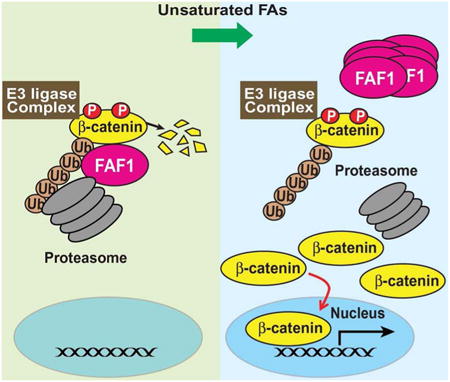
Summary
Some cancer cells exhibit elevated levels of free fatty acids (FAs) as well as high levels of β-catenin, a transcriptional co-activator that promotes their growth. Here we link these two phenomena by showing that unsaturated FAs inhibit degradation of β-catenin. Unsaturated FAs bind to the UAS domain of Fas-associated factor 1 (FAF1), a protein known to bind β-catenin, accelerating its degradation. FA binding disrupts the FAF1/β-catenin complex, preventing proteasomal degradation of ubiquitinated β-catenin. This mechanism for stabilization of β-catenin differs from that of Wnt signaling, which blocks ubiquitination of β-catenin. In clear cell renal cell carcinoma (ccRCC) cells, unsaturated FAs stimulated cell proliferation through stabilization of β-catenin. In tissues from biopsies of human ccRCC, elevated levels of unsaturated FAs correlated with increased levels of β-catenin. Thus, targeting FAF1 may be an effective approach to treat cancers that exhibit elevated FAs and β-catenin.
Graphical abstract
Introduction
Cancer cells alter their metabolism to provoke cell proliferation. One metabolic alteration in cancers is the accumulation of free fatty acids (FAs), which facilitate cell proliferation through a mechanism that remains elusive (Nomura et al., 2010). To pinpoint this mechanism, we studied FA-interacting proteins that may link free FAs to oncogenic signaling pathways. We previously identified UAS domains, which contain ∼160 amino acid residues, as the motifs that bind unsaturated but not saturated FAs (Kim et al., 2013). This domain polymerizes upon its interaction with unsaturated FAs (Kim et al., 2013). Mammalian cells express two homologous proteins that contain UAS domains (Kim et al., 2013): Ubxd8, a protein maintaining cellular FA homeostasis by stimulating degradation of Insig-1 (Ye and DeBose-Boyd, 2011), and Fas-associated factor 1 (FAF1), a protein that facilitates degradation of β-catenin (Zhang et al., 2012; Zhang et al., 2011).
Ubxd8 senses the cellular content of unsaturated FAs to regulate degradation of Insig-1, a protein that inhibits transcription of all genes required for synthesis of FAs (Kim and Ye, 2014; Ye and DeBose-Boyd, 2011). Through their direct binding to the UAS domain of Ubxd8, unsaturated FAs cause Ubxd8 to polymerize and dissociate from Insig-1 so that ubiquitinated Insig-1 cannot be delivered to proteasomes for degradation (Kim et al., 2013; Lee et al., 2010; Lee et al., 2008). Like Ubxd8, Unsaturated but not saturated FAs trigger polymerization of FAF1 upon their interaction with the UAS domain of the protein (Kim et al., 2013). The functional significance of the interaction between unsaturated FAs and FAF1 remains unknown.
FAF1 has been reported to be required for degradation of β-catenin (Zhang et al., 2012; Zhang et al., 2011), a transcriptional co-activator that stimulates expression of genes driving cell proliferation (Anastas and Moon, 2013). In normal cells the degradation of β-catenin is regulated by Wnt signaling: β-catenin is constitutively phosphorylated by the β-catenin destruction complex, which marks β-catenin for ubiquitination followed by rapid proteasomal degradation (Clevers and Nusse, 2012; Moon et al., 2002); Wnt signaling inactivates the β-catenin destruction complex, thereby inhibiting phosphorylation of β-catenin and consequently ubiquitination and degradation of the protein (Clevers and Nusse, 2012; Moon et al., 2002). Mutations that inactivate proteins required for degradation of β-catenin lead to various cancers as a result of aberrant accumulation of β-catenin (Clevers, 2006). However, some cancer cells contain elevated levels of β-catenin in the absence of these mutations (Barker and Clevers, 2006). Based on our previous observations with Ubxd8, we hypothesized that unsaturated FAs may bind to the UAS domain of FAF1, leading to inactivation of FAF1 and consequently stabilization of β-catenin.
In the current study, we report that unsaturated FAs indeed inhibit degradation of β-catenin by inactivating FAF1. We demonstrate the clinical significance of these findings by providing evidence that excess unsaturated FAs stabilize β-catenin in clear cell renal cell carcinoma (ccRCC), which represents a majority of kidney cancers (Li and Kaelin Jr, 2011). These results suggest that compounds blocking the interaction between FAF1 and unsaturated FAs may be useful in treating cancers whose proliferation is provoked by unsaturated FA-mediated stabilization of β-catenin.
Results
Unsaturated FAs inhibit degradation of β-catenin through their interaction with FAF1
We first used SRD-13A cells, a line of mutant CHO cells, to determine whether unsaturated FAs inhibit degradation of β-catenin. These cells are auxotrophic for FAs, and consequently their content of FAs can be controlled easily by the amount of FAs added into the culture medium (Rawson et al., 1999). We pre-incubated the cells in FA-depleted medium and then supplemented the medium with various FAs. The effect of these FAs on levels of β-catenin was determined by immunoblot analysis. As a positive control, we treated the cells with Wnt3a to block β-catenin degradation. β-Catenin was barely detectable in cells cultured in the absence of FAs (Figure 1A, lane 1). Addition of palmitate (C16:0), a saturated FA, did not raise the amount of β-catenin (Figure 1A, lane 2). However, oleate (C18:1) and other unsaturated FAs markedly increased the levels of β-catenin (Figure 1A, lanes 3-8), reaching levels that were comparable to those in cells treated with Wnt3a (Figure 1A, lane 9). In a pulse-chase experiment, we demonstrated that oleate increased the amount of β-catenin by inhibiting degradation of the protein (Figure 1B).
