Abstract
The maintenance of anxiety disorders is thought to depend, in part, on deficits in extinction memory, possibly due to reduced contextual control of extinction that leads to fear renewal. Animal studies suggest that the neural circuitry responsible fear renewal includes the hippocampus, amygdala, and dorsomedial (dmPFC) and ventromedial (vmPFC) prefrontal cortex. However, the neural mechanisms of context-dependent fear renewal in humans remain poorly understood. We used functional magnetic resonance imaging (fMRI), combined with psychophysiology and immersive virtual reality, to elucidate how the hippocampus, amygdala, and dmPFC and vmPFC interact to drive the context-dependent renewal of extinguished fear. Healthy human participants encountered dynamic fear-relevant conditioned stimuli (CSs) while navigating through 3-D virtual reality environments in the MRI scanner. Conditioning and extinction were performed in two different virtual contexts. Twenty-four hours later, participants were exposed to the CSs without reinforcement while navigating through both contexts in the MRI scanner. Participants showed enhanced skin conductance responses (SCRs) to the previously-reinforced CS+ in the acquisition context on Day 2, consistent with fear renewal, and sustained responses in the dmPFC. In contrast, participants showed low SCRs to the CSs in the extinction context on Day 2, consistent with extinction recall, and enhanced vmPFC activation to the non-reinforced CS−. Structural equation modeling revealed that the dmPFC fully mediated the effect of the hippocampus on right amygdala activity during fear renewal, whereas the vmPFC partially mediated the effect of the hippocampus on right amygdala activity during extinction recall. These results indicate dissociable contextual influences of the hippocampus on prefrontal pathways, which, in turn, determine the level of reactivation of fear associations.
Keywords: fear conditioning, amygdala, hippocampus, anterior cingulate cortex, ventromedial prefrontal cortex, extinction, virtual reality, functional magnetic resonance imaging
1. Introduction
Conditioning-based accounts of post-traumatic stress disorder (PTSD) hypothesize that the persistence of fear memories is due, in part, to failures of extinction mechanisms that render prior fear associations susceptible to recovery over time (Rauch et al., 2006). A major contributing factor to successful fear extinction is contextual control – knowing when and where it is appropriate to express fear responses to a potentially dangerous stimulus. Recent research has revealed that inhibitory interactions between the ventromedial prefrontal cortex (vmPFC) and amygdala are key to extinguishing conditioned fear responses, and dysfunction in this regulatory circuitry leads to fear persistence in PTSD (Garfinkel et al., 2014; Milad and Quirk, 2012). However, it is not well understood how contextual control mechanisms interface with this circuitry to guide the recovery of fear (Maren et al., 2013).
The return of fear following a shift away from the context of extinction training is called fear renewal (Bouton, 2004). Animal studies of fear renewal suggest a model of inter-connected brain areas for the contextual gating of extinguished fear that includes the amygdala, hippocampus, the dorsomedial prefrontal cortex (dmPFC) and the vmPFC (Maren, 2011). How the nodes of this network interact to promote fear renewal is not known, given that both direct hippocampal-amygdala and indirect hippocampal-PFC-amygdala pathways have been implicated (Maren, 2011; Milad and Quirk, 2012; Sotres-Bayon et al., 2004). For the indirect pathways, the vmPFC is assumed to inhibit the amygdala based on hippocampal signaling of the extinction context to promote extinction recall, whereas the dmPFC is thought to spur recovery of the fear memory based on hippocampal input that the current environment differs from the extinction context. Multivariate modeling of whole-brain human functional magnetic resonance imaging (fMRI) data in humans can evaluate the relative contributions of these direct and indirect pathways simultaneously. In this way, fMRI can help resolve current controversies in the animal literature and identify network dynamics that may go awry in PTSD to promote the contextual return of fear. However, human neuroscientific studies of context-dependent fear renewal are lacking to provide a translational bridge between the seminal animal work and their clinical implications (Holt et al., 2012).
One challenge to studying context-dependent fear renewal is determining how to modify the global configuration of the contexts during fMRI scanning (Maren et al., 2013). We have demonstrated that immersive (3-D) virtual reality is a powerful tool for the study of contextual modulation of fear conditioning (Ahs et al., 2015; Dunsmoor et al., 2014a; Huff et al., 2011; Huff et al., 2010). Here we adapt this technology for fMRI scanning to investigate the frontotemporal pathways involved in fear renewal, along with concurrent skin conductance response (SCR) as a dependent measure of conditioned fear.
Participants acquired and extinguished conditioned fear responses in different 3-D virtual worlds on Day 1 and returned 24 hours later for fear renewal testing in both the acquisition and extinction contexts in a counterbalanced order. We hypothesized that SCRs to the fear cue (CS+) relative the safe cue (CS−) would increase in the acquisition context compared to the extinction context on Day 2, indicating fear renewal (Vervliet et al., 2013). We tested two unrelated sets of hypotheses regarding the fMRI data. The first set tested predictions of local change in fMRI responses during extinction recall and renewal in our a priori ROIs (amygdala, hippocampus, vmPFC, dmPFC). The second set tested whether the same ROIs were part of a different functional circuitry between the two contexts. Based on previous studies of local change in fMRI response during extinction recall (Milad et al., 2005; Milad et al., 2007b; Phelps et al., 2004), we predicted greater vmPFC responses to the CS− relative to the CS+ in the extinction context on Day 2. We also expected the opposite pattern of results in the dmPFC, with greater responses to the CS+ than the CS− in the acquisition context on Day 2, as these regions are thought to modulate fear expression (Graham and Milad, 2011). In accordance with the animal models described above (Maren, 2011), we predicted that the dmPFC would statistically mediate the influence of the hippocampus on the amygdala during fear renewal whereas the vmPFC would mediate the same influence during extinction recall, and that these indirect pathways would predominate over the direct hippocampal-amygdala pathway in driving the context-dependent return of fear.
2. Materials and Methods
2.1.1 Participants
Forty-five right-handed healthy adults provided written informed consent in accordance with Duke University Medical Center Institutional Review Board guidelines. Two participants did not participate in the Day 2 assessments due to scanner failure and were removed from the analysis, leaving 43 participants in the final sample (Mean age: 28.7±10.4 yrs; 22 women).
