. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiological Reviews. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. The Journal of Comparative Neurology. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: Implications for localization and function. The Journal of Neuroscience. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: Multiple roles of calcium channel subunits. Current Opinion in Neurobiology. 2009a;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. The Journal of Neuroscience. 2009b;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. The Journal of Biological Chemistry. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- Hartwick AT, Lalonde MR, Barnes S, Baldridge WH. Adenosine A1-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Investigative Ophthalmology & Visual Science. 2004;45:3740–3748. doi: 10.1167/iovs.04-0214. [DOI] [PubMed] [Google Scholar]
- Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin) 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MG, Felix R, Campbell KP. Long-term regulation of voltage-gated Ca(2+) channels by gabapentin. FEBS Letters. 2002;528:177–182. doi: 10.1016/s0014-5793(02)03295-7. [DOI] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localization of GABA(B) receptors in neurons of the rat retina. The European Journal of Neuroscience. 1998;10:1446–1456. doi: 10.1046/j.1460-9568.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. The Journal of Neuroscience. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, Pinnock RD, Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–366. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacology. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mich PM, Horne WA. Alternative splicing of the Ca2+ channel beta4 subunit confers specificity for gabapentin inhibition of Cav2.1 trafficking. Molecular Pharmacology. 2008;74:904–912. doi: 10.1124/mol.108.045153. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Rock DM, Kelly KM, Macdonald RL. Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Research. 1993;16:89–98. doi: 10.1016/0920-1211(93)90023-z. [DOI] [PubMed] [Google Scholar]
- Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. doi: 10.1016/j.neuroscience.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic GABA receptors facilitate L-type and inhibit N-type calcium channels in single salamander retinal neurons. The Journal of Physiology. 1999;516:711–718. doi: 10.1111/j.1469-7793.1999.0711u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology. 1998;37:83–91. doi: 10.1016/s0028-3908(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Giacomini P, Lavaroni F, Bernardi G. The effects of gabapentin on different ligand- and voltage-gated currents in isolated cortical neurons. Epilepsy Research. 2001;43:239–248. doi: 10.1016/s0920-1211(00)00201-1. [DOI] [PubMed] [Google Scholar]
- Suman-Chauhan N, Webdale L, Hill DR, Woodruff GN. Characterisation of [3H]gabapentin binding to a novel site in rat brain: Homogenate binding studies. European journal of pharmacology. 1993;244:293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. British Journal of Pharmacology. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel OD, Di Guilmi MN, Urbano FJ, Gonzalez-Inchauspe C. Acute modulation of calcium currents and synaptic transmission by gabapentinoids. Channels (Austin) 2010;4:490–496. doi: 10.4161/chan.4.6.12864. [DOI] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: Role in channel function. Trends in Neurosciences. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]