Figure 1. Unsaturated FAs stabilize β-catenin through inactivating FAF1.
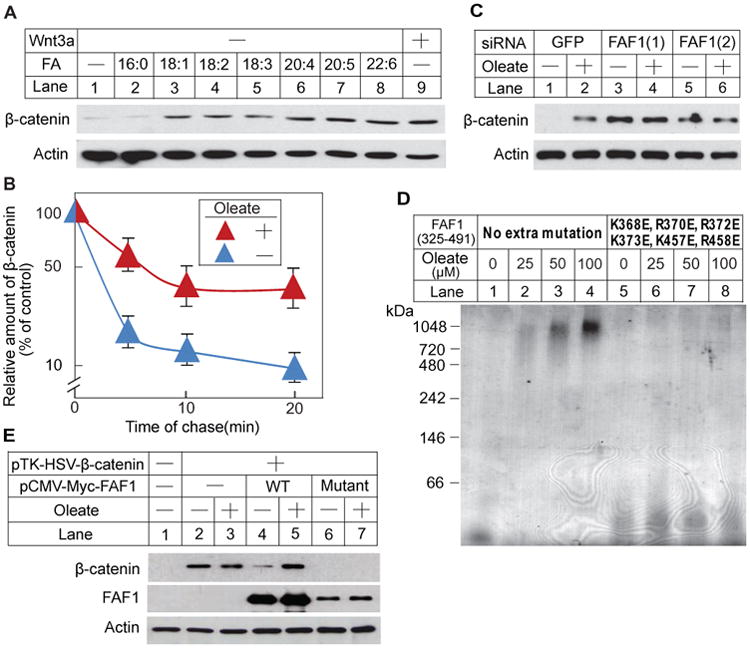
(A) SRD-13A cells were seeded at 3.5 × 105/60-mm dish on day 0. On day 1, cells were depleted of FAs by incubation in medium A supplemented with 5% DFCS, 5 μg/ml cholesterol, 1 mM sodium mevalonate for 14 h. On day 2, cells were switched to medium A supplemented with 0.5% DFCS, 5 μg/ml cholesterol, 1 mM sodium mevalonate in the absence or presence of the indicated FAs (100 μM) or mouse Wnt3a (5 ng/ml). Following incubation for 6 h, cells were harvested. Cell lysate was subjected to SDS-PAGE followed by immunoblot analysis.
(B) SRD-13A cells seeded as described in (A) were subjected to pulse-chase analysis as described in Experimental Procedures. Results are reported as means ± S.E. from three independent experiments.
(C) SRD-13A cells were seeded at 1.5 × 105/60-mm dish on day 0. On day 1, cells were transfected with 20 μM of the indicated siRNA. On day 3 and 4, cells were depleted of FAs and then treated with oleate as described in A. Cell lysate was subjected to SDS-PAGE followed by immunoblot analysis.
(D) Indicated purified proteins were incubated with oleate added as stock solutions dissolved in ethanol, subjected to BN-PAGE, and visualized with Coomassie blue staining.
(E) SRD-13A cells were seeded as described in (A). On day 1, cells were transfected with 1μg/dish pTK-HSV-β-catenin, 0.3 μg/dish pCMV-Myc-FAF1(WT) or 0.6 μg/dish pCMV-Myc-FAF1(Mutant). Following incubation for 8 h, cells were depleted of FAs and then treated with or without oleate on day 2 as described in A. Cell lysate was subjected to SDS-PAGE followed by immunoblot analysis.
If unsaturated FAs stabilize β-catenin through inactivation of FAF1, then knockdown of FAF1 should increase the amount of β-catenin regardless of the presence of unsaturated FAs. To test this hypothesis, we transfected SRD-13A cells with a control siRNA or siRNAs targeting FAF1. Oleate markedly raised the amount of β-catenin in cells transfected with the control siRNA (Figure 1C, lanes 1 and 2). Transfection of the cells with two siRNAs targeting different regions of FAF1 reduced its mRNA by 70-80% (Figure S1A) and raised the amount of β-catenin in cells cultured in the absence of FAs (Figure 1C, lanes 3 and 5). In the absence of FAF1 oleate did not further increase the amount of β-catenin (Figure 1C, lanes 4 and 6).
