Summary
Hepatocyte transplantation has the potential to cure inherited liver diseases, but its application is impeded by a scarcity of donor livers. Therefore, we explored whether transplantation of hepatocyte-like cells (iHeps) differentiated from human induced pluripotent stem cells (iPSCs) could ameliorate inherited liver diseases. iPSCs reprogrammed from human skin fibroblasts were differentiated to iHeps, which were transplanted into livers of uridinediphosphoglucuronate glucuronosyltransferase-1 (UGT1A1)-deficient Gunn rats, a model of Crigler-Najjar syndrome 1 (CN1), where elevated unconjugated bilirubin causes brain injury and death. To promote iHep proliferation, 30% of the recipient liver was X-irradiated before transplantation, and hepatocyte growth factor was expressed. After transplantation, UGT1A1+ iHep clusters constituted 2.5%–7.5% of the preconditioned liver lobe. A decline of serum bilirubin by 30%–60% and biliary excretion of bilirubin glucuronides indicated that transplanted iHeps expressed UGT1A1 activity, a postnatal function of hepatocytes. Therefore, iHeps warrant further exploration as a renewable source of hepatocytes for treating inherited liver diseases.
Highlights
-
•
Human skin fibroblast-derived iPSCs were differentiated to hepatocyte-like iHeps
-
•
iHeps were transplanted into Gunn rats, a model of Crigler-Najjar syndrome 1
-
•
Engraftment of the iHeps in Gunn rat livers reduced serum bilirubin levels
-
•
iHeps may be potentially useful in treating liver-based metabolic disorders
Roy-Chowdhury and colleagues show that human iPSCs differentiated to hepatocyte-like iHep cells engraft into the liver of UGT1A1-deficient Gunn rats, a model of Crigler-Najjar syndrome 1, where hyperbilirubinemia causes brain injury and death. After iHep transplantation, serum bilirubin declined by 30%–60% and bilirubin glucuronides appeared in bile, indicating that the engrafted iHeps expressed UGT1A1, a postnatal hepatocyte function.
Introduction
Stem cells offer enormous promise as a source of differentiated cells for curing human diseases. Although liver transplantation is curative for life-threatening metabolic liver disorders (Åberg et al., 2011), minimally invasive catheter infusion of isolated hepatocytes into the liver can partially correct metabolic liver diseases and can greatly reduce the risk of fatal complications (Fox et al., 1998; Lysy et al., 2008; Roy-Chowdhury et al., 2009; Fisher and Strom, 2006). Host conditioning regimens developed in our laboratories (Guha et al., 2002) allow the expansion of engrafted donor hepatocytes, leading to complete cures of animal models of metabolic liver diseases. This host conditioning strategy is now being tested in a clinical hepatocyte transplant trial (University of Pittsburgh, Institutional Review Board [IRB] number PRO09040497).
Clinical application of hepatocyte transplantation has been impeded by the scarcity of donor livers, which are prioritized for organ transplantation. Pioneering studies have shown that somatic cells can be reprogrammed into induced pluripotent stem cells (iPSCs) that resemble embryonic stem cells (ESCs) (Takahashi and Yamanaka, 2006; Yu et al., 2007). Recent successes in differentiating ESCs and iPSCs into hepatocyte-like cells (iHeps) (Basma et al., 2009; Lavon et al., 2004; Schwartz et al., 2005; Cai et al., 2007; Duan et al., 2010; Si-Tayeb et al., 2010; Song et al., 2009) has opened the possibility of using iPSCs as a renewable source of human and, possibly, autologous hepatocytes.
Although iPSCs at various stages of differentiation have been reported to engraft and improve the survival of mice with severe toxic injury of the liver, neither the cause of death from the toxic liver injury nor the correction of any specific liver function by the engrafted cells has been demonstrated (Liu et al., 2011). In this study, we examined the efficacy of transplanting human iHep cells into Gunn rats, a well characterized animal model of Crigler-Najjar syndrome 1 (CN1) (Roy-Chowdhury et al., 1991, 1993). CN1 is an autosomal recessive disorder in which genetic lesions of UGT1A1 cause life-long unconjugated hyperbilirubinemia because of a lack of uridinediphosphoglucuronate glucuronosyltransferase 1A1-mediated bilirubin glucuronidation by hepatocytes (Bosma et al., 1992). CN1 is lethal unless treated with life-long daily phototherapy to reduce bilirubin levels. Even with aggressive therapy, patients remain at risk of bilirubin encephalopathy and death. Liver transplantation is the only definitive therapy (Ozçay et al., 2009). Gunn rats are well characterized animal models of CN1, with genetic and metabolic abnormalities similar to CN1 patients.
In this study, we transplanted human iHeps into the livers of Gunn rats. Proliferation of the transplanted cells was induced by preconditioning a single liver lobe by hepatic X-irradiation (HIR). HIR enhances the engraftment of transplanted cells by transiently disrupting the sinusoidal endothelial barrier. Additionally, reduction of the mitotic capacity of the irradiated host hepatocytes provides a competitive proliferative advantage to the engrafted cells (Guha et al., 2002; Yamanouchi et al., 2009). Here, as with patients, to increase the safety of HIR, we treated only one liver lobe, representing 30% of the liver mass, to achieve regional hepatic repopulation by the transplanted cells.