2.1.2 Stimuli
Dynamic snake and spider CSs were created using the Maya graphic design application (Autodesk, San Rafael, California) and imported into Virtools software (Dassault Syteme, Paris, France). CSs were displayed in 3D through MRI-compatible goggles (VisuaStim, Resonance Technology Inc., Northridge, CA). Fear-relevant stimuli were used because of their relevance for anxiety disorders and because they are particularly resistant to extinction learning (Ohman and Mineka, 2001). CS duration was 4 sec. Stereoscopic presentation in 3-D was used to enhance the experience of environmental immersion, thereby increasing real world relevance and more direct translation from the animal literature in which organisms are physically placed into different 3-D contexts. The US consisted of an electrical stimulation applied to the right wrist for 16 ms through two disposable electrodes (EL507, BIOPAC systems, Goleta, CA). The electric stimulation was controlled with the MP-150 BIOPAC system (BIOPAC systems, Goleta, CA) using the STM-100 and STM-200 modules. US delivery co-terminated with the CS presentations. Prior to the start of the experimental session, the intensity of the electric shock (US) delivered to the wrist was calibrated for each participant using an ascending staircase procedure. Participants were always given at least two stimulations during the workup procedure to find a level deemed by the participant as being “highly annoying but not painful,” as we described previously (Dunsmoor et al., 2009).
2.1.3 Contexts
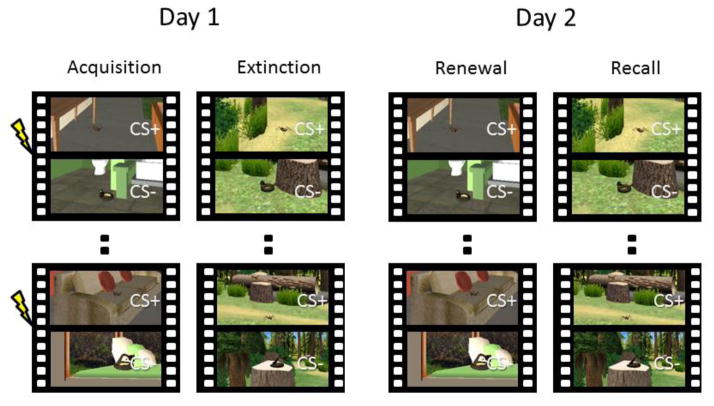
Two virtual environments were created using Maya graphic design application (Autodesk, San Rafael, California) imported into Virtools software (Dassault Syteme, Paris, France). One environment consisted of an outdoor forest and the other was an indoor apartment (Fig. 1). The trajectory and speed with which the participants traveled through each environment were matched using passive navigation. The software controlled the location and timing of the CSs in the environments, and the overall object density and navigation trajectory of the environments were matched. The environments were presented in first-person 3-D view using forward navigation. For additional details on the environments, see Huff et al. (2011).
Figure 1.
Experimental design. Participants were conditioned to dynamic cues (spiders or snakes) while travelling through a virtual reality environment (indoor or outdoor) presented in 3-D goggles. The fear cue was paired with shock on 5 out of the 16 trials (CS+) whereas the safe cue (CS−) was never paired with shock. Participants were not informed about which cue would serve as CS+ and CS−. Fear was extinguished in another environment following conditioning. Twenty-four hours later, responses to the fear cue and the safe cue were tested in the original acquisition context (Fear Renewal context) and the extinction context (Extinction Recall context), with presentation order counterbalanced between subjects. Trials were divided into Early (trials 1–8) and Late (9–16) phases for analysis purposes to map gross temporal dynamics in responses over time (not shown). CS, conditioned stimulus.
2.1.4 Conditioning procedure
Fear conditioning assessments were performed on two consecutive days. Prior to being positioned in the scanner on Day 1, participants were given the information that there was a pattern in how the CSs and the US were presented, but they had to learn the contingency through experience. The participants’ task across all phases was to estimate whether they would receive a shock when a snake or a spider was displayed using a button press response (0 = “no”, 0.5 = “unsure”, 1 = “yes”). The first functional scan consisted of a habituation phase during which 4 CS+’s and 4 CS−’s were presented in a pseudorandom order without reinforcement. Data from this phase were not analyzed. During the acquisition phase, 16 CS+’s and 16 CS−’s were presented in a pseudorandom sequence, subject to the constraint that no more than two trials of the same CS occur consecutively to avoid mood induction effects. The CS+ was reinforced on 31% of the trials (5 out of 16 trials). Partial reinforcement of the CS+ was used to delay rapid extinction that normally occurs in human participants following 100% CS+ reinforcement (LaBar et al., 1998; Phelps et al., 2004). Assignment of CS category (snake, spider) to the CS+ and CS− stimulus types was counterbalanced across subjects. After a 5-min break, participants underwent extinction training in which they experienced 16 unreinforced presentations of each CS type in a pseudorandom order, subject to the same constraints as during acquisition training. Extinction was always performed in a different 3-D virtual context from the context used during acquisition training (Fig. 1), counterbalanced across participants.
Participants returned to the MRI scanner the following day for a test of extinction recall and fear renewal. CSs were presented in both the conditioning context and the extinction context in a counterbalanced order across subjects (Fig. 1). This design permitted a within-subjects comparison of contextual effects on fear expression on Day 2, which is maximally efficient for fMRI designs due to the large variance in blood-oxygenated-level-dependent (BOLD) signal across individuals (Aguirre et al., 1998). A jittered inter-stimulus interval (ISI) of 12 ± 2 s was used during all experimental phases.
2.1.5 Anxiety ratings
Participants rated their level of state anxiety on a visual analog scale from 1–10 where 1 represented “not anxious at all” and 10 represented “worst imaginable anxiety.” Participants rated their anxiety level once at the end of each experimental phase.
