Abstract
Background
The effects of mammalian target of rapamycin (mTOR) inhibition are limited by feedback reactivation of receptor tyrosine kinase signaling in PTEN-null tumors, thus we tested the combination of mTOR inhibition (everolimus) and EGFR inhibition (gefitinib) in castration-resistant prostate cancer (CRPC).
Methods
In phase I, 12 patients (10 CRPC, 2 glioblastoma) received daily gefitinib (250 mg) with weekly everolimus (30, 50, or 70 mg). In phase II, 27 CRPC patients received gefitinib with everolimus 70 mg.
Results
Phase I revealed no pharmacokinetic interactions and no dose-limiting toxicities. In phase II, 18 of 27 (67%) patients discontinued treatment before the 12-week evaluation due to progression as evidenced by prostate-specific antigen (PSA) levels (n=6) or imaging (n=5), or grade ≥2 toxicity (n=7). Thirteen of the total 37 (35%) CRPC patients exhibited a rapidly rising PSA after starting treatment which declined upon discontinuation. Fluorodeoxyglucose positron emission tomography at 24 to 72 hours after starting treatment showed a decrease in standardized uptake value consistent with mTOR inhibition in 27 of 33 (82%) evaluable patients; there was a corresponding rise in PSA in 20 of these 27 patients (74%).
Conclusions
The combination of gefitinib and everolimus did not result in significant antitumor activity. The induction of PSA in tumors treated with mTOR inhibitors was consistent with preclinical data that PI3K pathway signaling feedback inhibits the androgen receptor (AR). This clinical evidence of relief of feedback inhibition promoting enhanced AR activity supports future studies combining PI3K pathway inhibitors and second-generation AR inhibitors in CRPC.
Keywords: prostatic neoplasms, sirolimus derivatives (everolimus), TOR serine-threonine kinases (mTOR), pharmacokinetics, quinazolines (gefitinib)
INTRODUCTION
A molecular profiling study of human prostate cancers showed that the phosphoinositide 3-kinase (PI3K) signaling axis was altered in 42% of primary and 100% of metastatic castration-resistant tumor samples.1 Therapies directed at inhibiting the PI3K axis such as LY294002 and wortmannin have been limited by untoward adverse events,2 leading to the evaluation of agents that inhibit key downstream components of the pathway such as mammalian target of rapamycin (mTOR). Rapamycin binds to mTORC1, one of the two protein complexes in which mTOR functions, and has been shown to inhibit cell proliferation in a variety of tumor models, including those in which PI3K/AKT signaling is deregulated by phosphatase and tensin homolog (PTEN) loss.3-5 Preclinical development of rapalogs with rapamycin-like mTORC1 inhibitory effects followed, but none induced tumor regressions in genetically engineered models of PTEN-deficient prostate cancer. This lack of activity has been mirrored in the clinic, where the effects of rapamycin-like TORC1 inhibitors in human prostate tumors in which loss of PTEN is prevalent have been modest at best.6-8
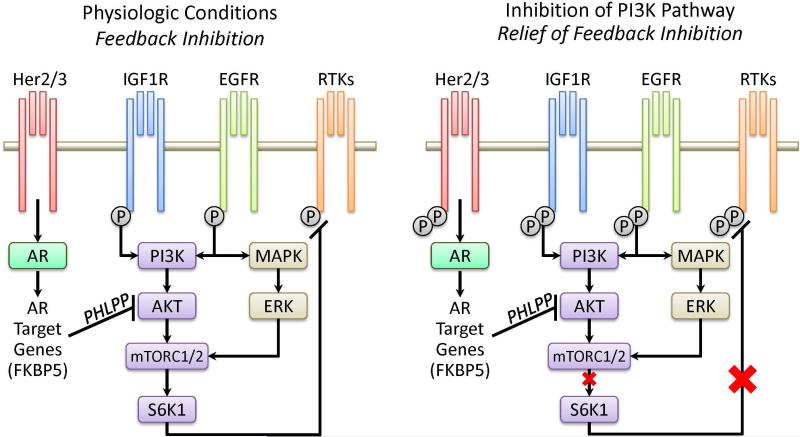
The lack of antitumor activity in prostate tumors treated with rapamycin and other PI3K pathway inhibitors may be due, in part, to activation of upstream signaling pathways (Figure 1). Activation of AKT and mTOR has been shown to feedback inhibit a variety of receptor tyrosine kinases (RTKs),9 but rapamycin-like drugs relieve this feedback and activate RTKs. It has been suggested that this attenuates or prevents the antitumor effects of these drugs.
