Abstract
Background
Smoking abstinence impairs executive function, which may promote continued smoking behavior and relapse. The differential influence of nicotine and non-nicotine (i.e. sensory, motor) smoking factors and related neural substrates is not known.
Methods
In a fully factorial, within-subjects design, 33 smokers underwent fMRI scanning following 24-h of wearing a nicotine or placebo patch while smoking very low nicotine content (VLNC) cigarettes or remaining abstinent from smoking. During scanning, blood oxygenation level-dependent (BOLD) signal was acquired while participants performed a verbal N-back task.
Results
Following 24-h placebo (vs. nicotine) administration, accuracy on the N-back task was significantly worse and task-related BOLD signal lower in dorsomedial frontal cortex. These effects were observed irrespective of smoking.
Conclusions
Our data provide novel evidence that abstinence-induced deficits in working memory and changes in underlying brain function are due in large part to abstinence from nicotine compared to non-nicotine factors. This work has implications both for designing interventions that target abstinence-induced cognitive deficits and for nicotine-reduction policy.
Keywords: addiction, fMRI, neuroimaging, nicotine, smoking, working memory
INTRODUCTION
Cessation of tobacco smoking results in a constellation of withdrawal signs and symptoms including subjective reports of difficulty concentrating and objective worsening of attention and working memory performance (Falcone et al., 2013a; Gilbert et al., 2004; Heishman, 1998; Heishman et al., 1994; Hendricks et al., 2006; Mendrek et al., 2006). These effects on cognition emerge within minutes of abstinence (Hendricks et al., 2006), persist for weeks following quitting smoking among some smokers (Gilbert et al., 2004) and are predictive of relapse (Patterson et al., 2010). Understanding the unique and interactive influence of various sensory, behavioral and pharmacological aspects of smoking on abstinence-induced cognitive impairments can guide the development of interventions that specifically target these deficits. The current study systematically investigates the effects of nicotine and non-nicotine factors on working memory performance and related brain function with the goal of delineating the role of these factors.
Smoking abstinence results in cessation of self-delivered nicotine—the primary psychoactive substance in cigarette smoke—and also non-nicotine factors including the sensory (e.g. taste, smell) and behavioral (e.g. ritualistic) aspects of smoking; and any of the potentially psychoactive, non-nicotine components of smoke (Caggiula et al., 2002; Rose, 2006). Research on the effects of nicotine (administered in the absence of smoking) or smoking denicotinized or very low nicotine content (VLNC) cigarettes (administered in the absence of nicotine) suggest a greater role for nicotine in modulating cognition. Nicotine administration compared to placebo has been shown to improve cognitive performance among abstinent smokers (Atzori et al., 2008; Beaver et al., 2011; Foulds et al., 1996; Froeliger et al., 2009) including one large and well-controlled study in which performance on a working memory N-back task was improved following administration of a 21 mg nicotine versus placebo patch to overnight-deprived smokers (Kleykamp et al., 2011). Consistent with these findings, administration of VLNCs has been shown to perpetuate abstinence-induced impairments in cognition (Gilbert et al., 1997; Wesnes and Warburton, 1983).
Neuroimaging studies of smokers performing tasks that require sustained attention and working memory suggest that frontal brain regions are modulated in conjunction with abstinence-induced performance deficits (Falcone et al., 2013b; Kozink et al., 2010; Xu et al., 2006). In a prior study, we observed decreased BOLD signal in dorsolateral prefrontal cortex (dlPFC) following 24-h abstinence during a task requiring sustained attention and working memory (Kozink et al., 2010). In another study of the effects of 24-h abstinence on working memory, abstinence decreased activation in working memory-related regions including dlPFC and dorsal medial frontal cortex (Falcone et al., 2013b). In both studies, effects were observed regardless of memory load suggesting they were due to disruption of non-working memory specific processes (e.g. sustained attention). Acute nicotine (compared to placebo) administration to overnight abstinent smokers has been shown to both increase (Beaver et al., 2011) and decrease (Ernst et al., 2001) cortical activation. The effects of longer-term smoking/nicotine manipulations have not been evaluated nor has there been systematic investigation of the effects of nicotine vs. non-nicotine factors on the neural substrates underlying cognition.