Results and Discussion
Characteristics of Adult Human Skin Fibroblast-Derived iPSCs
iPSCs had a typical ESC-like morphology; expressed pluripotency markers (Figure S1A) at levels similar to those of the H1 hESC line (WiCell); differentiated spontaneously to cells of mesodermal, endodermal, and ectodermal origin (Figure S1B); and gave rise to teratomas after injection in severe combined immunodeficiency (SCID) mice (Figure S1C). iPSCs were diploid and contained 46 intact chromosomes (Figure S1D), and RT-PCR showed that exogenous OCT-4, SOX2, KLF4, and c-MYC were silenced (data not shown).
Directed Differentiation of Human iPSCs and Characteristics of iHeps
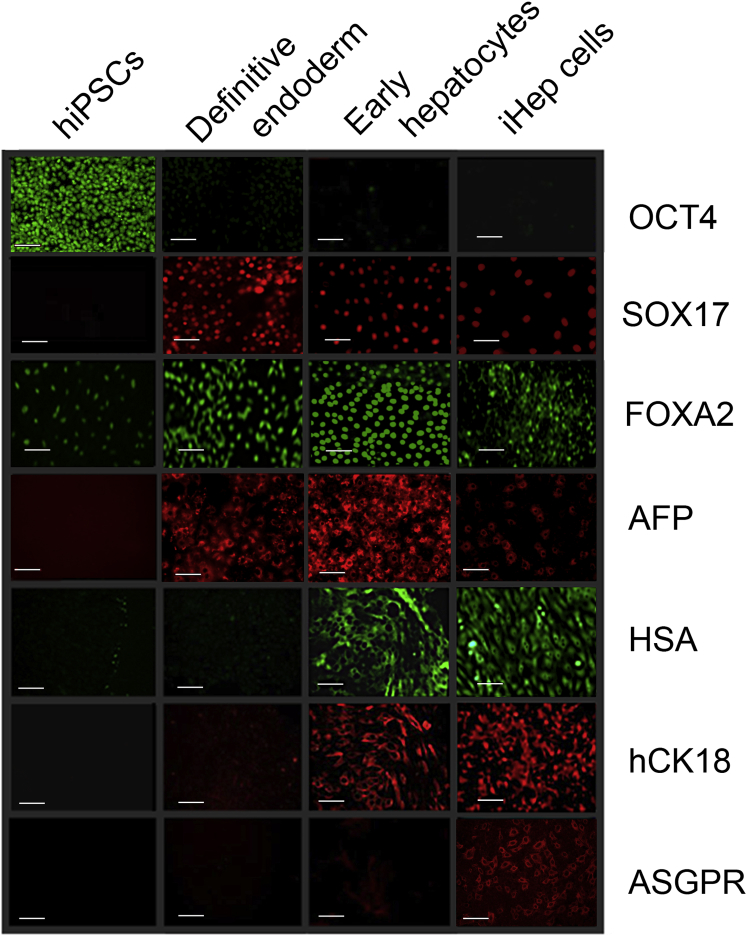
Undifferentiated iPSCs (Figure 1) expressed OCT-4 but there was very little expression of hepatocyte-preferred genes. After exposure to activin A and bFGF, OCT4 expression declined markedly, and the definitive endoderm marker SOX17 and the nuclear factor FOXA2 were expressed in 70%–85% of cells. The fetal hepatocyte marker α-fetoprotein (AFP) was expressed in 30%–50% of cells. After culturing with HGF and DMSO, AFP expression increased and human serum albumin (HSA) and CK18 were expressed. Following exposure to dexamethasone, the morphology of a majority of the cells changed toward that of primary human hepatocytes (Figures 2A–2C). A majority of these cells, termed iHeps, expressed HSA and cytokeratin 18, and less than half of the cells also expressed the asialoglycoprotein receptor (ASGPR), a marker of mature hepatocytes. AFP expression declined but was still seen in 1%–5% of cells (Figure 1). mRNA content measured by qRT-PCR showed gene expression of transcription factors that are important in hepatocyte development (e.g., HEX and PROX1) and maturation (e.g., HNF4α and C/EBPα) and secreted proteins as well as cytosolic, ER, plasma membrane, and peroxisomal proteins (Figure S2A). Confocal microscopy showed that ASGPR was distributed in both the plasma membranes and the cytoplasm (Figures S2B–S2D), whereas HSA was present in the cytoplasm (Figures S2E–S2G). In this cell population, some albumin-negative cells stained positive for SOX17, an early endodermal marker. SOX17 staining was absent or very faint in albumin-expressing cells (Figures S2H–S2K). Flow cytometry showed that 60%–90% of cells expressed HSA, whereas 28%–40% of cells expressed ASGPR (Figure 2D). At all stages, differentiation of iPSCs toward iHeps was similar to that seen with human embryonic stem cells (hESCs) (Basma et al., 2009). The iHeps exhibited hepatocyte-like characteristics, including glycogen storage (by periodic acid Schiff staining) (Figures 2E and 2F) and indocyanin green uptake (Figure 2I). They also showed uptake of dioctadecylindocarbocyanine (DiI)-labeled low-density lipoproteins (Figures 2G and 2H). Western blot analysis of cell homogenates confirmed that AFP appeared after iPSC exposure to activin A plus bFGF and increased after exposure to HGF and DMSO (Figure 2J). After exposure to dexamethasone, AFP decreased markedly. AFP was undetectable in mature primary hepatocytes. A trace of HSA was found in iPSCs and at the definitive endoderm stage (Figure 2J). HSA expression increased markedly after exposure to HGF plus DMSO. After exposure to dexamethasone, the HSA content reached 25% of that in freshly isolated primary human hepatocytes (Figure 2L).