Figure 1. Inhibition of PI3K pathway: relief of feedback inhibition on RTK signaling.
RTK: receptor tyrosine kinase; EGFR: epidermal growth factor receptor; AR: androgen receptor. Activation of AKT and mTOR has been shown to feedback inhibit the activation, expression and signaling of a variety of RTKs, including members of the EGFR family, IGF1R, HER2/3 and others.9 Rapamycin-like drugs can relieve this feedback and activate RTKs, thereby attenuating the anti-tumor effect of these drugs. Inhibition of PI3K can activate AR through relief of negative feedback on RTK. Conversely, inhibition of AR activates AKT by reducing levels of the AKT phosphatase PHLPP.
Although epidermal growth factor receptor (EGFR) inhibitors as monotherapy have been ineffective in patients with castration-resistant prostate cancer (CRPC),10-12 since mTOR inhibition reactivates HER kinase signals, we hypothesized that inhibition of EGFR signaling might enhance the effects of mTOR inhibition.13, 14 This study tested whether combined inhibition of the PI3K/mTOR and EGFR signaling pathways with everolimus and gefitinib would potentiate the response for patients with CRPC.
It is worth noting that, when this study was initially designed, the reciprocal feedback relationship between the androgen receptor (AR) and PI3K signaling pathways was not well defined; publications citing the preclinical data for this phenomenon came later.15, 16 Our hypothesis at the time of this trial was that a rising prostate-specific antigen (PSA) in the setting of mTOR inhibition was related to disease progression, potentially through relief of negative feedback inhibition on the EGFR pathway, which prompted this combination study.13, 14 We later realized that in some instances, the paradoxical rise of PSA in response to treatment was clinical evidence demonstrating the relief of feedback inhibition by everolimus on the AR signaling pathway.
MATERIALS AND METHODS
Study Design
This registered phase I/II study (clinicaltrials.gov NCT00085566) was approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board. All subjects (patients with progressive metastatic prostate cancer or progressive glioblastoma multiforme [GBM]) provided written informed consent.
Phase I consisted of a single-agent lead-in period to assess the pharmacokinetics of everolimus and gefitinib separately and in combination using a weekly oral dose of 30, 50 or 70 mg of everolimus (based on preclinical and clinical analysis17, 18) and a fixed oral daily dose of 250 mg of gefitinib. Twelve patients (10 CRPC, 2 GBM) were enrolled in phase I and received a single dose of everolimus on day 1 of week 1, then received only gefitinib 250 mg per day beginning on week 2 for 2 consecutive weeks. Combination therapy began on week 4. The 30- and 50-mg everolimus cohorts contained 3 patients each, while the 70-mg cohort had 6 patients. Patients were monitored for toxicity for 28 days from the beginning of combination therapy before treatment of a cohort began at the next everolimus dose level. The primary objective of the phase I portion was to define the tolerability of everolimus when administered in combination with a fixed dose of gefitinib. The probability of dose escalation depended on the dose-limiting toxicity. The 2 phase I GBM patients are included in the toxicity and pharmacokinetic data reported below in Results, but not in the efficacy data; their efficacy data have been reported separately.19
Phase II consisted of a combination of everolimus 70 mg weekly and gefitinib 250 mg daily based on the phase I results. It enrolled 27 CRPC patients and 20 GBM patients; the phase II GBM patients are not included in this report, having been reported separately.19 For the prostate cancer cohort, the primary objective of the phase II portion was to assess the efficacy of the dose combination in patients with metastatic CRPC. Efficacy was assessed by the proportion of patients who showed no change (≤50% rise) or a decline in PSA at 12 weeks, with no radiographic20 or clinical progression. A response rate of 25% was considered active and 10% inactive. A Simon two-stage design was used in which an initial 27 patients were treated and if 2 or fewer responses were observed, the trial was to be terminated. If 3 or more responses were observed, an additional 13 patients were to be accrued and the combination considered worthy of further study if 7 or more responses were observed from the 40 patients enrolled. The design had a power of .90 if the population response proportion is 0.25 using a 0.10 size test.
