Abstract
Chronic cardiovascular disease is associated with air pollution exposure in epidemiology and toxicology studies. Inhaled toxicants can induce changes in serum bioactivity that impact endothelial inflammatory gene expression in vitro and impair vasorelaxation ex vivo, which are common precursors to atherosclerosis. Comparisons between single pollutants and common combustion mixtures, in terms of driving such serum inflammatory and vasoactive effects, have not been characterized. Healthy C57BL/6 mice were exposed to a single 6h period of contrasting pollutant atmospheres: road dust, mixed vehicle emissions (MVE; a combination of gasoline and diesel engine emissions) particulate matter (MVE-PM), mixed vehicle emissions gases (MVE-G), road dust plus ozone, road dust plus MVE, and hardwood smoke. Serum obtained from mice 24h after these exposures was used as a stimulus to assess inflammatory potential in two assays: incubated with primary murine cerebrovascular endothelial cells for 4h to measure inflammatory gene expression, or applied to naïve aortic rings in an ex vivo myographic preparation. Road dust and wood smoke exposures were most potent at inducing inflammatory gene expression, while MVE atmospheres and wood smoke were most potent at impairing vasorelaxation to acetylcholine. Responses are consistent with recent reports on MVE toxicity, but reveal novel serum bioactivity related to wood smoke and road dust. These studies suggest that the compositional changes in serum and resultant bioactivity following inhalation exposure to pollutants may be highly dependent on the composition of mixtures.
Keywords: diesel, particulate matter, gasoline, exhaust, cardiovascular, wood smoke, endothelium
INTRODUCTION
Air pollution exposure is associated with adverse cardiovascular outcomes (Pope et al., 2004; Brook et al., 2013) despite limited direct systemic absorption or translocation of pollutant components into the body (Postlethwait et al., 1990; Postlethwait et al., 1994; Mercer et al., 2013). In recent studies, serum or plasma obtained after pollutant exposures has been shown to possess a pathologic bioactivity capable of inducing inflammation, arresting growth, and impairing vasodilation in cell culture or isolated organ experiments (Channell et al., 2012; Robertson et al., 2013; Liberda et al., 2014). Because these studies employed such varied pollutants as diesel exhaust, nitrogen dioxide, ozone, and nickel nanoparticles, it is difficult to gauge the relative potency of these pollutants in driving a serum-borne bioactivity without head-to-head experiments.
Ambient air pollution is comprised of a complex and dynamic mixture of gaseous and particulate substances that may all have varying cardiovascular impact (Vedal et al., 2013). Emissions arising from traffic have been shown to have a focused impact on cardiovascular outcomes in epidemiological studies (Sarnat et al., 2008; Hoffmann et al., 2009) and diesel exhaust inhalation impairs vasoreactivity in healthy individuals (Mills et al., 2005). However, overall ambient air pollution remains an important contributor to chronic cardiovascular disease and numerous source apportionment studies have drawn different conclusions regarding the relative potency of ambient airshed constituents. A recent examination of cardiovascular hospital admissions in the Northeast U.S. implicated road dust contributions, moreso than motor vehicles, oil combustion, sea salt, or regional sources, as being a principal driver (Bell et al., 2014). However, earlier studies suggested a stronger association with mobile source emissions and cardiovascular hospital admissions (Sarnat et al., 2008). The current lack of understanding of the pathogenesis on cardiovascular health impacts of inhaled materials substantially impedes our ability to derive congruous conclusions from large-scale population studies.
The overarching hypothesis of the present work is that inhaled materials lead to compositional changes in the blood that are then conveyed by the circulation to vulnerable sites, such as atherosclerotic regions of the coronary or cerebrovascular arterial beds, and there the bioactive serum may promote inflammatory responses or impair vasodilation. Given the myriad potential compositional changes of the circulation, the present study utilizes two related functional outcomes, inflammatory response and vasorelaxation, under an endothelial cell biosensor assay paradigm. Using healthy mice exposed to a battery of varied complex pollutant atmospheres, we examined the relative impact of serum changes on inflammatory and vasoactive impairments.
METHODS
Animals
Male C57BL/6 (Jackson Laboratories) and Apolipoprotein E-null (ApoE−/−; Taconic Laboratories) mice were obtained at 6–8 weeks of age and quarantined for 14 days prior to exposures. Mice were housed in standard shoebox caging under AAALAC-approved conditions for temperature and humidity, with food and water available ad libitum, except during exposures. Following exposures, mice were euthanized by humane means (pentobarbital) and tissues collected. For myograph experiments, male C57BL/6 mice were also used as donors for aortic rings. Donor mice were euthanized via exsanguination while under anesthesia (isoflurane; concentration 1.5–2%). All procedures were approved by both the University of New Mexico and the Lovelace Respiratory Research Institute animal care and use committees.
