Abstract
Background
We recently identified several highly specific bedside physical signs associated with impending death within 3 days among patients with advanced cancer. In this study, we developed and assessed a diagnostic model for impending death based on these physical signs.
Methods
We systematically documented 62 physical signs every 12 hours from admission to death or discharge in 357 patients with advanced cancer admitted to acute palliative care units (APCUs) at two tertiary care cancer centers. We used recursive partitioning analysis (RPA) to develop a prediction model for impending death in 3 days using admission data. We validated the model with 5 iterations of 10-fold cross-validation, and also applied the model to APCU days 2/3/4/5/6.
Results
Among 322/357 (90%) patients with complete data for all signs, the 3-day mortality was 24% on admission. The final model was based on 2 variables (palliative performance scale [PPS] and drooping of nasolabial fold) and had 4 terminal leaves: PPS≤20% and drooping of nasolabial fold present, PPS≤20% and drooping of nasolabial fold absent, PPS 30–60% and PPS ≥ 70%, with 3-day mortality of 94%, 42%, 16% and 3%, respectively. The diagnostic accuracy was 81% for the original tree, 80% for cross-validation, and 79%–84% for subsequent APCU days.
Conclusion(s)
We developed a diagnostic model for impending death within 3 days based on 2 objective bedside physical signs. This model was applicable to both APCU admission and subsequent days. Upon further external validation, this model may help clinicians to formulate the diagnosis of impending death.
Keywords: death, diagnosis, neoplasms, palliative care, physical examination, mortality
Introduction
The diagnosis of impending death is of practical significance to clinicians and families involved in the care of patients in the last days of life. Many important medical decisions, including hospital discharge, hospice referral, continuation of bloodwork, discontinuation of potentially futile medications and interventions, and in some hospitals, whether a patient should be moved to a single room are dependent on whether the patient has entered the final phase of life [1, 2]. The Liverpool Care Pathway was been discontinued in the United Kingdom since 2014, partly because clinicians had difficulty to tell with confidence that patients are imminently dying [1, 3]. Knowing whether the patient is imminently dying can also inform many personal decisions for family caregivers, such as whether a wife should stay overnight, whether a son still has time to fly in to see his father, and how long the daughter should take time off work to be at the bedside.
We recently reported our findings from the Investigating the Process of Dying study, a prospective longitudinal observational study that systematically documented 62 clinical signs every 12 hours from the time of admission to an acute palliative care unit (APCU) until death or discharge in consecutive patients [4]. We found that 2 early and 14 late physical signs were associated with impending death within 3 days (Table 1) [4, 5]. The early signs were Palliative performance scale ≤ 20% and Richmond Agitation Sedation Scale ≤−2. These signs were observed in a majority of patients before death, had an onset >3 days before death, and had moderate diagnostic value for impending death. The late signs were inability to close eyelids, non-reactive pupils, drooping of nasolabial fold (figure 1), hyperextension of neck, death rattle, grunting of vocal cords, decreased response to verbal stimuli, decreased response to visual stimuli, respiration with mandibular movement, Cheyne Stokes breathing, pulselessness of radial artery, peripheral cyanosis, decreased urine output and upper gastrointestinal bleed. In contrast to the early signs, late signs were observed less frequently and mostly in the last 3 days of life, and were highly specific for impending death. The late signs, when present, were highly suggestive of impending death; however, their absence could not rule out death in 3 days [4, 5].
Table 1.