This investigation sought to evaluate the effects of nicotine and non-nicotine factors on neurocognition. In a 2x2, within-subjects design, adult dependent smokers underwent scanning after 24-h of wearing a 21 mg/d nicotine or placebo patch while either smoking VLNCs or remaining abstinent from smoking. Blood oxygenation-level dependent (BOLD) signal was acquired during scanning. We hypothesized that performance on the placebo patch would be worse than performance on the nicotine patch, irrespective of smoking. Moreover we hypothesized that nicotine abstinence would decrease task-related BOLD signal in brain regions identified a priori that support working memory and sustained attention including dlPFC, dorsomedial frontal cortex (dmFC) and the parietal cortex. Based on research reviewed above in which smoking and nicotine were manipulated in the absence of the other factor, we hypothesized that smoking would have a relatively weaker or no impact on brain activation compared to nicotine, or would only modulate outcomes in interaction with nicotine.
METHODS AND MATERIALS
Participants
Thirty-five smokers between the ages of 18–55 were recruited from the community and completed all aspects of the study. Inclusion criteria for all subjects included being right-handed, free of serious health problems (e.g., hypertension), not currently undergoing treatment for a psychiatric illness, free of medications altering CNS functioning, not having any conditions making MRI research unsafe, testing negative for illicit drug use, and among females, having a negative serum pregnancy test.
Smokers were required to smoke ≥ 10 cigarettes/day of a brand delivering > 0.5 mg nicotine according to the standard Federal Trade Commission (FTC) method for at least 2 years, have an expired CO concentration of at least 10 ppm or a positive urine cotinine test (Nicalert™) of 100 ng/mL or greater, not currently use any nicotine products other than cigarettes and have no interest in quitting.
Procedures
All participants read and signed an IRB-approved informed consent form. Participants completed a 1.5-hr screening session during which they provided breath and saliva samples, underwent a medical evaluation that included a review of systems, EKG and vitals, and completed questionnaires regarding mood, smoking history, and suitability for MRI research. Participants who passed screening completed a training session during which they practiced a verbal working memory task (N-back, see below) while lying in a mock scanner. Participants were required to achieve at least 40% accuracy on the 2-back condition within 4 practice attempts. In a 2 (PATCH)×2 (SMOKE) design, smokers underwent four separate fMRI scanning sessions following 24-h wearing a nicotine (NIC) or placebo (PLAC) transdermal patch during which time they smoked either VLNC cigarettes ad libitum or remained abstinent (ABST). Participants were provided with all study-related materials: 21 mg Nicoderm CQ® patches, placebo patches, and Quest 3 brand VLNC cigarettes (Vector Tobacco Co.; nicotine delivery < .05 mg/cigarette; FTC method). Patches were repackaged so that neither the experimenter nor the participant was aware of the patch condition. Results from a post-session questionnaire indicated that more than half of participants (52%) were not able to correctly guess their patch condition more than 50% of the time across the four sessions. The order in which participants completed conditions was random. Menthol or non-menthol VLNC cigarettes were provided based on participant preference (43% = menthol smokers). For conditions in which smoking was prescribed, participants were required to smoke the assigned cigarette immediately before entering the hospital (i.e. < 30 min prior to scanning). Abstinence was verified by expired CO on the scan day. Each scanning session was held at least 4 days apart (mean=8.6 days, SD=3.5). During all scanning sessions, participants completed the N-back task.
Nicotine Dependence
At screening, participants completed the Fagerström Test of Nicotine Dependence (FTND)(Heatherton et al., 1991).
