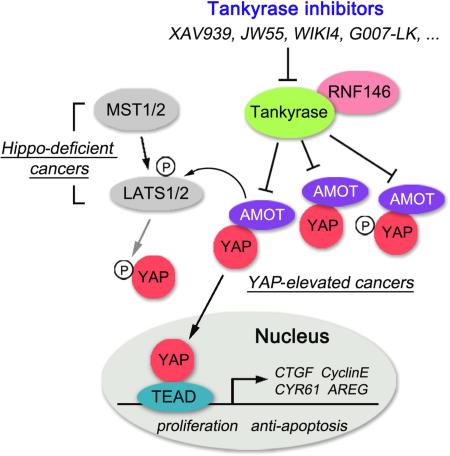
Summary
As the key effector in the Hippo pathway, YAP was identified as an oncoprotein whose expression is elevated in various human cancers. However, the development of potentially therapeutic compounds targeting YAP has been slow and limited. Here, we find that tankyrase inhibitors suppress YAP activity. This effect is mediated by anigomotin (AMOT) family proteins. Tankyrases associate with AMOT family proteins and promote their degradation through E3 ligase RNF146. By antagonizing tankyrase activity, tankyrase inhibitors stabilize AMOT family proteins, thereby suppressing YAP oncogenic functions. Together, our studies not only demonstrate the tankyrase-RNF146-AMOT axis as an upstream pathway regulating YAP, but also reveal a therapeutic opportunity in targeting YAP for cancer treatment.
Graphical Abstract
Introduction
The evolutionarily conserved Hippo pathway plays fundamental roles in tissue homeostasis and organ size control (Halder and Johnson, 2011; Pan, 2010; Zhao et al., 2010). Genetic mutations of Hippo pathway components lead to tissue/organ overgrowth and eventually tumorigenesis, which suggests that the Hippo pathway is a putative tumor suppressor pathway. In mammals, the Hippo pathway is composed of kinase cascades (MST and LATS), adaptor proteins (SAV1 for MST and MOB1 for LATS), a downstream effector (YAP) and nuclear transcription factors (TEADs). MST kinase phosphorylates and activates LATS kinase. The activated LATS kinase phosphorylates YAP at serine 127, providing the docking site for 14-3-3 proteins, which sequesters YAP in the cytoplasm. On the other hand, un-phosphorylated YAP translocates into the nucleus and functions as a transcriptional co-activator with TEAD family transcription factors. The YAP-TEAD transcriptional complex governs the transcription of downstream genes involved in cell proliferation and anti-apoptosis. The nuclear protein VGLL4 antagonizes the YAP-TEAD complex and consequently inhibits YAP's transactivation activity (Jiao et al., 2014; Koontz et al., 2013; Zhang et al., 2014). TAZ is a YAP paralog and is similarly regulated by the Hippo pathway (Lei et al., 2008; Zhang et al., 2009), although YAP and TAZ have exhibited different physiological functions based on the phenotypes observed in genetically modified mouse models (Kang et al., 2009; Makita et al., 2008).
As the key target in the Hippo pathway, YAP has been identified as an oncoprotein. Overexpression of YAP in mice led to liver enlargement and liver cancer formation (Camargo et al., 2007; Dong et al., 2007). Elevated expression of YAP has also been identified in various human cancers (Dong et al., 2007; Harvey et al., 2013; Mo et al., 2014). Notably, recent studies demonstrated that YAP overexpression promoted resistance to KRAS-, RAF-, and MEK-targeted cancer therapies (Kapoor et al., 2014; Lin et al., 2015; Shao et al., 2014), highlighting the need to target YAP for cancer treatment.
Efforts have been devoted to search for druggable targets within the Hippo-YAP pathway in order to develop pharmacological compounds that could inhibit YAP oncogenic activities. For example, the small molecule verteporfin was identified as an effective inhibitor of YAP because of its ability to block formation of the TEAD-YAP transcriptional complex (Liu-Chittenden et al., 2012). Moreover, recent studies identified GPCR receptors as upstream regulators for the Hippo-YAP pathway (Miller et al., 2012; Yu et al., 2012), which expanded the potential upstream targets for YAP suppression. Intriguingly, PPxY (PY) motif-containing proteins, angiomotin (AMOT) family proteins (Chan et al., 2011; Wang et al., 2011; Zhao et al., 2011) and PTPN14 (Huang et al., 2013; Liu et al., 2013; Michaloglou et al., 2013; Wang et al., 2012b) were also able to antagonize YAP oncogenic functions by translocating YAP into the cytoplasm. This ability to retain YAP in the cytosol is achieved through direct protein-protein interactions mediated by the AMOT/PTPN14-PY motif and YAP-WW domains. Thus, modulating the levels of AMOT and PTPN14 or the PY motif-WW domain interaction could be additional approaches for anti-YAP agents.
In this study, our aim was to identify other effective YAP-targeting strategies. We identified tankyrase inhibitors as compounds that potentially target YAP. Tankyrase inhibitors suppressed a series of YAP-dependent oncogenic functions and specifically targeted the three-dimensional (3D) acinar growth of YAP-transformed MCF10A cells. Moreover, the tankyrase inhibitors stabilized AMOT family proteins by suppressing their tankyrase-RNF146 axis–mediated degradation. These data not only reveal tankyrases and RNF146 as regulators of the Hippo-YAP pathway, but also indicate the potential therapeutic value of employing tankyrase inhibitors to target YAP for cancer treatment.