Patient Eligibility
Eligible prostate cancer patients had histologically confirmed metastatic CRPC with progression defined by one or more of these criteria: a) rising PSA levels showing at least a 25% increase: minimum of 3 rising levels obtained more than 1 week apart or 2 rising PSA values more than 1 month apart; b) transaxial imaging: new or progressive (20% increase in the sum of the indicator lesions) soft-tissue masses on computed tomography or magnetic resonance imaging scan; c) bone scan: new metastatic lesions. Patients needed to meet standard laboratory eligibility requirements to assure adequate bone marrow reserve and liver and kidney function and a Karnofsky Performance Status ≥70%. There were no restrictions to prior therapy for CRPC.
Patient Evaluation
Patients were assessed at biweekly intervals for toxicity based on standard National Cancer Institute Common Toxicity Criteria version 3.0. Patients were provided with medication diaries, which were reviewed at each physician visit. Dose-limiting toxicity was defined as any one of the following: a) grade 3 or 4 non-hematologic toxicity excluding nausea, vomiting, rash, and untreated hyperlipidemia; b) grade 3 diarrhea lasting more than 48 hours or grade 4 diarrhea; c) grade 3 fatigue lasting more than a week or grade 4 fatigue; d) neutropenic fever; e) grade 4 hematologic toxicity; or f) any toxicity causing treatment delay for longer than 2 weeks with one or both agents.
Patients were assessed for efficacy at week 12 (in phase I, this represented week 9 of combined therapy) and every 12 weeks thereafter (for phase I patients, every 8 weeks thereafter). Prostate cancer patients were assessed using a combination of changes in PSA, measurable disease, and lesions identifiable on bone scan. The primary endpoint was the proportion of patients who showed a decline or no change (≤50% rise) in PSA at 12 weeks.
Pharmacokinetics (Phase I Only)
Blood samples for pharmacokinetic studies of everolimus administered alone were obtained during the first week of treatment on day 1: pre-treatment and 1, 2, 5, and 8 hours after treatment. A single blood sample for pharmacokinetic studies of gefitinib alone was obtained on week 3 day 1 (7 days after initiation of gefitinib) in order to analyze gefitinib at steady-state. Blood samples for pharmacokinetic studies of both agents were obtained on week 4 day 1 (the first day of combination therapy): pre-treatment and 1, 2, 5, and 8 hours after treatment. At each designated sampling time, a whole blood sample (2 ml for everolimus alone; 4 ml for gefitinib alone; or 6 ml for everolimus and gefitinib coadministration) was drawn into a potassium-EDTA Vacutainer tube, then centrifuged to obtain plasma, which was shipped on dry ice to either the Novartis or Avantix Laboratories to measure drug concentrations. Liquid/liquid extraction of the blood samples was followed by analysis of the reconstituted extracts by HPLC/MS using atmospheric pressure chemical ionization. The lower limit of quantitation of the analytical method was 0.3 ng/ml, using 500 μl of blood.
FDG PET Scans
A secondary study objective was to explore the association between everolimus administration, clinical outcomes, and serial fluorodeoxyglucose positron emission tomography (FDG PET) imaging. To demonstrate the pharmacodynamic effects of mTOR inhibition in the clinic, and to assess the predictive value of an early FDG PET response in relation to time to progression, we utilized FDG PET imaging as an early measure of the on-target effect of mTOR inhibition in tumor.21, 22 A baseline scan was obtained within 30 days before the start of everolimus; an optional early post-treatment scan was obtained within 24 to 72 hours following treatment initiation; and a third scan was requested at the week 12 response assessment or treatment discontinuation, whichever came first. The definition of FDG PET for this trial followed the EORTC criteria which describe a decline as a decrease in standardized uptake value (SUV) > 25% of prior SUV values, and progression as increase > 25%. In this case, we used an average of SUVmax as the measured parameter, which was then converted into a fractional decline by normalizing the SUV with respect to the baseline SUVmax average in each individual patient.21
RESULTS
Patient Characteristics
Overall, 37 patients with progressive CRPC (10 phase I, 27 phase II) were treated on this study at MSKCC between July 2004 and March 2006. Baseline characteristics are summarized in Table 1. The majority of patients had bone metastases (34/37, 92%) and had received chemotherapy prior to study entry (23/37, 62%), including 30% (11/37) with 3 or more prior regimens. In the phase I portion, 2 patients with GBM were enrolled (1 each on the 30- and 50-mg everolimus cohorts) and have been included in the toxicity and pharmacokinetic analysis reported here. The 20 GBM patients participating in the phase II portion of the trial are not included in this paper, as their data have been previously reported.19 All phase II patients reported here had metastatic CRPC (n=27).
