Abstract
Chronic hepatitis B virus (HBV) infection in endemic areas usually starts since infancy and early childhood and persists lifelong. The clinical course varies among different chronic infected subjects. Majority of chronic HBV infected children present with immune-tolerant status initially, experience the immune clearance phase with various degree of liver injury during or beyond puberty, and then enter the inactive phase after hepatitis B e antigen (HBeAg) seroconversion. Part of them may have HBV DNA titers elevation with hepatitis flare after HBeAg seroconversion, the so call HBeAg-negative hepatitis flare. Liver cirrhosis, and even hepatocellular carcinoma may develop afterward.
The complex course of chronic HBV infection is associated with the age/route of viral acquisition, host factors such as immune and endocrine factors, viral factors, and host-viral interactions. The adrenarche and puberty onset modulate the start of immune clearance and the severity of liver inflammation in chronic HBV infected children. The genotype and phenotype of human cytokines, innate immunity, and human leukocyte antigens are also associated with the onset of immune clearance of HBV and severity of inflammation. Immune escape HBV mutant strains, emerged during the immune clearance phase under host immune surveillance, may cause different impacts on viral biosynthesis, host immune responses, and clinical course.
Early events in childhood during chronic HBV infection may serve as important predictors for the later outcome in adulthood. Understanding the mechanisms triggering liver inflammation and their long-term impacts may enhance the development of better and earlier therapeutic strategies for patients with chronic HBV infection.
Keyword: Hepatitis B virus, Immune-tolerance, Immune clearance, Host viral interaction, Endocrine system
Introduction
Human hepatitis B virus (HBV) is a member of hepadnavirus, a small enveloped virus with partially double stranded circular deoxyribonucleic acid (DNA) that replicate by reverse transcription [1]. The virus particles deliver their DNA into the hepatocyte nucleus at the time of infection, where the viral DNA is then converted to a covalently closed circular DNA (cccDNA) that serve as the transcriptional template for pre-genomic ribonucleic acid (RNA) and messenger RNA (mRNA) for hepatitis B surface antigen (HBsAg), hepatitis B e antigen/core antigen (HBeAg/HBcAg), polymerase, and X protein (HBx) [2].
HBV infection is the major pathogen causing chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) in the world [3]. The clinical course of HBV infection is diverse among individuals with different host genome, viral strains, and host-viral interactions. The earlier the HBV acquisition age is, the more likely the lifelong infection results [4–6]. In endemic areas, such as Taiwan, perinatal infection account for approximately half of the cases of chronic HBV infection before the universal HBV vaccination program, and the perinatal infection increased to 90 % after the program [7]. The natural course of chronic HBV infection was generally sub-divided into immune-tolerant, immune clearance/inflammatory, post HBeAg seroconversion inactive phases. Part of the infected subjects may enter the HBeAg-negative hepatitis reactivation phase, and even develop liver cirrhosis or HCC [8, 9]. Very minority of chronic HBV infected subjects may developed HBsAg seroconversion, and get rid off chronic infected status. The immune-tolerant phase is indicated by normal alanine aminotransferase (ALT) levels, high viral loads, and presence of HBeAg. In majority of the patients during or after the adolescent stage, the flare of ALT and the HBeAg seroconversion to its antibody (Anti-HBe) indicate the immune clearance/inflammatory phase. HBeAg seroconversion generally indicates the decrement of active viral replication and hepatitis activity, while delayed HBeAg seroconversion with persistently high viremia after the 4th decade of life indicates a higher risk of developing liver cirrhosis, and HCC [10–14]. Chronic HBV infected subjects usually experience the inactive phases with normal ALT levels, low viremia and negative HBeAg after HBeAg seroconversion. However, up to 10-25 % of chronic HBV infected adults subjects may suffer from HBeAg-negative hepatitis flare after HBeAg seroconversion, especially in those who experience late HBeAg seroconversion, and are associated with increased life-long risk of liver cirrhosis and HCC [13, 14]. HBV basal core promoter (BCP) and precore/core gene mutation emerge during the process of immune clearance phase and HBeAg seroconversion, and are associated with different viral replication ability and clinical outcomes [15, 16].
The triggering host and viral factors to end the immune-tolerant phase, modulating the course of immune-clearance/inflammatory phase, HBeAg/HBsAg seroclearance and seroconversion, and even the occurrence of HBeAg-negative hepatitis flare are the key determinants to the life-long risk of liver injuries, liver cirrhosis and HCC.
Review
Host factors
Clinical relevanxce of human endocrine influence
From a very long-term chronic HBV cohort followed from infants and young children to adult life, the spontaneous HBeAg seroconversion rate was low before 10 years of age, and accelerated since the second decade of life [17]. The annual spontaneous HBeAg seroconversion rate was 1.70 % (95 % CI 0.43 %–2.97 %) in the first decade of life, 3.78 % (95 % CI 2.61 %–4.94 %) in the second decade of life, and 4.02 % (95 % CI 1.61 %–6.23 %) in the third decade of life in genotype B and C chronic HBV infected cohort [17].