Results
Tankyrase inhibitors target YAP
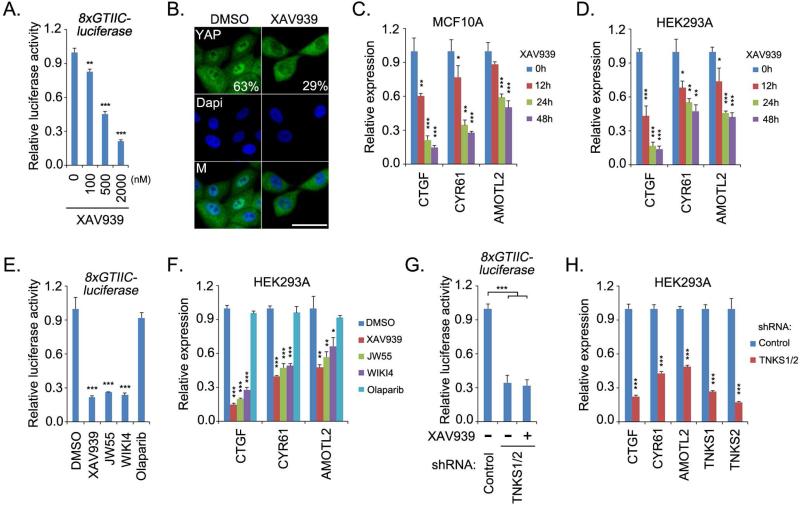
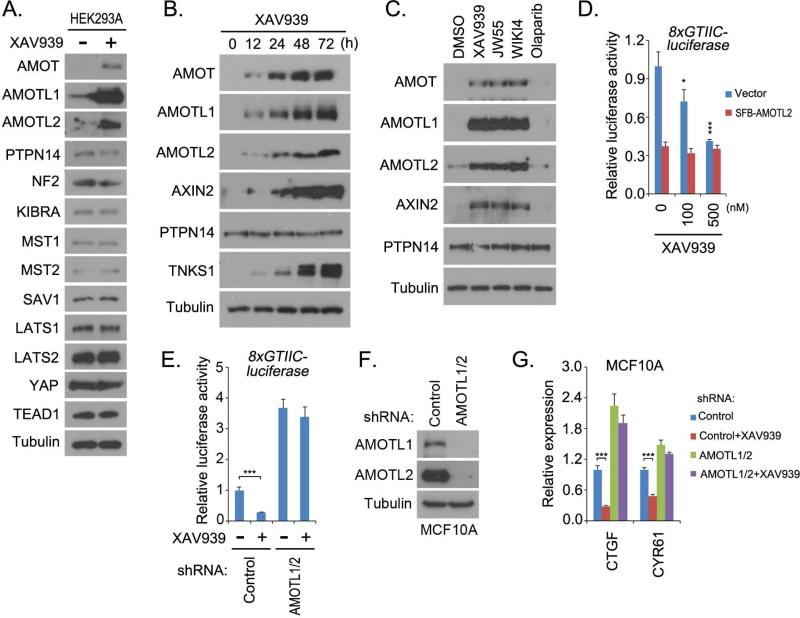
To explore the translational potential of targeting the Hippo-YAP pathway for cancer treatment, we performed a compound screen using YAP-TEAD luciferase reporter assay and YAP cellular localization as indications of YAP activity (Figure S1A). Interestingly, we identified the compound XAV939 as a putative inhibitor of YAP. XAV939 suppressed YAP/TEAD-based luciferase reporter activity (Figure 1A) and partially translocated YAP from the nucleus into cytoplasm in low-density MCF10A cells (Figure 1B). Consistent with this finding, the transcription of YAP target genes were also suppressed by XAV939 treatment in both MCF10A and HEK293A cells (Figure 1C and 1D, Figure S1B). Moreover, similar inhibitory effect on YAP activity mediated by XAV939 was also observed in several other types of cancer cell lines (Figure S1D). These data suggest that XAV939 can suppress YAP activity.
Figure 1. Tankyrase inhibitors suppressed YAP activity.
(A) XAV939 suppressed YAP/TEAD luciferase reporter activity. Luciferase reporter assay was performed in HeLa cells treated with XAV939 at indicated concentrations for 24 h. pSV40-Renilla was used as internal control. (B) XAV939 treatment partially translocated YAP from nucleus into cytoplasm. Low-density MCF10A cells were treated with dimethyl solfoxide (DMSO) or XAV939 (10 μM) for 48 h, and the percentages of nuclear YAP are shown. Nucleus is indicated by DAPI staining. M, merged. Scale bar, 20μm. (C-D) XAV939 treatment suppressed YAP transcriptional activity. The transcripts of YAP target genes were detected by quantitative PCR in MCF10A (C) and HEK293A (D) cells. Cells were treated with XAV939 (10 μM) for the indicated time periods. (E-F) Tankyrase inhibitors suppressed YAP activities. YAP/TEAD-luciferase reporter activity (E) and the transcripts of YAP target genes (F) were detected in cells treated with indicated inhibitors (10 μM) for 24h. (G-H) Double knockdown of tankyrase 1 and 2 suppressed YAP activities. YAP/TEAD-luciferase reporter activity (G) and the transcripts of YAP target genes (H) were detected in cells transduced with indicated shRNAs. For the luciferase reporter assay, HeLa cells were treated with XAV939 (10 μM) for 24 h. For all panels, * p<0.05, ** p<0.01 and *** p<0.001.