Exposures
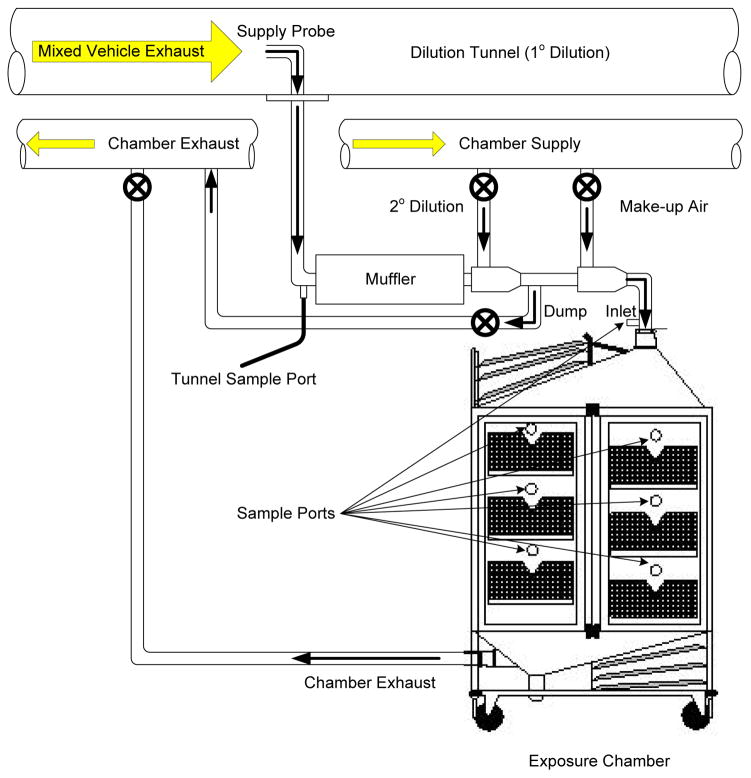
All exposures with C57BL/6 mice were conducted for a single 6h period, and serum was obtained 18h following the cessation of exposures (N=6 per group). All exposure systems have been rigorously characterized in terms of chemical composition and each is described in brief, below. Exposures were conducted in 1 or 2 m3 whole-body rodent inhalation chambers (Lab Products, Inc., Maywood, NJ) that were ventilated with exposure atmospheres at ~500 lpm, for a residence time of approximately 4 min. The chambers contain sampling ports above each cage unit to facilitate characterizing spatial homogeneity of exposures and to provide multiple sample locations for exposure characterization (Figure 1). During the exposures, concentrations of particulate matter were measured by gravimetric analysis of filter samples three times during each exposure. For each exposure condition, a matched cohort of mice exposed to filtered air (FA) was included as a sham control group.
Figure 1.
General schematic for inhalational exposures.
Road dust was obtained from roadway surfaces on residential streets and urban arterials in Phoenix and Tucson, AZ (Vedal et al., 2013). Material was vacuumed from street surfaces with a commercially- available, standardized, low-volume surface sampler (CS3, Inc., Sandpoint, ID). The sampler excluded particulate material >10 microns during collection. Particulate samples were subsequently sieved on an orbital shaker and the finest fraction (<38 μm bulk material diameter) was retained. Aerosol generation was conducted with a Wright-dust feeder that was coupled to a PM2.5 cyclone on the effluent stream to remove particles >2.5 microns. This size selective cut was used to ensure the material was in the respirable range for a rodent.
Ozone was generated using a Sander Ozonisator (Model IV, Sander Company, West Germany), diluted to 0.3 ppm, and monitored with a Teledyne Ozone Analyzer (Model 465L, Teledyne, San Diego, California).
Mixed vehicle emissions (MVE) were generated by combining exhausts from a diesel generator (single-cylinder, 5500-watt, Yanmar diesel-engine generator (McDonald et al., 2004) using Number 2 Diesel Certification Fuel) pulling a constant 90% load during operation with a gasoline engine (1996 General Motors 4.3 L V6 gasoline engine) connected to an eddy current dynamometer (McDonald et al., 2008). The ratio of diesel PM to gasoline engine PM was approximately 5:1, but gasoline contributed greater ratios of gaseous components. Oxides of nitrogen (NOx) and carbon monoxide (CO) concentrations were monitored and recorded at approximately 30–60 minute intervals throughout the exposures.
Woodsmoke was generated by a PineRidge wood-burning stove burning a supply of solid oak logs.