| Physical Signs | Frequency of sign in last 3 days of life, %* | Median Onset in days (Q1–Q3)† | Sensitivity‡ (95% CI) | Specificity‡ (95% CI) | Negative Likelihood Ratio‡ (95% CI) | Positive Likelihood Ratio‡ (95% CI) |
|---|---|---|---|---|---|---|
| Palliative performance scale ≤20% | 93 | 4.0 (3.5–6.0) | 64 (63.4–64.7) | 81.3 (80.9–81.7) | 0.44 (0.43–0.45) | 3.5 (3.4–3.6) |
| Richmond Agitation Sedation Scale ≤−2 | 90 | 4.5 (3–6) | 50.5 (49.9–51.1) | 89.3 (88.9–89.7) | 0.6 (0.5–0.6) | 4.9 (4.7–5) |
| Urine output over last 12 h <100 ml | 72 | 1.5 (1.0–2.5) | 24.2 (23.2–25.1) | 98.2 (98–98.5) | 0.77 (0.76–0.78) | 15.2 (13.4–17.1) |
| Death rattle | 66 | 1.5 (1.0–2.0) | 22.4 (21.8–22.9) | 97.1 (96.9–97.3) | 0.8 (0.79–0.81) | 9 (8.1–9.8) |
| Respiration with mandibular movement | 56 | 1.5 (1.0–2.0) | 22 (21.5–22.4) | 97.5 (97.3–97.6) | 0.8 (0.8–0.81) | 10 (9.1–10.9) |
| Peripheral cyanosis | 59 | 3.0 (2.0–4.5) | 26.7 (26.1–27.3) | 94.9 (94.7–95.2) | 0.77 (0.77–0.78) | 5.7 (5.4–6.1) |
| Cheyne Stokes breathing | 41 | 2.0 (1.0–2.0) | 14.1 (13.6–14.5) | 98.5 (98.4–98.7) | 0.9 (0.9–0.9) | 12.4 (10.8–13.9) |
| Pulselessness of radial artery | 38 | 1.0 (0.5–1.0) | 11.3 (10.9–11.8) | 99.3 (99.2–99.5) | 0.89 (0.89–0.9) | 15.6 (13.7–17.4) |
| Decreased response to verbal stimuli | 69 | 2.0 (1.5–4.0) | 30 (29.4–30.5) | 96 (95.8–96.3) | 0.73 (0.72–0.74) | 8.3 (7.7–9) |
| Decreased response to visual stimuli | 70 | 3.0 (2.0–4.0) | 31.9 (31.4–32.4) | 94.9 (94.6–95.1) | 0.72 (0.71–0.72) | 6.7 (6.3–7.1) |
| Non-reactive pupils | 38 | 2.0 (1.5–3.0) | 15.3 (14.9–15.7) | 99 (98.8–99.1) | 0.86 (0.85–0.86) | 16.7 (14.9–18.6) |
| Drooping of nasolabial fold | 78 | 2.5 (1.5–3.0) | 33.7 (33.2–34.3) | 95.5 (95.3–95.8) | 0.69 (0.69–0.7) | 8.3 (7.7–8.9) |
| Hyperextension of neck | 46 | 2.5 (2.0–3.0) | 21.2 (20.6–21.7) | 96.7 (96.5–96.9) | 0.82 (0.81–0.82) | 7.3 (6.7–8) |
| Inability to close eyelids | 57 | 1.5 (1.0–1.5) | 21.4 (20.9–21.8) | 97.9 (97.7–98.1) | 0.8 (0.8–0.81) | 13.6 (11.7–15.5) |
| Grunting of vocal cords | 54 | 1.5 (1.0–2.0) | 19.5 (19–19.9) | 97.9 (97.7–98.1) | 0.82 (0.82–0.83) | 11.8 (10.3–13.4) |
| Upper gastrointestinal bleed | 5 | 5.5 (0.5–17.0) | 2.9 (2.8–3) | 99.7 (99.6–99.7) | 0.97 (0.97–0.98) | 10.3 (9.5–11.1) |
any occurrence of the sign of interest within the last 3 days of life among patients who died in the APCU
median onset from death backwards (in days)
we computed the sensitivity, specificity, positive likelihood ratio (LR), and negative LR for each sign for death within 3 days using data from all 357 patients. We constructed a 2×2 table with one observation per patient based on the presence or absence of a particular sign during a randomly sampled nursing shift and whether that patient died within the next 3 days from that shift (i.e. gold standard), and then calculated the sensitivity, specificity, positive LR and negative LR. To account for the multiple observations for each patient, we re-sampled our data 100x to obtain the average and 95% confidence interval for each statistic
Abbreviations: CI, confidence interval; Q1–Q3, interquartile range
Figure 1. Drooping of Nasolabial Fold.