XAV939 is a tankyrase inhibitor (Huang et al., 2009). Tankyrases (TNKS1 and TNKS2) belong to the poly(ADP-ribose) polymerase (PARP) family and are known to induce the poly-ADP ribosylation (PARsylation) of their substrates (Riffell et al., 2012). The substrate is recognized by tankyrases through its tankyrase-binding domain (TBD), which has the consensus amino acid sequence RxxPxG (Guettler et al., 2011). The tankyrase-mediated PARsylation leads to at least two fates for the substrate: change of subcellular localization or proteasome-dependent degradation. It was reported that the E3 ligase RNF146 recognizes the tankyrase-mediated PAR modification of substrates and is responsible for their ubiquitination and degradation (Zhang et al., 2011). Tankyrases regulate diverse cellular functions through their substrates (Riffell et al., 2012), such as telomere length by degrading TRF1 (Smith et al., 1998), mitosis progress by changing the localization for Miki (Ozaki et al., 2012), bone development by degrading 3BP2 (Levaot et al., 2011), and Wnt signaling by degrading AXIN (Huang et al., 2009).
To validate the role of tankyrase inhibition in YAP suppression, we examined the effect of two other tankyrase inhibitors, JW55 and WIKI4. Just like XAV939, these two tankyrase inhibitors suppressed YAP/TEAD luciferase reporter activity (Figure 1E) and the transcription of YAP target genes (Figure 1F), while the control, PARP inhibitor olaparib, had no effect on these YAP-dependent assays. These data suggest that tankyrase inhibitors are putative YAP inhibitors. Moreover, loss of tankyrase 1/2 not only suppressed YAP/TEAD luciferase reporter activity (Figure 1G) and transcription of YAP target genes (Figure 1H) but also attenuated the inhibitory effect of XAV939 (Figure 1G). These results indicate that tankyrases are positive regulators of YAP and could be used to target this oncoprotein.
Tankyrases interact with angiomotin family proteins in the Hippo pathway
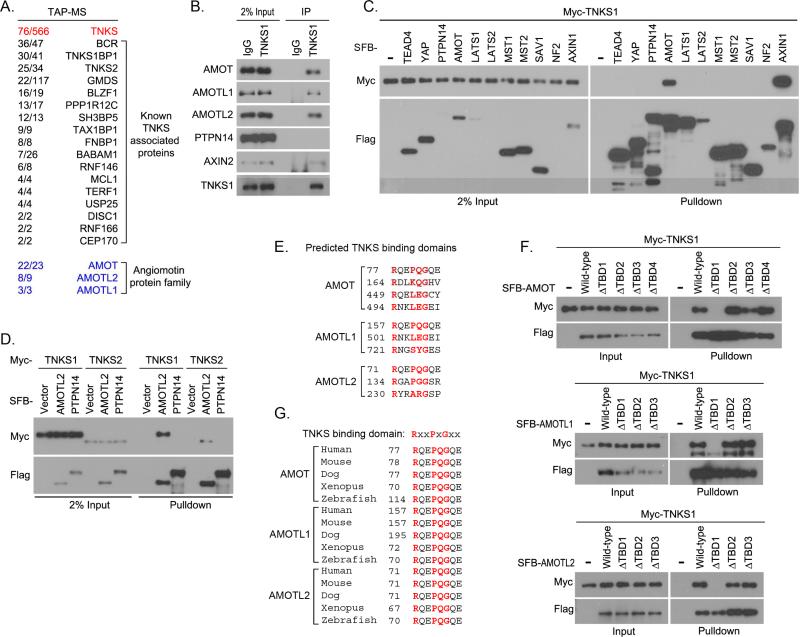
To determine the underlying mechanisms for the tankyrase inhibition–mediated YAP suppression, we isolated TNKS1 associated protein complexes from HEK293T cells using tandem affinity purification followed by mass spectrometry analysis. Surprisingly, all members of the angiomotin protein family (AMOT, AMOTL1, and AMOTL2) were identified on the prey list (Figure 2A and Table S1). The interactions between AMOT family proteins and tankyrase 1 were confirmed by endogenous immunoprecipitation assay (Figure 2B). As predicted, the known tankyrase substrate AXIN2 interacted with tankyrase 1, while another PY-motif containing protein PTPN14 did not (Figure 2B). The specific interaction between tankyrase 1 and AMOT was further confirmed by using other components of the Hippo pathway (Figure 2C). Moreover, both tankyrase 1 and tankyrase 2 bound to AMOTL2 (Figure 2D). Of the several TBDs that were predicted for each AMOT family protein (Figure 2E) (Guettler et al., 2011), the first predicted TBD in each AMOT family protein was confirmed to mediate the protein's association with tankyrase 1 (Figure 2F). This TBD is highly conserved among AMOT family proteins and among different species (Figure 2G). Together, these data suggest that AMOT family proteins are bona-fide binding partners of tankyrases. Of note, the interaction between AMOT family proteins and YAP was independent of this TBD, since AMOT TBD-deleted mutants still associated with YAP (Figure S2A).
