Abstract
Cystic fibrosis-associated liver disease (CFLD) is a chronic cholangiopathy that negatively affects the quality of life of cystic fibrosis patients. In addition to reducing biliary chloride and bicarbonate secretion, up-regulation of TLR4/NF-kB-dependent immune mechanisms plays a major role in the pathogenesis of CFLD, and may represent a therapeutic target. Nuclear receptors (NRs) are transcription factors that regulate several intracellular functions. Some NRs, including peroxisome proliferator-activated receptor-γ (PPAR-γ), may counter-regulate inflammation in a tissue-specific manner. In this study, we explored the anti-inflammatory effect of PPAR-γ stimulation in vivo in Cftr-KO mice exposed to DSS, and in vitro in primary cholangiocytes isolated from wild type and from Cftr-KO mice exposed to LPS. We found that in CFTR-defective biliary epithelium, expression of PPAR-γ is increased, but does not result in increased receptor activity because the availability of bioactive ligands is reduced. Exogenous administration of synthetic agonists of PPAR-γ (pioglitazone and rosiglitazone) upregulates PPAR-γ-dependent genes, while inhibiting the activation of NF-kB and the secretion of proinflammatory cytokines (LIX, MCP-1, MIP-2, G-CSF, KC) in response to LPS. PPAR-γ agonists modulate NF-kB-dependent inflammation by upregulating IkBα, a negative regulator of NF-kB. Stimulation of PPAR-γ in vivo (rosiglitazone) significantly attenuates biliary damage and inflammation in Cftr-KO mice exposed to a DSS-induced portal endotoxemia.
Conclusion
These studies unravel a novel function of PPAR-γ in controlling biliary epithelium inflammation and suggest that impaired activation of PPAR-γ contributes to the chronic inflammatory state of CFTR-defective cholangiocytes.
Keywords: Cholangiopathy, innate immunity, TLRs, cytokines, thiazolidinediones
Cystic Fibrosis (CF) is an autosomal recessive disease that severely affects the secretory epithelia of several organs, including pancreas, lungs, liver and gut. It is caused by mutations of the gene encoding for the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-regulated chloride channel expressed at the apical membrane of most epithelial cells (1, 2). About 30% of CF patients present liver abnormalities that may evolve into a clinically significant chronic liver disease (CFLD). Consistent with the expression of CFTR in the biliary epithelium, CFLD manifests as a chronic and progressive cholangiopathy that can eventually progress into sclerosing cholangitis and focal biliary cirrhosis (3, 4).
The pathogenesis of CFLD was thought to be uniquely associated with the ductal cholestasis caused by the impaired bile flow and biliary alkalinization consequent to the defective channel function of CFTR at the cholangiocyte level. The resulting hyperviscous secretions would accumulate into the bile ducts causing retention of hydrophobic bile acids and toxins that would damage the biliary epithelium (3, 5, 6). However, derangement of epithelial immunity has recently emerged as an important pathogenic component of CFLD and other manifestations of CF (7–10). Recent data have demonstrated that when defective in CFTR, the biliary epithelium upregulates TLR4/NF-kB-dependent innate immune responses when exposed to bacterial-derived endotoxins leading to peribiliary inflammation (11).
This novel interpretation of the pathogenesis of CFLD paves the way for new approaches to the management of CFLD. The current standard of care for CFLD is limited to the administration of ursodeoxycholate (UDCA), a bile acid, which is able to stimulate the choleretic function of hepatocytes and cholangiocytes and to modify the composition of the bile acid pool towards a reduced toxicity (12, 13). However a pathogenesis-based therapeutic approach should also target the TLR4/NF-kB-dependent inflammatory pathway in the biliary epithelium.
Nuclear receptors (NRs) constitute a superfamily of ligand-dependent transcription factors that regulate a variety of cellular functions, including metabolic homeostasis, apoptosis, cell proliferation and differentiation (14–16). Moreover, selected classes of NRs, such as glucocorticoid receptors (GR), liver X receptors (LXR) and peroxisome proliferator-activated receptors (PPARs), are also able to modulate inflammation by controlling TLR-dependent signaling pathways. However, the biological functions regulated by a given NR, as well as the underlying mechanisms, vary in a tissue-specific manner (17–23). In the biliary epithelium, NRs participate in the regulation of many cholangiocyte function, including protection from bile-circulating toxics (vitamin D receptor,VDR; farnesoid X receptor, FXR), secretion (GR, FXR) and cell proliferation (hormone receptors) (24). However, it is not known if PPAR-γ possesses anti-inflammatory properties in CF biliary epithelium or its potential mechanism of action.
In this study, we analyzed the pattern of NRs expressed in wild type and CFTR-defective cholangiocytes and investigated the ability of nuclear receptor PPAR-γ to modulate innate immune responses initiated by activation of TLRs. We show that PPAR-γ expression is upregulated in CFTR-defective cholangiocytes and provide evidence that stimulation of PPAR-γ is able to negatively regulate NF-kB-dependent innate immune responses in CF biliary epithelium, by stimulating the NF-kB negative regulator IkBα. We also show that administration of PPARγ agonist rosiglitazone ameliorates liver damage in CF mice in vivo. Our results suggest that PPAR-γ signaling may represent a novel target to control biliary inflammation in cystic fibrosis.
MATERIAL AND METHODS
Additional methods are detailed in the supplemental materials and methods.
Animals and experimental protocols
All procedures were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. Congenic C57BL/6J-Cftrtm1Unc mice (Cftr-KO), an accepted model for the human CF disease, and their wild type littermates were used for in vivo experiments and for the isolation of primary cholangiocytes cell lines. Animals were bred in our facility or provided by the CF Core Center Animal Core (Case Western Reserve University, Cleveland, OH) and were maintained as previously described (11, 13, 25). Cftr-KO mice and wild type littermates were exposed to dextran sodium sulfate (DSS) (11) alone or in combination with the PPAR-γ agonists pioglitazone (PIO) and rosiglitazone (ROSI) (10 mg/Kg, by i.p. daily). At the end of the treatment, mice were sacrificed and their liver tissues were harvested, fixed in formalin, and then embedded in paraffin for histochemical analysis.
Cell culture and treatments
Mouse cholangiocytes were isolated from wild type and Cftr-KO mice as described (11, 13). After the first passage, cells were plated into 25-cm2 tissue culture flasks coated with rat-tail collagen as previously described (11, 13). Before selected experiments, cells were cultured in transwell inserts with a 0.4 µm pore semipermeable membrane (Becton, Dickinson, and Co, Franklin Lakes, NJ). Under this condition, cells grow as a polarized monolayer that can be accessed both through the apical and basolateral domain separately. Establishment of a confluent monolayer was routinely checked, measuring transepithelial resistance and membrane potential difference (Millicell ERS System; Millipore, Billerica, MA). One week after confluence, transepithelial resistance was >1000 Ω∙cm2 and cells were ready for analysis. Wild type and Cftr-KO cholangiocytes were exposed to LPS (100 ng/ml), PIO (10–50 µM), ROSI (10–50 µM) and GW9662 (10 µM). PIO and ROSI were administered two hours before and during LPS stimulation. GW9662 was administered three hours before and during LPS treatment.