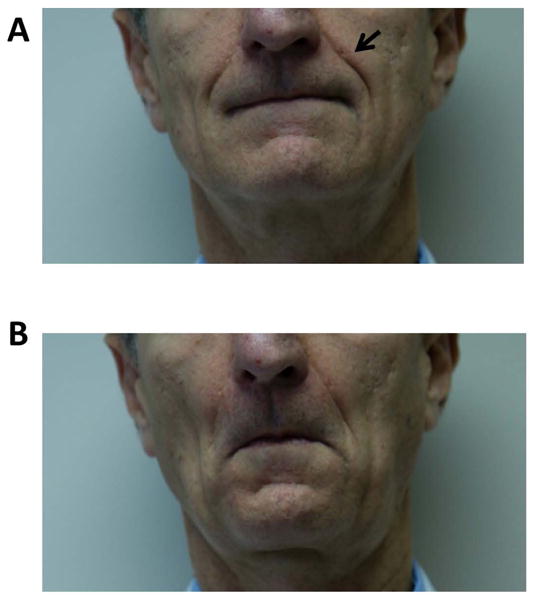
(A) Nasolabial folds are the skin folds that run from the nose to corners of the mouth (arrow). (B) In the last days of life, drooping of nasolabial fold may be noted in which they become less prominent because of the loss of facial muscle tone. The face appears to be more relaxed.
One practical question relates to how clinicians can take advantage of the combination of these signs to make the diagnosis of impending death. A diagnostic tool for impending death can potentially assist clinicians in making this important diagnosis with greater confidence, and ultimately, guide clinical decision making and prepare family caregivers. In this study, we derived and validated a diagnostic model for impending death.
Methods
Study Setting and Criteria
This study is based on data collected in the Investigating the Process of Dying Study. Details of the study methodology has been reported in detail [4, 5]. In brief, we enrolled consecutive advanced cancer patients who were ≥18 years of age and admitted to the APCUs at MD Anderson Cancer Center (MDACC) in Houston, Texas between 4/5/2010–7/6/2010 and Barretos Cancer Hospital (BCH) in Barretos, Brazil between 1/27/2011–6/1/2011. The study protocol was approved by the Institutional Review Boards at both hospitals. All participating clinicians signed the informed consent prior to enrollment. We obtained waiver of patient consent to minimize distress during the consent process and to ensure that we could collect data on consecutive patients regardless of their mental status.
This study was conducted in the APCUs because of the availability of experienced nurses and physicians who provide intensive routine monitoring and the high prevalence of impending death among this patient population. As reported previously, the two APCUs have similar patterns of practice.[4, 5] APCU patients receive comprehensive supportive care, and have access to a full array of laboratory and imaging investigations and medical interventions such as intravenous fluids, antibiotics and transfusions if indicated.
Data Collection
The data collection process for the physical signs has been reported in detail elsewhere [4, 5]. Briefly, physical signs were assessed by palliative care nurses twice a day using standardized forms from APCU admission until discharge or death. All participating nurses worked full time in the palliative care unit, and had extensive expertise in the care of dying patients. The median experience in nursing was 6 years (interquartile range 3–5 years), with 3 years (interquartile range 1–7 years) in palliative care. Before study initiation, all nurses had a 1 hour standardized training session to review the study objectives, design and data collection process, with the principal investigator demonstrating multiple physical signs, such as drooping of nasolabial fold and grunting of vocal cords, to ensure consistency in interpretation of the signs. The nurses also received longitudinal support and audits from the principal investigators and charge nurses during the study to ensure data collection was complete and accurate.. They also received longitudinal support and audits from the principal investigators and charge nurses during the study to ensure data collection is complete and accurate. The two study sites had weekly video conference to ensure data were collected systematically and accurately. The study forms were translated to Portuguese to facilitate data collection in Brazil and back-translated to ensure accuracy of translation.
Survival from time of APCU admission was collected from institutional databases and electronic health records.