Figure 2. Tankyrases associated with angiomotin family proteins.
(A) Association of angiomotin family proteins with tankyrase 1 was identified by tandem affinity purification–mass spectrometry (TAP-MS) in HEK293T cells. Bait protein is marked in red. Identified angiomotin family proteins AMOT, AMOTL1, and AMOTL2 are marked in blue. The left row of numbers represents unique peptide number/total peptide number. The known tankyrase 1 (TNKS1)–associated proteins are indicated. (B) TNKS1 interacted with AMOT proteins. Endogenous immunoprecipitation (IP) was performed using extract prepared from HEK293T cells; normal rabbit IgG was taken as control. (C) TNKS1 specifically associated with AMOT in the Hippo pathway. Myc-tagged TNKS1 was co-expressed with indicated SFB-tagged proteins in HEK293T cells and cell lysates were subjected to pulldown assays. (D) Both TNKS1 and TNKS2 associated with AMOTL2. Myc-tagged TNKS1 or TNKS2 was co-expressed with indicated SFB-tagged proteins in HEK293T cells, and cell lysates were subjected to pulldown assays. (E) The predicted tankyrase-binding domains (TBD) for AMOT family proteins are listed. The key amino acids for each TBD are indicated in red. (F) The first predicted TBD was identified as the bona-fide TBD for each AMOT proteins. Myc-tagged TNKS1 was co-expressed with indicated SFB-tagged AMOT proteins or their TBD-deleted mutants in HEK293T cells, and cell lysates were subjected to pulldown assays. (G) The sequence alignments for the TBD in AMOT family proteins from different species are shown. The key amino acids for each TBD are indicated in red.
Tankyrases promote degradation of AMOT family proteins through E3 ligase RNF146
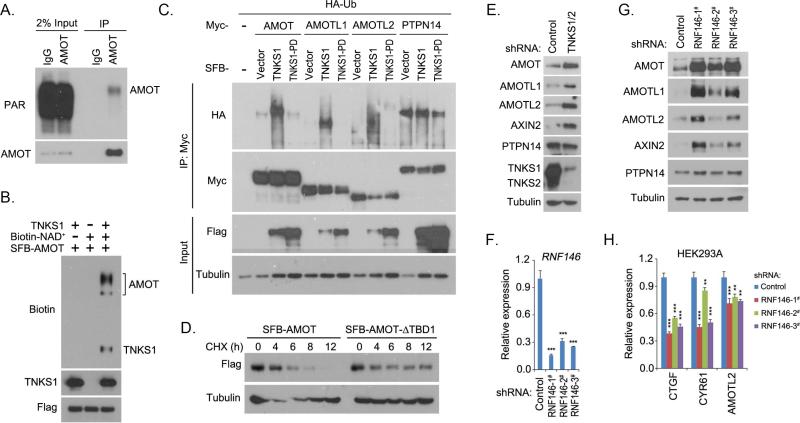
Since AMOT family proteins are found to associate with tankyrases, we examined whether AMOT family proteins can be ribosylated by tankyrases. Indeed, AMOT protein was ribosylated in vivo (Figure 3A). Next, we tested whether AMOT protein could be ribosylated by tankyrases. As shown in Figure 3B, tankyrase 1 not only ribosylated itself but also ribosylated AMOT protein, as indicated by the biotinylated NAD+ through in vitro ribosylation assay. These results indicate that AMOT proteins could be ribosylated by tankyrases.
Figure 3. Tankyrase-RNF146 axis promoted the degradation of AMOT proteins.
(A) AMOT protein was ribosylated in vivo. Endogenous immunoprecipitation (IP) was performed using extract prepared from HEK293T cells containing ADP-HPD (5 μM); normal rabbit IgG was taken as control. PAR, poly-ADP ribosylation polymer antibody. (B) Tankyrase 1 (TNKS1) promoted ribosylation of AMOT protein in vitro. In vitro ribosylation assay was performed and the ribosylated proteins were indicated by the incorporation of biotinylated NAD+ and detected by anti-biotin antibody. (C) Tankyrase 1 (TNKS1) induced the ubiquitination of AMOT proteins. HA-tagged ubiquitin (Ub) was co-expressed with indicated proteins in HEK293T cells for 24 h. Cells were treated with proteasome inhibitor MG132 (10 μM) for 6 h and subjected to immunoprecipitation (IP) assay. TNKS1-PD is the inactive mutant for TNKS1. (D) Tankyrase-binding domain (TBD) deletion mutation stabilized the AMOT protein. AMOT or its TBD-deleted mutant (AMOT-ΔTBD1) was expressed in HEK293T cells for 24 h. Cells were treated with cycloheximide (CHX; 100 μg/mL) and collected at different time points for Western blotting. (E) Loss of tankyrase 1/2 stabilized AMOT proteins. Indicated proteins were detected in control and tankyrase 1/2 knockdown (shRNA-transduced) HEK293A cells by Western blotting. (F-G) Loss of RNF146 stabilized AMOT proteins. RNF146 knockdown efficiency was shown by quantitative PCR (F). Indicated proteins were detected in control and three groups of RNF146 shRNA-transduced HEK293A cells by Western blotting (G). (H) Loss of RNF146 suppressed the transcripts of YAP target genes. The transcripts were detected by quantitative PCR in control and three groups of RNF146 shRNA-transduced HEK293A cells. For panels F and H, ** p<0.01 and *** p<0.001.