Another way to examine the role of FAF1 in FA-regulated degradation of FAF1 is to generate a mutant version of FAF1 that does not bind unsaturated FAs. Since the mutant FAF1 may not be inactivated by unsaturated FAs, degradation of β-catenin should not be inhibited by unsaturated FAs in cells expressing the mutant FAF1. Our previous structural analysis of the UAS domain of FAF1, which directly binds unsaturated FAs, identified a surface patch that is highly enriched in positively charged amino acid residues (Kim et al., 2013). We demonstrated that these positively charged residues are conserved in the UAS domain of Ubxd8 and that substitution of these residues with glutamate abolished the interaction between Ubxd8 and unsaturated FAs (Kim et al., 2013). We thus expressed and purified a mutant variant of the UAS domain of FAF1 (FAF1 325-491) with the corresponding amino acid substitutions (K368E, R370E, R372E, K373E, K457E, R458E). The interaction between oleate and the FAF1 UAS domain was detected by oleate-induced polymerization of the protein as revealed by blue native polyacrylamide gel electrophoresis (BN-PAGE) (Kim et al., 2013; Lee et al., 2010). Oleate induced the polymerization of the wild-type UAS domain (Figure 1D, lanes 1-4), but not the mutant UAS domain (Figure 1D, lanes 5-8). This observation indicates that the mutant UAS domain of FAF1 does not interact with unsaturated FAs. Next, we transfected SRD-13A cells with plasmids encoding β-catenin and wild-type FAF1 or mutant FAF1 with the mutations in the UAS domain described above. When β-catenin was overexpressed by transfection, the protein was present even in the absence of FAs, and there was no further stabilization by oleate (Figure 1E, lanes 2 and 3). Cotransfection of wild type FAF1 reduced the amount of β-catenin in FA-depleted cells, and this reduction was blocked by oleate (Figure 1E, lanes 4 and 5). This result suggests that FAF1 is a limiting factor for degradation of β-catenin when it is overexpressed. Cotransfection with a plasmid encoding mutant FAF1 reduced the amount of β-catenin, but this reduction was no longer reversed by addition of oleate (Figure 1E, lanes 6 and 7). Collectively, these findings suggest that FAF1 accelerates β-catenin degradation and that the interaction between unsaturated FAs and the UAS domain of FAF1 is required for these FAs to inhibit degradation of β-catenin.
The effect of unsaturated FAs on degradation of β-catenin is not restricted to SRD-13A cells. Unsaturated but not saturated FAs also stabilized the protein in HEK-293 cells that are not auxotrophic for FAs (Figure S1B). A difference between this experiment and that performed in SRD-13A cells is that in addition to incubating cells in FA-free medium, we treated HEK-293 cells with A939572, an inhibitor of stearoyl-CoA desaturase-1 (SCD1), which catalyzes the rate-limiting step in biosynthesis of unsaturated FAs (Paton and Ntambi, 2010), to deplete the cells of unsaturated FAs.
Unsaturated FAs stabilize β-catenin through a mechanism different from Wnt signaling
Since HEK-293 cells have been used to study Wnt-regulated degradation of β-catenin (Li et al., 2012), we used these cells to compare unsaturated FA versus Wnt-mediated stabilization of β-catenin. We first determined the effect of unsaturated FAs on phosphorylation of β-catenin. Both oleate and Wnt3a increased the amount of β-catenin in HEK-293 cells cultured in FA-depleted medium (Figure 2A, panel 4). To analyze phosphorylation of β-catenin, we treated the cells with a proteasome inhibitor MG132 to prevent the degradation of the phosphorylated protein (Figure 2A, panel 2). While Wnt3a inhibited phosphorylation of β-catenin (Figure 2A, panel 1, lane 3), oleate increased the amount of phosphorylated β-catenin (Figure 2A, panel 1, lane 2). To determine the impact of these treatments on ubiquitination of β-catenin, we immunoprecipitated β-catenin from lysates of cells treated with MG132, and measured ubiquitinated β-catenin as detected by high molecular weight smears in immunoblot analysis of the immunoprecipitates with anti-β-catenin and anti-ubiquitin. Ubiquitinated β-catenin was observed in untreated cells (Figure 2B, lane 1). In contrast to Wnt3a, which reduced the level of ubiquitinated β-catenin (Figure 2B, lane 3), oleate increased the amount of ubiquitinated β-catenin (Figure 2B, lane 2). The results of Wnt treatment are consistent with previous observations that Wnt inhibits phosphorylation and ubiquitination of β-catenin. In contrast, unsaturated FAs prevent the degradation of ubiquitinated β-catenin.
Figure 2. Unsaturated FAs inhibit degradation of β-catenin at a post-ubiquitination step.
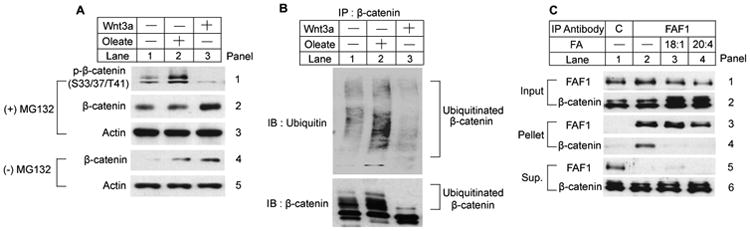
HEK-293 cells were seeded at 4.0 × 105/60-mm dish on day 0. On day 2, cells were incubated in medium B supplemented with 10% DFCS and 1 μM A939572 for 16 h. On day 3, cells were switched to serum-free medium B supplemented with 1 μM A939572 and treated with 100 μM oleate, 40 ng/ml human Wnt3a or 10 μM MG132 as indicated for 6 h.
(A) Cell lysate was subjected to SDS-PAGE followed by immunoblot analysis.
(B) β-Catenin was immunoprecipitated from lysate of the cells treated with MG132, and the immunoprecipitates were subjected to immunoblot analysis.
(C) Cytosolic fractions of the cells treated with MG132 were subjected to immunoprecipitation with a control antibody (C) or anti-FAF1. Aliquots of the cytosol fraction before the immunoprecipitation (input) and the pellet and supernatant fractions of the immunoprecipitation were loaded at a ratio of 1:10:1 on SDS-PAGE followed by immunoblot analysis.