Western Blotting
PPAR-γ protein expression was assessed by Western Blot in cytosolic and nuclear protein fractions of wild type and Cftr-KO cholangiocytes, using an antibody against PPAR-γ (Cell Signaling Technology, Rabbit, 1:1000, 5% milk in TBS-tween). IkBα was investigated on total lysates of WT an Cftr-KO cholangiocytes, treated as described above, using an antibody against total IkBα (Cell Signaling, Rabbit, 1:1000, 5% Bovine serum Albumin in TBS-tween). For additional details, refer to supplemental materials and methods.
Immunohistochemistry
Liver slides 4 µm thick from paraffin embedded tissues were processed and stained as previously described (11) with the cholangiocyte specific marker cytokeratin19 (K19) to visualize the ductular reaction and with the leukocyte specific marker CD45 to analyze the inflammatory cells infiltrate. Quantification of the K19 and CD45 positive areas by morphometric analysis was performed as described (11).
Statistical analysis
Results are shown as mean +/− SD. Statistical comparisons were made using one-way analysis of variance or the Wilcoxon–Mann–Whitney 2-sample rank sum test where appropriate. The statistical analysis was performed using SAS software (SAS Institute Inc, Cary, NC). P values less than .05 were considered significant.
RESULTS
Expression of nuclear receptor PPAR-γ is increased in CFTR-defective cholangiocytes
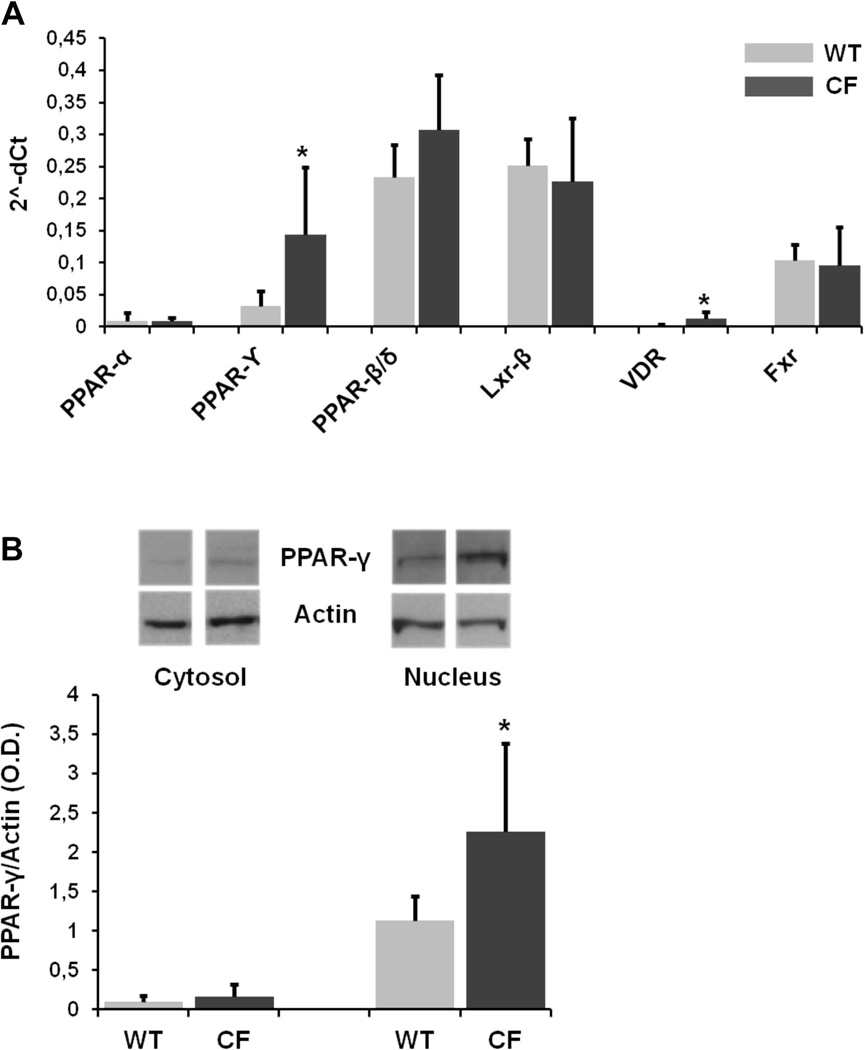
In the liver, NRs are expressed by different cellular subtypes, such as hepatocytes, stellate cells, macrophages and cholangiocytes, with each displaying a specific expression pattern (24, 26). By real time PCR analysis, we compared the expression of different NRs in primary cholangiocytes isolated from wild-type and Cftr-KO mice, cultured on transwell inserts and grown as polarized monolayers. Gene expression of multiple NRs was detected (figure 1A) including PPAR subgroup α, γ and δ, LXR subgroup β, FXR and VDR. Among these transcripts, PPAR-δ, LXR-β and FXR where expressed at higher levels, as compared to PPAR-α and VDR, in both groups of cells. On the contrary, the expression of PPAR-γ was significantly increased in Cftr-KO cholangiocytes as compared to wild type cells. Western Blot analysis on cytosolic and nuclear protein fractions showed that in both groups of cells the expression of PPAR-γ was mostly nuclear, and significantly increased in CF cells, which confirmed our gene expression results (figure 1B).
Figure 1.
A. Gene expression of nuclear receptor PPAR-γ is increased in CFTR-defective cholangiocytes. Real-time PCR analysis of nuclear receptors in WT and Cftr-KO (CF) cholangiocytes. Data were performed in duplicate in n=5 (WT) and n=6 (CF) different cell lines, normalized to HPRT housekeeping gene. *p<.05 vs WT. PPAR: peroxisome proliferator-activated receptor; LXR: liver X receptors; VDR: vitamin D receptor; FXR: farnesoid X receptor. B. Protein expression of nuclear receptor PPAR-γ is increased in CFTR-defective cholangiocytes. Western blot of PPAR-γ in cytosolic and nuclear protein fractions from WT and Cftr-KO (CF) cholangiocytes. Data were performed in n=3 experiments. *p<.05 vs WT.