Majority of children with chronic HBV infection entered the immune clearance/inflammatory phase after their puberty onset [17, 18]. Earlier onset of puberty and increased steroid 5-alpha reductase type II (SRD5A2) enzyme activity are associated with earlier HBeAg seroconversion in males with chronic HBV infection [19]. Earlier menarche, indicating earlier puberty-onset, is also associated with earlier HBeAg seroconversion in female subjects with chronic HBV infection [20].
The clinical courses of infection with various pathogens differ greatly between males and females; the difference is thought to result from cross-talk between different sex steroids and immune effectors [21, 22]. The main sex steroids at puberty are testosterone and estradiol in male and female subjects, respectively. Animal studies showed the androgen pathway can increase HBV transcription and suppress the tumor suppressor gene in early hepatocarcinogenesis [23, 24], the estrogen pathway can repress HBV genes’ transcription [25]. Hence, the association of puberty onset in both genders with the start of immune clearance/inflammation may not be answered by the sex steroids alone [19, 20]. Factors other than sex steroids and common to both genders, acting during the peri-puberty period, may contribute to the initiation of immune clearance/inflammatory phase.
Dehydroepiandrosterone sulphate (DHEAS), a adrenarche marker, elevated 2-3 years before puberty is significantly associated with the age of HBeAg seroconversion in both genders [26]. DHEAS is elevated between six and eight years of age in both genders and peaks at the third decade of life [27, 28]. It is regarded as a potent immune modulator in human immune responses to various infectious pathogens [27–31]. Higher serum DHEAS levels at mid-puberty was further showed to predict higher decay rate of HBV viral load and HBsAg titer from mid-puberty to young adulthood [26].
The endocrine factors, particularly the DHEAS, may be partially responsible for the initiation of immune clearance and inflammation in immune-tolerant subjects with chronic HBV infection (Fig. 1).
Fig. 1.
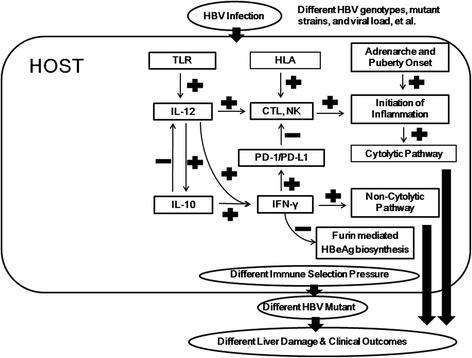
Fine tune interactions between the host and hepatitis B virus (HBV) during the chronic natural course of infection. CTL = cytotoxic T lymphocyte; HLA = human leukocyte antigen; IL = interleukin; IFN = interferon; NK = natural killer cell; PD-1 = program death – 1; PD-L1 = program death ligand-1; TLR = toll like receptor
Clinical relevance of human immune system
Host immune response is considered to play an important role in the course of HBV infection. Human T-lymphocytes may identify HBV viral peptides presented by human leukocyte antigen (HLA) on antigen presenting cells. Variations in immune response are often associated with polymorphisms of HLA antigens [32]. Previous cross section studies in Gambia showed major histocompatibility complex (MHC) class II alleles HLA-DRB1*1301-02 are associated with protection against persistent HBV infection, and subsequently confirmed by other two series in Germany and Korea. [33–35] Other series suggested a protective role of HLA-DR2, HLA-DR*0406, HLA-B*4001, and HLA-DR7 antigens for acute HBV infection [36, 37]. Recent genome-wide association cross-sectional study showed the association of HLA-DP with protection against chronic hepatitis B and with viral clearance in Japanese and Korean [38]. Our long-term cohort showed the HLA class I antigen B61 and class II antigen DQB1*0503 are associated with earlier HBeAg seroconversion in Taiwanese children with chronic HBV infection [39].
Cytokines play important roles in the defense mechanism directly by inhibiting viral replication and indirectly by determining the predominant pattern of host immune response, which is regulated by human genetic background and modulates outcomes of chronic viral hepatitis. Previous study showed interferon-γ and tumor necrosis factor-α (TNF-α) may contribute to the cell mediated anti-HBV response in children with chronic HBV infection entering the immune clearance/inflammatory phase [40, 41].
Our candidate gene approach analysis in a long-term HBV cohort, demonstrated that interleukin (IL)-10 and IL-12 correlate with the HBeAg seroconversion age and severity of inflammation during the immune clearance/inflammatory phase. IL-10 -1082 G/G genotype is associated with higher serum IL-10 levels, while the IL-12β -10993 C/G genotype is associated with higher HBcAg inducible IL-12 secretion of peripheral blood mononuclear cells. Both are associated with earlier spontaneous HBeAg seroconversion [42].