We next investigated whether unsaturated FAs disrupted the FAF1/β-catenin complex that is known to be required for degradation of β-catenin (Zhang et al., 2012). For this purpose, we treated HEK-293 cells with MG132 to prevent proteasomal degradation of β-catenin. We immunoprecipitated FAF1 and determined the amount of β-catenin that was co-precipitated. β-Catenin was co-immunoprecipitated with FAF1 in FA-depleted cells (Figure 2C, panel 4, lane 2), but not in cells treated with oleate or arachidonate (C20:4), another unsaturated FA (Figure 2C, panel 4, lanes 3 and 4).
Stabilization of β-catenin by unsaturated FAs is required for proliferation of ccRCC cells
ccRCC is characterized by excess lipid accumulation owing to increased synthesis of FAs and cholesterol (Drabkin and Gemmill, 2010; Li and Kaelin Jr, 2011). The increased synthesis of unsaturated FAs appears to be important for proliferation of ccRCC cells, as growth of mouse xenograft tumors was inhibited by treatment with A939572, an inhibitor of SCD1 required for synthesis of unsaturated FAs (von Roemeling et al., 2013). A recent study also reported increased β-catenin levels as a predictor of poor clinical outcomes in ccRCC patients (Krabbe et al., 2013). We thus hypothesized that accumulation of excess unsaturated FAs in ccRCC tumor cells may promote their proliferation through stabilization of β-catenin.
To test this hypothesis, we studied the impact of unsaturated FAs on β-catenin levels in the ccRCC cell line SW156. These cells contain much more triglyceride, cholesterol and free unsaturated FAs than HEK-293 cells (Figures S2A-C). Unlike SRD-13A cells in which β-catenin is not associated with membranes, in SW156 cells a significant fraction of β-catenin is membrane-associated (Figure S2D). This pool of β-catenin is known to be required for cell adhesion but not for stimulation of cell proliferation, and it is not subjected to rapid degradation (Fagotto, 2013). We therefore assessed the effect of FAs on stabilization of cytosolic β-catenin. As observed in SRD-13A cells, we found that addition of unsaturated but not saturated FAs stabilized cytosolic β-catenin in SW156 cells (Figure 3A). We then determined the effect of unsaturated FAs on expression of cyclin D1, a target gene of β-catenin that drives cell proliferation (Shtutman et al., 1999; Tetsu and McCormick, 1999). We found that unsaturated but not saturated FAs caused a marked increase in cyclin D1 mRNA expression (Figure 3B). To confirm that the increased expression of cyclin D1 was caused by elevated β-catenin, we treated the cells with NCX4040, which inhibits the transcriptional co-activating activity of β-catenin (Nath et al., 2003). Treatment with NCX4040 completely abolished oleate-induced expression of cyclin D1 mRNA (Figure 3C).
Figure 3. Excess unsaturated FAs promote proliferation of SW156 through stabilization of β-catenin.
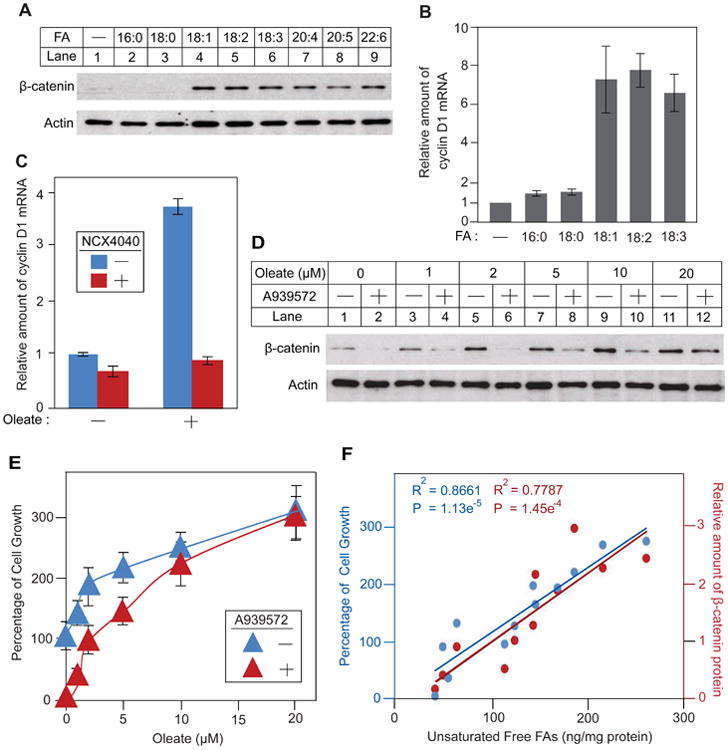
(A) SW156 cells were seeded at 3.5 × 105/60-mm dish on day 0. On day 1, cells were depleted of FAs by incubation in medium C supplemented with 10% DFCS and 1 μM A939572 for 24 h. On day 2, cells were switched to serum-free medium C supplemented with 1 μM A939572 and treated with 100 μM of the indicated FA for 6 h. Cytosolic fractions were subjected to SDS-PAGE followed by immunoblot analysis.
(B and C) SW156 cells were seeded on day 0 and depleted of FAs on day 1 as described in A. On day 2, cells were switched to serum-free medium C supplemented with 1 μM A939572 and treated with 100 μM of the indicated FA in the absence or presence of 10 μM NCX4040 for 9 h. The amount of cyclin D1 mRNA was determined by RT-QPCR with the value in untreated FA-depleted cells set at 1. Results are reported as means ± S.E. from three independent experiments.
(D) SW156 cells were seeded at 1.0 × 105/60-mm dish on day 0. On day 1, cells were switched to medium C supplemented with 10% DFCS and the indicated concentration of oleate in the absence or presence of 1 μM A939572. On day 3, after incubation for 60 h, cytosolic fractions of the cells were subjected to immunoblot analysis.