National Particulate Component Toxicity Initiative Study
Additionally, serum was available from previous studies using 50-day exposures in ApoE−/− mice. In the present manuscript, serum from road dust, road dust with MVE gases, and MVE gases was used in in vitro assays, described below. These exposures are described in detail elsewhere (Vedal et al., 2013) and are the basis for exposures above.
Serum Inflammatory Potential
Cell culture of murine cerebrovascular endothelial cells and isolation of RNA was performed similarly to previously described methods. Briefly, murine cerebrovascular endothelial cells (mCECs) were obtained from a commercial vendor (Cell Biologics). Serum from each subject was added to individual wells of a 24-well plate at a ratio of 1:40 (2.5%) with complete culture media and incubated for 4h at 37°C. Following incubation, cell supernatants were collected and mCECs were washed with PBS, lysed and immediately cell lysates were pooled and collected for RNA purification. Total RNA was isolated using RNeasy Mini Prep Kits (Qiagen) and RNA was reverse-transcribed using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems) prior to quantitative real-time PCR (qPCR) assessment of endothelial adhesion markers. Amplification of target message was performed in TaqmanR universal master mix following manufacturer’s recommended conditions with Taqman® Gene expression assays for inflammatory markers included the arachidonic acid metabolizing enzyme cyclooxygenase-2 (COX2 aka PTGS2), adhesion molecules (ICAM1, VCAM1), and cytokines (IL-6, CXCL1, CCL2, and CCL5) with endogenous TATA-box binding protein (TBP) as the housekeeping gene. Relative gene expression was analyzed by the 2−ΔΔCT method (Livak and Schmittgen, 2001) using a relative amount of mRNA for each sample normalized to TATA-box binding protein.
Ex Vivo Serum Effects on Vasorelaxation
Serum from exposed mice was applied to aortic rings from naïve mice to assess the impact on acetylcholine-mediated relaxation, similar to that previously described (Robertson et al., 2013). Briefly, thoracic aortas were isolated from naïve C57BL/6 mice and cleaned of connective tissue. Ring segments of aorta (2–3 mm length) were then mounted in a myograph system (610 M; DMT, Inc, Atlanta, USA) and submerged in physiological salt solution (composition in mM: 119.0 NaCl, 25.0 NaHCO3, 5.5 glucose, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.027 ethylenediaminetetraacetic acid, 2.5 CaCl2) bubbled at 37°C with 21%O2-5%CO2-balance N2, and left to equilibrate at 2 g of tension for approximately 30 min. Tension was gradually applied over 10 min to an optimal passive tension of 10 mN. Vessel viability was confirmed by a contractile response on addition of high potassium PSS (KPSS in mM: 64.9 NaCl, 25.0 NaHCO3, 5.5 glucose, 58.9 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.027 ethylenediaminetetraacetic acid, 2.5 CaCl2), repeated twice.
After a 30-min equilibration period, vessels were incubated with 1% serum obtained from WT mice following exposure to FA or other pollutant atmosphere (N=5–7 per group). To generate a control relaxation curve for the various atmospheres, serum from 1–2 mice per control group were used and the data from these ACh-response curves were pooled (n=7). This allowed for control data to be collected in parallel with all other atmospheres. Addition of serum to the myography bath induced contraction of aortic rings and cumulative concentration-response curves to ACh (10−9–10−4) were performed after response to serum had stabilized (approx. 20 min). Data were acquired by a MacLab/4e analogue-digital convertor displayed through Chart™ software (AD Instruments, USA). Relaxation data are expressed as a percentage of serum-induced contraction, with baseline tension subtracted.
Statistics
Serum effects on gene expression were compared by an unpaired Student’s t-test with each exposure linked to the simultaneously-conducted filtered air control group. Subchronic studies in ApoE−/− mice were compared with an ANOVA with Dunnett’s Multiple Comparison Test. Myographic studies were compared with a two-way analysis of variance considering exposure and acetylcholine concentration as the two factors, and post-hoc comparisons at specific acetylcholine concentrations were conducted using a Fisher’s Least Significant Difference test (GraphPad Prism, v 6.0). To generate a control relaxation curve for the various atmospheres, serum from 1–2 mice per control group were used and the data from these ACh-response curves were pooled (n=7). Other comparisons were conducted with a standard one-way analysis of variance.
RESULTS
Exposure Generation
Particulate matter and gas concentrations for each exposure atmosphere, both target and measured values, are shown in Table 1. Filtration of the MVE atmosphere effectively removed approximately 96% of particulate mass compared to the pre-filtration atmosphere. In contrast, denuding the MVE atmosphere to reduce the gaseous fraction effectively removed 84% of NOx and 85% of CO compared to the pre-denuded atmosphere.
Table 1.
