Abstract
A wealth of experimental evidence argues that infectious prions are composed largely, if not entirely, of the scrapie isoform of the prion protein. We attempted to restore scrapie infectivity after exposure to protein denaturants including urea, chaotropic salts, and SDS. None of the procedures restored infectivity. Dialysis to remove slowly chaotropic ions and urea failed to restore scrapie infectivity. Attempts to create monomers of the scrapie isoform of the prion protein under nondenaturing conditions using a wide variety of detergents have been unsuccessful, to date, except for one report claiming that scrapie infectivity could be recovered from 12% polyacrylamide gels after SDS/PAGE [Brown, P., Liberski, P. P., Wolff, A. & Gajdusek, D. C. (1990) Proc. Natl. Acad. Sci. USA 87, 7240-7244]. We found that < 0.001% of the infectious prion titer could be recovered from the region of a polyacrylamide gel where the denatured proteinase K-resistant core of the scrapie isoform of the prion protein and other 30-kDa proteins migrate. We conclude that under the denaturing conditions used for SDS/PAGE, the scrapie isoform of the prion protein is denatured and little or no renaturation occurs upon injection of fractions eluted from gels into animals for bioassays.
Full text
PDF

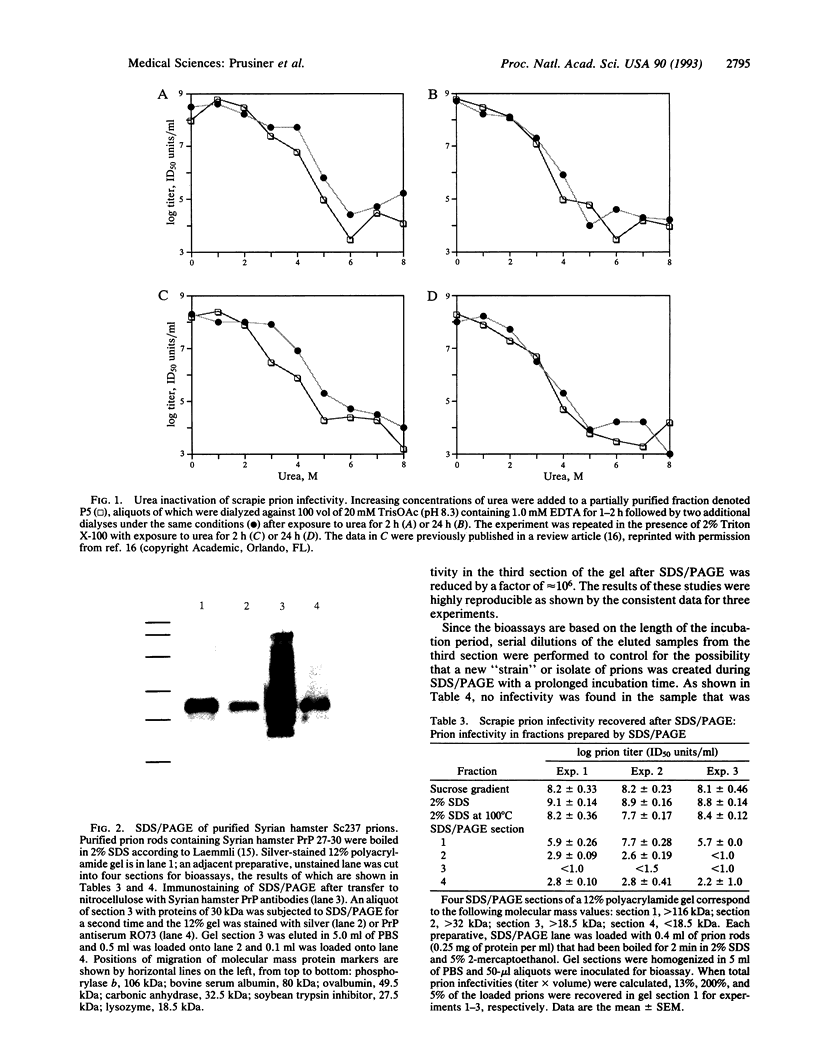


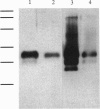
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinger-Kawahara C. G., Kempner E., Groth D., Gabizon R., Prusiner S. B. Scrapie prion liposomes and rods exhibit target sizes of 55,000 Da. Virology. 1988 Jun;164(2):537–541. doi: 10.1016/0042-6822(88)90569-7. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Liberski P. P., Wolff A., Gajdusek D. C. Conservation of infectivity in purified fibrillary extracts of scrapie-infected hamster brain after sequential enzymatic digestion or polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7240–7244. doi: 10.1073/pnas.87.18.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. R., Preece M. A., Milner R. D. Mortality, neoplasia, and Creutzfeldt-Jakob disease in patients treated with human pituitary growth hormone in the United Kingdom. BMJ. 1991 Apr 6;302(6780):824–828. doi: 10.1136/bmj.302.6780.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B. Spongiform encephalopathies. PrP and the scrapie agent. Nature. 1992 Apr 16;356(6370):560–560. doi: 10.1038/356560a0. [DOI] [PubMed] [Google Scholar]
- Czub M., Braig H. R., Diringer H. Pathogenesis of scrapie: study of the temporal development of clinical symptoms, of infectivity titres and scrapie-associated fibrils in brains of hamsters infected intraperitoneally. J Gen Virol. 1986 Sep;67(Pt 9):2005–2009. doi: 10.1099/0022-1317-67-9-2005. [DOI] [PubMed] [Google Scholar]
- Czub M., Braig H. R., Diringer H. Replication of the scrapie agent in hamsters infected intracerebrally confirms the pathogenesis of an amyloid-inducing virosis. J Gen Virol. 1988 Jul;69(Pt 7):1753–1756. doi: 10.1099/0022-1317-69-7-1753. [DOI] [PubMed] [Google Scholar]
- Diener T. O., McKinley M. P., Prusiner S. B. Viroids and prions. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5220–5224. doi: 10.1073/pnas.79.17.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradkin J. E., Schonberger L. B., Mills J. L., Gunn W. J., Piper J. M., Wysowski D. K., Thomson R., Durako S., Brown P. Creutzfeldt-Jakob disease in pituitary growth hormone recipients in the United States. JAMA. 1991 Feb 20;265(7):880–884. [PubMed] [Google Scholar]