Figure 1.
Expression of Cellular Marker Proteins during Differentiation of Human iPSCs
iPSCs were differentiated sequentially to definitive endoderm, early hepatocytes, and iHep cells as described in the text. Immunofluorescence staining for human OCT4, SOX17, FOXA2, AFP, HSA, CK18, and ASGPR is shown. Scale bars, 100 μm. See also Figure S1.
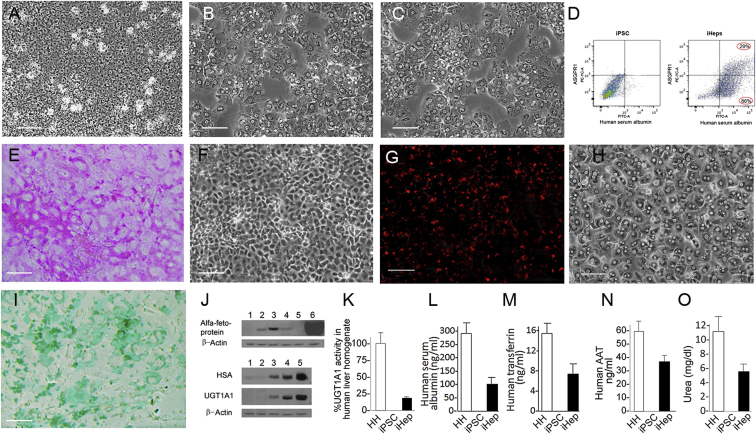
Figure 2.
Characterization of the iHep Cells
The cells were differentiated in culture as described in Experimental Procedures.
(A–C) Phase contrast image of iPS cells, iHeps, and human primary hepatocytes, respectively. Scale bars, 100 μm.
(D) Flow cytometric analysis of HSA and ASGPR in iPSCs and iHeps. Scale bars, 100 μm.
(E) Periodic acid Schiff staining for glycogen content. Scale bars, 100 μm.
(F) Phase contrast image of (E).
(G) Uptake of DiI-LDL.
(H) Phase contrast image of (G).
(I) ICG uptake.
(J) Western blot for α-fetoprotein, human serum albumin, and UGT1A1: 1, undifferentiated human iPS; 2, definitive endoderm; 3, early hepatocytes; 4, hepatocyte-like cells (after exposure to dexamethasone); 5, human liver primary hepatocytes; 6, human hepatoma cell line HepG2.
(K) UGT1A1 activity toward bilirubin. The values are shown as percentage of the mean enzyme activity in homogenates of normal human liver specimens. Means of four determinations ± SD are shown. HH, primary human hepatocytes. The values are shown per million cells (means ± SD of data from four different iHep preparations).
(L–N) Secretion of human proteins into the media. After a fresh change of the serum-free culture medium, the cells were cultured for 24 hr, the media were harvested, and specific protein concentrations were determined by ELISA. The values are shown per million cells (means ± SD of data from four different iHep preparations).
(O) Urea secreted into the media. Cell culture and media harvesting was as in (L–N). The values are shown per million cells (means ± SD of data from four different iHep preparations).
See also Figure S2.
In contrast to AFP and HSA, significant levels of UGT1A1 appeared only after exposure to HGF plus DMSO. Developmentally, UGT1A1 is expressed only with hepatocyte maturation after birth. After exposure to dexamethasone, the UGT1A1 content in iHeps increased markedly (Figure 2J), and UGT1A1 activity toward bilirubin was 18.8 ± 3.4 to 25.00 ± 4.6 pmole/min/mg protein (mean ± SD, n = 4), which is 18%–22% of the mean UGT1A1 activity in isolated normal primary human hepatocytes (Figure 2K).
As assessed by ELISA, the iHeps secreted the liver-specific proteins HSA, transferrin, and α-1-antitrypsin (ΑΑT) into the culture medium at 33%, 46%, and 60% of the rate of secretion by primary human hepatocytes under identical conditions (Figures 2L–2N). iHeps secreted urea at approximately half the rate of primary human hepatocytes (Figure 2O). Undifferentiated iPSCs did not secrete HSA, AAT, transferrin, or urea (Figures 2K–2O).