Concentrations of major atmosphere components, PM, NOx, and CO in the 7 test atmospheres. For Nox and CO, values for “pre” and “post” indicate values upstream and downstream of the denuder or filter, when in use.
| PM (μg/m3) | NOx (ppm) | CO (100 ppm) | ||||
|---|---|---|---|---|---|---|
| EXPOSURE | Target | Actual | Pre | Post | Pre | Post |
| Road Dust | 300 | 349 | - | - | - | - |
| Road Dust + MVE | 200+100 | 342 | 10.2 (2.5) | - | 25.6 (8.2) | - |
| MVE Gases | 0 | 13 | 28.6 (5.1) | 25.6 (6.3) | 66.2 (24.4) | 58.5 (26.6) |
| MVE PM | 300 | 328 | 24.6 (8.0) | 4.0 (1.2) | 54.6 (9.3) | 8.0 (3.1) |
| Road Dust + 0.33 ppm Ozone | 300 | 344 | - | - | - | - |
| Woodsmoke | 300 | 380 | - | - | - | - |
| MVE (for ApoE−/− mice) | 300 | 349 | 17.8 (4.8) | - | 32.4 (8.2) | - |
Values are given and mean and (SD).
In Vitro Serum Inflammatory Potential
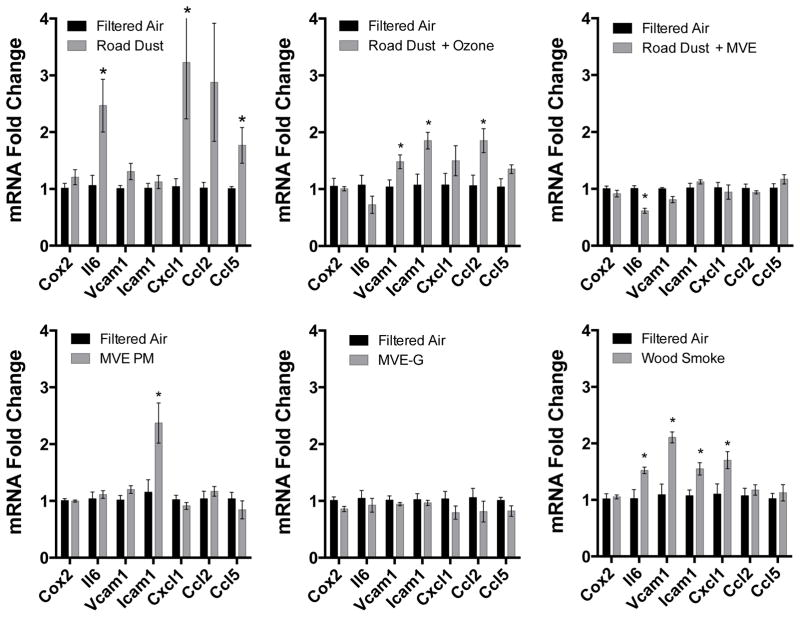
We analyzed a panel of inflammatory markers expressed in cultured endothelial cells at the mRNA level, as an indicator of the cumulative inflammatory potential of serum from exposed mice. Serum obtained from C57BL/6 mice exposed for a single 6 h period to Road Dust was able to potently induce several inflammatory genes, namely cytokines IL-6, CXCL1, CCL2, and CCL5 (Figure 2). Interestingly, serum from mice exposed to Road Dust + MVE did not display this inflammatory induction on endothelial cells. Consistent responses were observed with serum from Road Dust + O3, however, with endothelial transcription of CCL2, ICAM-1, and VCAM-1 being significantly elevated. Serum from mice exposed to MVE PM or MVE gases failed to elicit any induction of the inflammatory genes that were assessed in cultured mCECs.
Figure 2.
Serum-induced endothelial cell gene expression in an in vitro assessment of inflammatory potential. Serum was obtained 24h after C57BL/6 mice were exposed for 6h to one of 6 atmospheres. Each exposure group (N=6) had a unique filtered air control group (N=6). Results for each gene are compared by a Student’s t-test, with asterisks indicating p<0.05.