Table 1.
Baseline characteristics for the prostate cancer patients
| Characteristic | Phase I (n = 10) | Phase II (n = 27) | Total (n = 37) |
|---|---|---|---|
| Age, y | |||
| Median | 70 | 73 | 70 |
| Range | 59–76 | 52–87 | 52–87 |
| Karnofsky Performance Status | |||
| Median | 90 | 80 | 90 |
| Range | 80–90 | 70–90 | 70–90 |
| Baseline PSA, ng/mL | |||
| Median | 59 | 197 | 194 |
| Range | 7–1013 | 1–5447 | 1–5447 |
| Primary Gleason score | |||
| Median | 7 | 8 | 8 |
| Range | 6–9 | 5–9 | 5–9 |
| Baseline LDH, IU/L | |||
| Median | 204 | 207 | 207 |
| Range | 157–336 | 139–615 | 139–615 |
| Primary treatment, n | |||
| Surgery | 0 | 6 | 6 |
| Radiation | 4 | 10 | 14 |
| Untreated | 6 | 11 | 17 |
| Sites of disease, n | |||
| Bone only | 5 | 12 | 17 |
| Soft tissue only | 0 | 3 | 3 |
| Bone and soft tissue | 5 | 12 | 17 |
| Prior chemotherapy regimens, n | |||
| 0 | 4 | 10 | 14 |
| 1 | 3 | 6 | 9 |
| 2 | 1 | 2 | 3 |
| ≥ 3 | 2 | 9 | 11 |
Abbreviations: PSA, prostate-specific antigen; LDH, lactate dehydrogenase.
Pharmacokinetics
The phase I portion of this trial had a 3-week single-agent lead-in phase, in which the individual pharmacokinetic profiles of everolimus and gefitinib were measured. The ratio of the area under the curve (AUC) for everolimus in combination with gefitinib compared to the AUC for the same dose of everolimus as a single agent was 1.32 for the 30-mg everolimus dose (n = 3), 0.99 for the 50-mg everolimus dose (n = 3), and 1.03 for the 70-mg everolimus dose (n = 6). Similarly, the ratio of the AUC for gefitinib in combination with either 30- or 50-mg everolimus compared to the AUC of the same dose of gefitinib as a single agent was 1.17 (n = 6). These results suggest no significant pharmacokinetic interactions between everolimus and gefitinib.
Toxicity
There was no dose-limiting toxicity observed in phase I. Table 2 lists common toxicities for all 12 phase I patients (10 CRPC, 2 GBM) as well as the 27 CRPC patients treated in phase II. The most common toxicities grade 2 or higher were fatigue (grade 2: 38%, grade 3: 5%), prompting 4 patients to discontinue treatment early, and hyperglycemia (grade 2: 23%, grade 3: 8%), which did not require hospitalization.
Table 2.
Selected toxicities for all patients (phase I and phase II; n = 39a)
| Clinical | Common Toxicity Criteria by Grade, Number of Patients (%) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Anorexia | 14 (36) | 2 (5) | 0 | 0 |
| Constipation | 15 (38) | 1 (3) | 0 | 0 |
| Diarrhea | 24 (62) | 1 (3) | 0 | 0 |
| Fatigue | 19 (49) | 15 (38) | 2 (5) | 0 |
| Mucositis | 12 (31) | 3 (8) | 0 | 0 |
| Nausea | 10 (26) | 4 (10) | 0 | 0 |
| Sensory neuropathy | 16 (41) | 2 (5) | 0 | 0 |
| Acneiform rash | 19 (49) | 2 (5) | 0 | 0 |
| Urinary urgency/frequency | 23 (59) | 1 (3) | 0 | 0 |
| Weight loss | 11 (28) | 1 (3) | 0 | 0 |
| Renal failureb | 0 | 0 | 2 (5) | 1 (3) |
| Laboratory | 1 | 2 | 3 | 4 |
| Lymphopenia | 0 | 0 | 14 (36) | 0 |
| Thrombocytopenia | 16 (41) | 0 | 1 (3) | 1 (3) |
| Hyperglycemia | 23 (59) | 9 (23) | 3 (8) | 0 |
| INR too high or low | 6 (15) | 2 (5) | 2 (5) | 0 |
| Elevated AST or ALT | 29 (74) | 3 (8) | 1 (3) | 0 |
| Hypertriglyceridemia | 21 (54) | 3 (8) | 0 | 0 |
Abbreviations: INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Includes toxicity data from 2 glioblastoma multiforme patients in phase I.