Transplantation and Repopulation of Gunn Rat Livers with iHeps
Gunn rats were transplanted with 2 × 106 viable HSA-positive iHeps by intrasplenic injection as described previously (Zhou et al., 2012). Because the rats are immune-competent, they were injected with tacrolimus (2 mg/kg subcutaneously daily) starting 7 days before transplantation to prevent xenograft rejection (Basma et al., 2009). For comparison, two rats received 2 × 106 human hepatocytes isolated from the livers of fumaryl acetoacetate hydrolase-deficient, Rag2−/−, IL2-γc−/− (FRG) mice repopulated with human hepatocytes (purchased from Yecuris). To induce the preferential proliferation of transplanted cells, recipient rats received preparative HIR (50 Gy) to the median liver lobe, constituting 30% of the liver mass, on the day before transplantation and were injected with an adenoviral vector expressing HGF (1012 particles) 24 hr after the transplant (Zhou et al., 2012). Control rats (n = 4) received immunosuppression and HIR but were injected with saline instead of iHeps or primary hepatocytes.
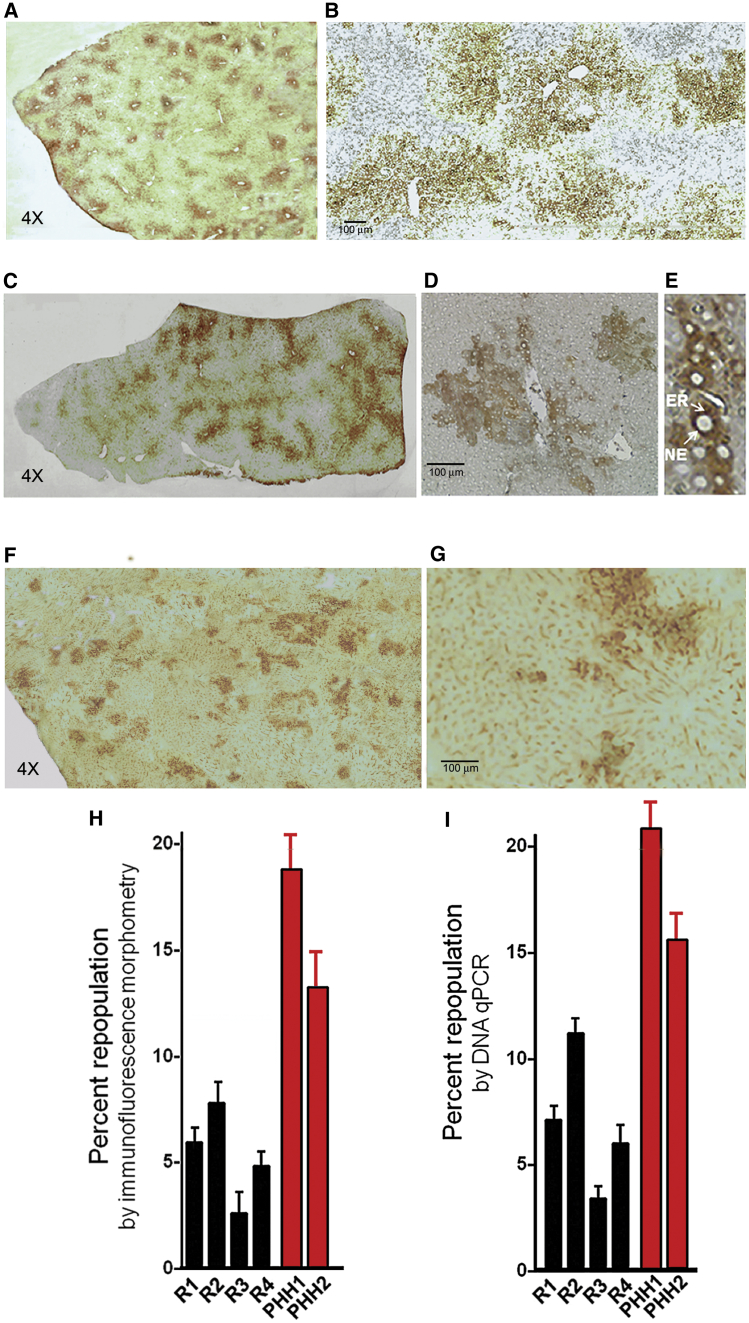
Immunohistochemistry of liver sections of the preconditioned median lobe using a human UGT1A group-specific antibody showed clusters of human hepatocytes (Figures 3A and 3B) or iHep cells (Figures 3C–3E). A similar level of repopulation with iHeps was found by staining for HSA (Figures 2F and 2G). Image analysis using the ImageJ program showed that, 4-6 months after transplantation, the transplanted hepatocytes and iHeps comprised 17% ± 3.1% and 5.1% ± 2.6% (mean ± SD) of the hepatocyte mass, respectively (Figure 3H). In non-irradiated liver lobes, engrafted iHeps or primary human hepatocytes were seen as single cells or in groups of two to three cells (data not shown). Because hepatocytes engraft initially as single cells, the formation of human cell clusters indicated the proliferation of the engrafted cells in the preconditioned lobe. As expected, no human UGT1A-positive cells were found in control Gunn rat livers (data not shown). Liver repopulation was also assessed by real-time genomic DNA PCR for HLA-A54 (Bick et al., 2008) using primers that did not yield any amplicons from the rat genomic DNA template. As an internal control, we used the 36B4 gene (O’Callaghan et al., 2008), which is amplified with equal efficiency from rat and human genomic DNA (see details in the Supplemental Experimental Procedures). By this measurement, human hepatocytes and iHeps constituted 18.2 ± 5.5 and 7.1 ± 4.8 (means ± SD) of the cells in the preconditioned median lobe (Figure 3I) but were below the detectable level in non-irradiated liver lobes. To assess protein secretion by the engrafted human hepatocytes and iHeps, we measured HSA and human transferrin in recipient Gunn rat sera by ELISA. Four to six months after transplantation, mean HSA and human transferrin levels in the four iHep recipients were 48–73 (60 ± 14) μg/ml and 3.2–8.3 (5.75 ± 2.7) μg/ml, respectively (Figures S3A and S3B). In recipients of primary human hepatocytes, the levels were 232 ± 25 μg/ml and 27 ± 5 μg/ml, respectively (Figures S3A and S3B). These levels were lower than what might be predicted from the extent of liver repopulation, which may reflect the limitations of xenografting in immunocompetent recipients. Both proteins were undetectable in pre-transplant Gunn rat sera and in sham-treated controls.