Increasing evidences indicate that innate immune responses, especially the toll-like receptor (TLR) signaling pathway, are essential to the defense mechanism against various pathogens, including HBV, by activating downstream inflammatory cascades like nuclear factor κB, interferon regulatory factor, mitogen activated protein kinases, and pro-inflammatory cytokines [43, 44]. Chronic HBV infected patients with TLR5 rs5744174 (p.Phe616Leu) and C allele at TLR9 rs5743836 promoter area polymorphism are also noted to have earlier spontaneous HBeAg seroconversion [43]. TLR5 rs5744174 (p.Phe616Leu) associated with higher interferon-γ production in chronic HBV infected patients, and C allele at the TLR9 rs5743836 promoter SNP site was reported to increase TLR9 receptor expression levels, which may mediate stronger signals from thymosin α-1 to suppress HBV replication through downstream cytokines [43].
The A-allele of IL-10 SNP rs1800872, and the G-allele of IL-12β SNP rs3212217 were predictors of spontaneous HBsAg seroconversion and HBV recovery in HBV infected patients [45]. In human liver tissue, IL-10 and IL-12β mRNA abundances were positively correlated with interferon-γ mRNA expression levels during the immune clearance/inflammatory phase [46]. The G allele carriers at TLR4 rs4986790 (p.Asp299Gly) was demonstrated to associate with spontaneous HBsAg seroconversion in our cohort. A recent animal study demonstrated that the TLR4-dependent pathway altered the gut microbiota in mice and stimulated liver immune response and resulted in rapid HBV clearance [43, 47].
The interferon-γ mRNA abundance in human liver was also associated with lower furin, and higher program death 1 (PD-1)/program death ligand -1 (PD-L1) mRNA levels in liver tissue from HBeAg-positive patients [45, 46]. The intra-hepatic interferon-γ may modulate the inflammatory response to avoid excessive hepatocyte damage through the enhancement of PD-1 and PD-L1 expression, whereas interferon-γ mediated furin suppression may contribute to a reduction in HBeAg and HBsAg biosynthesis [45–49].
These evidences implied both the innate immune factors and human cytokine may modulate the interferon-γ mediated HBV suppression pathway to promote earlier HBeAg seroconversion, and clear the HBV in human.
Viral factors
Clinical relevance of HBV genotype and viral mutants emerging during immune clearance/inflammatory phase, and viremia profile
Different HBV genotypes have been documented to be an important predictor of the clinical course of chronic HBV infection. Chronic genotype D HBV was reported to associate with more severe liver damage than genotype A HBV, and predict HCC in India [50]. Genotype B and C are the predominant HBV strains in far-eastern Asian countries, while the genotype C is noted to associate with more severe liver disease and delayed spontaneous HBeAg seroconversion than genotype genotype B HBV in both adults and children in Taiwan [51, 52]. The genotype Ba HBV was reported to associate with the development of HCC in young non-cirrhotic patients in Taiwan, while the genotype Bj HBV does not shown to associate with increased HCC risk as compared with genotype C HBV infection in Japan [51–53].
In our pediatric HBV cohort followed since immune-tolerant phase, mutations of core promoter at nucleotide position 1752, 1775, and 1799 have significant correlations with HBeAg seroconversion; precore 1896 mutant existed in half of childhood HBeAg seroconverters; and genotype C HBV is associated with basal core promotor (BCP) 1762 + 1764 mutations during the process of HBeAg seroconversion [54]. The prevalence of HBV precore/core mutation strains increased significantly in the immune clearance/inflammatory phase than in the immune-tolerant phase [55]. The increased proportion of BCP mutant strain were reported to increase the risk of liver cirrhosis and HCC in chronic genotype B and C HBV infected adults [56, 57]. A recent quantitative analysis of precore G1896A and BCP mutants in interferon-treated patients demonstrated that the distinct HBV evolution/mutation patterns during HBeAg seroconversion may present with different HBV viremia pattern after HBeAg seroconversion [58].
Young HBeAg seroconverters in a pediatric cohort showed decreased viral loads, persistent normal ALT levels, and uneventful courses after HBeAg seroconversion [59]. However, HBeAg seroconversion beyond the 4th decade of life in adults is associated with increased risk of increased HBV viral load, HBeAg-negative hepatitis flare, liver cirrhosis and HCC [13, 14, 60]. These evidences implied that, different immune mechanism and possible different HBV mutants selected during the immune clearance/inflammatory phase between young and old HBeAg seroconverters may results in different clinical courses and life-long outcomes.