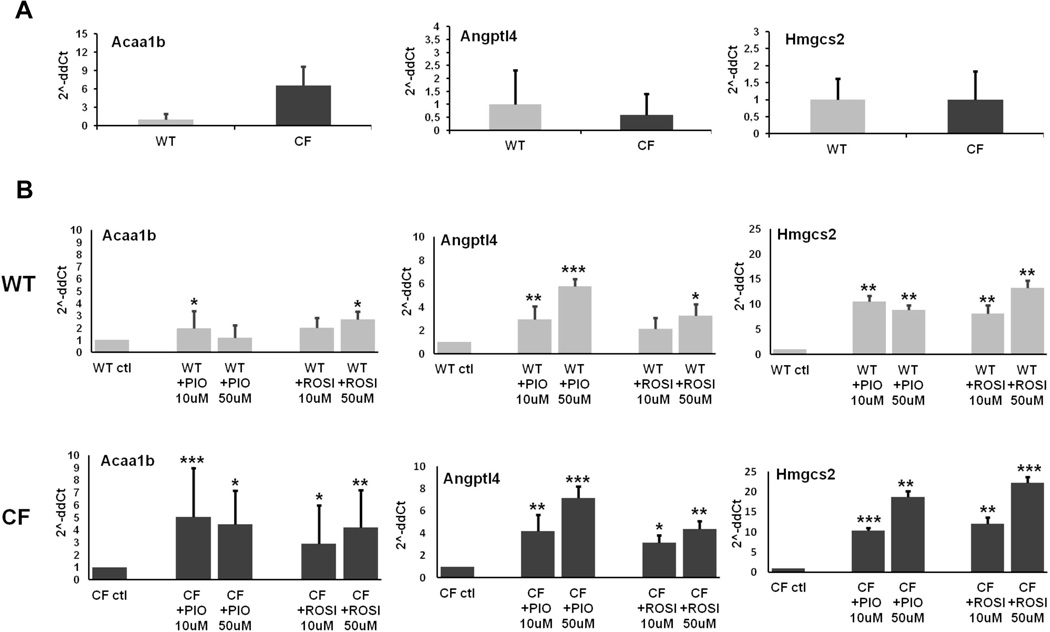
Expression of PPAR-γ target genes is not increased in Cftr-KO cholangiocytes but is upregulated after stimulation with PPAR-γ agonists
PPARs are constitutively bound to the DNA, repressing the transcription of specific genes. When bound to the proper ligands (endogenous or synthetic), these receptors activate the transcription of specific target genes that regulate several cellular functions (27). The expression level of these signature genes is considered indicative of the receptor activation rate. To investigate whether the increased expression of PPAR-γ in CF compared to WT cells is associated to a higher transcriptional activity of the receptor, we studied by RT-PCR the gene expression of metabolic target genes known to be transcriptionally modulated by PPAR-γ: acetyl-Coenzyme A synthase 1B (Acaa1b), angiopoietin-like 4 (Angptl4), and 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (Hmgcs2) (28). Expression of these genes in Cftr-KO cells was similar to that of wild type cells, indicating a comparable basal activity of PPAR-γ (figure 2A). Treatment with specific PPAR-γ agonists PIO and ROSI (10–50 µM) significantly increased the expression of PPAR-γ target genes (figure 2B), indicating that PPAR-γ receptor in both wild type and Cftr-KO cells can be activated by exogenous ligands. These findings show that PPAR-γ in CF-defective cholangiocytes can be readily activated by exogenous agonists and suggest that an imbalance in the level of endogenous activators might explain why the increased expression of PPAR-γ does not correlate with an increased activity of the receptor.
Figure 2. Expression of PPAR-γ target genes is not increased in Cftr-KO cholangiocytes but is upregulated after stimulation with PPAR-γ agonists.
Gene expression of PPAR-γ target genes in WT and Cftr-KO (CF) cholangiocytes before (A) and after 12h of treatment with 10–50 µM of the agonists PIO and ROSI (B). Data were generated in duplicate in n=4–6 different experiments, normalized to HPRT housekeeping gene, and are expressed as 2^-ddCt to the respective internal control. Acaa1b: acetyl-Coenzyme A acyltransferase 1B; Angptl4: angiopoietin-like 4; Hmgcs2: 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2; *p<.05; **p<.01; ***p<.001.
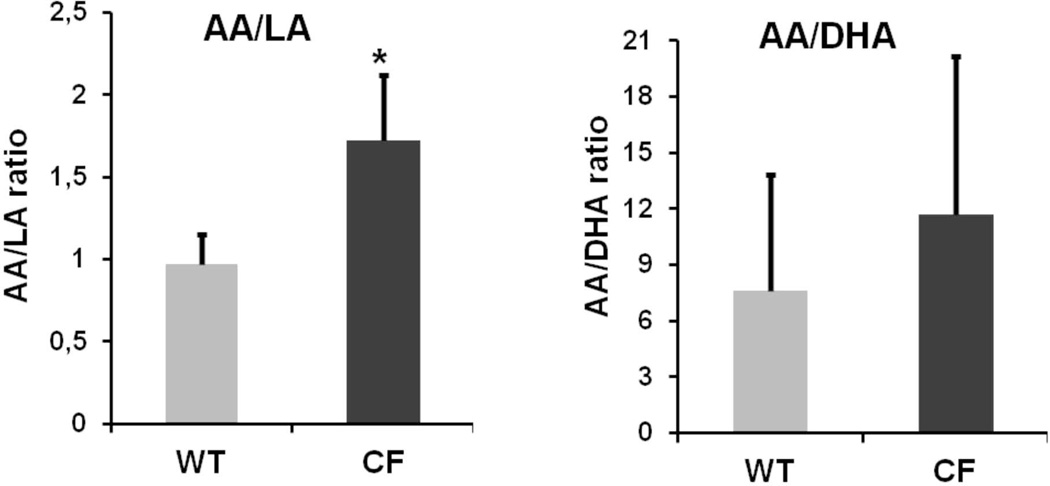
Consistent with this hypothesis, we documented an imbalance between ω-3 and ω-6 poly-unsaturated fatty acids (PUFAs) in Cftr-KO cholangiocytes. We extracted total lipids from WT and Cftr-KO cholangiocytes and performed a lipidomic analysis by gas chromatography of the major ω-3 and ω-6 PUFAs involved in the metabolic cascade that leads to the production of natural ligands of PPAR-γ (21, 29–32). As shown in figure 3, we detected a significant increase in the ratio of arachidonic/linoleic acid (AA/LA) and a trend towards an increased ratio of arachidonic/docosahexaenoic acid (AA/DHA).
Figure 3. Cftr-KO cholangiocytes show an imbalance between ω-3 and ω-6 poly-unsaturated fatty acids (PUFAs).
Lipidomic analysis of total lipids extraction from WT and Cftr-KO (CF) cholangiocytes. AA: arachidonic acid; DHA: docosahexaenoic acid; LA: linoleic acid. Data were performed in n=4 experiments. *p<.01.