Statistical Analysis
We used a recursive partitioning analysis (RPA) to develop a diagnostic predictor for impending death in 3 days. RPA is a non-parametric multivariate algorithm that generates a decision tree for classification of patients into different risk groups [6, 7]. RPA divides a group of patients into two groups with maximum separation with respect to the outcome (i.e. 3-day mortality) by scanning each candidate variable to identify the best split (optimal cut-points for continuous variables and optimal combinations of categories for categorical variables). We used the likelihood ratio test statistic as the criterion for best split. This splitting process is repeated in each subset created until the subsets become too small to split further (i.e. <15 patients). It has been used to develop various clinical prediction tools, such as prognostication of survival in patients with brain tumors [8] and unknown primary [9] and the probability of discharge from an APCU in cancer patients [10].
Using data on APCU admission (first shift), we fitted a classification tree to predict the 3-day mortality (37% at baseline). Only patients with complete data were included in the model. Among the 62 physical signs, we entered 13 late signs and 2 early signs in the RPA (Table 1) [4, 5]. The 13 late signs all had a positive likelihood ratio (LR+) >5 for impending death within 3 days: inability to close eyelids, non-reactive pupils, drooping of nasolabial fold, hyperextension of neck, death rattle, grunting of vocal cords, decreased response to verbal stimuli, decreased response to visual stimuli, respiration with mandibular movement, Cheyne Stokes breathing, pulselessness of radial artery, peripheral cyanosis, and upper gastrointestinal bleed. Decreased urine output also had a high LR+ of 15.2 for impending death, but was excluded from the analysis because it was not routinely collected at one study site. We also included 2 early signs, palliative performance scale (PPS) and Richmond Agitation Sedation Scale (RASS), as two additional variables because of their relevance and relatively high sensitivity for impending death. PPS (10% to 100%) and RASS (−5 to +4) were coded as continuous variables, and all late signs were coded as binary variables (absent=0 or present=1).
Palliative Performance Scale (PPS) is a validated 11-point scale that ranges from 0% (death) to 100% (completely asymptomatic) based on the patient’s function, oral intake and cognitive status, and has high inter-rater reliability (intraclass correlation coefficients >0.9) [11, 12]. It is widely used in oncology and palliative care to estimate survival, [13] and is interchangeable with other performance scales.[14] A score of ≤ 20% indicates patient is completely bed bound and has a limited survival [15].
Validation Procedures
We provided internal validation by conducting 5 iterations of 10-fold cross-validation to set the optimal tree size as one with 4 terminal nodes (i.e. leaves).[6] Specifically, 10-fold cross validation was conducted by (1) randomly splitting the cohort into 10 datasets of equal sizes, (2) training on 9 datasets and testing on 1 dataset, then (3) repeating this procedure 10 times to obtain an average. We then computed the diagnostic accuracy (i.e. [True positive + True negative]/[True positive + True negative + False positive + False negative] = 1 − misclassification rate) using 2 × 2 diagnostic tables with the predicted outcome (death in 3 days or not) against the gold standard (actual 3-day mortality) for the initial model and for each cross-validation run.[16] By convention, all patients in a leaf with the probability of death in 3 days >50% were classified as deaths and all patients in a leaf with the probability of death in 3 days <50% were classified as non-deaths.
To further validate our model, we applied the models to the data on subsequent shifts 3, 5, 7, 9 and 11, corresponding to day 2, 3, 4, 5 and 6 of the APCU admission.
We also repeated the same RPA procedures above but also include the number of late signs (ranging from 0 to 13) as an additional variable in the model in addition to the 15 signs. The optimal tree size was one with 6 leaves.
We used the tree library in S+ (Version 8.2 for Windows, TIBCO Software Inc.) to perform RPA.
Results
Patient Demographics
Patient characteristics have been reported previously [4]. In brief, we enrolled 151 patients from MDA and 203 patients from BCH. The average age was 58 (range 18–88), 233 (65%) were of Hispanic origin, 195 (55%) were female, and 101 (28%) had a diagnosis of gastrointestinal cancer. The median APCU admission length was 6 days (interquartile range 4–9 days), and the median survival from time of admission was 10 days (95% confidence interval 8–12 days).