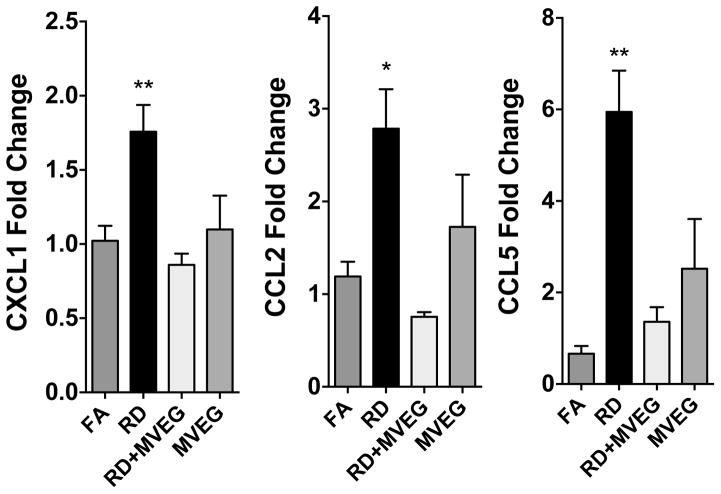
Given the divergent responses between Road Dust and Road Dust + MVE, we retrieved banked serum from previous studies on these pollutant atmospheres (Vedal et al 2013). This previous research involved the exposure of ApoE−/− mice to Road Dust (the same source material and concentration used in the single day exposure), Road Dust + MVE gases, and MVE gases, albeit for a subchronic study design (6 h/d × 50d) (Vedal et al., 2013). In co-incubations with mCECs, we also noted that serum obtained from Road Dust had a substantial impact on expression of CXCL1, CCL2, and CCL5 (Figure 3). Combined with the gaseous fraction of MVE, however, Road Dust failed to confer such inflammatory potential on the serum, consistent with the 1-d studies (Figure 2). The MVE gas fraction alone was similarly ineffective at increasing serum inflammatory potential. These data suggest that certain gases in the complex mixture may act to inhibit inflammatory responses of endothelial cells or the formation of inflammatory intermediates in the serum.
Figure 3.
Serum-induced endothelial cell gene expression in an in vitro assessment of inflammatory potential. Serum was obtained 24h after ApoE−/− mice were exposed for 6h/d × 50d to one of 3 atmospheres. Exposure groups (N=6 per group) were conducted in parallel with a single filtered air control group (N=6). Results for each gene are compared by an ANOVA with Dunnett’s Multiple Comparison Test. Asterisks indicate significant difference from control (*p<0.05; **p<0.01).
Serum from Exposed Mice Impacts on Vasorelaxation
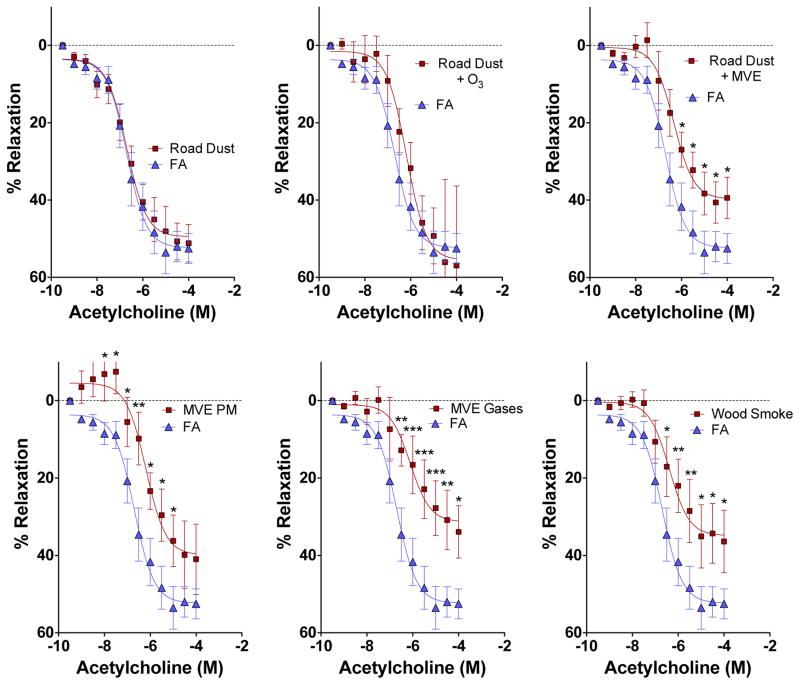
In contrast to inflammatory markers in cultured cells, serum from Road Dust and Road Dust + O3 exposure mice had no apparent effect on vasorelaxation in co-incubated aortic rings from naïve mice, compared to serum from control (filtered air exposed) mice (Figure 4). However, all atmospheres that contained complex emissions gases induced changes in serum that impaired relaxation ex vivo. Additionally, PM from MVE, with copollutant gases removed by denudation, was also able to reduce vasorelaxation responses to acetylcholine. In terms of magnitude of response, the atmospheres MVE PM, MVE Gases, wood smoke, and Road Dust +MVE could not be statistically differentiated, with all inducing a 20–40% reduction to the maximal effect in FA controls (Supplemental Fig 1). Additionally, all serum applications in the ex vivo bath led to some degree of contraction from the baseline tension. Serum from mice exposed to MVE PM or Road Dust + MVE induced the greatest contraction, relative to FA controls (Figure 5), even at a substantial dilution, suggesting the induction of pro-constrictive factors in the serum.