Figure 3.
Repopulation of Gunn Rat Livers with Transplanted Human iHep Cells
(A–G) Four to six months after transplantation, livers were harvested from Gunn rats receiving iHeps or primary human hepatocytes (PHH), and cryosections were immunostained with a monoclonal antibody (WP1) that recognizes the carboxy-terminal region of human UGT1A isoforms (A–E) or an HSA-specific antibody (F and G). Representative sections of HIR-preconditioned median lobes are shown.
(A) recipient of PHH. Magnification, 4×.
(B) Section of the same lobe as in (A). Magnification, 10×.
(C) An iHep recipient. Magnification, 4×.
(D) The same lobe. Magnification, 10×.
(E) The same lobe showing characteristic cytoplasmic and nuclear envelope distribution of UGT1A1. Magnification, 40×.
(F) An iHep recipient, stained for HSA. Magnification, 4×.
(G) The same lobe. Magnification, 10×.
(H) Morphometric determination of percent repopulation by human UGT1A1-positive engrafted iHep cells. Each liver lobe was cut into six pieces, and cryosections from each piece were immunostained for human UGT1A1 as in (A). The proportion of hepatocytes that were positively stained was determined by counting ten fields representing 15,000 cells. Data from the median lobes of four individual iHep recipient rats (R1, R2, R3, and R4) and two PHH recipient rats (PHH1 and PHH2) are shown.
(I) Quantification of liver repopulation by iHeps by genomic DNA PCR. Genomic DNA was extracted from homogenates of liver lobes of the four recipient Gunn rats. The repopulating human cells were quantified by qPCR of the HLA-A54 gene as described in the Supplemental Experimental Procedures. The highly conserved 36B4 gene was amplified as an internal control for both human and rat genes. Data are shown from the median lobes of recipient Gunn rats as in (F).
See also Figure S3.
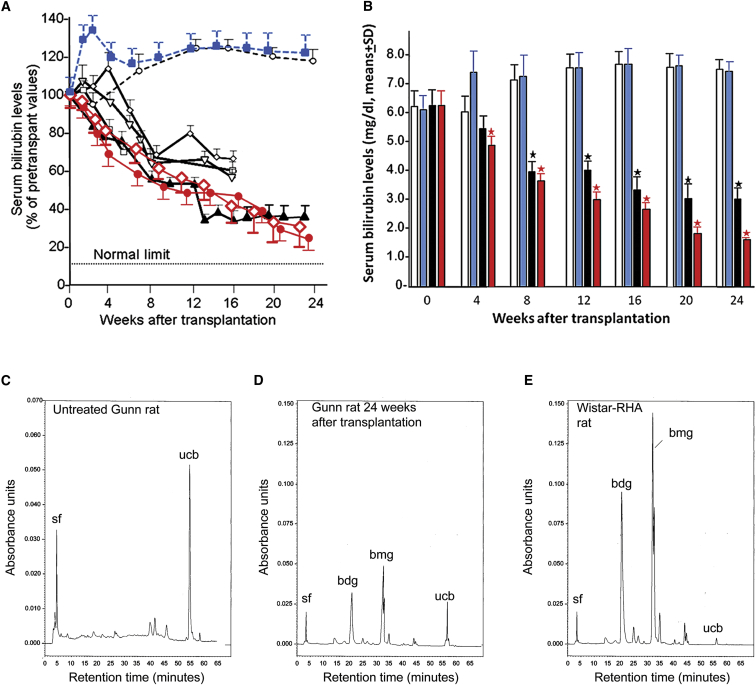
To assess the correction of the metabolic abnormality in Gunn rats, we determined hepatic UGT1A1 activity toward bilirubin and serum bilirubin levels in transplant recipients. UGT1A1 activity was undetectable in livers of control Gunn rats. In homogenates of preconditioned median lobes of Gunn rats receiving human hepatocytes, UGT1A1 activities were 3.2 ± 0.4 and 3.6 ± 0.6 nmol/g wet weight/min−1 (mean of three determinations ± SD). UGT1A1 activities in iHep recipients were 0.52 ± 0.25 to 2.19 ± 0.38 nmol/g wet weight/min−1 (mean ± SD, n = 6), which was 1.5% to 5% of the mean UGT1A1 activity in homogenates of normal human liver (n = 4). In untreated control Gunn rats, serum bilirubin levels increased with age (Figure 4A). In sham-treated controls, serum bilirubin increased to levels above those in untreated Gunn rats for 4–8 weeks, after which the levels equalized. In recipients of human hepatocytes and iHeps, serum bilirubin decreased over time by 70%–80% and 30%–61%, respectively, from pre-transplant levels of 5–7 mg/dl (85–119 μM) (n = 4, p < 0.01). The bilirubin levels were lower (p < 0.01) than in age-matched controls (n = 6) 6 weeks after transplantation and at all time points thereafter (Figures 4A and 4B).