Derivation and Validation of the Main RPA Model
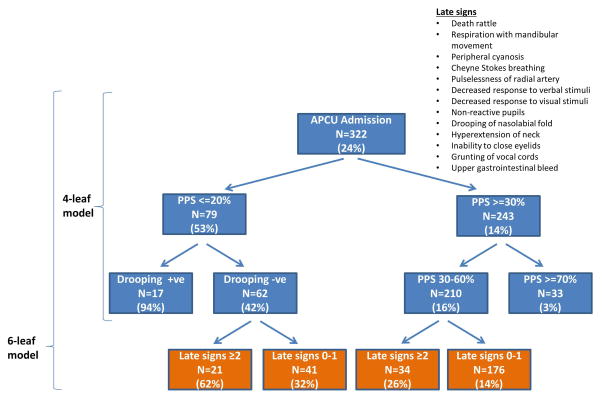
Of the 357 patients, 322 (90%) had complete dataset on APCU admission and were included in the derivation of the model. The main RPA model included 2 variables (PPS and drooping of nasolabial fold) and had 4 leaves. As shown in Figure 2 (exclude the bottom level), the 3-day mortality rates varied between 3% and 94%. The observed diagnostic accuracy was 81%. The average accuracy over 5 rounds of 10-fold cross-validation was 80%.
Figure 2. Recursive Partitioning Model for Impending Death in 3 Days.
The main model (exclude bottom level) is based on 2 variables (drooping of nasolabial fold and palliative performance scale) and has 2 levels and 4 leaves. The diagnostic accuracy was 81%. Inclusion of the variable “number of late signs” resulted in a 6-leaf model that included 3 variables and 3 levels. The diagnostic accuracy was 82%. For each node, the number of patients that fulfill the criteria is shown along with the 3-day mortality rate (in parentheses).
Table 2 shows that the number of patients in each node and 3-day mortality rate when the recursive partitioning model was applied to data collected on admission and subsequent days during the APCU stay. The diagnostic accuracy varied between 79% and 84%.
Table 2.
Application of Main 4-Leaf Model and Alternative 6-Leaf Model on Admission and Subsequent Days
| 4-Leaf Model | ||||||
|---|---|---|---|---|---|---|
| Shift | N* | PPS≤20%, drooping present N (%)† |
PPS ≤20%, drooping absent N (%)† |
PPS 30–60% N (%)† |
PPS ≥70% N (%)† |
Diagnostic accuracy, %‡ |
| 1 | 351 | 19 (84) | 68 (40) | 227 (15) | 37 (3) | 81 |
| 3 | 322 | 29 (72) | 63 (43) | 199 (17) | 31 (3) | 79 |
| 5 | 282 | 30 (77) | 50 (38) | 172 (13) | 30 (10) | 82 |
| 7 | 243 | 23 (78) | 44 (41) | 150 (13) | 25 (4) | 82 |
| 9 | 202 | 16 (69) | 40 (40) | 121 (10) | 25 (4) | 83 |
| 11 | 161 | 16 (62) | 28 (29) | 101 (11) | 16 (0) | 84 |
| 6-Leaf Model | ||||||||
|---|---|---|---|---|---|---|---|---|
| Shift | N* | PPS≤20%, drooping present N (%)† |
PPS≤20%, drooping absent, ≥2 late signs N (%)† |
PPS ≤20%, drooping absent, 0–1 late signs N (%)† |
PPS 30–60%, ≥2 late signs N (%)† |
PPS 30–60%, 0–1 late signs N (%)† |
PPS ≥70% N (%)† |
Diagnostic accuracy, %‡ |
| 1 | 328 | 19 (84) | 21 (62) | 41 (32) | 34 (26) | 176 (14) | 37 (3) | 82 |
| 3 | 307 | 29 (72) | 17 (65) | 42 (36) | 32 (22) | 156 (15) | 31 (3) | 80 |
| 5 | 270 | 30 (77) | 16 (62) | 30 (27) | 27 (26) | 137 (10) | 30 (10) | 83 |
| 7 | 235 | 23 (78) | 16 (69) | 25 (28) | 26 (31) | 119 (8) | 26 (4) | 85 |
| 9 | 191 | 16 (69) | 10 (80) | 25 (28) | 18 (39) | 97 (4) | 25 (4) | 86 |
| 11 | 153 | 16 (62) | 7 (43) | 20 (25) | 11 (45) | 83 (6) | 16 (0) | 84 |
The number of patients varies over time because (1) for each row, we only included patients with complete data for the two variables (PPS and drooping of nasolabial fold), and (2) some patients died or were discharged from the hospital
The total number of patients (N) and the proportion of patients who died in 3 days (%) were shown for each leaf in the recursive partitioning model
Accuracy = 1 − misclassification rate = [True positive + True negative]/[True positive + True negative + False positive + False negative] in 2 × 2 diagnostic tables with the predicted outcome (death in 3 days or not) against the gold standard (actual 3-day mortality).[14]
Abbreviations: PPS, palliative performance scale
The 6-Leaf RPA Model
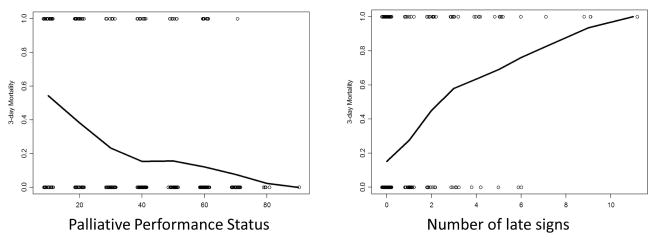
Figure 3a shows that the 3-day mortality increased with decreasing performance status. As shown in Figure 3b, the probability of death in 3 days also increased with the number of late signs on admission.