The 2 cases of grade 3 renal failure were attributed to dehydration.
There were only 2 episodes of grade 4 toxicity: thrombocytopenia in a heavily pre-treated patient with advanced bone disease, and renal failure likely related to dehydration in another patient. Grade 3 non-hematologic toxicities included renal failure attributed to dehydration (2 patients) and fatigue (2 patients). Grade 3 lymphopenia was noted in 14 (36%) patients without clinical significance.
Patient Outcomes
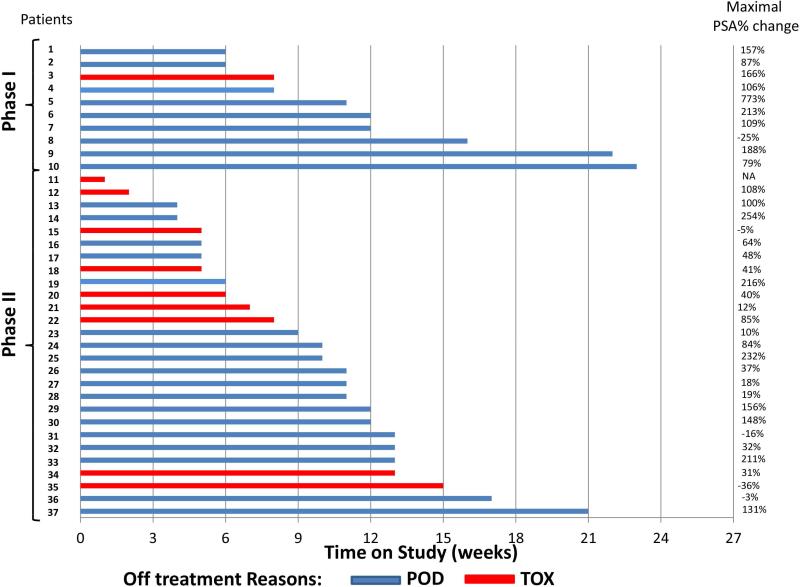
More than half of the CRPC patients discontinued treatment early, prior to the scheduled week 12 reassessment (23/37, 62%; phase II only: 18/27, 67%). Time on study and reasons for discontinuation (progression versus toxicity) are given in Figure 2. In phase II, 18 out of 27 (67%) CRPC patients discontinued treatment prior to the 12-week evaluation due to disease progression as evidenced by a rise in PSA (6 patients) or imaging (5 patients), or grade ≥ 2 toxicity (7 patients). Fifteen of the 18 patients who discontinued treatment early in phase II had a rising PSA. The median time on study was 12 weeks (range 6−23) in phase I, and 11 weeks (range 1−21) in phase II.
Figure 2. Time on study, maximal percent PSA change from baseline during study, and reason for discontinuation in prostate cancer patients (n=37).
PSA: prostate-specific antigen, POD: progression of disease; TOX: toxicity; NA: not available. Thirty-seven patients with castration-resistant prostate cancer were treated on study (10 phase I and 27 phase II). Median time on study was 12 weeks in phase I and 11 weeks in phase II. The majority of patients (5 phase I, 18 phase II) discontinued treatment prior to the planned 12-week evaluation due to disease progression determined by imaging and/or rise in PSA (15 patients: 4 phase I, 11 phase II), or grade ≥ 2 toxicity (8 patients: 1 phase I, 7 phase II). We examined the greatest percent PSA change from baseline for each patient at any time during the study: this constituted a PSA rise for 31 patients (ranging from 10% to 773%), a PSA decline for 5 patients (ranging from −3% to −36%), and maximal percent change could not be calculated for one patient who discontinued after only 1 week on study. Of the 5 patients whose maximal PSA change was a decline, median time on study was 15 weeks.