Figure 4.
Metabolic Effect of Repopulation
(A) Serum bilirubin levels in the four Gunn rat iHeps (black lines) at the indicated time points after transplantation are shown as percent of pretransplant levels (means ± SD of three determinations for each time point). Dashed black line, untreated age-matched controls (means ± SD of 6 rats); dashed blue line, bilirubin levels in the sham-treated controls; red lines, bilirubin levels after transplantation of primary human hepatocytes; Dotted line, upper limit of serum bilirubin levels in congeneic normal Wistar-RHA rats.
(B) Serum bilirubin levels (mean ± SD) at indicated intervals after transplantation. Open bar, untreated control (n = 6 rats); light blue, sham-operated control (n = 4 rats); black, iHep transplant recipients (n = 4 rats); red, recipients of primary human hepatocytes (n = 2 rats). ∗p < 0.02.
(C–E) High-pressure liquid chromatographic analysis of bilirubin species excreted in the bile of a control Gunn rat, a rat receiving iPSC-derived hepatocytes, and a congeneic normal Wistar RHA rat. sf, solvent front. The absorbance unit scale in (E) is different from that in (F and G).
See also Figure S3.
To confirm the UGT1A1 activity of the engrafted iHeps in vivo, we analyzed the bile from untreated control and transplanted Gunn rats by high-pressure liquid chromatography (HPLC). Untreated Gunn rat bile contained only an unconjugated bilirubin (ucb) peak and no bilirubin glucuronides (Figure 4C). In contrast, bile from the iHep recipients contained bilirubin diglucuronide (bdg) and monoglucuronide (bmg), and the ucb peak was reduced (Figure 4D). bdg and bmg are the predominant species in congeneic normal Wistar-RHA rat bile, which contains only a small amount of ucb (Figure 4E).
Although, in this study, we did not enrich iHeps for ASGPR-expressing cells before transplantation to reduce the risk of transplanting cells with tumor potential, at autopsy, recipient rats did not exhibit teratomas or any other type of tumors despite significant proliferation of the engrafted cells after transplantation, suggesting the absence of residual pluripotent cells after in vitro differentiation of iPSCs.
Therefore, we demonstrated engraftment, proliferation, and function of iHeps following transplantation by immunohistochemical morphometry, qPCR of genomic DNA, UGT1A1 activity in liver homogenates, reduction of serum bilirubin, and excretion of bilirubin glucuronides in the bile. The slightly lower values observed by immunostaining for human UGT1A1 could be because all engrafted iHeps did not express UGT1A1. The function of engrafted iHeps in vivo was also evidenced by the appearance of human proteins in the recipient rat serum. Importantly, because UGT1A1 activity develops postnatally (Odell, 1967), the finding of UGT1A1 activity in the iHeps in vitro and in vivo indicates that, at least in this respect, the iHeps had matured beyond the level of fetal hepatocytes. Although our iHep cells did not exhibit all features of mature hepatocytes, their engraftment and proliferation in the Gunn rat liver provided sufficient UGT1A1 activity to ameliorate jaundice. Human stem cell- or fibroblast-derived hepatocytes have been reported to engraft in murine livers after severe liver injury (Liu et al., 2011). Very recently, iHeps generated by a protocol similar to that used in this study has been shown to engraft in the livers of urinary plasminogen activator transgenic mice and be infectable with hepatitis virus C in vivo (Carpentier et al., 2014). In contrast to these studies, we provide definitive evidence of the engraftment and function of iHeps by reconstitution of a missing hepatocyte-specific enzyme activity and significant amelioration of a metabolic liver defect.