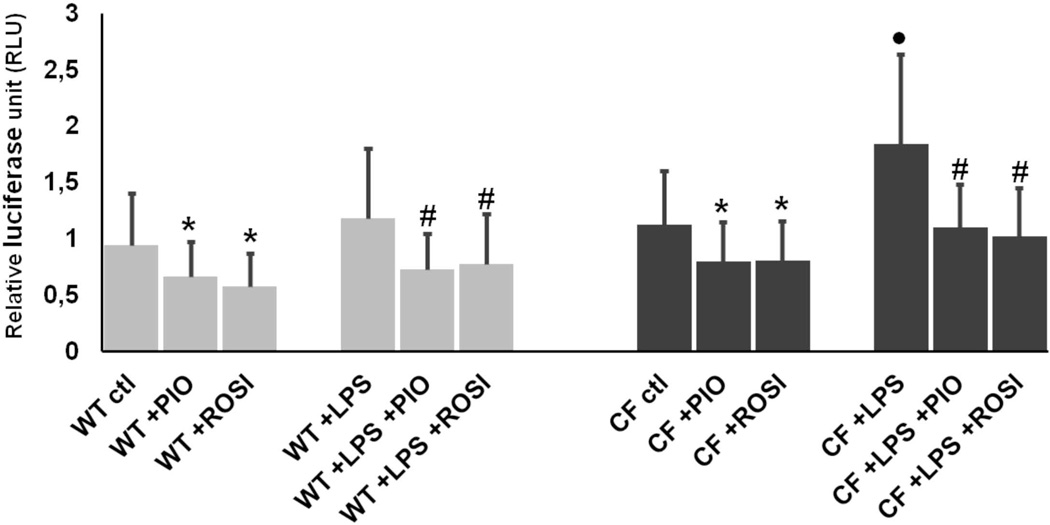
PPAR-γ stimulation inhibits LPS-induced NF-kB activation in Cftr-KO cholangiocytes
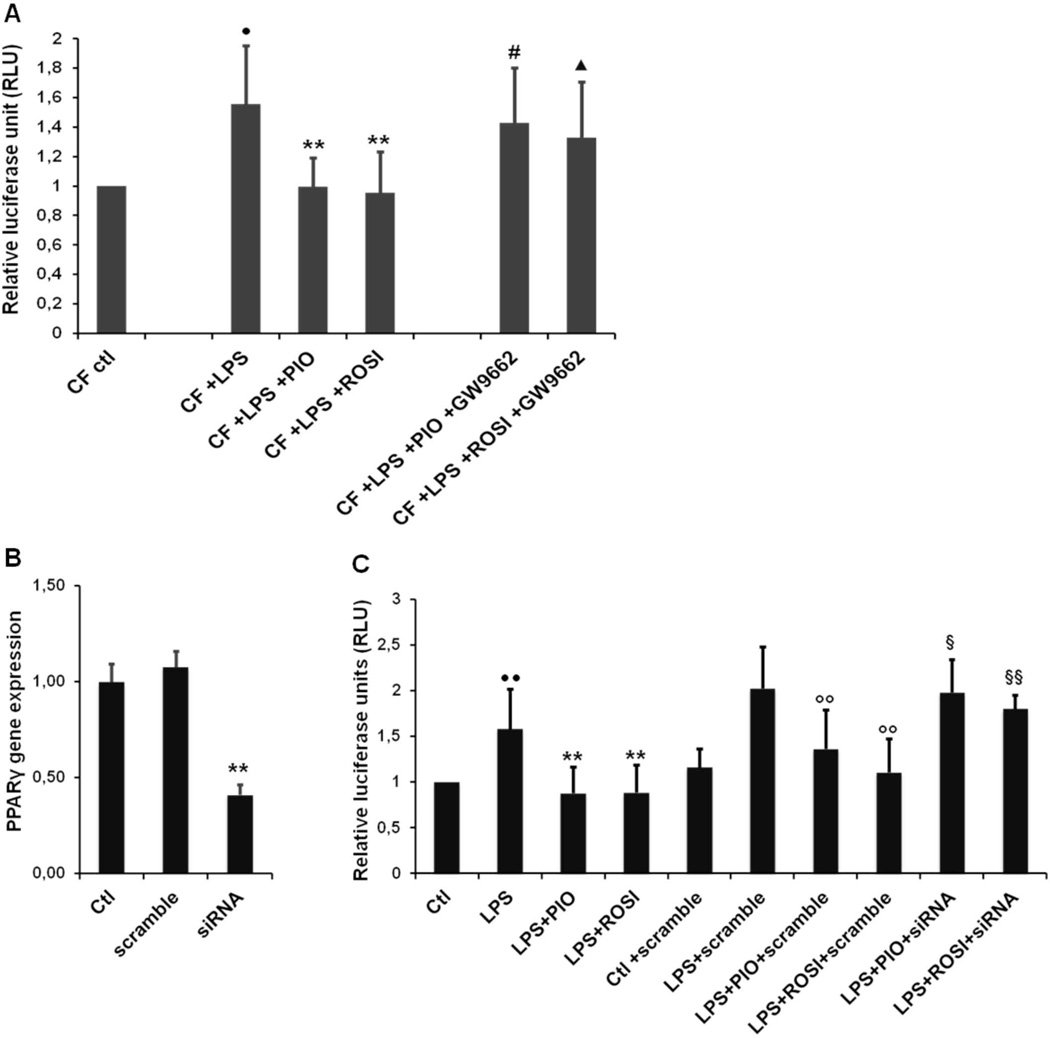
We have previously shown that CFTR-defective cholangiocytes respond to LPS with an increased NF-kB activation, as compared to wild type cells (11). To understand whether activation of PPAR-γ may regulate the LPS-dependent inflammatory response in the biliary epithelium, we treated wild type and Cftr-KO cholangiocytes with PIO or ROSI (50 µM) alone or in combination with LPS (100ng/ml) for 6 hours, and NF-kB transcriptional activity was assessed by a luciferase-based gene reporter. As previously reported (11), Cftr-KO cholangiocytes responded to LPS with a higher NF-kB transcriptional activity with respect to control cells. Moreover, in Cftr-KO cells, treatment with PIO or ROSI significantly inhibited NF-kB activation in both group of cells at basal level and after LPS challenge (figure 4). Toxic cellular effects of PIO and ROSI at the experimental concentration used were excluded by performing a LDH assay (Supplementary figure 1).
Figure 4. PPAR-γ stimulation inhibits LPS-induced NF-kB activation in Cftr-KO cholangiocytes.
Luciferase-based NF-κB gene reporter of WT and Cftr-KO (CF) cells before and after 6h of treatment with LPS (100 ng/ml) alone or in combination with pioglitazone (PIO) (50 µM) or rosiglitazone (ROSI) (50 µM). Bar graph represents the ratio between the NF-kB-dependent expression of luciferase and the constitutive expression of renilla. Data were performed in n=4 experiments. *p<.05 vs ctl; #p<.05 vs LPS; ●p<.05 vs WT+LPS.
The inhibitory effect of PPAR-γ agonists on NF-kB activity in CF cells is dependent on a direct activation of PPAR-γ
Thiazolidinediones may have also receptor-independent effects (33). To understand whether the inhibitory effect of PIO and ROSI on NF-kB activity in CF cells was dependent on a direct activation of PPAR-γ, we used two different approaches. First, polarized Cftr-KO cholangiocytes were treated with LPS (100ng/ml) or the combination of LPS with PIO or ROSI (50 µM), in the presence or absence of the chemical antagonist GW9662 (10µM) (34). We found that pretreatment with GW9662 significantly prevented the inhibitory effect of both agonists on the transcriptional activation of NF-kB (figure 5A). Secondly, experiments were repeated after PPAR-γ gene silencing. Gene expression analysis in silencing conditions confirmed a significant decrease of PPAR-γ gene (figure 5B). As shown in figure 5C, PPAR-γ gene silencing significantly abolished the anti-inflammatory effect of PIO and ROSI. Collectively, these findings indicate that the anti-inflammatory property of PIO and ROSI is receptor-dependent.
Figure 5. The inhibitory effect of PPAR-γ agonists on NF-kB activity in CF cells is receptor dependent.
(A) Luciferase-based NF-kB gene reporter of Cftr-KO (CF) cells before and after 6h of LPS (100 ng/ml) alone or in combination with pioglitazone (PIO) and rosiglitazone (ROSI) (50 µM) with or without the PPAR-γ antagonist GW9662 (10 µM). Data were generated from n=4 experiments. Bar graph represents the ratio between the NF-kB-dependent expression of luciferase and the constitutive expression of renilla. (B) Gene expression analysis confirmed a significant reduction of PPAR-γ mRNA in silencing condition. (C) Luciferase-based NF-kB gene reporter of Cftr-KO (CF) cells before and after 6h of LPS (100 ng/ml) alone or in combination with pioglitazone (PIO) and rosiglitazone (ROSI) (50 µM) with or without small interference RNA specific for PPAR-γ (siRNA) (30nM) or with scrambled oligonucleotides (scramble) (30nM). Data were generated from n=5 experiments. Bar graph represents the ratio between the NF-kB-dependent expression of luciferase and the constitutive expression of renilla.