Host-virus interaction
Clinical relevance of host and virus interaction
Gender is a key determinant of chronic HBV clinical outcome, and the relative risk of HBV-related HCC and liver disease related death are consistently several fold (1.5 to 7.6 times) higher in males than females [61–63]. Animal studies showed the androgen pathway can increase HBV transcription, while the estrogen pathway may repress the efficacy of HBV genes transcription [23–25]. Our previous study also demonstrated the severity of liver inflammation is closely associated with the serum testosterone levels in chronic HBV infected males [19]. The different outcomes between chronic HBV infected males and females are closely related to the effect of sex steroids on HBV biosynthesis.
Furin, a proprotein peptidase located at the endoplasmic reticulum membrane in human hepatocytes, is used by HBV to facilitate the biosynthesis and maturation of HBeAg from 25-kDa proprotein to 17-kDa mature HBeAg [64]. The inhibition of furin either by the interferon-γ, small molecular weight antagonist and even the knock-down experiment, were all demonstrated to inhibit the biosynthesis of mature HBeAg both in vivo and in vitro studies [46, 64, 65].
PD-1 and PD-L1 are considered as markers of immunologic tolerance and T-cell dysfunction in the presence of infectious pathogens, including HBV [66, 67]. Blockage of PD-1/PD-L1 was found to enhance the re-activation of HBV-specific cytotoxic T lymphocytes (CTLs) and secretion of interferon-γ by circulating intrahepatic lymphocytes in subjects with chronic HBV infection and HBeAg seroconversion [68]. Strikingly, the non-expression of the PD-1/PD-L1 pathway was associated with fulminant hepatic failure in acute HBV-infected patients [69]. Hence, the up-regulation of the PD-1/PD-L1 pathway may efficiently mitigate pathogenic T-cell responses, limit liver damage, and avoid massive hepatocyte damage and fulminate hepatic failure in patients with HBV infection [68, 69]. PD-1/PD-L1, induced in the liver, is thus considered to play key regulatory roles in avoiding excessive tissue damage when the inflammatory response is programed to turn off or the immune response fails to clear the pathogen [46, 48, 70]. Most patients with chronic HBV infection may experience hepatitis flare-up at the immune clearance/inflammatory phase, followed by an inactive phase after HBeAg seroconversion, with a decline in viral load and normalization of liver-function profile. The interferon-γ mediated circus, including the up-regulation of PD-1/PD-L1 and down-regulation of furin, may serve important roles on transition from cytolytic to non-cytolytic HBV suppression inside the liver to avoid excessive liver damage and hepatic failure (Fig. 1) [46]. The interaction between host factors and virus, both directly on the viral biosynthesis or immune selection pressure, play significant roles on the life-long risk of chronic HBV infected patients.
HBV precore/core gene mutations during the immune clearance/inflammatory phase are known to be the result of host immune selection pressure [15, 71]. Mutations in the HBV precore/core gene may change the amino acid sequence, protein structure, antigenicity, and the biological function of both HBeAg and HBcAg. The alternation of HBcAg sequence and structure may change the stability of HBV nucleocapsid, HBV pgRNA packaging, and the efficacy and accuracy of HBV replication [72, 73]. Our previous study showed the IL-10 -1082 polymorphism site G/G genotype carrying subjects is associated with higher HBV C2189A mutations during the immune clearance/inflammatory phase, and results in lower HBV viral load [55]. The HBV core protein P135Q mutant and the precore 1896 mutant were the most prevalent mutants before HBeAg seroconversion in genotype B and C HBV chronic infected subjects [55, 74]. The HBV P135Q mutant strain was further demonstrated to altered the normal HBV capsid assembly, HBeAg biosynthesis, and reduced human immune responses following HBeAg seroconversion [74]. Hence, different immune selection pressure in different host results in divergent HBV immune escape mutant strains, which leads to different viral life cycle and clinical course/outcomes of chronic HBV infection.
Significance of childhood events on the life-long chronic HBV disease course
The HBeAg seroconversion age and the severity of liver damage during the immune clearance phase are both important outcome determine factors during the natural course of chronic HBV infection [75]. Extremely early HBeAg seroconversion before 3 years of age with severe liver damage was noted to increase the risk of childhood HCC [76, 77]. On the other hand, HBeAg seroconversion during childhood without severe liver damage have been demonstrated to associate with a relatively uneventful course with low viremia profile, lower incidence of hepatitis reactivation after HBeAg seroconversion, and higher chance of spontaneous HBsAg seroconversion [45, 59, 75]. Furthermore, the delay in HBeAg seroconversion after the 4th decade of life was regarded as an important risk factors of HBeAg-negative hepatitis falre, liver cirrhosis, and HCC [10–12, 14, 75]. Recently, we demonstrated earlier breakthrough of immune-tolerance and earlier HBeAg seroconversion in children with chronic HBV infection are both important predictors of spontaneous HBsAg seroconversion [45].