To examine the regulation of AMOT family proteins by tankyrases, we found that tankyrase 1, but not its enzyme-inactive mutant (H1184A/E1291A) (tankyrase 1-PD), promoted ubiquitination of all the AMOT family proteins (Figure 3C). Notably, we did not detect any localization change of AMOT family proteins with tankyrase 1 overexpression (data not shown), suggesting that tankyrases may only modulate the stability of AMOT family proteins. Moreover, compared to wild-type AMOT, deletion of the TBD stabilized the AMOT protein (Figure 3D), which further confirmed that tankyrases are involved in the regulation of AMOT family protein stability.
Since the E3 ligase RNF146 has been shown to recognize tankyrase substrate and promote its proteasome-dependent degradation (Zhang et al., 2011), we next examined the role of RNF146 in the regulation of AMOT family proteins. Indeed, loss of tankyrase1 and 2 stabilized all the AMOT family proteins (Figure 3E). Similar findings were observed in RNF146 knockdown cells (Figure 3F and 3G). The WWE domain of RNF146, which has been shown to recognize the ADP-ribosylated proteins (Zhang et al., 2011), was required for the association between RNF146 and AMOT family proteins (Figure S2B and S2C). Moreover, overexpression of RNF146 induced the ubiquitination of AMOT protein, which was suppressed by XAV939 treatment (Figure S2D). In addition, loss of RNF146 inhibited the ubiquitination of AMOT protein (Figure S2E). These data suggest that RNF146 is an E3 ligase for AMOT proteins. Loss of RNF146 also inhibited the transcription of YAP downstream target genes (Figure 3H), suggesting the positive role of RNF146 in YAP regulation. Together, these data indicate that the tankyrase-RNF146 axis controls the stability of AMOT family proteins.
Tankyrase inhibitors stabilize AMOT family proteins
The observation that the tankyrase-RNF146 axis promoted the degradation of AMOT family proteins (Figure 3) suggested that targeting tankyrases with tankyrase inhibitors would stabilize AMOT proteins. Notably, treatment with tankyrase inhibitor XAV939 specifically increased the levels of all the AMOT family proteins but did not affect other Hippo pathway components (Figure 4A and Figure S1C). Moreover, the levels of AMOT family proteins were regulated by XAV939 treatment in a dose-dependent manner (Figure S3A) and also in a time-dependent manner (Figure 4B). The other two tankyrase inhibitors, JW55 and WIKI4, also stabilized AMOT family proteins, while this was not the case for PARP inhibitor olaparib (Figure 4C). These data suggest that, in the Hippo pathway, tankyrase inhibitors specifically regulate AMOT family protein levels.
Figure 4. Tankyrase inhibitors targeted YAP through AMOT family proteins.
(A) XAV939 treatment specifically increased the levels of AMOT family proteins in the Hippo pathway. Western blot was performed in dimethyl sulfoxide (control)– and XAV939– treated HEK293A cells with indicated antibodies. (B) XAV939 treatment increased the levels of AMOT family proteins in a time-dependent manner. HEK293A cells treated with XAV939 (10 μM) were collected at indicated time points for Western blotting with indicated antibodies. (C) AMOT proteins were stabilized by treatment with tankyrase inhibitors. HEK293A cells treated with the indicated inhibitors (10 μM) for 24 h were subjected to Western blotting with indicated antibodies. (D) Overexpression of AMOTL2 attenuated XAV939-induced suppression of YAP. YAP/TEAD luciferase reporter assay was performed in HeLa cells transfected with vector or SFB-AMOTL2 and treated with indicated doses of XAV939 for 24 h. pSV40-Renilla was used as internal control. (E-G) Loss of AMOTL1/2 attenuated XAV939-induced suppression of YAP. Control and AMOTL1/2 shRNA-transduced HeLa and MCF10A cells were subjected to YAP/TEAD luciferase reporter assay (E) and transcription analysis of YAP target genes by quantitative PCR (G), respectively. The downregulation of AMOTL1 and AMOTL2 in MCF10A cells is shown (F). Both HeLa and MCF10A cells were pre-treated with XAV939 (10μM) for 24 h before collection. For panels D, E, and G, * p<0.05 and *** p<0.001.
Consistently, XAV939 treatment only stabilized wild-type AMOT proteins but not their TBD1-deleted mutants (Figure S3C). Loss of RNF146 attenuated the effect of XAV939 on the stabilization of AMOT family proteins (Figure S3D). Notably, AMOT family proteins were previously shown to be regulated by NEDD4.2 E3 ligase (Skouloudaki and Walz, 2012; Wang et al., 2012a). However, another known NEDD4.2 substrate, SMAD2 (Kuratomi et al., 2005), and NEDD4.2 upstream regulator SGK1 (Lamothe and Zhang, 2013) were not affected by XAV939 treatment (Figure S3E), suggesting that NEDD4.2 E3 may not be involved in this process. Together, these results further demonstrated that tankyrase inhibitors stabilize AMOT family proteins through tankyrase-RNF146 axis.