2.1.6 Skin conductance responses (SCRs)
Skin conductance recording was controlled with the MP-150 BIOPAC system (BIOPAC Systems, Goleta, CA). MRI-compatible Ag/AgCl SCR electrodes were placed on the palmar surface of the left hand. The BIOPAC system was grounded through the radiofrequency filter panel and shielded from magnetic interference. SCR analysis was carried out using AcqKnowledge software (BIOPAC Systems) using procedures previously described (Dunsmoor et al., 2009). Briefly, SCR was scored if the trough-to-peak response occurred 1–4 s following stimulus onset, lasted between 0.5 and 5.0 s, and was greater than 0.02 microSiemens. A trial that did not meet these criteria was scored as a zero. Because this method normalizes SCRs relative to the skin conductance level taken just prior to CS onset, baseline differences in arousal across contexts are relatively well controlled. However, to explicitly address the potential confound of baseline skin conductance level on the return of fear, we also compared skin conductance levels averaged across the 9-sec post-CS onset intervals between the Fear Renewal context and the Extinction Recall context on Day 2. Our prior behavioral work has shown that this baseline contextual fear index does not influence phasic responses to CSs during fear retention tests (Huff et al., 2011).
2.1.7 Magnetic resonance imaging
Scanning was performed on a General Electric Signa EXCITE HD 3.0 Tesla MRI scanner with 40-mT/m gradients and an eight channel head coil for parallel imaging (General Electric, Waukesha, Wisconsin, USA). Sixty-eight high-resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 500 ms; TE = 31 ms; image matrix = 2562; voxel size = 0.9375 × 0.9375 × 1.9 mm) and used for coregistration with the functional data. Functional images were acquired parallel to the AC-PC line using a SENSETM spiral-in sequence: acquisition matrix, 64 × 64; field of view, 256 × 256; flip angle, 60°; 34 slices with interleaved acquisition; slice thickness, 3.8 mm, with no gaps between slices; repetition time, 2 s; echo time, 27 ms. Functional data were preprocessed using SPM 8 software (Wellcome Department of Cognitive Neurology, University College London, www.fil.ion.ucl.ac.uk) implemented in MATLAB (The Mathworks Inc, Natick, MA). The first 4 functional images from each scanning run were discarded to account for magnetic equilibration effects, and remaining images were corrected for head motion using a threshold of 3 mm in any direction. Preprocessing included realignment, spatial normalization to the Montreal Neurological Institute (MNI) template using DARTEL, and smoothing using an isotropic 8-mm3 Gaussian full width half maximum kernel. To remove low-frequency drifts, high pass temporal filtering was applied using a 128-s cutoff.
2.1.8 Regions of interest (ROIs)
The choice of ROIs followed the model proposed by Maren (Maren, 2011) and included bilateral amygdalae and dorsal (posterior) hippocampi, as well as the vmPFC and the dmPFC (Fig. 2). The ROIs were defined anatomically using the Wake Forest University PickAtlas software (Maldjian et al., 2003). The dorsal (posterior) part of the hippocampus was used because previous animal studies have indicated that this region may be especially involved in processing spatial contextual information of relevance to fear memories (Ji and Maren, 2005; Zelikowsky et al., 2012). The dorsal hippocampus was defined as the part of the AAL library definition of the hippocampus posterior to y = −24mm in MNI space in accordance with a recent definition (Poppenk et al., 2013). The left and right amygdala ROIs were defined in the TD library of the Wake Forest University PickAtlas (Maldjian et al., 2003). The dmPFC was defined as the part of the anterior cingulate cortex in the AAL library superior to the genu (z > 8mm). This region corresponds to cluster 3 in the parcellation of the medial prefrontal cortex by Beckman et al (Beckmann et al., 2009) and is thought to modulate the expression of fear memories (Milad et al., 2007a). Finally, the vmPFC was defined as BA 25 dilated by 2mm to include all voxels that were circumscribed by this region as defined in the TD library. This region corresponds to cluster 1 in the parcellation by Beckman et al. (Beckmann et al., 2009) and is the region of the medial PFC with the strongest white matter connectivity to the amygdala (Johansen-Berg et al., 2008). Thus ROI definitions were anatomically-based and were independent of the functional activation maps themselves.
Figure 2.
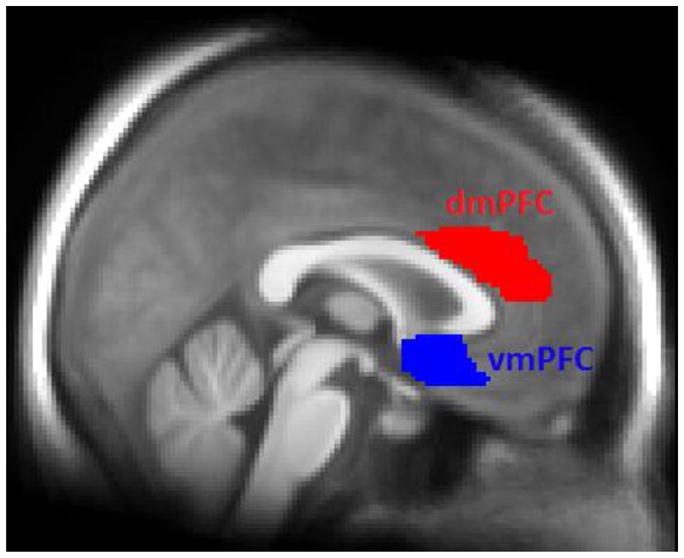
Prefrontal anatomical regions of interest overlaid on participants’ average T1-weighted MR image. These regions were used for extraction of fMRI data. dmPFC, dorsomedial prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
2.1.9 Statistical analysis and extraction of fMRI data
Following pre-processing of the functional imaging data, first-level analysis was performed in SPM8 (Wellcome Department of Cognitive Neurology, University College London, www.fil.ion.ucl.ac.uk). Each of the phases (Acquisition, Extinction, Renewal and Extinction Recall) was modeled as a separate session. Realignment parameters were entered as regressors to correct for head movements during scanning. Onsets of CS− and CS+ presentations were modeled separately and compared to the implicit baseline fMRI signal during the inter trial interval. Responses to the CS+ and the CS− during different phases of the experiment are thus normalized with respect to baseline activity in the ROIs and are thus not contaminated by baseline contextual fear responses. The onset of the US was also included in the statistical modeling of the Acquisition phase. Within each phase, early (trials 1–8) and late (trials 9–16) trials were modeled separately, resulting in contrast images for the CS+ and the CS− for early and late trial epochs to show gross temporal changes (see for example Linnman et al., 2012; Milad et al., 2009). Average contrast values for each of the 6 ROIs (bilateral amygdalae, bilateral hippocampi, dmPFC and vmPFC) were extracted for early and late trials during each phase. Extracted data were analyzed using SPSS (IBM Corporation, New York, USA).