Conclusions
HBV infection in endemic area mostly occurred in infant and young childhood, and resulted in chronic infection status. The viral factors, host factors, and host-virus interactions performed as an orchestra, and acting together to modulate the natural course of chronic HBV infection (Table 1). The early events of chronic HBV infection occurring during childhood, reflecting the complex interactions between the host and virus, are key earlier predictors of the life-long outcomes of chronic HBV infection. Careful monitoring of host and viral markers, and providing early and effective intervention may improve the long-term outcome of chronic HBV infected patients.
Table 1.
Host and viral factors associated with the natural course of chronic hepatitis B virus (HBV) infection
| Clinical events | Associate factors | References |
|---|---|---|
| Hepatitis B e antigen (HBeAg) seroconversion | Host factors | |
| Puberty onset | [19, 20] | |
| Steroid 5-alpha reductase type II | [19] | |
| Dehydroepiandrosterone sulphate | [26] | |
| Human leukocyte antigen (HLA)-B61 and HLA-DQB1*0503 | [39] | |
| Interleukin-10 and 12 | [42] | |
| Toll-like receptor-5 and -9 | [43] | |
| Furin | [68, 69] | |
| Program death 1 and program death ligand-1 pathway | [46, 48, 70] | |
| Virus factors | ||
| HBV Genotype | [40–52] | |
| HBV mutant strains (core-promotor, precore, core gene) | [54, 57, 58, 67] | |
| HBV viral load | [26] | |
| HBV viral titer decrement | Host factors | |
| Puberty onset | [19] | |
| Dehydroepiandrosterone sulphate | [26] | |
| Virus factors | ||
| HBV mutant strains (core-promotor, precore, core gene) | [54, 57, 58, 67] | |
| Hepatitis B surface antigen (HBsAg) seroclearance/seroconversion | Host factors | |
| Dehydroepiandrosterone sulphate | [26] | |
| Gut microbiota | [47] | |
| Menarche onset (in females) | [20] | |
| HLA (DRB1*1301-02, DR2, DR7, DR*0406, B*4001, DPA1 and DPB1) | [33–38] | |
| Interleukin-10 and 12 | [45] | |
| Tumor necrosis factor alpha | [41] | |
| Toll-like receptor-4 | [43, 47] | |
| Program death 1 and program death ligand-1 pathway | [46, 48, 70] | |
| Breakthrough of immune tolerance | [45] | |
| HBeAg seroconversion at childhood | [45] | |
| Virus factors | ||
| HBV viral load | [45] | |
| HBsAg titer | [45] | |
| HBeAg-negative hepatitis | HBeAg seroconversion age | [10–12, 60, 75] |
| HBV mutant | [57, 75] |
Acknowledgements
The authors thank Prof. Hong-Yuan Hsu, Prof. Yen-Hsuan Ni, and Prof. Huey-Ling Chen from the Department of Pediatrics, National Taiwan University Children’s Hospital for long-term contribution.
Funding
The study was supported by the National Taiwan University Hospital (NTUH 104-S2663).
Footnotes
Competing interests
The authors have no potential, perceived, or real competing interests.
Authors’ contributions
JF Wu conceived of the study, and participated in its design and coordination and to draft the manuscript. MH Chang, the corresponding author is the principle investigator of this study. All authors read and approved the final manuscript.
References
- 1.Ganem D, Varmus HE. The molecular biology of the hepatitis B virus. Annu Rev Biochem. 1987;56:651–93. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 2.Tuttleman JS, Pugh JC, Summers JW. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22,707 men in Taiwan. Lancet. 1981;2:1129–33. doi: 10.1016/S0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–4. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, McMahon AJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 6.Beasley RP. Rocks along the road to the control of HBV and HCC. Ann Epidemiol. 2009;19:231–4. doi: 10.1016/j.annepidem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, et al. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287–93. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 8.Chen DS. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science. 1993;262:369–70. doi: 10.1126/science.8211155. [DOI] [PubMed] [Google Scholar]
- 9.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 10.Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829–34. doi: 10.1016/j.amjmed.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat. 2007;14:147–52. doi: 10.1111/j.1365-2893.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–74. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 13.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–17. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435–44. doi: 10.1002/hep.23348. [DOI] [PubMed] [Google Scholar]