Tankyrase inhibitors target YAP through AMOT family proteins
AMOT proteins have been shown to inhibit YAP's activities by translocating YAP from nucleus into cytoplasm, which provides a potential mechanism for the tankyrase inhibition–mediated YAP suppression through stabilization of all AMOT proteins. As a matter of fact, overexpression of AMOT family proteins, (i.e., AMOTL2), which mimics the stabilization of AMOT proteins by tankyrase inhibitors, neutralized the capacity of XAV939 to suppress YAP/TEAD luciferase activity (Figure 4D and Figure S3H). Moreover, loss of AMOTL1 and AMOTL2 attenuated XAV939's inhibitory effect on YAP, as indicated by YAP/TEAD-luciferase reporter activity (Figure 5E) and the transcription of YAP target genes (Figure 5F and 5G). These results demonstrate that tankyrase inhibitors target YAP by stabilizing AMOT family proteins.
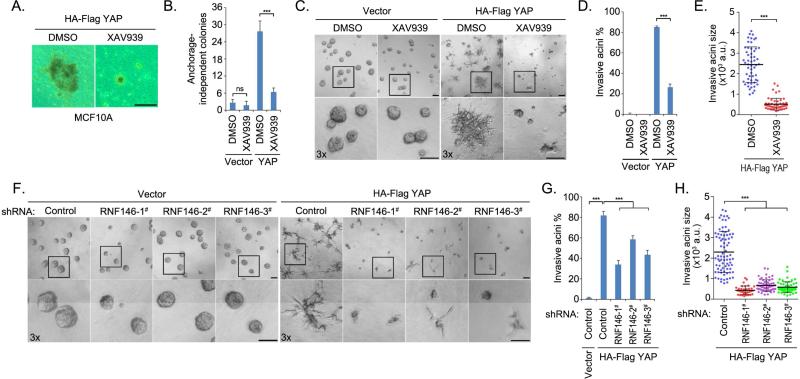
Figure 5. Tankyrase inhibitors targeted the oncogenic functions of YAP.
(A-B) XAV939 treatment suppressed the anchorage-independent growth of YAP-transformed MCF10A cells. Vector- or YAP-overexpressing MCF10A cells were subjected to soft agar assay and treated dimethyl sulfoxide (DMSO) or XAV939 (30 μM) for 4 weeks. Representative colonies are shown (A) and were quantified (B). Data are presented as means ± standard deviation (s.d.) from three independent experiments. (C-E) XAV939 treatment inhibited YAP-induced MCF10A invasive acini formation. Vector- or YAP-overexpressing MCF10A cells were subjected to 3D culture in matrigel and were treated with DMSO or XAV939 (30 μM) for 5 days. Representative acini are shown (C). The percentage (D) and the size (E) of invasive acini are quantified. Data are presented as means ± s.d. from three independent experiments. a.u., arbitrary unit. (F-H) Loss of RNF146 suppressed YAP-induced MCF10A invasive acini formation. Vector- or YAP-overexpressing MCF10A cells transduced with control or one of three RNF146 shRNAs were subjected to 3D culture in matrigel and treated with DMSO or XAV939 (30 μM) for 5 days. Representative acini (F) and quantification of percentage (G) and size (H) of invasive acini are shown. Data are presented as means ± s.d. from three independent experiments. a.u., arbitrary unit. For all the panels, ns, not significant; *** p<0.001; Scale bar, 100μm.
Notably, XAV939 treatment did not affect YAP phosphorylation at S127 or the kinase activity of LAST and MST (Figure S3G), suggesting that XAV939 targets YAP independent of YAP phosphorylation. These findings are consistent with previous studies that AMOT family proteins suppressed YAP activity by translocating YAP from nucleus into cytoplasm through direct protein-protein interaction but independent of YAP phosphorylation status (Chan et al., 2011; Wang et al., 2011; Zhao et al., 2011). Moreover, overexpression AMOT protein also did not affect YAP phosphorylation at S127 (Figure S3H).
Tankyrase inhibitors inhibit the 3D growth of YAP-transformed MCF10A cells
YAP was first identified as an oncoprotein with the capacity to transform normal mammary epithelial MCF10A cells (Overholtzer et al., 2006), which provides a model to study YAP's oncogenic functions. We found that, consistent with previous finding (Overholtzer et al., 2006), overexpression of YAP increased the anchorage-independent growth of MCF10A cells, and this was suppressed by the tankyrase inhibitor XAV939 treatment (Figure 5A and 5B). Moreover, in mammary epithelial MCF10A cells, which form acinar structures when grown in 3D conditions, recapitulating numerous features of breast epithelium in vivo (Debnath et al., 2003), overexpression of YAP led to the formation of branch-like invasive acini in matrigel (Figure 5C), confirming YAP's oncogenic activities (Overholtzer et al., 2006). Intriguingly, XAV939 suppressed this formation of invasive acini induced by YAP overexpression in 3D matrigel culture, decreasing both the number and size of invasive acini (Figure 5C-5E). Notably, XAV939 had only a moderate effect on acini growth for vector-transduced MCF10A cells (Figure 5C), suggesting that tankyrase inhibitors could specifically target YAP in 3D growth with minimal effect on normal acini formation.