N-back task
A verbal N-back task was comprised of 60 sec blocks during which participants were presented with a series of letters on a screen at a rate of 1/sec. Participants were required to identify whether the current stimulus was the same as the stimulus presented N-back. In 1-back blocks, participants identified whether the current letter was the same as the letter that preceded it (i.e. 1 letter back). In the 2-back block, participants identified whether the letter was the same as the letter presented 2 letters back. During the ‘0-back’ blocks, participants pressed a button whenever they saw the letter ‘X’. Nine targets per block were presented (mean inter-target-interval = 6 sec) and each block level (0-, 1-, 2-back) was presented four times over the course of 2 runs (total task time = 18 min). N-back blocks were separated by 25 sec rest periods.
Imaging Methods
Scanning was performed on a 3.0 T GE EXCITE HD scanner equipped with 40 mT/m gradients (Waukesha, WI). BOLD images were collected for 30 contiguous slices (3.8 mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures. An inverse SENSE spiral pulse sequence sensitive to BOLD contrast was used, with TR=1.5 s, TE=30 ms, FOV=25.6 cm, matrix=64×64, flip angle=60°, and in-plane resolution=4 mm2. After completion of the functional data collection, a T1-weighted 3D fast spoiled gradient-recalled (FSPGR) structural image was collected for 166 slices (1 mm thick), with TR=7.5 ms, TE=3 ms, FOV=25.6 cm, matrix=256×256, flip angle=12°, and in-plane resolution=1 mm2.
Analysis of Behavioral Data
Two participants were excluded from the behavioral data analyses due to non-compliance (high CO on non-smoking day, n=1; < 50% accuracy on the 0-back, n=1). Analyses of the effects of condition on N-back task accuracy, reaction time (RT) and reaction time standard deviation (RTSD) were conducted using general linear mixed models in SAS 9.3 with subject-level Gaussian random effects. The models included SMOKE (VLNC, ABST), PATCH (NIC, PLAC) and N-back LEVEL (0,1,2) as factors.
Analysis of Brain Data
Preprocessing
Preprocessing was conducted using SPM8 to attenuate noise and artifacts. The first four volumes of each run were discarded to allow for T1 stabilization. All functional images underwent correction for acquisition timing and for head motion using rigid-body rotation and translation (Friston et al., 1994). Each participant’s data was then subsequently warped into a standard stereotaxic space (Montreal Neurological Institute) with an isotropic 2 mm voxel size and smoothed with a 6 mm FWHM Gaussian filter. As in our previous work (Sweitzer et al., 2013), artifact detection tools implemented in SPM (ART; www.nitrc.org/projects/artifact_detection) were used to identify and adjust for image artifacts related to intensity spiking and motion (Chai et al., 2014; Redcay et al., 2013; see Supplement for details). In addition to those excluded from the behavioral analyses, participants were excluded from imaging analyses if > 10% of the total volumes were identified as outliers (n=3); or signal loss/other artifact (n=2).
Statistical Analysis
Participant’s data from each session was entered into a first-level, whole-brain analysis using the General Linear Model (Friston et al., 1994) to examine BOLD response to each of the 3 N-back trial types (0-back; 1-back, 2-back). Blocks of each trial type were modeled as a box car function (60 sec) convolved with the canonical hemodynamic response function (HRF). Problematic volumes were removed from the analysis by including regressors generated by the ART program. A high-pass filter (128 seconds) was applied to remove slow signal drift.