- 15.Lim SG, Cheng Y, Guindon S, Seet BL, Lee LY, Hu P, et al. Viral quasi-species evolution during hepatitis Be Antigen seroconversion. Gastroenterology. 2007;133:951–8. doi: 10.1053/j.gastro.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Lin CL, Liao LY, Liu CJ, Yu MW, Chen PJ, Lai MY, et al. Hepatitis B viral factors in HBeAg-negative carriers with persistently normal serum alanine aminotransferase levels. Hepatology. 2007;45:1193–8. doi: 10.1002/hep.21585. [DOI] [PubMed] [Google Scholar]
- 17.Wu JF, Su YR, Chen CH, Chen HL, Ni YH, Hsu HY, et al. Predictive effect of serial serum alanine aminotransferase levels on spontaneous HBeAg seroconversion in chronic genotypes B and C HBV-infected children. J Pediatr Gastroenterol Nutr. 2012;54:97–100. doi: 10.1097/MPG.0b013e31822a033e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang MH, Sung JL, Lee CY, Chen CJ, Chen JS, Hsu HY, et al. Factors affecting clearance of hepatitis B e antigen in hepatitis B surface antigen carrier children. J Pediatr. 1989;115:385–90. doi: 10.1016/S0022-3476(89)80836-4. [DOI] [PubMed] [Google Scholar]
- 19.Wu JF, Tsai WY, Hsu HY, Ni YH, Chen HL, Tsuei DJ, et al. The effect of puberty onset on spontaneous hepatitis B virus e antigen seroconversion in men. Gastroenterology. 2010;138:942–8. doi: 10.1053/j.gastro.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Wu JF, Tsai WY, Tung YC, Chen HL, Ni YH, Hsu HY, et al. Effect of menarche onset on the clinical course in females with chronic hepatitis B virus infection. J Pediatr. 2014;165:534–8. doi: 10.1016/j.jpeds.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 21.Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J Steroid Biochem Mol Biol. 1990;35:157–75. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 22.Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PLoS One. 2013;8:e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology. 2009;50:1392–402. doi: 10.1002/hep.23163. [DOI] [PubMed] [Google Scholar]
- 24.Chen PJ, Yeh SH, Liu WH, Lin CC, Huang HC, Chen CL, et al. Androgen pathway stimulates microRNA-216a transcription to suppress the tumor suppressor in lung cancer-1 gene in early hepatocarcinogenesis. Hepatology. 2012;56:632–43. doi: 10.1002/hep.25695. [DOI] [PubMed] [Google Scholar]
- 25.Wang SH, Yeh SH, Lin WH, Yeh KH, Yuan Q, Xia NS, et al. Estrogen receptor a represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4a. Gastroenterology. 2012;142:989–98. doi: 10.1053/j.gastro.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 26.Wu JF, Tsai WY, Tung YC, Chen HL, Ni YH, Hsu HY, et al. Role of serum dehydroepiandrosterone sulfate level on the clearance of chronic hepatitis B virus infection. J Gastroenterol. 2014;49:900–6. doi: 10.1007/s00535-013-0831-0. [DOI] [PubMed] [Google Scholar]
- 27.Nawata H, Yanase T, Goto K, Okabe T, Nomura M, Ashida K, et al. Andropause. Horm Res. 2004;62:110–4. doi: 10.1159/000080509. [DOI] [PubMed] [Google Scholar]
- 28.Hazeldine J, Arlt W, Lord JM. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol. 2010;120:127–36. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Chittiprol S, Kumar AM, Shetty KT, Kumar HR, Satishchandra P, Rao RS, et al. HIV-1 clade C infection and progressive disruption in the relationship between cortisol, DHEAS and CD4 cell numbers: a two-year follow-up study. Clin Chim Acta. 2009;409:4–10. doi: 10.1016/j.cca.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.la TorreB D, VonKroghG SM, Holmberg V. Blood cortisol and dehydroepiandrosterone sulfate (DHEAS) levels and CD4 T cell counts in HIV infection. Clin Exp Rheumatol. 1997;15:87–90. [PubMed] [Google Scholar]
- 31.Kutlu NO, Akinci A, So¨nmezgo¨z E, Temel I, Evliyaog˘lu E. The effects of androstenediol and dehydroepiandrosterone on the immune response toBCGat puberty. J Trop Pediatr. 2003;49:181–185. [DOI] [PubMed]
- 32.Bertoni R, Sidney J, Fowler P, Chesnut RW, Chisari FV, Sette A. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J Clin Invest. 1997;100:503–13. doi: 10.1172/JCI119559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thursz MR, Kwiatkowski D, Allsopp CE, Greenwood BM, Thomas HC, Hill AV. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N Engl J Med. 1995;332:1065–9. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 34.Hohler T, Gerken G, Notghi A, Lubjuhn R, Taheri H, Protzer U, et al. HLA-DRB1*1301 and *1302 protect against chronic hepatitis B. J Hepatol. 1997;26:503–7. doi: 10.1016/S0168-8278(97)80414-X. [DOI] [PubMed] [Google Scholar]
- 35.Ahn SH, Han KH, Park JY, Lee CK, Kang SW, Chon CY, et al. Association between Hepatitis B virus infection and HLA-DR type in Korea. Hepatology. 2000;31:1371–3. doi: 10.1053/jhep.2000.7988. [DOI] [PubMed] [Google Scholar]