- Gabizon R., McKinley M. P., Prusiner S. B. Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4017–4021. doi: 10.1073/pnas.84.12.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabizon R., Prusiner S. B. Prion liposomes. Biochem J. 1990 Feb 15;266(1):1–14. doi: 10.1042/bj2660001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasset M., Baldwin M. A., Fletterick R. J., Prusiner S. B. Perturbation of the secondary structure of the scrapie prion protein under conditions that alter infectivity. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):1–5. doi: 10.1073/pnas.90.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker R., Taraboulos A., Scott M., Pan K. M., Yang S. L., Torchia M., Jendroska K., DeArmond S. J., Prusiner S. B. Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 1992 Jul;6(7):1213–1228. doi: 10.1101/gad.6.7.1213. [DOI] [PubMed] [Google Scholar]
- Jendroska K., Heinzel F. P., Torchia M., Stowring L., Kretzschmar H. A., Kon A., Stern A., Prusiner S. B., DeArmond S. J. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology. 1991 Sep;41(9):1482–1490. doi: 10.1212/wnl.41.9.1482. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malone T. G., Marsh R. F., Hanson R. P., Semancik J. S. Membrane-free scrapie activity. J Virol. 1978 Mar;25(3):933–935. doi: 10.1128/jvi.25.3.933-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R. F., Kimberlin R. H. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. doi: 10.1093/infdis/131.2.104. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Malone T. G., Semancik J. S., Lancaster W. D., Hanson R. P. Evidence for an essential DNA component in the Scrapie agent. Nature. 1978 Sep 14;275(5676):146–147. doi: 10.1038/275146a0. [DOI] [PubMed] [Google Scholar]
- Pocchiari M., Peano S., Conz A., Eshkol A., Maillard F., Brown P., Gibbs C. J., Jr, Xi Y. G., Tenham-Fisher E., Macchi G. Combination ultrafiltration and 6 M urea treatment of human growth hormone effectively minimizes risk from potential Creutzfeldt-Jakob disease virus contamination. Horm Res. 1991;35(3-4):161–166. doi: 10.1159/000181894. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Chemistry and biology of prions. Biochemistry. 1992 Dec 15;31(49):12277–12288. doi: 10.1021/bi00164a001. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Cochran S. P., Groth D. F., Downey D. E., Bowman K. A., Martinez H. M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bildstein C., Masiarz F. R., McKinley M. P., Cochran S. P. Electrophoretic properties of the scrapie agent in agarose gels. Proc Natl Acad Sci U S A. 1980 May;77(5):2984–2988. doi: 10.1073/pnas.77.5.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions: novel infectious pathogens. Adv Virus Res. 1984;29:1–56. doi: 10.1016/s0065-3527(08)60404-2. [DOI] [PubMed] [Google Scholar]
- Safar J., Wang W., Padgett M. P., Ceroni M., Piccardo P., Zopf D., Gajdusek D. C., Gibbs C. J., Jr Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6373–6377. doi: 10.1073/pnas.87.16.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Taraboulos A., Jendroska K., Serban D., Yang S. L., DeArmond S. J., Prusiner S. B. Regional mapping of prion proteins in brain. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7620–7624. doi: 10.1073/pnas.89.16.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. M., Dickinson A. G., Fraser H., Robertson P. A., Salacinski P. R., Lowry P. J. Preparation of growth hormone free from contamination with unconventional slow viruses. Lancet. 1985 Aug 3;2(8449):260–262. doi: 10.1016/s0140-6736(85)90302-2. [DOI] [PubMed] [Google Scholar]
- Xi Y. G., Ingrosso L., Ladogana A., Masullo C., Pocchiari M. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature. 1992 Apr 16;356(6370):598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]
- Zhi W., Landry S. J., Gierasch L. M., Srere P. A. Renaturation of citrate synthase: influence of denaturant and folding assistants. Protein Sci. 1992 Apr;1(4):522–529. doi: 10.1002/pro.5560010407. [DOI] [PMC free article] [PubMed] [Google Scholar]