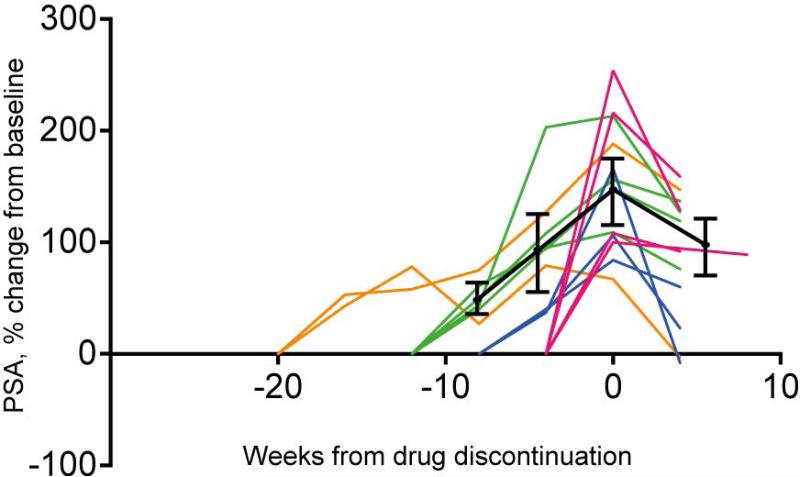
Notably, 13 of the 37 (35%) CRPC patients treated on study experienced an early and rapid increase in the rate of rise in PSA on treatment which declined after treatment was stopped (Figure 3).
Figure 3. Clinical evidence of relief of feedback inhibition on AR in response to mTOR inhibition.
AR: androgen receptor, PSA: prostate-specific antigen.
Change from baseline PSA level for 13 patients on study who experienced a rise in PSA on treatment and then a PSA decline following discontinuation of treatment. Week 0 indicates discontinuation of treatment. Two patients (orange lines) were on treatment ≥ 20 weeks; 4 patients (green lines) were on treatment for 12 weeks; 3 patients (blue lines) were on treatment for 8-10 weeks (graphed as 8 weeks); and 4 patients (red lines) were on treatment for 2−6 weeks (graphed as 4 weeks). Black lines show the average percent change in PSA from baseline at each time point along with a corresponding 95% confidence interval.
Overall, 14 CRPC patients in phase I and phase II remained on study for at least 12 weeks, of whom 8 (56%) showed a best response of stable disease on imaging. Of these, 6 (2 phase I, 4 phase II) met the protocol criteria for a favorable outcome of decline or no change (≤50% rise) in PSA at 12 weeks, with no other signs of radiographic or clinical progression. No patient had >30% decline in PSA and this was not durable, as 3 patients (2 phase I, 1 phase II) showed disease progression on bone scan by week 23, and 1 phase II patient discontinued due to rising PSA at week 13. The remaining 2 patients discontinued treatment by week 15 on account of toxicity. Since the overall outcomes demonstrated limited anti-tumor activity with a best response of stable disease and the majority of patients discontinued treatment prior to the scheduled 12-week reassessment, a decision was made by the investigators to terminate the study instead of expanding accrual.
FDG PET Scan Results
Since mTOR directly stimulates glucose uptake and glycolysis by enhancing both transcription and translation of glucose transporter 1 mRNA, FDG PET scans were performed as an exploratory pharmacodynamic marker of mTOR inhibition.23-25 The FDG PET scans in the trial were performed at baseline; 24 to 72 hours after start of treatment to assess the pharmacodynamic effect of everolimus alone; and at 12 weeks. The majority of patients discontinued the study before the 12-week assessment, due to disease progression or an adverse event.
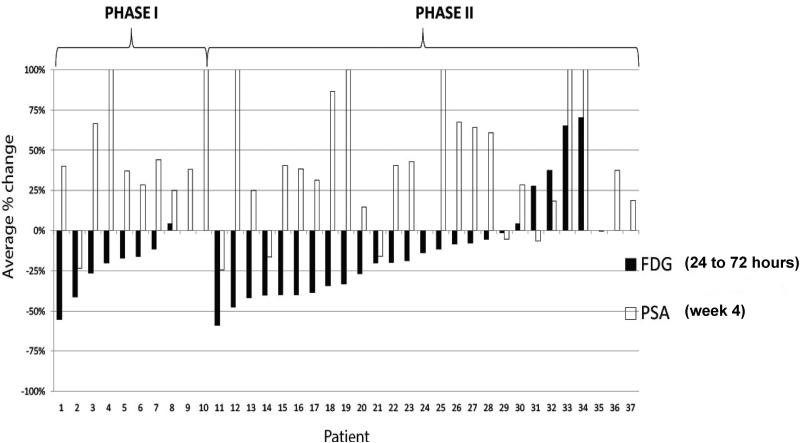
Overall, baseline and 24 to 72 hour FDG PET imaging was performed on 33 out of 37 patients, of whom 21 also had end-of-treatment FDG PET imaging performed. At the 24 to 72 hour FDG PET assessment, 27 of 33 (82%) evaluable patients showed some degree of SUV decline; a corresponding 20 of these 27 (74%) patients with an early SUV decline demonstrated a rise in PSA (Figure 4). The early declines in FDG accumulation at 24 to 72 hours were consistent with mTOR inhibition, but the declines were not durable as SUV increased to baseline or above at the next imaging timepoint and were not associated with favorable PSA outcomes or overall response. Both early responders (those experiencing SUV decline) and non-responders remained on study for an average of 11 weeks.