This study required several technical innovations. The ability to limit the delivery of conditioning HIR of the median lobe permitted repopulation of a single liver lobe, leaving the remaining liver unperturbed. Clinical studies using conformal HIR indicate that partial liver irradiation causes much less radiation-induced liver injury than whole-liver irradiation, permitting the safe delivery of higher radiation doses (Dawson and Ten Haken, 2005). Because the engrafted iHeps proliferated significantly only in the preconditioned lobe, 5.1% ± 2.6% repopulation of the median lobe was equivalent to about 1.7% ± 0.99% repopulation of the entire liver. Nonetheless, this level of repopulation was sufficient to significantly reduce the serum bilirubin levels. Although serum bilirubin was not normalized, an equivalent reduction in a clinical setting would be sufficient to protect CN1 patients from developing brain injury and could possibly circumvent the need for prolonged phototherapy.
The ratio between HSA and human transferrin levels in recipient rat sera was lower than that in the culture media of iHeps. This could be because albumin is a negative acute-phase reactant, and its synthesis could be downregulated in the xenotransplanted cells.
Translating this approach to the clinic will require several modifications. The iPSCs will need to be generated under Good Manufacturing Practice conditions, using newer methods of generating iPSCs without transgene integration (Yoshida and Yamanaka, 2011). Because of the small size of the rodent recipients, we delivered regional HIR by surgical exposure of the liver, but, for clinical application, regional HIR is delivered by conformal irradiation without requiring surgery. Regional irradiation for hepatic repopulation is now in a phase I trial. These readily achievable adaptations should permit the safe application of this transplant strategy for treating CN1 and other inherited liver-based disorders.
Experimental Procedures
iPSC Generation
Normal adult human skin fibroblasts were reprogrammed by retroviral transduction with pluripotency factors, OCT-4, SOX2, KLF4, and c-MYC, as described previously (Takahashi and Yamanaka, 2006).
Differentiation of iPSCs to iHep Cells
The iPSCs were differentiated to iHeps as previously described for human ESCs with some modifications (Basma et al., 2009). Briefly, embryoid bodies were not produced, and iPSCs dispersed using dispase were plated in monolayers. Definitive endoderm was generated by exposure to recombinant activin-A (R&D Systems) and fibroblast growth factor 2 (Invitrogen). During the next 8 days, the cells were exposed to HGF (R&D Systems) and DMSO, followed by culturing for 3 days in dexamethasone-containing media (Sigma). At each step, the expression of a series of markers was determined by immunofluorescence staining (see Supplemental Experimental Procedures and Figure 1).
Characterization of iHep Cells
To evaluate the hepatocyte-like characteristics of the iHep cells, we determined the glycogen content and low-density lipoprotein (LDL) and indocyanine green (ICG) uptake by the cells. We also determined the HSA, AFP, and UGT1A1 content; UGT1A1 activity toward bilirubin; as well as secretion of HSA and AAT in the media (see details in the Supplemental Experimental Procedures). For comparison, control primary hepatocytes were isolated from an explanted liver during liver transplantation in a patient with metabolic liver disease.
Statistical Analysis
Serum bilirubin levels in individual rats were determined in triplicate and expressed as percent of pre-transplant values. Data at each time point were compared with those in the control group at the same time point using Student’s unpaired two-tailed t test. The number of mice in the control group is represented by n.
Author Contributions
N.R.C., J.R.C., I.J.F., E.E.B., S.S., and C.G. designed experiments. Y.C., Y.L., X.W., W.Z., V.S., K.T., Z.P., T.T., Y.A., E.T., B.H., C.J.C., and M.G.S. performed cellular, biochemical, and animal experiments. N.R.C., J.R.C., and I.J.F. analyzed the data and wrote the paper.
Acknowledgments
This work was supported by grants NIH RO1 1RO1 DK092469-01 and a grant from the Oxalosis and Hyperoxaluria Foundation (to N.R.C.); NYSTEM CO24346 and CO26440 (to J.R.C.); NIH RO1 DK48794, AI49472, and DOD W81XWH-09-1-0658 (to I.J.F.); NIH 1PO1 DK 096990-01 (to I.J.F. and J.R.C.); NIH RO1 DK64670 and R21/R33 CA121051 (to C,G,), and NIH P30 DK 41296-26 (to the Liver Pathobiology and Gene Therapy Research Core Center).
Published: June 11, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.04.017.
Contributor Information
Jayanta Roy-Chowdhury, Email: jayanta.roy-chowdhury@einstein.yu.edu.
Namita Roy-Chowdhury, Email: namita.roychowdhury@einstein.yu.edu.