Figure 3. Decreasing palliative performance status and increasing number of late signs were associated with an increased probability of death in 3 days.
We plotted the (A) palliative performance status, and (B) number of late signs against a moving-average estimate of the probability of death in 3 days (y-axis).
When we repeated the RPA procedures and included the number of late signs, a third level was added to the main model. Specifically, the presence of 2 or more late signs was associated with higher 3-day mortality among patients with PPS ≤ 20% and no drooping and among patients with PPS 30–60% compared to patients with only 0 to 1 late signs (Figure 2, include the bottom level). The 3-day mortality rates varied between 3% and 94%. The diagnostic accuracy was 82% with the admission data, and 80% with cross-validation.
When this model was applied to data on subsequent days, the diagnostic accuracy varied between 80% and 86% (Table 2).
Discussion
Building on the previously reported early and late physical signs on impending death, we developed and validated a RPA model to predict impending death in 3 days among cancer patients admitted to APCUs. This model incorporated an early sign and a late sign to assess the risk of 3-day mortality, is easy to apply, and has a relatively high accuracy. Upon further validation, this risk score may facilitate the diagnosis of impending death.
Patients admitted to APCUs have a high mortality rate (20–40%) [10, 17, 18]. The ability to distinguish patients who are imminently dying and those who may be discharged alive has important clinical, practical, logistical, social and emotional implications. The Investigating the Process of Dying study systematically examined the signs and symptoms that occurred in the last days of life among cancer patients, and identified physical signs associated with impending death [4, 5, 19]. These signs were classified as early and late signs based on their time of occurrence in relation to impending death.
Although these signs may useful on their own, one practical concern is how clinicians can combine them in clinical practice to predict impending death in 3 days. To address this issue, we derived and validated the main RPA model. This model only included PPS and drooping of nasolabial fold. This model took advantage of the higher sensitivity of an early sign to help rule out impending death, and the higher specificity of a late sign to help rule in impending death. Specifically, patients with PPS ≥30% had an average probability of death of 14%, which decreased further to 3% if PPS was ≥70%. In contrast, patients with PPS ≤20% had a much higher (54%) 3-day mortality. Among patients with PPS ≤20%, those who also presented with drooping of nasolabial fold had a very high risk of 3-day mortality (94%). Given the diagnosis of impending death, this subgroup of patients would benefit from staying in the hospital, and the healthcare team’s focus should be on maximizing comfort and minimizing invasive investigations and treatments. We conducted both cross-validation analyses and applied the model to other APCU days, and found the model to remain accurate in predicting 3-day mortality.
Drooping of nasolabial fold has been studied in the context of plastic surgery (i.e. correction of prominent naoslabial fold or reconstruction) and neurology (i.e. facial nerve palsy with flattening of the nasolabial fold).[20–23] Multiple studies have demonstrated that changes in nasolabial fold is a physical sign that can be assessed with good inter-rater reliability.[24, 25] For example, Buchner et al. reported that the intraclass correlation coefficient for nasolabial fold assessment was 0.88 among 5 physicians.[24] Further research is needed to examine the inter-rater reliability for the assessment of nasolabial fold and other physical signs in the palliative care setting among physicians, nurses and family caregivers.