Our method of selecting ROIs was patterned after prior studies of smoking and executive function (Falcone et al., 2013b; Loughead et al., 2010; Loughead et al., 2009) and consisted of identifying functional ROIs in our dataset that matched a priori-specified working memory areas: First, main effects of working memory were explored in a whole-brain repeated measures analysis of variance that identified activation across working memory load (2-0 and 1-0) and conditions. ROIs were functionally defined using a voxel threshold of p < 0.05 FWE-corrected and a cluster extent threshold of k=200. Five functional clusters, each matching a priori regions of interest associated with working memory were selected from among activation clusters (location of peak cluster listed, see Figure S1): dmFC comprised of the dorsal anterior cingulate cortex (dACC), supplementary motor area (SMA) and pre-supplementary motor area (preSMA) (x=0, y=2, z=60), bilateral dorsolateral prefrontal cortex (R/L dlPFC; x=32, y=−2, z=58 and x=−44, y=6, z=32, respectively), and bilateral parietal lobule (R/L PL; x=34, y=−60, z=46 and x=−32, y=−46, z=42 respectively). Regions were identified via visual inspection and in comparison with regions identified in prior meta-analyses (Owen et al., 2005; Wager and Smith, 2003) and prior studies of smoking and working memory (Falcone et al., 2013b; Loughead et al., 2010; Loughead et al., 2009).
Average percent signal change was extracted from these regions for each participant during each condition for each working memory load condition using MarsBar (Brett et al., 2002). As with behavioral analyses, percent signal change in each of the ROIs was included in a general linear mixed model with subject-level Gaussian random effects. Patch condition, smoking status, and working memory load were used to predict percent signal change. Since different working memory loads and pharmacological conditions result in variable RTs, as in previous working memory studies (Loughead et al., 2010) we sought to control for the amount of time spent processing and responding to stimuli by controlling for RT at each working memory load level and condition in analyses of BOLD signal. Effect sizes (Cohen’s d) were calculated for significant main effects of nicotine condition based on means, pooled variance and the correlation between measurements.
RESULTS
Participant Characteristics
Participant characteristics are presented in Table 1 for the full sample (n=33) and the sample that provided analyzable fMRI datasets (n=28).
Table 1.
Demographic data for the full sample (n=33) and the subsample available for fMRI analysis (n=28).
| Full Sample (n=33) |
fMRI Sub- Sample (n=28) |
|
|---|---|---|
| Mean (SD) | ||
| Age | 36.6 (9.2) | 37.7 (9.2) |
| Cigarettes/Day | 16.5 (5.1) | 17.0 (4.9) |
| Years Smoked | 18.0 (9.0) | 19.2 (8.6) |
| FTND | 5.0 (2.1) | 5.3 (2.0) |
| n (%) | ||
| Female | 17 (51.5) | 14 (50.0) |
| Non-White | 15 (45.5) | 13 (46.4) |
Biochemical Assessment
Breath CO levels were compared as a function of experimental condition using a general linear mixed model with Gaussian random level subject effects. Across patch conditions, CO levels were significantly lower in the ABST (mean=3.6 ; SD=2.7) as compared to the DENIC (mean=15.3; SD=10.4) condition (F1,96=126.82, p < .0001) suggesting a high level of compliance. CO levels did not vary as a function of nicotine (as compared to placebo) patch administration.
N-back Task Performance
Across conditions, increased task difficulty was associated with decreased accuracy (% correct) (F2,352=222.89, p < .0001) and longer RTs (F2,352=260.15, p < .0001) and greater variability in RT (SD of RT) (F2,352=182.03, p < .0001); see Table S1 for means and SDs. As shown in Figure 1, wearing a placebo (vs. nicotine) patch was associated with significantly worse accuracy (main effect of PATCH, F1,352=11.04, p=.001; d = .38). Placebo patch administration was also associated with increased variability in RTs (F1,352=11.28, p=.0009; d = .4). Effects of SMOKE or interactions involving SMOKE and/or LEVEL were not observed.
Figure 1. N-back task accuracy as a function of working memory load, nicotine administration and smoking.