Figure 4. FDG PET (%SUV) and PSA (%) change from baseline in cycle 1 of treatment.
FDG: fluorodeoxyglucose, PET: positron emission tomography, SUV: standardized uptake value, PSA: prostate-specific antigen.
FDG PET imaging was performed at 24 to 72 hours following initiation of treatment as a pharmacodynamic marker of mTOR inhibition. The early FDG SUV declines in 27 of 33 (82%) evaluable patients were consistent with mTOR inhibition and corresponded to a week 4 rise in PSA in 20 of 27 (74%) patients. FDG PET data at 24 to 72 hours was not available for 2 phase I patients and 2 phase II patients. Week 4 PSA data was not available for 1 phase II patient.
DISCUSSION
This phase I/II trial of everolimus in combination with gefitinib in patients with metastatic CRPC was based on preclinical evidence demonstrating that resistance to EGFR inhibition could be reversed through pharmacologic down-regulation of constitutive PI3K/Akt pathway activation. Although 6 patients in phase II met the protocol-defined criteria for a favorable outcome of decline or no change (≤50% rise) in PSA at 12 weeks, in the setting of limited anti-tumor activity with a best response of stable disease in 8 patients (22%) and with a high rate of treatment discontinuation prior to the 12-week reassessment interval (62%), a decision was made by the investigators to terminate the study early.
Early discontinuation of treatment was frequent and most often due to disease progression in this heavily pretreated population (30% of patients had ≥ 3 prior chemotherapy regimens) as opposed to drug-related toxicity, offering some further evidence of a lack of clinical efficacy. It should be noted, however, that this trial was conducted before the Prostate Cancer Working Group 2 recommendation that patients not be taken off a therapy for PSA progression alone26; in retrospect it is clear that our efficacy data were limited by the early discontinuation of many patients due to PSA progression and that this may in part have been related to reciprocal feedback on AR in response to mTOR inhibition.
Importantly, 13 out of 37 (35%) CRPC patients exhibited a rapidly rising PSA after starting everolimus and gefitinib which declined upon discontinuation of treatment (Figure 3). The rise and fall of PSA in these patients in response to treatment initiation and discontinuation suggests that therapy caused an increase in tumor growth and/or an increase in AR-dependent or -independent expression of PSA. Subsequent to the generation of these clinical data, we showed that AR transcriptional activity is repressed in prostate tumors with PI3K activation.15 Moreover, in preclinical models we showed this is due to PI3K-induced feedback inhibition of AR which is relieved by inhibitors of PI3K signaling. In these models, mTOR inhibition reactivates AR activity and AR-dependent transcription in an RTK-dependent manner. The induction of PSA seen in this study in response to treatment is consistent with preclinical data that PI3K/AKT/mTOR signaling feedback inhibits AR in prostate cancer and suggests the potential efficacy of combining PI3K pathway inhibitors and second generation AR inhibitors.
Since induction of AR activation in response to PI3K inhibition is likely to prevent or weaken any anti-tumor response, the recent development of improved AR inhibitors in combination with potent and selective inhibitors of the PI3K signaling pathway offers great promise for patients with CRPC. In addition, preclinical work by our group has shown that reactivation of AR in tumors exposed to PI3K pathway inhibition is mediated, at least in part, by reactivation of receptor tyrosine kinases.9, 15, 27 In this study, combining gefitinib with everolimus did not prevent PSA induction nor was the combination associated with antitumor activity. It is possible that inhibition of other, more functionally important receptors, alone or in combination with EGFR inhibition will be more effective.