One limitation of this model is that only a small proportion of patients had a high risk of death, and that some patients who do not present with drooping of nasolabial fold but have several other late signs may be misclassified as having low risk. Thus, we developed a second model in which the number of late signs was included as a variable. Interestingly, inclusion of this variable did not affect the overall structure of the first model. Instead, the number of late signs helped to identify patients among the 2 leaves who had an intermediate probability of 3-day mortality (i.e. PPS ≤20% and drooping absent, and PPS 30–60%). Patients with 2 or more late signs had a higher 3-day mortality rate. Upon external validation, this alternate model may also assist clinicians to make the diagnosis of impending death.
To our knowledge, these two models represent the first tools to diagnose impending death. The main advantages include the relatively high accuracy, ease of application, and the reliance on objective bedside physical signs. Furthermore, our models could be applied to multiple days throughout the admission. Most recently, Chen et al. conducted a retrospective study to develop the objective palliative prognostic score, which predicted 7-day survival in a hospice palliative care unit. This model included 6 variables: heart rate, leukocyte count, platelet count, serum creatinine and serum potassium, and history of chemotherapy [26]. Further research is needed to examine if these variables were also useful to predict 3-day mortality, and whether they remain independent of the physical signs found in this study.
This study has several limitations. First, we only included cancer patients in two APCUs. Thus, this model may not be generalizable in other study populations and settings. Second, the sample size was relatively small. Thus, we minimized the tree size to avoid over fitting of the model. Third, our model was generated based on admission data only. It is possible that the model may be different if developed using data on subsequent days. When applying the model to the subsequent days, we found the accuracy to be acceptable. Fourth, we were not able to include decreased urine output, which is an important late sign because it was not routinely collected at one study site. Fifth, although our model was able to differentiate patients with different risks of impending death, a large proportion of patients had intermediate probability, so the utility of RPA is somewhat limited in this dataset. Novel variables may be useful to help with impending death. Finally, external validation is needed to confirm findings from this study in various settings (e.g. intensive care units, inpatient and home hospices) and patient populations (e.g. non-cancer. Future studies should also characterize how clinicians and family caregivers can accurately monitor the changes in these physical signs to help them predict the timing of death.
In summary, we derived and provided validation of the RPA models to facilitate the diagnosis of impending death in 3 days. These models included only a few simple variables: PPS, drooping of nasolabial fold. With further external validation, these models may be useful to help clinicians to make the diagnosis of impending death.
Synopsis.
In this prospective observational study of hospitalized cancer patients, we developed a diagnostic recursive partitioning model for impending death in 3 days based on bedside physical signs. This model was accurate, applicable both at admission and during the hospitalization, and may help clinicians to formulate the diagnosis of impending death.
Acknowledgments
Funding: This research is supported in part by a University of Texas MD Anderson Cancer Center Support Grant (CA 016672), which provided the funds for data collection at both study sites. EB is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA122292-01, and R01CA124481-01. DH is supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and a National Institutes of Health grant (R21CA186000-01A1).
We would like to thank all clinicians who supported this study.