Data were first analyzed using repeated measures ANOVA with CS-type (CS+, CS−), Time (early trials, late trials), ROI and Context (Renewal, Extinction Recall) as within-subject factors. The results were analyzed with respect to main effects of Context and the interaction between Context and combinations of the other factors. Significant interaction effects were followed up with repeated measures ANOVAs within each context to characterize the nature of the interaction effects.
2.2.1 Mediation analysis
Statistical mediation analysis was performed to test the hypothesis that the dmPFC mediated the effect of the hippocampus on the amygdala during Renewal on Day 2 and that the vmPFC mediated the effect of the hippocampus on the amygdala during Extinction Recall on Day 2. Average contrast values from the contrast CS+ > CS− for each of the six ROIs were extracted from early trials (trials 1–8) of each CS-type. The analysis focused on early trials because these trials are the ones where renewal effects generally occur before they re-extinguish (Vervliet et al., 2013). The contrast CS+ > CS− was chosen to control for nonassociative effects of context shifts on brain reactivity to sensory cues. Mediation analysis was performed using structural equation modeling (SEM) implemented in the Mplus software (Muthén and Muthén, 1998–2010). Separate analyses were performed for data from the left and right amygdala and hippocampus, whereas data from the dmPFC and vmPFC were bilateral in both analyses due to the activation being focused along the midline. Mplus uses the Delta method to calculate the standard error of the indirect effect (Bollen, 1989). The statistical criterion was set to p < .05 for the indirect effect.
2.2.2 Statistical analysis of behavioral data
Statistical analysis of behavioral data was performed in SPSS (IBM Corporation, New York, USA). Anxiety ratings were analyzed using paired t-tests across phases separately for each day.
The analysis of SCR data during Acquisition and Extinction on Day 1 was performed using the averaged response to the CS+ and the CS− divided according to early (trials 1–8) and late (trials 9–16) trials (Fig. 1). Repeated measures ANOVAs were computed separately for the acquisition and extinction phases using CS-type (CS+, CS−) and trial (early, late) as within-group factors.
The effect of context shifts on fear renewal was tested by a repeated measures ANOVA with Context (Fear Renewal, Extinction Recall), CS-type (CS+, CS−) and Time (Early, Late Trial Blocks) as within-group factors, as suggested by Vervliet et al. (Vervliet et al., 2013). Planned t-tests were used to test for effects within each context. Shock expectancy data were analyzed the same way as SCR data.
3. Results
3.1 Fear Renewal and Extinction Recall
Because the focus of the study was on contextual control of extinction recall, these results are reported here. Results from Day 1 indicated successful fear conditioning (Fig. S1) and extinction (Fig. S2). Details can be found in the Supplemental Information.
3.1.1 Anxiety ratings
Anxiety ratings were greater during Fear Renewal than Extinction Recall (mean ± SEM, Renewal: 3.3 ± .30; Recall: 2.8 ± .30; t42 = 2.18, p = .04).
3.1.2 SCRs: Contextual renewal of fear
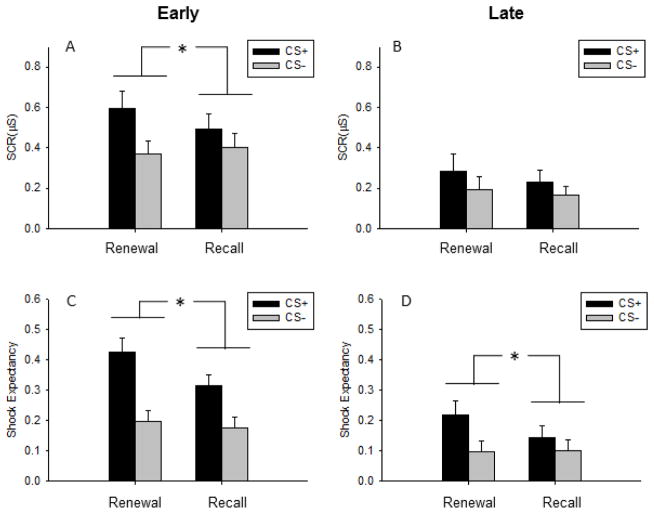
SCR differed between the CSs across the Fear Renewal and Extinction Recall contexts, as indicated by the three-way interaction in the ANOVA (Context x CS-type x Time: F1,42 = 4.49, p = .04). The three-way interaction was driven by greater SCRs to the CS+ than the CS− in the Fear Renewal than in the Extinction Recall context during Early trials (Context x CS-type: F1,42 = 4.49, p = .03, Fig. 3). In fact, we only observed increased responding to the CS+ than the CS− in the Renewal context (t42 = 4.16, p <.001) whereas SCR to the CS+ was similar to SCR to CS− during Extinction Recall (t42 = 1.71, p =.09). No interaction was observed between CS-type and Contexts during Late trials (Context x CS-type: F1,42 = 0.26, p = .61). This test of fear renewal, as recommended by (Vervliet et al., 2013), indicates that our virtual reality paradigm was successful in eliciting contextual control of fear memories. The difference in SCRs between contexts was not attributable to differences in baseline skin conductance level (contextual fear) during the interstimulus intervals, as confirmed by a repeated measures ANOVA, which yielded non-significant effects of both Context (F1,42 = .47, p = .50) and the CS x Context interaction (F1,42 = .04, p = .84) on these baseline response measures.
Figure 3.