(E) SW156 cells were seeded at 800/well in a 96-well plate on day 0. On day 1, cell number was determined in some wells of the cells. The rest of the cells were treated the same as described in D. On day 3, after incubation for 60 h, the cell number in each well was determined, and the percentage increase in the cell number compared to that in day 1 was presented. Results are reported as means ± S.E. from three independent experiments.
(F) Densitometry quantification of β-catenin protein in D (red) and relative cell growth observed in E (blue) were plotted against intracellular concentration of unsaturated FAs measured by mass spectroscopy in all of the treatment conditions in D and E.
We next analyzed the effect of unsaturated FA-mediated stabilization of β-catenin on cell proliferation. We incubated SW156 cells in FA-depleted medium in the absence or presence of the SCD1 inhibitor A939572, and then treated the cells with various concentrations of oleate. In the absence of exogenous oleate, a small amount of β-catenin was detected in cells incubated in the absence of A939572 (Figure 3D, lane 1). The protein disappeared upon treatment with A939572 (Figure 3D, lane 2). In parallel with the amount of β-catenin, the cells proliferated in the absence but not in the presence of A939572 (Figure 3E, oleate at 0 μM). Addition of exogenous oleate increased the amount of β-catenin in cells treated with or without A939572 (Figure 3D, lanes 3-12). At 20 μM the added oleate raised the amount of β-catenin in cells treated with A939572 to the same level as that in cells incubated in the absence of the inhibitor (Figure 3D, lanes 11 and 12). In parallel with the amount of β-catenin, exogenous oleate increased the proliferation rate of the cells treated with or without A939572 (Figure 3E). At 20 μM of added oleate, cell proliferation rate was the same in the presence or absence of A939572 (Figure 3E). To make a more direct comparison, we used mass spectroscopy to measure the amount of free unsaturated FAs in cells subjected to all of these treatments. We plotted the amount of β-catenin and the rate of cell growth against the content of unsaturated FAs. The amount of β-catenin (red) and the rate of cell growth (blue) were positively correlated with the intracellular content of unsaturated FAs, and the linear fitting of both data sets was almost superimposable (Figure 3F). These results strongly suggest that unsaturated FA-mediated stabilization of β-catenin is responsible for cell proliferation.
In addition to SW156 cells, we observed accumulation of lipids including unsaturated FAs in 786-O cells, another line of ccRCC cells (Figures S2A-C). In these cells unsaturated but not saturated FAs also stabilized β-catenin (Figure S2E) in a FAF1-dependent manner, as β-catenin was stabilized regardless of the presence of the FAs in the cells in which FAF1 was knocked down by the transfected siRNA (Fig. S2F). Similar to SW156 cells, the stabilization of β-catenin in 786-O cells was correlated to cell proliferation (Figure S2G).
Excess unsaturated FAs increase oncogenic activity of β-catenin in specimens of ccRCC tumors
To further substantiate the clinical relevance of our findings, we analyzed the correlation between unsaturated FAs and the intracellular distribution of β-catenin in biopsies exercised from patients with ccRCC. We determined the subcellular localization of β-catenin in 25 tumors by immunohistochemistry. While most tumor cells showed β-catenin staining on plasma membranes (Figure 4A), a few of them, for example tumor #22383, had intracellular staining of the protein (Figure 4B). The strong intracellular staining of β-catenin (> 60%) was only observed in grade 4 ccRCC cells, which constitute the most aggressive form of the tumors (Figure 4C, red bars, #21886, #22383, #24152, and #21470). We then used mass spectroscopy to measure free unsaturated FAs in all of the 25 tumors and found that only these four tumors accumulated higher levels of the FAs compared to their benign controls (Figure 4C, blue bars, #21886, #22383, #24152 and #21470). Other tumors had no or low intracellular staining of β-catenin (< 35%) (Figure 4C, red bars), and most of them contained lower levels of unsaturated FAs compared to their benign controls (Figure 4C, blue bars). The probability of the correlation between increased intracellular distribution of β-catenin and increased levels of unsaturated FAs in tumor cells occurring by chance is 10-5 as determined by Pearson analysis. These results suggest that in ccRCC increased levels of unsaturated FAs stabilize intracellular β-catenin.
Figure 4. Increased levels of unsaturated FAs is correlated to elevated levels of intracellular β-catenin in ccRCC patient specimens.
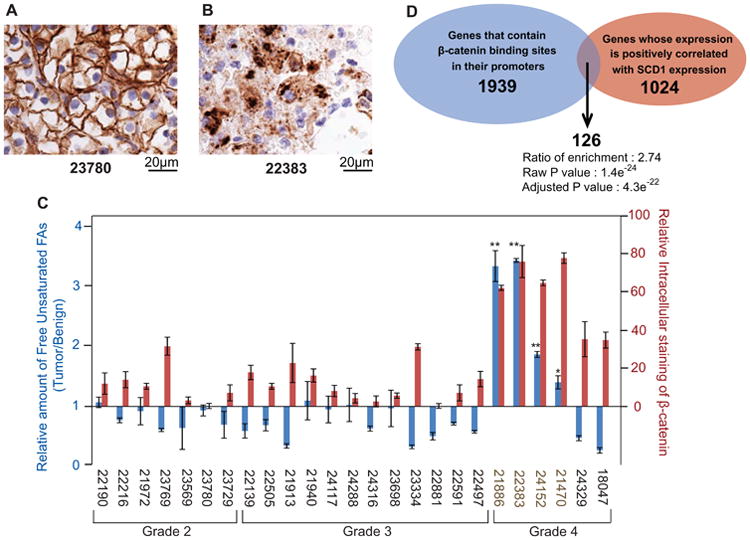
(A and B) Immunohistochemistry staining of β-catenin in the indicated ccRCC patient specimen was performed as described in Experimental Procedures.