Footnotes
Disclosures: No potential conflict of interest
References
- 1.Hui D, Con A, Christie G, et al. Goals of care and end-of-life decision making for hospitalized patients at a canadian tertiary care cancer center. J Pain Symptom Manage. 2009;38:871–81. doi: 10.1016/j.jpainsymman.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Hwang IC, Ahn HY, Park SM, et al. Clinical changes in terminally ill cancer patients and death within 48 h: when should we refer patients to a separate room? Support Care Cancer. 2012;21:835–40. doi: 10.1007/s00520-012-1587-4. [DOI] [PubMed] [Google Scholar]
- 3.Ellershaw J, Ward C. Care of the dying patient: the last hours or days of life. BMJ. 2003;326:30–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D, Dos Santos R, Chisholm G, et al. Clinical Signs of Impending Death in Cancer Patients. Oncologist. 2014;19:681–7. doi: 10.1634/theoncologist.2013-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Dos Santos R, Chisholm G, et al. Bedside clinical signs associated with impending death in patients with advanced cancer: Preliminary findings. Cancer. 2015;121:960–7. doi: 10.1002/cncr.29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breiman L, Friedman J, Olshen RA, et al. Classification and Regression Trees. New York: Chapman and Hall; 1984. [Google Scholar]
- 7.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods. 2009;14:323–48. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 9.Hess KR, Abbruzzese MC, Lenzi R, et al. Classification and regression tree analysis of 1000 consecutive patients with unknown primary carcinoma. Clin Cancer Res. 1999;5:3403–10. [PubMed] [Google Scholar]
- 10.Hui D, Elsayem A, Palla S, et al. Discharge outcomes and survival of patients with advanced cancer admitted to an acute palliative care unit at a comprehensive cancer center. J Palliat Med. 2010;13:49–57. doi: 10.1089/jpm.2009.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho F, Lau F, Downing MG, et al. A reliability and validity study of the Palliative Performance Scale. BMC palliative care. 2008;7:10. doi: 10.1186/1472-684X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson F, Downing GM, Hill J, et al. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 13.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–8. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 14.Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer. 2010;46:3175–83. doi: 10.1016/j.ejca.2010.06.126. [DOI] [PubMed] [Google Scholar]
- 15.Morita T, Tsunoda J, Inoue S, et al. Validity of the palliative performance scale from a survival perspective. J Pain Symptom Manage. 1999;18:2–3. doi: 10.1016/s0885-3924(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 16.Parshall MB. Unpacking the 2 × 2 table. Heart Lung. 2013;42:221–6. doi: 10.1016/j.hrtlng.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–61. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagman R, Rivera N, Walsh D, et al. Acute inpatient Palliative Medicine in a cancer center: clinical problems and medical interventions--a prospective study. The American Journal of Hospice & Palliative Care. 2007;24:20–28. doi: 10.1177/1049909106295292. [DOI] [PubMed] [Google Scholar]
- 19.Hui D, Dos Santos R, Chisholm G, et al. Symptom Expression in the Last 7 Days of Life among Cancer Patients Admitted to Acute Palliative Care Units. J Pain Symptom Manage. 2015 doi: 10.1016/j.jpainsymman.2014.09.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt MG, Franklin JH, Moore CC. Direct nasolabial lift, a technique for palliation of oncologic lower-face paralysis. J Otolaryngol Head Neck Surg. 2010;39:476–8. [PubMed] [Google Scholar]
- 21.Pessa JE, Brown F. Independent effect of various facial mimetic muscles on the nasolabial fold. Aesthetic Plast Surg. 1992;16:167–71. doi: 10.1007/BF00450609. [DOI] [PubMed] [Google Scholar]
- 22.Pogrel MA, Shariati S, Schmidt B, et al. The surgical anatomy of the nasolabial fold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:410–5. doi: 10.1016/s1079-2104(98)90365-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Tang MY, Jin R, et al. Classification of nasolabial folds in Asians and the corresponding surgical approaches: By Shanghai 9th People’s Hospital. J Plast Reconstr Aesthet Surg. 2015 doi: 10.1016/j.bjps.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Buchner L, Vamvakias G, Rom D. Validation of a photonumeric wrinkle assessment scale for assessing nasolabial fold wrinkles. Plast Reconstr Surg. 2010;126:596–601. doi: 10.1097/PRS.0b013e3181de243b. [DOI] [PubMed] [Google Scholar]
- 25.Shoshani D, Markovitz E, Monstrey SJ, et al. The modified Fitzpatrick Wrinkle Scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. Dermatol Surg. 2008;34 (Suppl 1):S85–91. doi: 10.1111/j.1524-4725.2008.34248.x. discussion S91. [DOI] [PubMed] [Google Scholar]
- 26.Chen YT, Ho CT, Hsu HS, et al. Objective Palliative Prognostic Score Among Patients With Advanced Cancer. J Pain Symptom Manage. 2014 doi: 10.1016/j.jpainsymman.2014.08.017. [DOI] [PubMed] [Google Scholar]