Behavioral test of fear renewal. A) During Early trials (first half of the trial blocks), the difference in skin conductance response (SCR) between the reinforced cue (CS+) and the control cue (CS−) was greater in the Fear Renewal context than the Extinction Recall context. B) During late trials, SCRs to the conditioned cues were similar, suggesting re-extinction of the conditioned response. C) The difference in shock expectancy between the CS+ and CS− was greater in the Fear Renewal context than the Extinction Recall context during early trials. D) The CS-difference in shock expectancy was also greater in the Fear Renewal context during late trials. Bars represent mean and standard error of the mean. *P < 0.05.
3.1.3 Shock expectancy
The difference in shock expectancy between the CS+ and the CS− was greater in the Fear Renewal context than in the Extinction Recall context (Context x CS-type interaction: F1,42 = 7.28, p = .01; Fig. 3). Overall shock expectancy was also higher in the Fear Renewal context than the Extinction Recall context (F1,42 = 6.85, p = .01), and, across contexts, expectancy was overall higher to the CS+ than the CS− (F1,42 = 19.54, p < .001). We did not observe a three-way interaction between CS, Context and Time (F1,42 = .44, p = .83), indicating sustained difference in shock expectancy between CSs from Early to Late trials in the Fear Renewal context compared to the Extinction Recall.
3.1.4 Functional MRI data
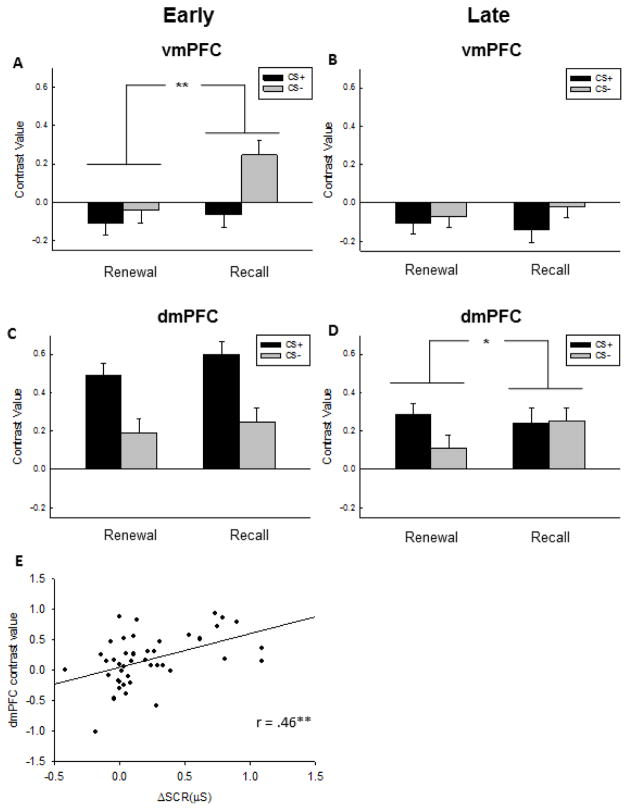
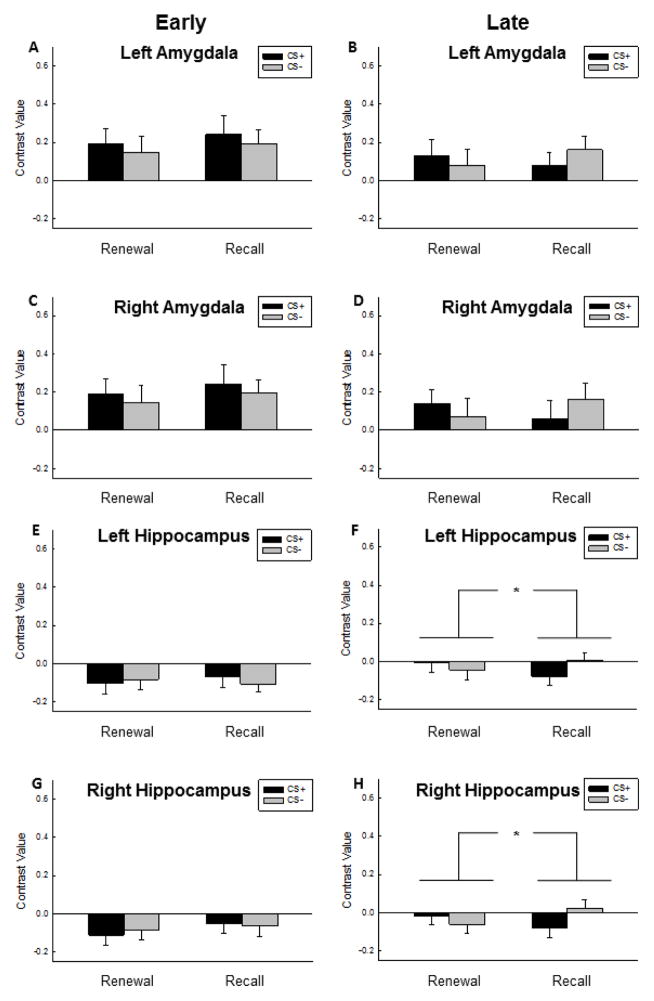
We observed a significant four-way interaction in the ANOVA [Context x CS-type x Time x ROI (F5,210 = 3.96, p = .002)], with no other effects reaching significance (all p’s > .08). To decompose the interaction, we conducted follow-up analyses with separate repeated-measures ANOVAs for each ROI divided into Early and Late trial blocks. During Early trials, responses in the vmPFC were greater to the CS− than the CS+ during Extinction Recall than during Fear Renewal, as reflected in the Context x CS interaction (Fig. 4, F1,42 = 7.89, p = .007). In contrast, the dmPFC exhibited greater responses to the CS+ than the CS− across both contexts during Early trials (F1,42 = 29.49, p < .001). We did not observe any effect of CS-type or Context in the amygdala (p’s > .20) or the hippocampus (p’s > .33) during early trials (Fig. 5).
Figure 4.