(A) **p<.01 vs LPS; ●p<.05 vs ctl; #p<.05 vs LPS+PIO; ▲p<.05 vs LPS +ROSI; (B) **p<.01 vs scramble. (C) ●●p<.01 vs ctl; **p<.01 vs LPS; § p<.05 vs LPS+PIO+scramble; §§p<.01 vs LPS+ROSI+scramble; ∞p<.01 vs LPS+scramble.
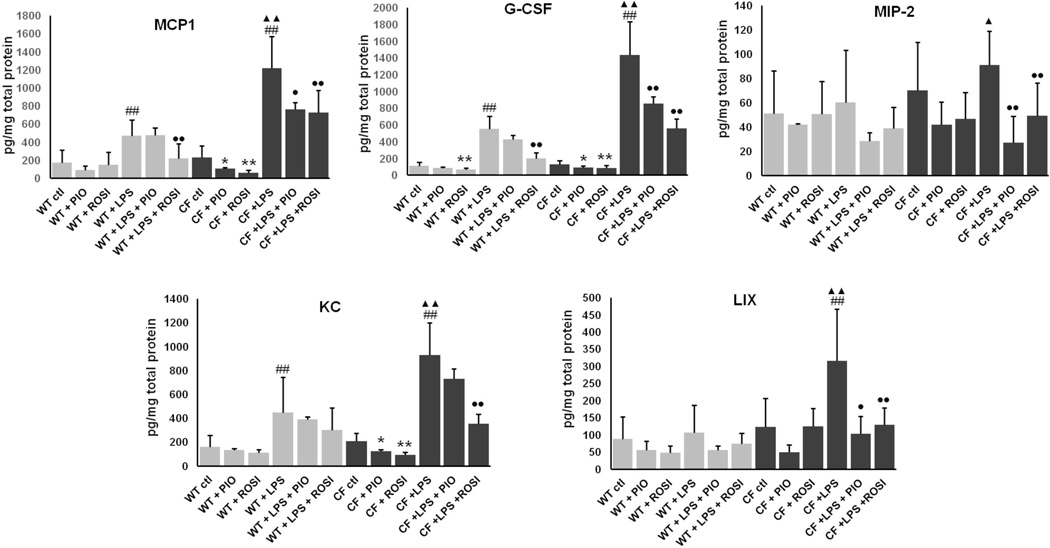
PPAR-γ stimulation inhibits LPS-induced secretion of NF-kB-dependent proinflammatory cytokines in Cftr-KO cholangiocytes
Sustained activation of NF-kB in endotoxin-stimulated Cftr-KO biliary cells is responsible for a robust production of specific pro-inflammatory cytokines/chemokines as described in our previous studies (11). Based on the inhibitory effect on NF-kB activation, we sought to investigate whether PPAR-γ activation also decreases the secretion of NF-kB-dependent inflammatory cytokines. The effect of PIO and ROSI was analyzed for the following panel of pro-inflammatory cytokines: interleukin (IL)-1 isoform α and β, interleukin (IL)-6, granulocyte colony-stimulating factor (G-CSF), keratinocyte chemo-attractant (KC), LPS-induced CXC chemokine (LIX), monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein 2 (MIP-2). After treatment with PIO or ROSI (50 µM), LPS (100ng/ml) or their combination for 12 hours, the medium was collected, and proteins harvested to normalize for the number of cells. Cytokine concentrations were assessed using a microsphere-based multiplex immunoassay (Luminex, MILLIPLEX™ MAP). In line with our previous studies, CF cells secreted a higher amount of cytokines, respect to WT cells. Moreover, treatment with both agonists significantly reduced the secretion of MCP-1, G-CSF, MIP-2, KC and LIX (figure 6).
Figure 6. PPAR-γ stimulation inhibits LPS-induced secretion of NF-kB-dependent proinflammatory cytokines in Cftr-KO cholangiocytes.
Luminex assay of proinflammatory cytokines monocyte chemotactic protein-1 (MCP1), macrophage inflammatory protein 2 (MIP-2), keratinocyte chemo-attractant (KC), granulocyte colony-stimulating factor (G-CSF) and LPS-induced CXC chemokine (LIX) secreted in culture medium of WT and Cftr-KO cholangiocytes, before and after 12h of treatment with LPS (100 ng/ml) alone or in combination with pioglitazone (PIO) or rosiglitazone (ROSI) (50 µM). Data presented were generated from n=4 experiments and are normalized for the total cellular protein content. *p<.05 vs ctl; **p<.01 vs ctl; #p<.05 vs ctl; ##p<.01 vs ctl; ●p<.05 vs LPS; ●●p<.01 vs LPS; ▲p<.05 vs WT+LPS; ▲▲p<.01 vs WT+LPS.
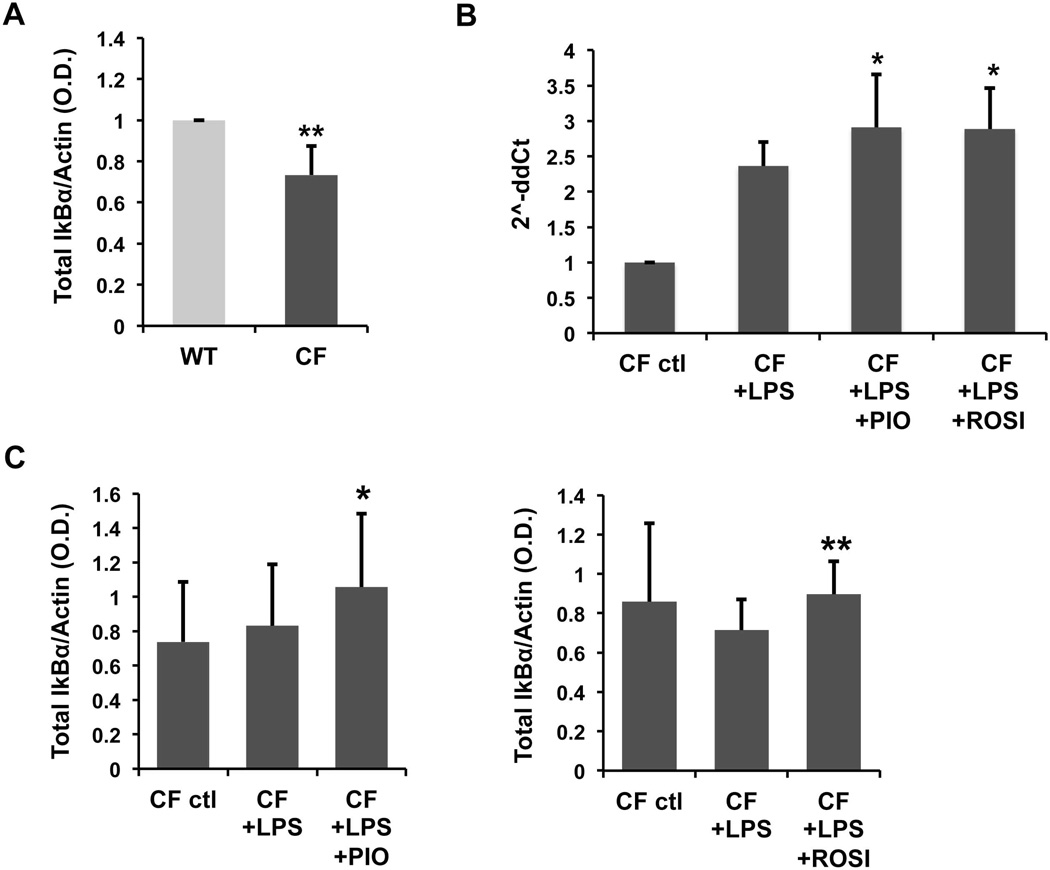
PPAR-γ stimulation inhibits the activation of NF-kB via an IkBα-dependent mechanism
To study the mechanism by which ligand-activated PPAR-γ blocks inflammation in CF cholangiocytes, we analyzed the effect of PIO and ROSI on crucial steps that lead to activation of the NF-kB pathway. In resting cells, the nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IkBα) retains the NF-kB subunits p50/p65 in a cytoplasmic inactive complex. In the presence of inducers of NF-kB, IkBα is phosphorylated by the kinase complex IKK and degraded, so that the activated p65 subunit can translocate to the nucleus (35). We measured the protein expression of total IkBα and found that was significantly reduced in CF, as compared to WT cells, which is consistent with a higher activation of NF-kB (figure 7A).