Supplemental Information
References
- Åberg F., Isoniemi H., Höckerstedt K. Long-term results of liver transplantation. Scand. J. Surg. 2011;100:14–21. doi: 10.1177/145749691110000104. [DOI] [PubMed] [Google Scholar]
- Basma H., Soto-Gutiérrez A., Yannam G.R., Liu L., Ito R., Yamamoto T., Ellis E., Carson S.D., Sato S., Chen Y. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick S.L., Bick D.P., Wells B.E., Roesler M.R., Strawn E.Y., Lau E.C. Preimplantation HLA haplotyping using tri-, tetra-, and pentanucleotide short tandem repeats for HLA matching. J. Assist. Reprod. Genet. 2008;25:323–331. doi: 10.1007/s10815-008-9233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma P.J., Chowdhury N.R., Goldhoorn B.G., Hofker M.H., Oude Elferink R.P.J., Jansen P.L.M., Chowdhury J.R. Sequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type I. Hepatology. 1992;15:941–947. doi: 10.1002/hep.1840150531. [DOI] [PubMed] [Google Scholar]
- Cai J., Zhao Y., Liu Y., Ye F., Song Z., Qin H., Meng S., Chen Y., Zhou R., Song X. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Carpentier A., Tesfaye A., Chu V., Nimgaonkar I., Zhang F., Lee S.B., Thorgeirsson S.S., Feinstone S.M., Liang T.J. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J. Clin. Invest. 2014;124:4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson L.A., Ten Haken R.K. Partial volume tolerance of the liver to radiation. Semin. Radiat. Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Duan Y., Ma X., Zou W., Wang C., Bahbahan I.S., Ahuja T.P., Tolstikov V., Zern M.A. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28:674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- Fisher R.A., Strom S.C. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- Fox I.J., Chowdhury J.R., Kaufman S.S., Goertzen T.C., Chowdhury N.R., Warkentin P.I., Dorko K., Sauter B.V., Strom S.C. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- Guha C., Parashar B., Deb N.J., Garg M., Gorla G.R., Singh A., Roy-Chowdhury N., Vikram B., Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354–362. doi: 10.1053/jhep.2002.34516. [DOI] [PubMed] [Google Scholar]
- Lavon N., Yanuka O., Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Kim Y., Sharkis S., Marchionni L., Jang Y.-Y. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci. Transl. Med. 2011;3:82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysy P.A., Najimi M., Stéphenne X., Bourgois A., Smets F., Sokal E.M. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J. Gastroenterol. 2008;14:3464–3470. doi: 10.3748/wjg.14.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan N., Dhillon V., Thomas P., Fenech M. A quantitative real-time PCR method for absolute telomere length. Biotechniques. 2008;44:807–809. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- Odell G.B. “Physiologic” hyperbilirubinemia in the neonatal period. N. Engl. J. Med. 1967;277:193–195. doi: 10.1056/NEJM196707272770406. [DOI] [PubMed] [Google Scholar]
- Ozçay F., Alehan F., Sevmiş S., Karakayali H., Moray G., Torgay A., Arslan G., Haberal M. Living related liver transplantation in Crigler-Najjar syndrome type 1. Transplant. Proc. 2009;41:2875–2877. doi: 10.1016/j.transproceed.2009.07.025. [DOI] [PubMed] [Google Scholar]
- Roy-Chowdhury J., Huang T.J., Kesari K., Lederstein M., Arias I.M., Roy-Chowdhury N. Molecular basis for the lack of bilirubin-specific and 3-methylcholanthrene-inducible UDP-glucuronosyltransferase activities in Gunn rats. The two isoforms are encoded by distinct mRNA species that share an identical single base deletion. J. Biol. Chem. 1991;266:18294–18298. [PubMed] [Google Scholar]
- Roy-Chowdhury N., Kondapalli R., Roy-Chowdhury J. The Gunn rat: an animal model for inherited deficiency of bilirubin glucuronidation. In: Cornelius C.E., editor. Animal Models in Liver Research. Academic Press; 1993. pp. 149–171. [Google Scholar]
- Roy-Chowdhury J., Roy-Chowdhury N., Horslen S., Fox I.J. Experimental Therapies: Hepatocytes transplantation, gene therapy, and liver assist devices. In: Yamada T., editor. Text Book of Gastroenterology. Wiley-Blackwell; 2009. pp. 2432–2448. [Google Scholar]
- Schwartz R.E., Linehan J.L., Painschab M.S., Hu W.S., Verfaillie C.M., Kaufman D.S. Defined conditions for development of functional hepatic cells from human embryonic stem cells. Stem Cells Dev. 2005;14:643–655. doi: 10.1089/scd.2005.14.643. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C., North P.E., Dalton S., Duncan S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Cai J., Liu Y., Zhao D., Yong J., Duo S., Song X., Guo Y., Zhao Y., Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Zhou H., Roy-Chowdhury N., Macaluso F., Liu L., Yamamoto T., Yannam G.R., Enke C., Solberg T.D., Adelson A.B. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–267. doi: 10.1002/hep.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Yamanaka S. iPS cells: a source of cardiac regeneration. J. Mol. Cell. Cardiol. 2011;50:327–332. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhou H., Dong X., Kabarriti R., Chen Y., Avsar Y., Wang X., Ding J., Liu L., Fox I.J., Roy-Chowdhury J. Single liver lobe repopulation with wildtype hepatocytes using regional hepatic irradiation cures jaundice in Gunn rats. PLoS ONE. 2012;7:e46775. doi: 10.1371/journal.pone.0046775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.