Functional MRI responses in the medial prefrontal cortex (mPFC) during Early and Late trials in the Fear Renewal and Extinction Recall contexts. A) During Early trials, ventromedial PFC (vmPFC) safety signaling (response to the CS− > CS+) was pronounced in the Extinction Recall context relative the Renewal context. B) Early trials were associated with an increased dorsomedial PFC (dmPFC) response to the CS+ in both contexts. C) During Late trials, the vmPFC response was low and non-differential across cues or contexts. D) During Late trials, the dmPFC signaling of fear responses (CS+ > CS−) was significant only in the Fear Renewal context, suggesting faster habituation in the Extinction Recall context. E) Fear expression is correlated with dmPFC reactivity during Early trials in the Fear Renewal context. Contrast values from Early trials are derived from the comparison CS+ > CS− and are plotted against the same comparison in skin conductance response (ΔSCR), averaged across subjects. A–D) Bars represent mean and standard error. * p < .05; ** p < .01..
Figure 5.
Functional MRI responses in the amygdala and hippocampus during Early and Late trials in the Fear Renewal and Extinction Recall contexts. During Early trials, similar mean levels of responding to the CS+ relative the CS− in the Extinction Recall context and the Fear Renewal context was observed in the A) left and C) right amygdala. Also during Late trials, the B) left and D) right amygdala response was low and non-differential across cues or contexts. A lack of differential responding across cues or contexts was further noted in the E) left and G) right hippocampus during Early trials. During Late trials however, hippocampal signaling of fear responses was attenuated relative safety responses (CS− > CS+) in the Extinction Recall context compared to the Fear Renewal context when considering both the F) left and H) right hippocampus signal. Bars represent mean and standard error.
During late trials, the dmPFC exhibited a CS-type x Context interaction (F1,42 = 5.02, p = .03) such that responses to the CS+ were elevated relative to the CS− only in the Fear Renewal context. In contrast, the vmPFC exhibited greater responses to the CS− relative to the CS+ across both contexts (Fig. 4, F1,42 = 4.70, p = .04), without any interaction between CS-type and Context (p > .22). Responses in the hippocampus were also elevated to the CS− relative to the CS+ but only in the Extinction Recall context (Fig. 5, Context x CS-type interaction, F1,42 = 8.06, p = .007).
3.1.5 Correlation between SCR and reactivity in the dmPFC
To test the prediction that dmPFC activity reflects fear expression, average contrast values from the contrast of CS+ > CS− during Early Renewal trials were extracted from the dmPFC and correlated to the difference in SCR between the CS+ and CS− on the same trials (Fig. 4, r = 0.46, p = 0.024, Bonferroni corrected). This finding was selective to Renewal, as the same correlation during Extinction Recall was not significant (r = −0.02, p = 0.89). The only other region that showed a relationship with fear expression during Renewal was the right amygdala (r = .31, p = 0.04, uncorrected), although this region did not survive Bonferroni correction for multiple comparisons.
3.1.6 Mediation analysis of the effect of dmPFC and hippocampus on the amygdala during Fear Renewal
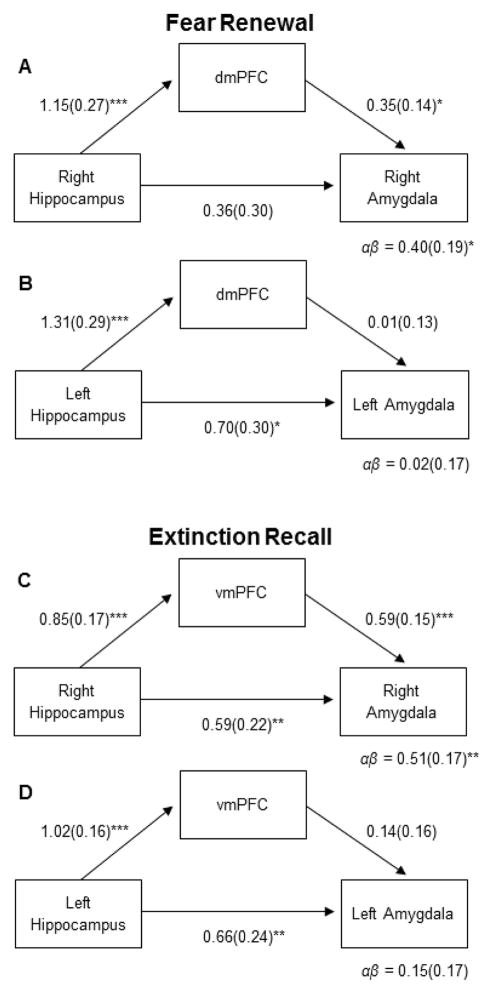
Average contrast values from the contrast CS+ > CS− during Fear Renewal were extracted from the amygdala, hippocampus, and dmPFC and entered into a statistical mediation model. This model tested both the direct effects of the hippocampus on the amygdala as well as the potential mediation of this effect via the indirect pathway going from the hippocampus through the dmPFC to the amygdala. In the right hemisphere, we observed a correlation between the hippocampus and the amygdala (r = .40, p = .01). When testing the hypothesis that dmPFC mediated the effect of the hippocampus on the amygdala using SEM, we found significant paths from the hippocampus to the dmPFC (Fig. 6, p < .001) as well as from the dmPFC to the amygdala (p < .05). Importantly, the indirect effect of the hippocampus on the amygdala via the dmPFC was significant (p < .05). When this pathway was taken into account, the direct path from the hippocampus to the amygdala was no longer significant, indicating full mediation. When the same model was tested using data from the Extinction Recall context, we did not observe any mediation effect (p > .28), suggesting that the mediation was specific to data from the Fear Renewal context.
Figure 6.
Results from the statistical mediation analysis investigating hippocampal influences on the amygdala via direct projections and through indirect dorsal and ventral medial prefrontal pathways during Fear Renewal and Extinction Recall. This analysis targeted Early trials, for which the psychophysiological effects were maximal (see Fig. 4). A) During Fear Renewal, the dmPFC fully mediated the effect of the right hippocampus on the right amygdala. B) In the left hemisphere, the effect of the hippocampus on the amygdala was not mediated by the dmPFC. C) During Extinction Recall, the vmPFC partially mediated the effect of the right hippocampus on the right amygdala. D) In the left hemisphere, we did not observe mediation of the effect of the hippocampus on the amygdala by the vmPFC. * p < .05; ** p < .01; *** p < .001.