- 36.Almarri A, Batchlor JR. HLA and hepatitis B infection. Lancet. 1994;344:1994–5. doi: 10.1016/S0140-6736(94)90510-X. [DOI] [PubMed] [Google Scholar]
- 37.Wu YF, Wang LY, Lee TD, Lin HH, Hu CT, Cheng ML, et al. HLA phenotypes and outcomes of hepatitis B virus infection in Taiwan. J Med Virol. 2004;72:17–25. doi: 10.1002/jmv.10557. [DOI] [PubMed] [Google Scholar]
- 38.Nishida N, Sawai H, Matsuura K, Sugiyama M, Ahn SH, Park JY, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS One. 2012;7(6):e39175. doi: 10.1371/journal.pone.0039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu JF, Chen CH, Hsieh RP, Shih HH, Chen YH, Li CR, et al. HLA typing associated with hepatitis B e antigen seroconversion in children with chronic hepatitis B virus infection: a prospective sibling cohort study in Taiwan. J Pediatr. 2006;148:647–51. doi: 10.1016/j.jpeds.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 40.Hsu HY, Chang MH, Ni YH, Lee PI. Cytokine release of peripheral blood mononuclear cells in children with chronic hepatitis B virus infection. J Pediatr Gastroenterol Nutr. 1999;29:540–5. doi: 10.1097/00005176-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Kao PC, Wu JF, Ni YH, Lin YT, Chen HL, Hsu SH, et al. Tumour necrosis factor-α promoter region polymorphisms affect the course of spontaneous HBsAg clearance. Liver Int. 2010;30:1448–53. doi: 10.1111/j.1478-3231.2010.02340.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu JF, Wu TC, Chen CH, Ni YH, Chen HL, Hsu HY, et al. Serum levels of interleukin 10 and 12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138:165–72. doi: 10.1053/j.gastro.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Wu JF, Chen CH, Ni YH, Lin YT, Chen HL, Hsu HY, et al. Toll-like receptor and hepatitis B virus clearance in chronic infected patients: a long-term prospective cohort study in Taiwan. J Infect Dis. 2012;206:662–8. doi: 10.1093/infdis/jis420. [DOI] [PubMed] [Google Scholar]
- 44.Lange NE, Zhou X, Lasky-Su J, et al. Comprehensive genetic assessment of a functional TLR9 promoter polymorphism: no replicable association with asthma or asthma-related phenotypes. BMC Med Genet. 2011;12:26. doi: 10.1186/1471-2350-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu JF, Hsu HY, Chiu YC, Chen HL, Ni YH, Chang MH. The effects of cytokines on spontaneous hepatitis B surface antigen seroconversion in chronic hepatitis B virus infection. J Immunol. 2015;194:690–6. doi: 10.4049/jimmunol.1401659. [DOI] [PubMed] [Google Scholar]
- 46.Wu JF, Hsu HY, Ni YH, Chen HL, Wu TC, Chang MH. Suppression of furin by interferon-γ and the impact on hepatitis B virus antigen biosynthesis in human hepatocytes. Am J Pathol. 2012;181:19–25. doi: 10.1016/j.ajpath.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Chou HH, Chien WH, Wu LL, Cheng CH, Chung CH, Horng JH, et al. Age-related immune clearance of hepatitis B virus infection requires the establishment of gut microbiota. Proc Natl Acad Sci U S A. 2015;112:2175–80. doi: 10.1073/pnas.1424775112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–8. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–50. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thakur V, Guptan RC, Kazim SN, Malhotra V, Sarin SK. Profile, spectrum and significance of HBV genotypes in chronic liver disease patients in the Indian subcontinent. J Gastroenterol Hepatol. 2002;17:165–70. doi: 10.1046/j.1440-1746.2002.02605.x. [DOI] [PubMed] [Google Scholar]
- 51.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554–9. doi: 10.1016/S0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 52.Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733–8. doi: 10.1053/j.gastro.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 53.Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, et al. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590–4. doi: 10.1053/jhep.2001.27221. [DOI] [PubMed] [Google Scholar]
- 54.Ni YH, Chang MH, Hsu HY, Tsuei DJ. Longitudinal study on mutation profiles of core promoter and precore regions of the hepatitis B virus genome in children. Pediatr Res. 2004;56:396–9. doi: 10.1203/01.PDR.0000136282.20470.87. [DOI] [PubMed] [Google Scholar]
- 55.Wu JF, Ni YH, Lin YT, Lee TJ, Hsu HJ, Chen HL, et al. Human interleukin-10 genotypes are associated with different precore/core gene mutation patterns in children with chronic hepatitis B virus infection. J Pediatr. 2011;158:808–13. doi: 10.1016/j.jpeds.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 56.Tseng TC, Liu CJ, Yang HC, Chen CL, Yang WT, Tsai CS, et al. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut. 2015;64:292–302. doi: 10.1136/gutjnl-2014-306977. [DOI] [PubMed] [Google Scholar]