Figure 4.
Serum-induced impairments of vasorelaxation response to acetylcholine. Results are compared by a two-factor ANOVA, considering exposure and acetylcholine concentration as factors, with a Fisher’s Least Significant Difference test to compare specific concentrations (GraphPad Prism, v 6.0). Asterisks indicate significant difference from control (*p<0.05; **p<0.01, ***p<0.001).
Figure 5. Serum-induced contraction in aortic rings.
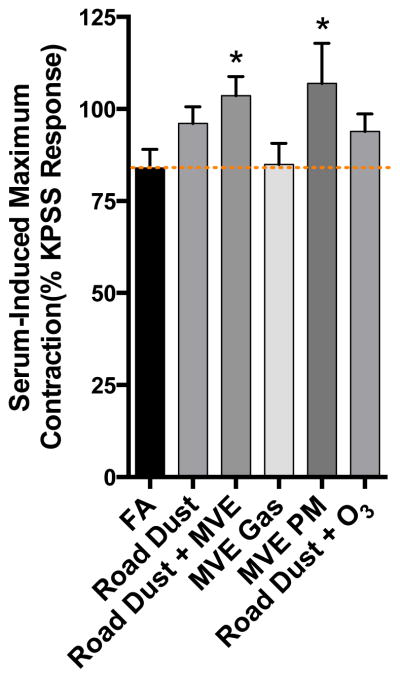
Upon incubation with serum in the tissue bath, aortic rings developed spontaneous tone relative to baseline that was comparable to that induced by KPSS. Asterisk indicates significant difference from filtered air (FA) controls by ANOVA with an Uncorrected Fisher’s Least Significant Difference test (P<0.05).
DISCUSSION
This study represents the first head-to-head comparison of inflammatory potential and vasoactivity of serum resulting from various complex pollutant exposures. Several conclusions can be inferred from the outcomes. First, a single day of exposure to these complex pollutant atmospheres in healthy C57BL/6 mice readily drove a varied bioactivity that was carried by the serum. Second, impairment of acetylcholine-induced vasorelaxation was not induced by the same serum modifications that caused inflammation, as there was very little overlap in these outcomes across different atmospheres. This suggests that specific serum compositional modifications may result from exposures to varied pollutants, resulting in divergent biological impacts. The exception to this was wood smoke exposure, which caused serum bioactivity that induced both inflammatory and vasoactivity changes. Specific differences in the responses to the atmospheres belie simplistic interpretation, but the overall bioactivity of the serum is consistent with recent studies in mice and humans (Channell et al., 2012; Robertson et al., 2013; Liberda et al., 2014).
Divergent inflammatory effects from the three atmospheres containing Road Dust are the most difficult to explain. The potent induction of inflammatory chemokine transcripts by serum from Road Dust exposures was confirmed with serum obtained from a sensitive model (ApoE−/− mice) exposed subchronically (50d). While it is not clear form our study design whether the serum inflammatory potential was increased by the repeated exposure or the hypercholesterolemic model, the end result is coherent with the single day exposures. Additionally, the lack of effect of combined RD and MVE or MVE gases in both models helps to strengthen this unexpected finding. Speculatively, one or more components of the combustion mixture could diminish the pulmonary interactions with the Road Dust particles, such as anti-inflammatory gases like carbon monoxide (Otterbein et al., 2003). In the analysis of aortic pathological outcomes of the 50d Road Dust and MVE gas exposures, we did not see protective effects, but the deleterious interactions seen between MVE gases and other particles (MVE PM, sulfate PM and nitrate PM) were invariably more pronounced than for Road Dust (Vedal et al., 2013).
Serum-induced impairments in vasorelaxation were evident in 4 of the 6 atmospheres tested. Road Dust alone failed to induce any changes, along with Road Dust + O3. Serum obtained from mice exposed to O3 at higher levels (1 ppm) induced substantial diminution of vasorelaxation to acetylcholine in a similar model, a response that did not appear to be dependent on lung inflammation but was dependent on vascular CD36 (Robertson et al., 2013). MVE PM, however, did lead to the induction of bioactivity in serum that impaired vascular responses to acetylcholine. Both MVE PM and MVE gases caused similar effects on serum in a 50d study, but the relative potency was reversed, with MVE PM eliciting the strongest loss of relaxation (Campen et al., 2014). The substitution of a portion of MVE PM with Road Dust PM in the whole MVE led to similar changes in serum bioactivity as MVE PM alone. As recently postulated (Vedal et al., 2013), combining combustion-source gases from fresh emissions with aged or secondary particles may enhance particle toxicity. Furthermore, we have confirmed the clinical relevance of diesel-induced serum bioactivity in human studies, showing not only induction of genes used in the present study (IL-8, ICAM-1, VCAM-1), but also a more complete microarray analysis of the endothelial cell response to plasma post-exposure (Channell et al., 2012; Schisler et al., 2015). The microarray analysis noted induction of more global inflammatory pathways in the endothelial cells treated with plasma post-diesel exposure, as well as induction of transcription factors previously unassociated with air pollution effects, including FOXO4, FOXF2, and TCF3 (Schisler et al., 2015).