In the left hemisphere, we also observed a correlation between the hippocampus and the amygdala (r = .40, p = .01). However, in this hemisphere the indirect mediation effect through the dmPFC was not supported (p > .91).
3. 1.7 Mediation analysis of the effects of the vmPFC and hippocampus on the amygdala during Extinction Recall
We observed a correlation between the hippocampus and the amygdala in the right hemisphere (r = .63, p < .001). This effect was mediated by the vmPFC (Fig. 6, p < .01). The path between the hippocampus and the amygdala remained significant (p < .01) when the indirect effect was included in the model, indicating that the direct pathway was only partially mediated by the vmPFC. The mediation effect was not significant when data from the Fear Renewal context were fitted to the same model (p >.06).
In the left hemisphere, amygdala reactivity was also correlated with hippocampal activity (r = .57, p < .001). However, the vmPFC did not mediate this effect (p = .39).
4. Discussion
Using a novel immersive virtual reality fMRI paradigm, we found an increased conditioned fear response in the Fear Renewal context relative to the Extinction Recall context when fear memory was tested 24 hrs following extinction learning. The enhanced recall of the extinction memory in the extinction context was paralleled by an increased response to the safety cue in the vmPFC. In the dmPFC, fear responses did not differ between contexts during early trials but showed reduced habituation in the renewal context during late trials. At a circuit level, we found that contextual gating of fear renewal was co-ordinated by a brain circuit implicated in animal studies, including the hippocampus, dmPFC and amygdala, especially in the right hemisphere. During extinction recall, our data instead suggest that the vmPFC plays a role as a mediator between the hippocampus and the amygdala in the right hemisphere. Thus, the competition between fear and extinction processes could be resolved by the hippocampal orchestration of a dmPFC-amygdala circuit during fear renewal or a vmPFC-amygdala circuit during extinction recall, resulting in activation or inhibiton of fear expression. Importantly, many of these insights were gained through statistical mediation modeling at the multi-regional level, as univariate activity in the medial temporal lobe regions themselves (and consideration of their direct connections) did not distinguish the experimental conditions.
In the vmPFC, we found increased signaling to the CS− relative to the CS+ during extinction recall, consistent with some prior studies (Phelps et al., 2004; Apergis-Schoute et al., 2014). However, other studies show the opposite pattern, with greater activity to the CS+ relative to the CS− (Kalisch et al., 2006; Garfinkel et al., 2014 (control data)). Milad et al. (2007b), Milad et al. (2009) (control data) and Holt et al. (2012) (control data) do not report results from a CS+ vs. CS− contrast during extinction recall, but instead they report more activation during extinction recall to the CS+ than another CS+ that had not undergone extinction. Thus, across the few existing human fMRI studies, the signature of vmPFC activation during extinction recall differs. We add a cautionary note here that it is difficult to directly compare studies, given that fMRI signals are always taken relative to a pre-stimulus baseline, and thus any given contrast can show either relative increases or decreases in activation depending on baseline activity levels. A second cautionary note is that the vmPFC is broadly defined, and there may be different results in different subregions of the vmPFC.
If one interprets the vmPFC as reflecting a relative ‘safety signaling’ mechanism that supports extinction recall, then the results are consistent across the majority of studies. Other studies have found greater vmPFC activity to the CS− relative to the CS+ during acquisition, extinction, and/or generalization testing (e.g., Phelps et al., 2004; Schiller et al., 2008; Dunsmoor et al., 2011; Apergis-Schoute et al., 2014; Dunsmoor et al., 2014b; Lissek et al., 2014; Icenhour et al., 2015), and Schiller et al. (2008) reported that the vmPFC activation patterns to a CS+ and CS− flip during reversal learning. Theoretically, safety signaling can support extinction recall by modifying the CS−/US, CS−/context, or CS−/CS+ associations, which, in turn, can affect the CS+/US and CS+/context associations. These associations during differential fear conditioning paradigms are more complex than those during single-cue paradigms, which predominate the animal literature on extinction recall, making direct comparisons across species challenging. The other possibility is that there are multiple mechanisms at play, perhaps mediated by different subregions of vmPFC, that target either CS+ or CS− representations and are engaged to a different extent across paradigms or stimuli. One potentially important difference between our task and prior studies that emphasize CS+ changes in the vmPFC is that we use dynamic, fear-relevant stimuli, which may make safety signaling more relevant during Extinction Recall. Our novel observation that the safe cue was associated with a greater vmPFC response in the Extinction Recall context than the Fear Renewal context suggests that the safe cue presented in a context that never had been paired with shocks is valued as safer than when presented in a context where shocks have been experienced, indicating a summation effect between context and cue in the extinction context. Thus, the value of a safe cue as signaled by the vmPFC is dependent on the value of the context in which the cue is experienced, which is consistent with a theory that the vmPFC works as a neural hub to give meaning to experiences and to link them to actions and emotional responses (Roy et al., 2012).
In contrast to the increased response to the safe cue in the vmPFC, we observed increased responses to the fear cue in the dmPFC. The function of the dmPFC in fear memory has been previously linked to fear expression during extinction recall as a marker of retention failure (Graham and Milad, 2011; Milad et al., 2007a). The dmPFC also signals fear cues during initial learning, a robust finding in the fear conditioning literature (Sehlmeyer et al., 2009). Here we add to this literature the novel finding that the dmPFC correlates with the difference in the conditioned response to the fear vs. safe cue in the Fear Renewal (but not the Extinction Recall) context, such that an increased response in the dmPFC was associated with increased autonomic fear expression. The response pattern in the dmPFC does not directly translate, however, to our observed overall increase in autonomic fear responses in the Fear Renewal context as a main effect. A possible explanation is that the dmPFC fear signal generalizes across contexts, but that the functional neural circuitry in which dmPFC operates changes between contexts, giving different meaning to the dmPFC signal. In other words, the dmPFC could be connected with a fear circuitry during Fear Renewal but not during Extinction Recall, as suggested by our structural equation modeling results. Alternatively, the dmPFC may perform different functions in the Extinction Recall context than in the Fear Renewal context such as evaluating the expected value of emotional control (Shenhav et al., 2013).