- 57.Liu CJ, Chen BF, Chen PJ, Lai MY, Huang WL, Kao JH, et al. Role of hepatitis B virus precore/core promoter mutations and serum viral load on noncirrhotic hepatocellular carcinoma: a case-control study. J Infect Dis. 2006;194:594–9. doi: 10.1086/505883. [DOI] [PubMed] [Google Scholar]
- 58.Yang HC, Chen CL, Shen YC, Peng CY, Liu CJ, Tseng TC, et al. Distinct evolution and predictive value of hepatitis B virus precore and basal core promoter mutations in interferon-induced hepatitis B e antigen seroconversion. Hepatology. 2013;57:934–43. doi: 10.1002/hep.26121. [DOI] [PubMed] [Google Scholar]
- 59.Ni YH, Chang MH, Chen PJ, Tsai KS, Hsu HY, Chen HL, et al. Viremia profiles in children with chronic hepatitis B virus infection and spontaneous e antigen seroconversion. Gastroenterology. 2007;132:2340–5. doi: 10.1053/j.gastro.2007.03.111. [DOI] [PubMed] [Google Scholar]
- 60.Chu CM, Liaw YF. Predictive factors for reactivation of hepatitis B following hepatitis B e antigen seroconversion in chronic hepatitis B. Gastroenterology. 2007;133:1458–65. doi: 10.1053/j.gastro.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 61.Lee CM, Lu SN, Changchien CS, Yeh CT, Hsu TT, Tang JH, et al. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86:1143–50. doi: 10.1002/(SICI)1097-0142(19991001)86:7<1143::AID-CNCR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 62.Evans AA, Chen G, Ross EA, Shen FM, Lin WY, London WT. Eight-year follow-up of the 90000-person Haimen city cohort: I. hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11:369–76. [PubMed] [Google Scholar]
- 63.Chang MH, Shau WY, Chen CJ, Wu TC, Kong MS, Liang DC, et al. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA. 2000;284:3040–42. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- 64.Lei RX, Shi H, Peng XM, Zhu YH, Cheng J, Chen GH. Influence of a single nucleotide polymorphism in the P1 promoter of the furin gene on transcription activity and hepatitis B virus infection. Hepatology. 2009;50:763–71. doi: 10.1002/hep.23062. [DOI] [PubMed] [Google Scholar]
- 65.Yang HY, Zheng NQ, Li DM, Gu L, Peng XM. Entecavir combined with furin inhibitor simultaneously reduces hepatitis B virus replication and e antigen secretion. Virol J. 2014;11:165. doi: 10.1186/1743-422X-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans A, Riva A, Cooksley H, Phillips S, Puranik S, Nathwani A, et al. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759–69. doi: 10.1002/hep.22419. [DOI] [PubMed] [Google Scholar]
- 67.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–70. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 68.Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–93. doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–49. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 70.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–6. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Chang MH, Hsu HY, Ni YH, Tsai KS, Lee PI, Chen PJ, et al. Precore stop codon mutant in chronic hepatitis B virus infection in children: its relation to hepatitis B e antigen seroconversion and maternal hepatitis B surface antigen. J Hepatol. 1998;28:915-22. [DOI] [PubMed]
- 72.Schlicht HJ, Wasenauer G. The quaternary structure, antigenicity, and aggregational behavior of the secretory core protein of human hepatitis B virus are determined by its signal sequence. J Virol. 1991;65:6817-25. [DOI] [PMC free article] [PubMed]
- 73.Wynne SA, Crowther RA, Leslie AGW. The crystal structure of human hepatitis B virus capsid. Mol Cell. 1999;3:771-80. [DOI] [PubMed]
- 74.Wu JF, Ni YH, Chen HL, Hsu HY, Chang MH. The impact of hepatitis B virus precore/core gene carboxyl terminal mutations on viral biosynthesis and the host immune response. J Infect Dis. 2014;209:1374-81. [DOI] [PubMed]
- 75.Wu JF, Chiu YC, Chang KC, Chen HL, Ni YH, Hsu HY, et al. Predictors of hepatitis B e antigen-negative hepatitis in chronic hepatitis B virus infected patients from childhood to adulthood. Hepatology. 2015. doi:10.1002/hep.28222. [DOI] [PubMed]
- 76.Chang MH, Hsu HY, Hsu HC, Ni YH, Chen JS, Chen DS. The significance of spontaneous hepatitis B e antigen sero-conversion in childhood: with special emphasis of the clearance of hepatitis B e antigen before 3 years of age. Hepatology. 1995;22:1387–92. [PubMed] [Google Scholar]
- 77.Tseng YR, Wu JF, Kong MS, Hu FC, Yang YJ, Yeung CY, et al. Infantile hepatitis B in immunized children: risk for fulminant hepatitis and long-term outcomes. PLoS One. 2014;9:e111825. doi: 10.1371/journal.pone.0111825. [DOI] [PMC free article] [PubMed] [Google Scholar]