Figure 7. PPAR-γ stimulation inhibits the activation of NF-kB via an IkBα-dependent mechanism.
Protein expression of total IkBα was analyzed by western blot. (A) Data represent the average of densitometric analysis of n=7 experiments in WT and CF cells, normalized for actin. **p<.01 vs WT. (B) Real-time PCR analysis of IkBα gene in CF cells treated for 6h with LPS (100 ng/ml) alone or in combination with pioglitazone (PIO) or rosiglitazone (ROSI) (50 µM). Bar graph represents the average of n=4 independent experiments in duplicate, normalized to GAPDH. *p<.05 vs LPS. (C) CF cells were treated as in (B). Bar graph represents the average of densitometric analysis of n=3 experiments, normalized for actin. *p<.05 vs CF+LPS; **p<.01 vs CF+LPS.
However, treatment with PIO and ROSI significantly increased IkBα protein expression after LPS challenge (figure 7C), indicating that PPAR-γ stimulation controls NF-kB activation by inducing its negative regulator IkBα. In addition, we found that, both PIO and ROSI significantly upregulated IkB-α gene expression (figure 7B), suggesting that PPAR-γ agonists modulate IkB-α protein at the transcriptional level.
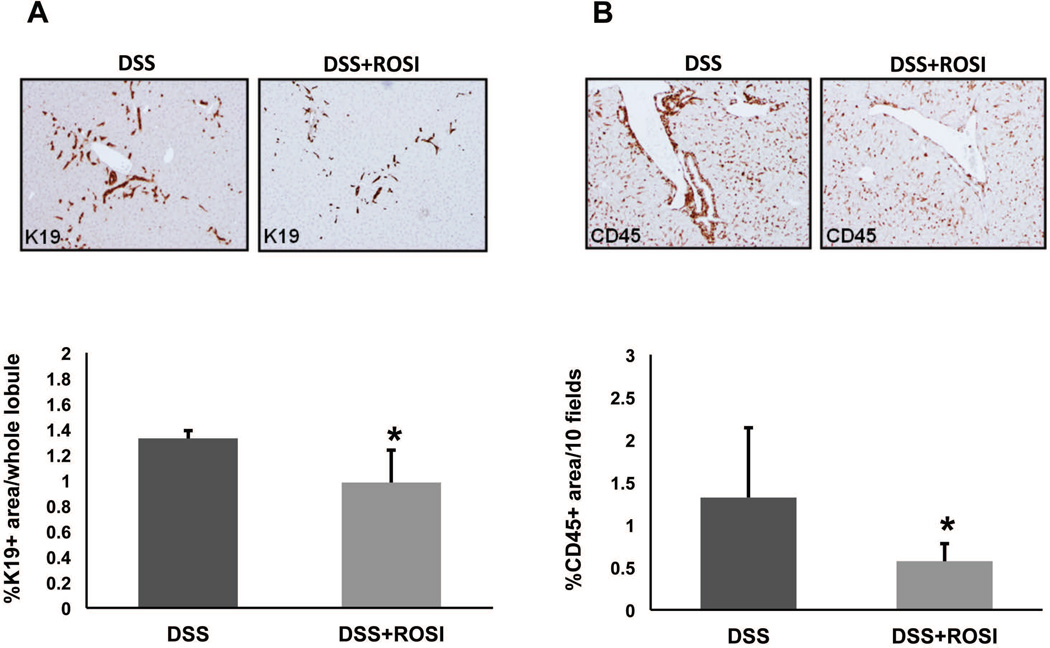
PPAR-γ stimulation in vivo reduces biliary damage and inflammation in Cftr-KO mice treated with DSS
To confirm in vivo the anti-inflammatory properties of PPAR-γ activation in CF cholangiopathy, we treated Cftr-KO mice with DSS to induce a portal endotoxemia, as previously described (11), in presence or absence of PPAR-γ agonists. At the end of the treatment, liver tissues were harvested and stained with K19, to quantify the biliary and progenitor cell compartment expansion as a marker of biliary damage, and with CD45, to quantify the amount of leukocyte infiltration in the portal space as a marker of inflammation. As shown previously (11, 36), DSS-treated Cftr-KO mice show bile duct proliferation and a significant infiltration of inflammatory cells in the portal space. Unfortunately, treatment with PIO showed a significant liver toxicity in both CF and WT mice treated with DSS (data not shown) and was not considered for further investigation. Conversely, in Cftr-KO mice treated with DSS and ROSI (10 mg/Kg by i.p. daily), bile duct proliferation (figure 8A) and inflammatory cell infiltration (figure 8B) were significantly reduced, as determined by computer-assisted morphometric analysis of K19 and CD45 positive areas, consistent with a protective effect of PPAR-γ activation in vivo.
Figure 8. PPAR-γ stimulation reduces biliary damage and inflammation in Cftr-KO mice treated with DSS.
Cftr-KO mice were treated with dextran sodium sulfate (DSS) (n=5) or with DSS and rosiglitazone (ROSI) (n=5) (10 mg/Kg by i.p. daily,). At the end of the treatment, liver tissues were harvested and stained with the cholangiocyte-specific marker cytokeratin19 (K19) (A) or the leukocyte specific marker CD45 (B). Computer-assisted morphometric analysis of K19 and CD45 positive areas shows that rosiglitazone treatment significantly reduced the bile duct proliferation and inflammatory cell infiltration in Cftr-KO mice treated with DSS. *p<0.05 vs DSS only.
DISCUSSION
CFLD is the third cause of death in patients with cystic fibrosis. Little is known about the pathogenesis and etiology of CFLD and current clinical management is limited to supportive care and the administration of choleretic bile acids, such as UDCA (3, 4, 12, 13). Treatment with UDCA has shown some benefit, however recent data on the pathophysiology of the disease suggest that treatment of CFLD should also aim at controlling innate immune responses and NF-kB-dependent inflammation (7, 8, 10, 37). Studies from our group demonstrated that TLR4/NF-kB-dependent innate immune responses are deregulated in cholangiocytes that lack CFTR and are exposed to bacterial-derived endotoxins (11).