(C) The relative amount of unsaturated FAs in tumors was measured through mass spectroscopy analysis, with the value in their benign controls set at 1 (blue bars). The relative intracellular staining of β-catenin in ccRCC patient specimens was determined as described in Experimental Procedures (red bars). The pathological grade of the tumors is indicated. The sample numbers for the tumors with higher levels of intracellular staining of β-catenin and unsaturated FAs are highlighted in brown. The results are reported as means ± S.E. from three independent measurements. Paired student t-test was performed to determine the statistical significance of the increase in the amount of unsaturated fatty acids in the highlighted tumors compared to their benign controls: *p = 0.02; ** p < 0.01.
(D) Venn diagram displaying overlap in putative target genes of β-catenin and genes whose expression is positively correlated with SCD1 expression. Adjusted p = 4.3 × 10-22.
We then performed gene expression analyses using RNA-Seq data from 532 human ccRCC samples generated by The Cancer Genome Atlas project. Since SCD1 catalyzes the rate-limiting step in the biosynthesis of unsaturated FAs, tumors with higher expression of the gene are expected to produce more unsaturated FAs. Thus, if unsaturated FAs stabilize β-catenin, tumors with higher expression of SCD1 should also express higher levels of β-catenin target genes. We identified 1024 genes as the top 5% of genes whose expression is positively correlated with SCD1 expression (Table S1). When we analyzed these genes for enrichment in functional annotation categories, we found enrichment in biological processes that are relevant to cell proliferation and cancer signaling (Figure S3). When we analyzed transcription factor motifs in the promoter regions of these genes we found enrichment for a LEF1 motif, which is typical for the gene-specific transcription factors that use β-catenin as a co-activator (Arce et al., 2006; Schuijers et al., 2014). The LEF1 motif was the second most significantly enriched motif in promoters for genes whose expression is positively correlated with SCD1 expression (adjusted p=4.3e-22). Out of the 1939 human genes with this motif in their promoter regions, 126 genes were found in the set of 1024 genes whose expression was positively correlated with SCD1 expression (Figure 4D and Table S1). This number is 2.74 times higher than expected from random overlap between the two gene sets. These analyses suggest that the potential target genes of β-catenin are overexpressed in ccRCC cells that express high levels of SCD1 mRNA.
Discussion
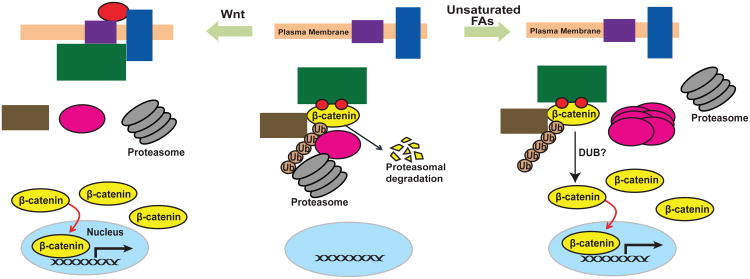
The current study supports a model shown in Figure 5, illustrating how unsaturated FAs and Wnt independently inhibit proteasomal degradation of β-catenin. Previous studies have demonstrated that β-catenin is constitutively phosphorylated by the β-catenin destruction complex, which marks β-catenin for ubiquitination by a β-Trcp-containing E3 ubiquitin ligase complex (Clevers and Nusse, 2012; Moon et al., 2002). The ubiquitinated β-catenin may then be targeted by FAF1 to proteasomes for degradation (Figure 5, middle panel). Activation of Wnt signaling recruits the destruction complex to plasma membranes, thereby preventing phosphorylation and ubiquitination of β-catenin, and resulting in stabilization of the protein (Clevers and Nusse, 2012; Moon et al., 2002) (Figure 5, left panel). In contrast to Wnt signaling, unsaturated FAs do not affect phosphorylation or ubiquitination of β-catenin. Instead, the FAs disrupt the FAF1/β-catenin complex by triggering polymerization of FAF1. Consequently, ubiquitinated β-catenin is not targeted to proteasomes for degradation, thereby stabilizing β-catenin (Figure 5, right panel).
Figure 5. Model illustrating that unsaturated FAs and Wnt stabilize β-catenin via different mechanism.
In the absence of Wnt and unsaturated fatty acids, β-catenin is phosphorylated by the β-catenin destruction complex, a reaction marking the protein for ubiquitination followed by rapid degradation by proteasomes. Activation of Wnt signaling stabilizes β-catenin by inhibiting phosphorylation and ubiquitination of the protein through recruiting the destruction complex to plasma membranes. In contrast to Wnt, unsaturated FAs do not affect phosphorylation or ubiquitination of β-catenin. Instead, the FAs trigger polymerization of FAF1, causing dissociation of the protein from β-catenin. As a result, β-catenin is stabilized as ubiquitinated β-catenin is not delivered to proteasomes for degradation. In the absence of proteasomal degradation, polyubiquitin chains on β-catenin may be cleaved off by reactions catalyzed by deubiquitinating enzymes (DUB), allowing β-catenin to activate its target genes in nucleus.
Exactly how FAF1 targets ubiquitinated β-catenin to proteasomes for degradation remains unclear. A previous study reported that recognition of ubiquitinated Insig-1 by proteasomes required recruitment of p97 to Insig-1, a reaction mediated by Ubxd8 that is a homolog of FAF1 (Ikeda et al., 2009). Inasmuch as FAF1 also binds p97 (Ewens et al., 2014), the mechanism through which FAF1 facilitates degradation of ubiquitinated β-catenin may be similar to that through which Ubxd8 stimulates degradation of ubiquitinated Insig-1.