Contextual fear renewal has been challenging to study in the scanner environment, and most previous attempts have used 2D-backgrounds as contexts. However, Kalisch et al. (Kalisch et al., 2006) noted that a static 2D-background during extinction could be interpreted as a discrete cue signaling that the CS+ is safe rather than a physical context. Experimental designs using 2-D backgrounds as contexts could thus be more readily interpreted in terms of conditioned inhibition than contextual modulation of fear extinction (see for example Jovanovic et al., 2005). In the present study, by presenting cues at different locations as participants navigate through a virtual environment, the background constantly changes between cue presentations, which minimizes the role of conditioned inhibition in our paradigm. Moreover, the rich contextual manipulation may provide stronger behavioral evidence of context-mediated fear renewal effects, which have been difficult to observe psychophysiologically and have typically been tested on the same day as extinction training (see review in Vervliet et al., 2013).
The current study has limitations. First, we studied the involvement of the dorsal hippocampus in our circuit analysis, while there is also reason to believe that the ventral hippocampus may be relevant for contextual regulation of extinction memory, given that it relays information from the dorsal hippocampus to the amygdala (Fanselow, 2010). From a methodological perspective, the ventral hippocampus is adjacent to the amygdala making it difficult to distinguish fMRI responses in these regions using standard fMRI resolution1. Therefore a correlation in fMRI responses between the ventral hippocampus and the amygdala could be artefactual and simply be influenced by the spatial proximity between the regions. Correlations between the dorsal hippocampus and the amygdala, on the other hand, are highly unlikely to be caused by such counfounds. Future studies could resolve these methodological issues by using high resolution fMRI to study the involvement of the ventral hippocampus in contextual regulation of extinction memory. Second, it could be argued that if a region (e.g. amygdala in this study) does not exhibit a differential response to cue presentations in the renewal and extinction context, a mediation analysis is not informative because the region is not likely to be contextually regulated We feel that this argument is overly simplistic and that a region can be active to a similar degree in both contexts, but that the function of the region is defined by the circuitry in which the region operates. This line of thought is in accordance with network theories of brain function (see for example Baronchelli, et al., 2013) which are becoming increasingly important for understanding complex brain functions.
Our observation that synergistic actions among the hippocampus, the amygdala and the vmPFC safeguard the organism from fear renewal might be of relevance to understand trauma-induced anxiety conditions such as PTSD. Reduced vmPFC volumes have been observed in patients with PTSD (Hartley et al., 2011; Milad et al., 2005), which could be a risk factor (as well as a maintaining factor) in PTSD due to the role it plays in mediating effects of the hippocampus on the amygdala. Morphologic alterations in PTSD have also been found in the amygdala (Morey et al., 2012) and the hippocampus (Bremner et al., 1995), further pointing to the usefulness of circuit-level analysis to understand neural dysfunctions in this disorder. The circuit-level approach to understand contextual control of fear might also inform the representation of fear memories in the brain such that the orchestration of a hippocampal-dmPFC-amygdala network represents a core, systems-level engram of the contextual retrieval of threat cues.
Supplementary Material
Highlights.
The neural circuitry serving contextual control of fear memory influences anxiety.
We studied neural response patterns during fear renewal and extinction recall.
Fear renewal engaged a circuitry consisting of the hippocampus, dmPFC and amygdala.
Extinction recall engaged a circuitry consisting of hippocampus, vmPFC and amygdala.
These pathways could determine the level of reactivation of fear associations.
Acknowledgments
This study was supported by grants NIH R01 DA027802 and NSF BCS 1460909 to KSL, as well as a Swedish Research Council Postdoctoral Fellowship award and a Sweden-America Foundation Postdoctoral Fellowship award to FA.
Footnotes
In response to a reviewer’s request, we conducted a univariate analysis of a ventral hippocampal ROI and found no contextual modulation of the CS+ vs. CS− contrast on Day 2 (all P’s>.27).
Conflict of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Ahs F, Dunsmoor JE, Zielinski D, LaBar KS. Spatial proximity amplifies valence in emotional memory and defensive approach-avoidance. Neuropsychologia. 2015;70:476–485. doi: 10.1016/j.neuropsychologia.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Schiller D, LeDoux JE, Phelps EA. Extinction resistant changes in the human auditory association cortex following threat learning. Neurobiology of Learning and Memory. 2014;113:109–114. doi: 10.1016/j.nlm.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronchelli A, Ferrer-i-Cancho R, Pastor-Satorras R, Chater N, Christiansen MH. Networks in cognitive science. Trends in Cognitive Sciences. 2013;17:348–360. doi: 10.1016/j.tics.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. John Wiley & Sons; New York, New York: 1989. [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Ahs F, Zielinski DJ, LaBar KS. Extinction in multiple virtual reality contexts diminishes fear reinstatement in humans. Neurobiol Learn Mem. 2014a;113:157–164. doi: 10.1016/j.nlm.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kragel PA, Martin A, LaBar KS. Aversive learning modulates cortical representations of object categories. Cereb Cortex. 2014b;24:2859–2872. doi: 10.1093/cercor/bht138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Mem. 2009;16:460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65 (1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. Am J Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of Neural Responses to Safety Cues in Schizophrenia. Archives of General Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Hernandez JA, Fecteau ME, Zielinski DJ, Brady R, Labar KS. Revealing context-specific conditioned fear memories with full immersion virtual reality. Front Behav Neurosci. 2011;5:75. doi: 10.3389/fnbeh.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Zeilinski DJ, Fecteau ME, Brady R, LaBar KS. Human fear conditioning conducted in full immersion 3-dimensional virtual reality. J Vis Exp. 2010 doi: 10.3791/1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, Forsting M, Elsenbruch S. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:114–127. doi: 10.1111/nmo.12489. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biol Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169:415–423. doi: 10.1176/appi.ajp.2011.10121780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry. 2014;75:909–915. doi: 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007a;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007b;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, California: 1998–2010. [Google Scholar]
- Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. Plos One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biol Psychol. 2013;92:51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Pham DL, Fanselow MS. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. 2012;22:1096–1106. doi: 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.