Wood smoke findings are relatively novel and few studies have addressed cardiovascular health effects of biomass combustion. Epidemiological studies related to wildfires are suggestive of a possible cardiovascular effect, but roughly equal numbers of positive and negative findings reflect a high variability of exposures associated with such sporadic natural events (Bell et al., 2014). Forchhammer and colleagues observed no adverse effects in exposed human subjects at up to 354 μg PM/m3 wood smoke for 3 hours (Forchhammer et al., 2012). Microvascular function assays were conducted 6 h after and blood samples were taken at 0, 6, and 20 hours after exposure for measures of oxidative stress and inflammation, with no obvious changes detected. Unosson and colleagues reported increased arterial stiffness in response to a comparable wood smoke exposure in health human subjects (Unosson et al., 2013), although in a follow-up study in firefighters, wood smoke exposure did not compromise vasodilatory capacity (Hunter et al., 2014). Utilizing filtrations systems to reduce indoor wood smoke PM levels in participating households in British Columbia, however, significantly improved vascular hyperemic response along with a 30% decrease in circulating C-reactive protein levels (Allen et al., 2011). However, controlled wood smoke exposures in mice have not resulted in substantial cardiovascular toxicity (Seilkop et al., 2012; Mauderly and Seilkop, 2014). Thus, the current findings showing that serum contains some potentially adverse bioactivity suggest that further research may be needed to identify specific factors responsible, and possibly factors that confer vulnerability.
The value of utilizing serum from exposed mice to test the inflammatory potential on endothelial cells lies in the rigor and pertinence of the inhalation methodology. As opposed to treating endothelial cells in vitro with unjustifiably high concentrations of particulates – with no consideration of co-pollutants – the present approach incorporates true inhalation exposures and then exposes endothelial cells to factors that would definitely be in direct contact with the endothelium in vivo. Furthermore, the treatments are at a highly dilute manner, owing to the requisite conditions for cell culture and isolated organ techniques. We do not propose what factor(s) may be responsible for these outcomes, as thousands of biomolecules are present in the serum and the present study is designed to assess the cumulative impact. Furthermore, the subsequent step of extrapolating the present outcomes to whole animal pathophysiology is less clear. We have recently shown that similar serum bioactivity exists in clinical syndromes, such as coronary artery disease, and clinical trials with the polyphenol resveratrol showed that reductions in serum inflammatory potential could be elicited in otherwise healthy human subjects (Cung et al., 2015). Quantitative links between the inflammatory potential in humans and laboratory species, in addition to linkages between short-term endothelial responses and chronic inflammatory diseases simply do not exist at present, owing to the novelty of the approach.
Overall, the findings of the present study reveal a consistent pattern of serum modifications following inhalation exposure to complex pollutants that lead to altered bioactivity. Both outcomes assessed – inflammatory pathways and impaired relaxation – are presumed to be part of a continuum of the early stages leading to atherosclerosis (Ross, 1999). It was somewhat surprising that there was not more overlap between the pollutant atmospheres and serum-induced outcomes. The main gap in the present study is a lack of serum compositional characterizations to examine potential candidate molecules to drive the varied effects. However, a thorough investigation, via proteomic or metabolomic approaches, is likely to yield more candidates than can be adequately confirmed in a single study. Future research will need to link complex compositional data sets with functional assay outcomes such as were developed in the present study to better delineate causal components.
Supplementary Material
Acknowledgments
Funding: This study was funded by grants from the National Institutes of Health (R01 ES014639, R01 HL114839, HL115106, T32 HL007736) and the Environmental Protection Agency (RD-83479601-0). The views expressed in this document are solely those of the authors and the U.S. EPA does not endorse any products or commercial services mentioned in this publication.
Footnotes
Compliance with Ethical Standards: The authors confirm that they have no conflicts of interest, financial or otherwise, with the contents of this manuscript. Studies were conducted with full approval by the Institutional Animal Care and Use Committees of both the University of New Mexico and Lovelace Respiratory Research Institute.