The strongest evidence indicating that unsaturated FAs inhibit degradation of β-catenin through a mechanism different from Wnt signaling comes from the observations that in contrast to activation of Wnt signaling, these FAs do not affect phosphorylation or ubiquitination of β-catenin. This mechanism is different from an earlier report showing the correlation between FA synthesis and stabilization of β-catenin: In that study FA synthesis was shown to be required for palmitoylation of Wnt (Fiorentino et al., 2008), a post-translational modification of Wnt critical for its signaling function. Instead of inhibiting ubiquitination of β-catenin, unsaturated FAs prevent degradation of ubiquitinated β-catenin. In the absence of proteasomal degradation, the polyubiquitin chains on β-catenin may be removed from the protein by deubiquitinating enzymes. The presence of the strong deubiquitination activity in mammalian cells may explain why the effect of unsaturated FAs on stabilization of nonubiquitinated β-catenin is more pronounced than that of ubiquitinated β-catenin.
The current study establishes that accumulation of unsaturated FAs can act as an oncogenic mechanism to increase β-catenin levels, which is different from the well-established mechanism caused by genetic inactivation of proteins in Wnt signaling. We determined that accumulation of excess unsaturated FAs was responsible for stabilization of β-catenin in some ccRCC tumors. We observed that aberrant stabilization of β-catenin in several grade 4 ccRCC tumors, the most aggressive form of the cancer, was correlated to their increased levels of unsaturated FAs. Through bioinformatics analysis we showed that the potential target genes of β-catenin were overexpressed in ccRCC cells that express high levels of SCD1 mRNA, which encodes the enzyme catalyzing the rate-liming step in synthesis of unsaturated FAs. These genes include Cyclin D1, met proto-oncogene and matrix metallopeptidase 14 that have been previously determined to be the target gene of β-catenin (Herbst et al., 2014; Shtutman et al., 1999; Tetsu and McCormick, 1999). Among these genes we showed that Cyclin D1 was activated by β-catenin in ccRCC cells. In addition to ccRCC, it will be interesting to determine whether accumulation of unsaturated FAs is also responsible for aberrant stabilization of β-catenin in other cancers that do not contain genetic mutations affecting the Wnt pathway.
Our finding provides mechanistic insights into the observations that accumulation of free FAs facilitates development and progression of certain cancers (Nomura et al., 2011; Nomura et al., 2010). Similar to ccRCC, these cancer cells may acquire FAs through enhancing their endogenous synthesis. They may also obtain FAs from plasma, which may explain why obesity, a condition associated with increased amount of free FAs in circulation, is a risk factor for cancer development (Park et al., 2014).
The current study further demonstrates the critical roles played by the UAS domain in transmitting signals elicited by unsaturated FAs. We have shown previously that binding of unsaturated FAs to the UAS domain of Ubxd8 is crucial for feedback inhibition of FA synthesis through inhibiting degradation of Insig-1 (Ye and DeBose-Boyd, 2011). In the current study, we show that binding of unsaturated FAs to the UAS domain of FAF1 is required for these FAs to inhibit degradation of β-catenin. These results suggest that compounds blocking the interaction between unsaturated FAs and the UAS domain of FAF1 should destabilize β-catenin. It will be interesting to determine whether the UAS domain of FAF1 could be targeted by drugs to treat cancers whose proliferation dependent on unsaturated FA-mediated stabilization of β-catenin.
Experimental Procedures
Measurement of lipids
Free FAs from approximately 0.5 million cells or 5 mg of tumors were quantified using gas chromatography – electron capture negative ionizacion – mass spectrometry as previously described (Morselli et al., 2014; Quehenberger et al., 2011). Triglycerides and free cholesterol were isolated from lipid extracts using Biotage isolute® NH2 SPE cartridges according to the instruction of the manufacturer. The amount of triglycerides was determined by the amount of free fatty acids released from triglycerides following saponification as previously described (Quehenberger et al., 2011). The amount of cholesterol was determined by Infinity ™ Cholesterol according to the instruction of the manufacturer.
Immunohistochemistry
Immunohistochemistry staining for β-catenin in ccRCC tumors was performed exactly as previously described (Krabbe et al., 2013). Intensity of intracellular (Ii) and membrane staining (Im) of β-catenin as well as percentage of the cells showing intracellular (Pi) and membrane staining (Pm) of the protein was determined. Relative intracellular staining of β-catenin was calculated as Ii × Pi / (Ii × Pi + Im × Pm) × 100%.
Supplementary Material
Highlights.
Unsaturated fatty acids (uFAs) inhibit β-catenin degradation by inactivating FAF1.
β-Catenin degradation can be independently inhibited by Wnt signaling and uFAs.
uFAs promote growth of kidney cancers by stabilization of β-catenin.
Acknowledgments
We thank Drs. Joseph Goldstein and Michael Brown for constant advices and critical evaluations of our manuscript; Lisa Beatty, Ijeoma Dukes, Muleya Kapaale, and Hue Dao for help with tissue culture; Jeff Cormier for RT-QPCR; and Saada Abdalla for technical assistance. This work was supported by grants from the National Institutes of Health (HL-20948, CA-154475) and Welch Foundation (I-1832). C.R-N. is supported by Clayton Foundation for Research.
Footnotes
Supplemental Information: Supplemental information including supplemental experimental procedures can be found with this article online at ____.