Among druggable pathways that can be targeted to decrease innate immune responses, nuclear receptors are attracting attention, and several specific drugs are in development or already in the market. In this study, we sought to investigate the ability of PPAR-γ to modulate the innate inflammatory responses initiated by activation of TLRs. In particular, our results provide evidence that activation of PPAR-γ signaling is able to repress the NF-kB-dependent inflammation in CF biliary epithelium, by stimulating the gene and protein expression of NF-kB negative regulator IkBα. We also provide evidence that administration of PPAR-γ agonist ROSI ameliorates liver biliary damage and inflammation in vivo in CFTR-defective mice providing direct evidence that this mechanism is of pathogenetic relevance.
Nuclear receptors include a wide range of ligand-activated transcription factors that influence cellular responses by altering gene expression (14–16). Although initially recognized for their role in metabolism and cellular homeostasis, some NRs, including PPARs, are now emerging as important negative regulators of inflammation even though the knowledge of their mechanisms of action is still limited (17–23).
NRs are differentially expressed in distinct liver cell types (24, 26). We found that primary cholangiocytes isolated from wild type and CFTR-defective mice express multiple receptors, including VDR, LXR-β, FXR and all the three isoforms of PPARs, consistent with previously published data (26). Conflicting reports have been described concerning PPAR-γ expression in CF (38). PPAR-γ was found decreased in CF airways epithelial cells (39) and unchanged in CF whole liver (40). Interestingly, we observed that Cftr-KO cholangiocytes had a significant higher gene and protein expression of PPAR-γ as compared to control cells in which PPAR-γ is expressed at low levels. This finding led us to further investigate whether the increased PPAR-γ expression was correlated with an increase in the receptor activity in CF cells. PPARs are constitutively bound to the DNA, where they repress the transcription of specific target genes. Upon binding of activator ligands, the transactivation pathway is switched on and the transcription of these genes is activated (27). We analyzed the expression of classical genes activated by PPAR-γ and found that this was comparable between CF and control cells indicating that, in spite of an increased expression of PPAR-γ in CF cholangiocytes, the transcriptional activity of the receptor was not different from WT cells.
Similar to other PPAR members, in a given tissue, the activation state of the receptor is modulated by the relative amount of endogenous PPAR-γ ligands (29, 32). We reasoned that proper activation of PPAR-γ in CF might be impaired by a limited availability of endogenous activators. Eicosanoids are signaling molecules derived from key fatty acids precursors present at the cell membrane level, such as LA, or from the intermediate metabolite AA and DHA. Selected classes of anti-inflammatory PUFAs can act as PPAR-γ endogenous ligands (21, 29–32). Altered ratios of AA/LA and AA/DHA have been reported in the blood and mucosal samples of CF patients and Cftr-KO mice (41–43). Consistent with the above findings, our lipidomic analysis showed an increase in AA/LA and AA/DHA ratios, in Cftr-defective cells, compared to their controls. Recent data showed that CFTR may alter AA/LA metabolism through an AMPK-mediated increase in Δ5 and Δ6 desaturases (44). Altered AA/LA ratio and a lower production of DHA-mediated endogenous activators of PPAR-γ in CF biliary cells may account for the increased expression of the receptor as a compensatory mechanism to counteract a lower activation state.
We next investigated whether the stimulation of PPAR-γ with synthetic ligands controls TLR4/NF-kB-driven inflammation in CFTR-defective biliary epithelium. We have shown that CFTR-defective biliary epithelial cells, in response to endotoxins, activate a strong TLR4-dependent inflammatory response, characterized by a higher NF-kB activity, which in turn is responsible for a sustained and increased production of several pro-inflammatory cytokines (11). To study the effect of PPAR-γ stimulation on NF-kB activation, we tested the effect of two different thiazolidinedione compounds. Thiazolidinedione are a family of potent synthetic agonists that activate PPAR-γ, and include troglitazone, ciglitazone, rosiglitazone and pioglitazone. Of these, troglitazone has been removed from the market due to significant hepatotoxicity. However, rosiglitazone and pioglitazone are currently FDA approved in the US for type-2 diabetes treatment and act as strong insulin sensitizers (45, 46). We found that treatment of WT and CF cells with PIO and ROSI alone or in the presence of LPS, significantly reduced the activation of NF-kB in CF cells, as shown by the significant reduction in the transcriptional activity of the NF-kB promoter. Moreover, the effect of thiazolidinediones on the activation of NF-kB was significantly reversed by pretreatment with GW9662, a selective PPAR-γ antagonist that irreversibly blocks the ligand-binding pocket of the receptor, and by experiment of gene silencing, indicating the specificity of the effects and the activation of a receptor-dependent pathway.
Furthermore, PPAR-γ activation significantly reduced the secretion of pro-inflammatory cytokines downstream to the NF-kB pathway. As previously described, CF cells secreted a higher amount of cytokines, respect to WT cells, after stimulation with LPS. After treatment with PIO and ROSI, the secretion of MCP-1, G-CSF, MIP-2, KC and LIX was significantly reduced in both groups of cells, with a more prominent effect in CF cells. The weaker effect in control cells, where PPAR-γ is less expressed, suggests that the anti-inflammatory effect was dependent on the amount of receptor available. Interestingly, G-CSF and especially KC (the murine homologue of human IL-8) are strongly involved in proliferation, survival and chemotaxis of neutrophils, which we have previously described as the major constituents of the inflammatory infiltrate in Cftr-KO livers, in the setting of endotoxin-induced damage (11).
PPARs may control inflammation through multiple mechanisms unique to a given cell type. Some of these mechanisms occur in the cytoplasm by interfering with the NF-kB activating machinery (47). The activation of NF-kB requires the inactivation by phosphorylation of its cytoplasmic inhibitor IkBα, by the IKK kinase complex (35). To dissect the mechanism by which ligand-activated PPAR-γ blocked inflammation in CF cholangiocytes, we analyzed the effect of PIO and ROSI on different steps of the NF-kB pathway. The gene and protein expression of IkBα in CF cells increased significantly after treatment with both agonists in combination with LPS. These findings indicate that the anti-inflammatory effect of PPAR-γ activation occurs, at least in part, through a transcriptional control of the NF-kB negative regulator.
We next investigated the effect of PPAR-γ activation in vivo in Cftr-KO mice treated with DSS, a model previously used by others and ourselves to induce an inflammatory cholangiopathy, caused by the translocation of intestinal bacteria into the portal circulation (11). PIO treatment in WT and CF mice showed a significant hepatotoxic effect and, therefore, was not used for further experiments. Conversely, Cftr-KO mice treated with DSS in combination with ROSI showed a significant decrease in biliary damage and portal inflammation, when compared to the control group where only DSS was administered. Liver toxicity by pioglitazone was clearly unrelated to the stimulation of PPAR-γ because no liver toxicity was seen with ROSI, a more PPAR-γ-specific agonist (48). PIO is commonly used in humans for the treatment of diabetes, and therefore our hypothesis is that liver toxicity in our experimental conditions is due to a species/strain-related off target effect of PIO. However, further toxicology studies will eventually be needed to clarify this aspect.