References
- Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, Wong I, Brauer M. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183:1222–1230. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, Wang Y, Dominici F, Peng RD. Associations of PM(2).(5) constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons >/= 65 years of age. Environ Health Perspect. 2014;122:138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajagopalan S, Goldberg MS, Pope CA, 3rd, Burnett RT. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36:3313–3320. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen M, Robertson S, Lund A, Lucero J, McDonald J. Engine exhaust particulate and gas phase contributions to vascular toxicity. Inhal Toxicol. 2014;26:353–360. doi: 10.3109/08958378.2014.897776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci. 2012;127:179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cung H, Aragon M, Anderson J, Nawarskas J, Roldan C, Sood A, Qualls C, Campen MJ. Characterization of a novel endothelial biosensor assay reveals increased cumulative serum inflammatory potential in stabilized coronary artery disease patients. J Translational Med. 2015 doi: 10.1186/s12967-015-0457-5. minor revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer L, Moller P, Riddervold IS, Bonlokke J, Massling A, Sigsgaard T, Loft S. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Particle and fibre toxicology. 2012;9:7. doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Dragano N, Mohlenkamp S, Memmesheimer M, Erbel R, Jockel KH Heinz Nixdorf Recall Investigative G. Residential traffic exposure and coronary heart disease: results from the Heinz Nixdorf Recall Study. Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals. 2009;14(Suppl 1):74–78. doi: 10.1080/13547500902965096. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Unosson J, Bosson JA, Langrish JP, Pourazar J, Raftis JB, Miller MR, Lucking AJ, Boman C, Nystrom R, Donaldson K, Flapan AD, Shah A, Pung L, Sadiktsis I, Masala S, Westerholm R, Sandstrom T, Blomberg A, Newby DE, Mills NL. Effect of wood smoke exposure on vascular function and thrombus formation in healthy fire fighters. Particle and fibre toxicology. 2014;11:62. doi: 10.1186/s12989-014-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberda EN, Cuevas AK, Qu Q, Chen LC. The acute exposure effects of inhaled nickel nanoparticles on murine endothelial progenitor cells. Inhal Toxicol. 2014;26:588–597. doi: 10.3109/08958378.2014.937882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, Seilkop SK. The National Environmental Respiratory Center (NERC) experiment in multi-pollutant air quality health research: III. Components of diesel and gasoline engine exhausts, hardwood smoke and simulated downwind coal emissions driving non-cancer biological responses in rodents. Inhal Toxicol. 2014;26:668–690. doi: 10.3109/08958378.2014.920440. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Barr EB, White RK. Design, characterization, and evaluation of a small-scale diesel exhaust exposure system. Aerosol Sci Tech. 2004;38:62–78. [Google Scholar]
- McDonald JD, Barr EB, White RK, Kracko D, Chow JC, Zielinska B, Grosjean E. Generation and Characterization of Gasoline Engine Exhaust Inhalation Exposure Atmospheres. Inhalation Toxicology. 2008;20:1157–1168. doi: 10.1080/08958370802449696. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Particle and fibre toxicology. 2013;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Postlethwait EM, Langford SD, Bidani A. Reactive absorption of nitrogen dioxide by pulmonary epithelial lining fluid. Journal of applied physiology. 1990;69:523–531. doi: 10.1152/jappl.1990.69.2.523. [DOI] [PubMed] [Google Scholar]
- Postlethwait EM, Langford SD, Bidani A. Determinants of inhaled ozone absorption in isolated rat lungs. Toxicol Appl Pharmacol. 1994;125:77–89. doi: 10.1006/taap.1994.1051. [DOI] [PubMed] [Google Scholar]
- Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, Campen MJ. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci. 2013;134:304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, Mulholland JA, Hopke PK, Tolbert PE. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Ronnebaum SM, Madden M, Channell MM, Campen MJ, Willis MS. Endothelial inflammatory transcriptional responses to an altered plasma exposome following inhalation of diesel emissions. Inhal Toxicol. 2015 doi: 10.3109/08958378.2015.1030481. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilkop SK, Campen MJ, Lund AK, McDonald JD, Mauderly JL. Identification of chemical components of combustion emissions that affect pro-atherosclerotic vascular responses in mice. Inhal Toxicol. 2012;24:270–287. doi: 10.3109/08958378.2012.667455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unosson J, Blomberg A, Sandstrom T, Muala A, Boman C, Nystrom R, Westerholm R, Mills NL, Newby DE, Langrish JP, Bosson JA. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Particle and fibre toxicology. 2013;10:20. doi: 10.1186/1743-8977-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedal S, Campen MJ, McDonald JD, Kaufman JD, Larson TV, Sampson PD, Sheppard L, Simpson CD, Szpiro AA. National Particle Component Toxicity (NPACT) Initiative Report on Cardiovascular Effects. Research report. 2013;178:238. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.