Author Contribution: J. Y. and H. K. designed and orchestrated the entire study and wrote the manuscript. H. K. and C. R-N. performed the experiments; R. K. K. and R. K. performed bioinformatics and statistical analysis; P. K. performed pathological analysis; J. B. and I. P. are involved in design of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF//TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-Catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Drabkin HA, Gemmill RM. Obesity, cholesterol, and clear-cell renal cell carcinoma (RCC) Adv Cancer Res. 2010;107:39–56. doi: 10.1016/S0065-230X(10)07002-8. [DOI] [PubMed] [Google Scholar]
- Ewens CA, Panico S, Kloppsteck P, McKeown C, Ebong IO, Robinson C, Zhang X, Freemont PS. The p97-FAF1 protein complex reveals a common mode of p97 adaptor binding. J Biol Chem. 2014;289:12077–12084. doi: 10.1074/jbc.M114.559591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F. Looking beyond the Wnt pathway for the deep nature of β-catenin. EMBO Report. 2013;14:422–433. doi: 10.1038/embor.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, Nguyen PL, Migita T, Zamponi R, Di Vizio D, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of β-catenin in prostate cancer. Lab Invest. 2008;88:1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Jurinovic V, Krebs S, Thieme S, Blum H, Goke B, Kolligs F. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/beta-catenin signaling. BMC Genomics. 2014;15:74. doi: 10.1186/1471-2164-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, DeMartino GN, Brown MS, Lee JN, Goldstein JL, Ye J. Regulated endoplasmic reticulum-associated degradation of a polytopic protein: p97 recruits proteasomes to Insig-1 before extraction from membranes. J Biol Chem. 2009;284:34889–34900. doi: 10.1074/jbc.M109.044875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ye J. Cellular responses to excess fatty acids: focus on ubiquitin regulatory X domain-containing protein 8. Curr opin Lipidol. 2014;25:118–124. doi: 10.1097/MOL.0000000000000048. [DOI] [PubMed] [Google Scholar]
- Kim H, Zhang H, Meng D, Russell G, Lee JN, Ye J. UAS domain of Ubxd8 and FAF1 polymerizes upon interaction with long-chain unsaturated fatty acids. J Lipid Res. 2013;54:2144–2152. doi: 10.1194/jlr.M037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe LM, Westerman ME, Bagrodia A, Gayed BA, Darwish OM, Haddad AQ, Khalil D, Kapur P, Sagalowsky AI, Lotan Y, et al. Dysregulation of β-Catenin is an independent predictor of oncologic outcomes in patients with clear cell renal cell carcinoma. J Urol. 2013 doi: 10.1016/j.juro.2013.11.052. 10.1016/j.juro.2013.1011.1052. [DOI] [PubMed] [Google Scholar]
- Lee JN, Kim H, Yao H, Chen Y, Weng K, Ye J. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc Natl Acad Sci USA. 2010;107:21424–21429. doi: 10.1073/pnas.1011859107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JN, Zhang X, Feramisco JD, Gong Y, Ye J. Unsaturated fatty acids inhibit proteasomal degradation of Insig-1 at a postubiquitination step. J Biol Chem. 2008;283:33772–33783. doi: 10.1074/jbc.M806108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kaelin WG., Jr New insights into the biology of renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:667–686. doi: 10.1016/j.hoc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Vivian SW, Ng Ser S, Boersema Paul J, Low Teck Y, Karthaus Wouter R, Gerlach Jan P, Mohammed S, Heck Albert JR, Maurice Madelon M, Mahmoudi T, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas Carlos R, Gordillo R, Neinast M, Kalainayakan Sarada P, et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014;9:633–645. doi: 10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath N, Kashfi K, Chen J, Rigas B. Nitric oxide-donating aspirin inhibits β-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear β-catenin–TCF association. Proc Natl Acad Sci USA. 2003;100:12584–12589. doi: 10.1073/pnas.2134840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Lombardi DP, Chang JW, Niessen S, Ward AM, Long JZ, Hoover HH, Cravatt BF. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer -mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton CM, Ntambi JM. Loss of stearoyl-CoA desaturase activity leads to free cholesterol synthesis through increased Xbp-1 splicing. Am J Physiol Endocrinol Metab. 2010;299:E1066–E1075. doi: 10.1152/ajpendo.00388.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Dennis EA. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography–mass spectrometry. Biochim Biophys Acta. 2011;1811:648–656. doi: 10.1016/j.bbalip.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RB, DeBose-Boyd R, Goldstein JL, Brown MS. Failure to cleave sterol regulatory element-binding proteins (SREBPs) causes cholesterol auxotrophy in Chinese hamster ovary cells with genetic absence of SREBP cleavage-activating protein. J Biol Chem. 1999;274:28549–28556. doi: 10.1074/jbc.274.40.28549. [DOI] [PubMed] [Google Scholar]
- Schuijers J, Mokry M, Hatzis P, Cuppen E, Clevers H. Wnt-induced transcriptional activation is exclusively mediated by TCF/LEF. EMBO J. 2014;33:146–156. doi: 10.1002/embj.201385358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, Tan WW, Tun HW, Copland JA. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013;19:2368–2380. doi: 10.1158/1078-0432.CCR-12-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004754. 10.1101/cshperspect.a004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Li Y, Drabsch Y, Zhang J, van Dam H, ten Dijke P. Fas-associated factor 1 is a scaffold protein that promotes β-transducin repeat-containing protein (β-TrCP)-mediated β-catenin ubiquitination and degradation. J Biol Chem. 2012;287:30701–30710. doi: 10.1074/jbc.M112.353524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou F, van Laar T, Zhang J, van Dam H, ten Dijke P. Fas-associated factor 1 antagonizes Wnt signaling by promoting β-catenin degradation. Mol Biol Cell. 2011;22:1617–1624. doi: 10.1091/mbc.E10-12-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.