To confirm the role of the tankyrase-RNF146 axis in YAP-dependent invasive acini formation, we knocked down RNF146 in both vector- and YAP- overexpressing MCF10A cells and subjected those cells to 3D culture (Figure 5F). Similar to treatment with XAV939, loss of RNF146 specifically suppressed invasive acini formation of YAP-transformed MCF10A cells, decreasing both the number and size of YAP-induced invasive acini (Figure 5F-5H). Similarly, loss of RNF146 had only a slight effect on acini formation of cells transfected with vector control (Figure 5F). These data indicate that the tankyrase-RNF146 axis was required for YAP-dependent invasive acini formation in 3D culture and that tankyrase inhibitors could be utilized to target YAP-dependent oncogenesis.
Discussions
In this study, we discovered that tankyrase inhibitors as a group of compounds target YAP's oncogenic activities. Interestingly, the tankyrase inhibitors specially suppressed YAP-induced MCF10A invasive acini formation but did not affect normal MCF10A acini formation, suggesting their therapeutic potential in the treatment of YAP-dependent cancers. This inhibitory effect may not require the Wnt pathway, since MCF10A cells have very low Wnt activity (Figure S4A) and it is difficult to detect AXIN protein expression in MCF10A cells (Figure S4B). Notably, tankyrase inhibitors only exhibited moderate inhibitory effect on the growth of YAP-overexpressing MCF10A cells in 2D culture, indicating that the microenvironment plays a critical role in this process, an implication that deserves further investigation.
AMOT family proteins are known to be part of the Hippo pathway. AMOT family proteins employ at least two mechanisms to negatively regulate YAP/TAZ. Firstly, AMOT family proteins translocate YAP/TAZ from nucleus into cytoplasm through direct protein-protein interaction, which is independent of YAP/TAZ phosphorylation status (Chan et al., 2011; Wang et al., 2011; Zhao et al., 2011). Secondly, AMOT family proteins have also been shown to activate LATS kinase and suppress YAP/TAZ's activity in a phosphorylation-dependent manner (DeRan et al., 2014; Paramasivam et al., 2011). In addition, AMOT proteins form multiple complexes with other Hippo components. For example, LATS kinase associates with and phosphorylates AMOT proteins, which in turn stabilizes AMOT proteins (Adler et al., 2013), and disrupts the association between AMOT proteins and actin cytoskeleton (Chan et al., 2013; Dai et al., 2013). AMOT proteins also associate with NF2/Merlin and regulate Ras-MAPK pathway (Yi et al., 2011). In this study, we showed that tankyrase inhibitors can stabilize AMOT family proteins by interrupting the tankyrase-RNF146 axis, which not only advances our understanding about the regulation of AMOT family proteins, but also provides useful tools, i.e. tankyrase inhibitors, to further study this protein family.
The discovery of the role of tankyrase inhibitors in YAP regulation led to identification of the RNF146-tankyrase-AMOT axis as an upstream signaling pathway regulating YAP in the Hippo pathway. Previously, we and others showed that tankyrase targeted AXIN (Huang et al., 2009) and PTEN (Li et al., 2015) for degradation and that tankyrase inhibitors stabilized these tumor suppressors. Together with our data presented here, these studies highlight the therapeutic potential of tankyrase inhibitors in cancer, since they target at least three different oncogenic proteins/pathways (YAP, Wnt and AKT). Besides, two tankyrase inhibitors (JW55 and G007-LK) have shown excellent oral availability and plasma/tissue distribution in mice (Lau et al., 2013; Waaler et al., 2012), making them likely candidate compounds for future translational studies.
Experimental Procedures
The information about antibody, cell culture, plasmid construction, Immunofluorescent staining, tandem affinity purification, mass spectrometry, reverse transcription in this study is described in the Supplemental Experimental Procedures.
In vitro ribosylation assay
Recombinant baculovirus-derived GST-TNKS1 (amino acids 1000-1328) (0.2 μg, Sigma #SRP0422) and HEK293T-purified SFB-AMOT proteins were incubated in 30 μL reaction buffer (50 mM Tris-HCl at pH 8.0, 4 mM MgCl2, 0.2 mM dithiothreitol) with or without 25 mM biotinylated NAD+ (Trevigen) at 25°C for 30 min. Reactions were terminated and subjected to Western blotting.
Anchorage independent growth on soft agar
MCF10A cells (2 × 104) transduced with HA-Flag vector or HA-Flag YAP constructs were added to growth medium (1.5 mL) of growth medium with 0.33% agar and layered onto beds of 0.5% agar (2 mL) in six-well plates. Cells were fed with medium (1 mL) with 0.33% agar every 7 days for 4 weeks, after which colonies were photographed. Assays were duplicated in three independent experiments.