In conclusion, these studies show a novel function of the nuclear receptor PPAR-γ in controlling biliary epithelium inflammation. PPAR-γ is expressed more in CF biliary epithelium, but a defective production of bioactive ligands impairs the proper activation of the receptor, contributing to the chronic inflammatory state of CFTR-defective cholangiocytes. On the other hand, exogenous stimulation of PPAR-γ has anti-inflammatory properties in the CF biliary epithelium and limits the TLR4/NF-kB-dependent innate immune responses to endotoxins. Finally, our in vivo results strongly indicate the pathophysiological relevance of the described mechanism and the potential value of PPAR-γ as a therapeutic target in CF and possibly in other inflammatory cholangiopathies.
Supplementary Material
Acknowledgments
The authors thank Chiara Gabbi, University of Houston (TX), for helpful discussions and Gary Cline, Yale University, for support with the lipidomic analysis and to Blake Acquarulo for proofreading the paper.
Financial support
This work was supported by the NIH grant RO1 DK096096, by NIH grant DK34989, Silvio O. Conte Digestive Disease Research Core Centers; by Fondazione Fibrosi Cistica (grant #18-2012) and by Telethon (grant #GGP12133) to M.S., by PSC Partners Seeking a Cure Foundation to R.F and partially by NIH grant U24 DK-059635.
List of abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- CFLD
cystic fibrosis-associated liver disease
- TLR
toll-like receptor
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NR
nuclear receptor
- PPAR
peroxisome proliferator-activated receptor
- DSS
dextran sodium sulfate
- PIO
pioglitazone
- ROSI
rosiglitazone
- LPS
lipopolysaccharide
- IkBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- K19
cytokeratin19
- AA
arachidonic acid
- LA
linoleic acid
- DHA
docosahexaenoic acid
- G-CSF
granulocyte colony-stimulating factor
- KC
keratinocyte chemo-attractant
- LIX
LPS-induced CXC chemokine
- MCP-1
monocyte chemotactic protein-1
- MIP-2
macrophage inflammatory protein 2.
Footnotes
Potential conflict of interests: Nothing to disclose
REFERENCES
- 1.Riordan JR. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 3.Feranchak AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Semin Liver Dis. 2001;21:471–488. doi: 10.1055/s-2001-19030. [DOI] [PubMed] [Google Scholar]
- 4.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 5.Colombo C, Battezzati PM. Liver involvement in cystic fibrosis: primary organ damage or innocent bystander? J Hepatol. 2004;41:1041–1044. doi: 10.1016/j.jhep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Colombo C, Battezzati PM, Strazzabosco M, Podda M. Liver and biliary problems in cystic fibrosis. Semin Liver Dis. 1998;18:227–235. doi: 10.1055/s-2007-1007159. [DOI] [PubMed] [Google Scholar]
- 7.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol. 2007;292:L383–L395. doi: 10.1152/ajplung.00403.2005. [DOI] [PubMed] [Google Scholar]
- 9.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, et al. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 10.Verhaeghe C, Delbecque K, de Leval L, Oury C, Bours V. Early inflammation in the airways of a cystic fibrosis foetus. J Cyst Fibros. 2007;6:304–308. doi: 10.1016/j.jcf.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13:529–536. doi: 10.1097/MCP.0b013e3282f10a16. [DOI] [PubMed] [Google Scholar]
- 13.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 14.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 15.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tata JR. Signalling through nuclear receptors. Nat Rev Mol Cell Biol. 2002;3:702–710. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- 17.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 18.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 19.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 20.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 21.Harmon GS, Lam MT, Glass CK. PPARs and lipid ligands in inflammation and metabolism. Chem Rev. 2011;111:6321–6340. doi: 10.1021/cr2001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613–864. doi: 10.1155/2013/613864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firrincieli D, Zuniga S, Poupon R, Housset C, Chignard N. Role of nuclear receptors in the biliary epithelium. Dig Dis. 2011;29:52–57. doi: 10.1159/000324129. [DOI] [PubMed] [Google Scholar]
- 25.Spirli C, Fiorotto R, Song L, Santos-Sacchi J, Okolicsanyi L, Masier S, et al. Glibenclamide stimulates fluid secretion in rodent cholangiocytes through a cystic fibrosis transmembrane conductance regulator-independent mechanism. Gastroenterology. 2005;129:220–233. doi: 10.1053/j.gastro.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Xia X, Jung D, Webb P, Zhang A, Zhang B, Li L, et al. Liver × receptor beta and peroxisome proliferator-activated receptor delta regulate cholesterol transport in murine cholangiocytes. Hepatology. 2012;56:2288–2296. doi: 10.1002/hep.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 28.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, et al. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nat Med. 16:313–318. doi: 10.1038/nm.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Giudetti AM, Cagnazzo R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012;99:57–67. doi: 10.1016/j.prostaglandins.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Ott J, Hiesgen C, Mayer K. Lipids in critical care medicine. Prostaglandins Leukot Essent Fatty Acids. 2011;85:267–273. doi: 10.1016/j.plefa.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int Arch Allergy Immunol. 2003;132:310–321. doi: 10.1159/000074898. [DOI] [PubMed] [Google Scholar]
- 33.McKinnon B, Bersinger NA, Mueller MD. Peroxisome proliferating activating receptor gamma-independent attenuation of interleukin 6 and interleukin 8 secretion from primary endometrial stromal cells by thiazolidinediones. Fertil Steril. 2012;97:657–664. doi: 10.1016/j.fertnstert.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 35.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 36.Blanco PG, Zaman MM, Junaidi O, Sheth S, Yantiss RK, Nasser IA, et al. Induction of colitis in cftr™/™ mice results in bile duct injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G491–G496. doi: 10.1152/ajpgi.00452.2003. [DOI] [PubMed] [Google Scholar]
- 37.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, et al. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol. 2007;73:1982–1994. doi: 10.1016/j.bcp.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Dekkers JF, van der Ent CK, Kalkhoven E, Beekman JM. PPARgamma as a therapeutic target in cystic fibrosis. Trends Mol Med. 2012;18:283–291. doi: 10.1016/j.molmed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB. Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;295:L303–L313. doi: 10.1152/ajplung.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pall H, Zaman MM, Andersson C, Freedman SD. Decreased peroxisome proliferator activated receptor alpha is associated with bile duct injury in cystic fibrosis transmembrane conductance regulator™/™ mice. J Pediatr Gastroenterol Nutr. 2006;42:275–281. doi: 10.1097/01.mpg.0000189368.37535.42. [DOI] [PubMed] [Google Scholar]
- 41.Al-Turkmani MR, Freedman SD, Laposata M. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2007;77:309–318. doi: 10.1016/j.plefa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 43.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftR™/™) mice. Proc Natl Acad Sci U S A. 1999;96:13995–14000. doi: 10.1073/pnas.96.24.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umunakwe OC, Seegmiller AC. Abnormal n-6 fatty acid metabolism in cystic fibrosis is caused by activation of AMP-activated protein kinase. J Lipid Res. 2014;55:1489–1497. doi: 10.1194/jlr.M050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karak M, Bal NC, Bal C, Sharon A. Targeting peroxisome proliferator-activated receptor gamma for generation of antidiabetic drug. Curr Diabetes Rev. 2013;9:275–285. doi: 10.2174/15733998113099990065. [DOI] [PubMed] [Google Scholar]
- 47.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright MB, Bortolini M, Tadayyon M, Bopst M. Minireview: Challenges and opportunities in development of PPAR agonists. Mol Endocrinol. 2014;28:1756–1768. doi: 10.1210/me.2013-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.