Recognizing the difficulty of obtaining metastatic CRPC from bone for molecular analysis, disease heterogeneity, and the lack of validation assays at the time of this study for the targets of interest in bone specimens (ie, PTEN), we performed FDG PET imaging to assess the on-target effect of everolimus. During the FDG PET assessment at 24 to 72 hours, 27 of 33 (82%) evaluable patients showed some degree of SUV decline compared with baseline consistent with mTOR inhibition (Figure 4), but this early SUV decline seen in the majority of patients was not durable and did not predict for overall response. Twenty of these 27 (74%) patients with an early decline in SUV had a corresponding rise in PSA at the 4-week assessment suggesting the possibility of activation of compensatory pathways contributing to cell survival in response to abrogation of mTOR signaling. However, our ability to collect and usefully interpret the FDG PET data was limited by the large number of patients who stopped treatment early due to PSA progression.
The data from this study present confirmatory evidence that reciprocal feedback inhibition is a clinically relevant phenomenon. Although the combination of everolimus and gefitinib was ineffective, we postulate that everolimus-induced relief of feedback inhibition enhanced AR activity in a subset of patients; this supports the need for rational combinations of targeted agents to overcome compensatory signaling pathways and provide meaningful anti-tumor activity. Future interventions targeting the PI3K axis in patients with CRPC include selective PI3K and AKT inhibitors which are now in early clinical development, mTOR kinase inhibitors that are effective against both mTORC1 and mTORC2, and further exploration of combined AR/growth factor/AKT pathway inhibition.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers. Supported in part by the MSKCC SPORE in Prostate Cancer (NIH grant P50 CA092629), the Department of Defense Prostate Cancer Research Program (PC051382), The Prostate Cancer Foundation, Novartis (drug supply and funding), and AstraZeneca (drug supply).
Footnotes
Part of this work has been published in abstract form in J Clin Oncol, 2006 ASCO Annual Meeting Proceedings Part I, Abstract #14520 (published in proceedings but not presented at ASCO).
The following financial disclosures or potential conflicts of interest were disclosed by the contributing authors: None
REFERENCES
- 1.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fruman DA, Rommel C. PI3Kdelta inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 2011;1:562–572. doi: 10.1158/2159-8290.CD-11-0249. [DOI] [PubMed] [Google Scholar]
- 3.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 4.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2008;6:97–102. doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 7.George DJ, Armstrong AJ, Creel P, et al. A phase II study of RAD001 in men with hormone-refractory metastatic prostate cancer (HRPC). ASCO Genitourinary Cancers Symposium; San Francisco, CA. February 14-16, 2008; 2008. Abstract 181. [Google Scholar]
- 8.Templeton AJ, Dutoit V, Cathomas R, et al. Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol. 2013;64:150–158. doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boccardo F, Rubagotti A, Conti G, et al. Prednisone plus gefitinib versus prednisone plus placebo in the treatment of hormone-refractory prostate cancer: a randomized phase II trial. Oncology. 2008;74:223–228. doi: 10.1159/000151391. [DOI] [PubMed] [Google Scholar]
- 11.Lorusso PM. Phase I studies of ZD1839 in patients with common solid tumors. Semin Oncol. 2003;30:21–29. doi: 10.1053/sonc.2003.50029. [DOI] [PubMed] [Google Scholar]
- 12.Pezaro C, Rosenthal MA, Gurney H, et al. An open-label, single-arm phase two trial of gefitinib in patients with advanced or metastatic castration-resistant prostate cancer. Am J Clin Oncol. 2009;32:338–341. doi: 10.1097/COC.0b013e31818b946b. [DOI] [PubMed] [Google Scholar]
- 13.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 14.She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin Cancer Res. 2003;9:4340–4346. [PubMed] [Google Scholar]
- 15.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulholland DJ, Tran LM, Li Y, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka C, O'Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 18.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 19.Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92:99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Majumder PK, Yeh JJ, George DJ, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 23.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 24.Ma WW, Jacene H, Song D, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–2704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross RW, Manola J, Oh WK, et al. Phase I trial of RAD001 (R) and docetaxel (D) in castration resistant prostate cancer (CRPC) with FDG-PET assessment of RAD001 activity.. ASCO Annual Meeting; Chicago, IL.. May 30 - June 3, 2008; 2008. Abstract 5069. [Google Scholar]
- 26.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]