Three-dimensional culture of MCF10A cell for acini formation
MCF10A cells (5 × 103) stably transfected with HA-Flag vector or HA-Flag YAP constructs were grown in growth factor–reduced matrigel matrix in the 8-well chamber slide system (Fisher Scientific). Cultured cells were analyzed for invasive acini after 5 days of growth in matrigel and at least four replicates were performed as described (Debnath et al., 2003). Dimethyl sulfoxide or XAV939 (30 μM) was added to the medium and refreshed every 2 days.
Supplementary Material
Acknowledgements
We thank all of our colleagues in Dr. Chen's laboratory for insightful discussion and technical assistance, especially Drs. Jingsong Yuan and Lin Feng. We thank Dr. Yutong Sun and the staff of the shRNA-ORFeome core facility at MD Anderson Cancer Center for the ORFs and shRNAs. We thank Dr. Susan Smith (New York University) for providing the TNKS1, TNKS1-PD, and TNKS2 plasmids. We thank Kathryn Hale for proof-reading the manuscript. This work was supported in part by a Department of Defense Era of Hope Research scholar award to J.C. (W81XWH-09-1-0409). J.C. is also a recipient of an Era of Hope Scholar award from the Department of Defense (W81XWH-05-1-0470) and a member of M.D. Anderson Cancer Center (CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
W.W. and J.C. designed the experiments. W.W. performed all of the experiments with assistance from N. L., X.L., M.K.T., X.H. and J.C. J.C. supervised the study. W.W. and J.C. wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, Conwell MD, Sun Y, Hudmon A, Wells CD. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc Natl Acad Sci U S A. 2013;110:17368–17373. doi: 10.1073/pnas.1308236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Chong YF, Pobbati AV, Huang C, Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo F, Tan I, Leung T, Hong W. Actin-binding and cell proliferation activities of angiomotin family members are regulated by Hippo pathway-mediated phosphorylation. J Biol Chem. 2013;288:37296–37307. doi: 10.1074/jbc.M113.527598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, She P, Chi F, Feng Y, Liu H, Jin D, Zhao Y, Guo X, Jiang D, Guan KL, et al. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J Biol Chem. 2013;288:34041–34051. doi: 10.1074/jbc.M113.518019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettler S, LaRose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, Sicheri F. Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell. 2011;147:1340–1354. doi: 10.1016/j.cell.2011.10.046. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI, Wang Q. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–2229. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Kang HS, Beak JY, Kim YS, Herbert R, Jetten AM. Glis3 is associated with primary cilia and Wwtr1/TAZ and implicated in polycystic kidney disease. Mol Cell Biol. 2009;29:2556–2569. doi: 10.1128/MCB.01620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratomi G, Komuro A, Goto K, Shinozaki M, Miyazawa K, Miyazono K, Imamura T. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor. Biochem J. 2005;386:461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe SM, Zhang S. The serum- and glucocorticoid-inducible kinases SGK1 and SGK3 regulate hERG channel expression via ubiquitin ligase Nedd4-2 and GTPase Rab11. J Biol Chem. 2013;288:15075–15084. doi: 10.1074/jbc.M113.453670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levaot N, Voytyuk O, Dimitriou I, Sircoulomb F, Chandrakumar A, Deckert M, Krzyzanowski PM, Scotter A, Gu S, Janmohamed S, et al. Loss of Tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell. 2011;147:1324–1339. doi: 10.1016/j.cell.2011.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Y, Han X, Liang K, Wang J, Feng L, Wang W, Songyang Z, Lin C, Yang L, et al. Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth. Genes Dev. 2015;29:157–170. doi: 10.1101/gad.251785.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, Zhang J. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–1273. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Lehmann W, Martin T, Delaunay C, Hueber A, Barys L, Niu H, Billy E, Wartmann M, Ito M, et al. The tyrosine phosphatase PTPN14 is a negative regulator of YAP activity. PLoS One. 2013;8:e61916. doi: 10.1371/journal.pone.0061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki Y, Matsui H, Asou H, Nagamachi A, Aki D, Honda H, Yasunaga S, Takihara Y, Yamamoto T, Izumi S, et al. Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome maturation. Mol Cell. 2012;47:694–706. doi: 10.1016/j.molcel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22:3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov. 2012;11:923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouloudaki K, Walz G. YAP1 recruits c-Abl to protect angiomotin-like 1 from Nedd4-mediated degradation. PLoS One. 2012;7:e35735. doi: 10.1371/journal.pone.0035735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012;72:2822–2832. doi: 10.1158/0008-5472.CAN-11-3336. [DOI] [PubMed] [Google Scholar]
- Wang C, An J, Zhang P, Xu C, Gao K, Wu D, Wang D, Yu H, Liu JO, Yu L. The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitin-dependent degradation. Biochem J. 2012a;444:279–289. doi: 10.1042/BJ20111983. [DOI] [PubMed] [Google Scholar]
- Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012b;26:1959–1971. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Troutman S, Fera D, Stemmer-Rachamimov A, Avila JL, Christian N, Persson NL, Shimono A, Speicher DW, Marmorstein R, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin's regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–540. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, Feng Y, Zheng C, Wang Z, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Mickanin C, Feng Y, Charlat O, Michaud GA, Schirle M, Shi X, Hild M, Bauer A, et al. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol. 2011;13:623–629. doi: 10.1038/ncb2222. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.