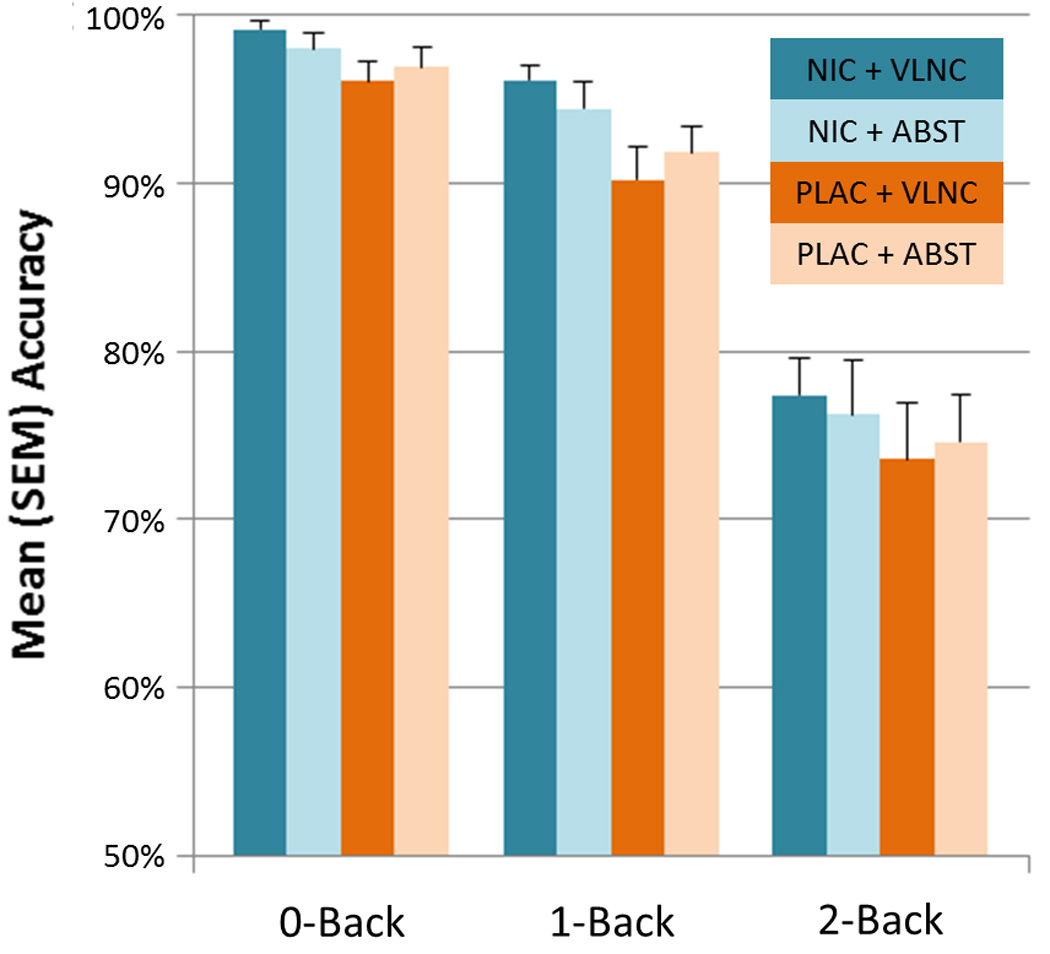
A main effect of PATCH (p < .001) was characterized by greater accuracy when smokers were wearing a nicotine (shaded in blue) as compared to placebo (shaded in orange) patch, irrespective of smoking behavior. See text for additional statistical details and the online Supplement for means and SDs.
Imaging Results
In each of the five ROIs, main effects of LEVEL were observed (2-back > 1-Back > 0-Back; p’s < 0.0001). In dmFC, BOLD signal was lower following 24 h placebo (compared to nicotine) administration across LEVEL and SMOKE factors (main effect of PATCH; F1,296=5.01, p=0.026; d = .26; see Figure 2). In the R PL, a significant SMOKE×PATCH interaction (F1,296=4.17, p=0.042) was observed. This interaction was characterized by lower BOLD signal in the PLAC+VLNC condition compared to the NRT+VLNC and PLAC+ABST conditions (p’s < .05). An evaluation of whether FTND score moderated the effects of nicotine patch on performance or BOLD signal did not reveal any significant findings.
Figure 2. Task-related BOLD signal as a function of nicotine administration and smoking in dACC/preSMA and right PL.
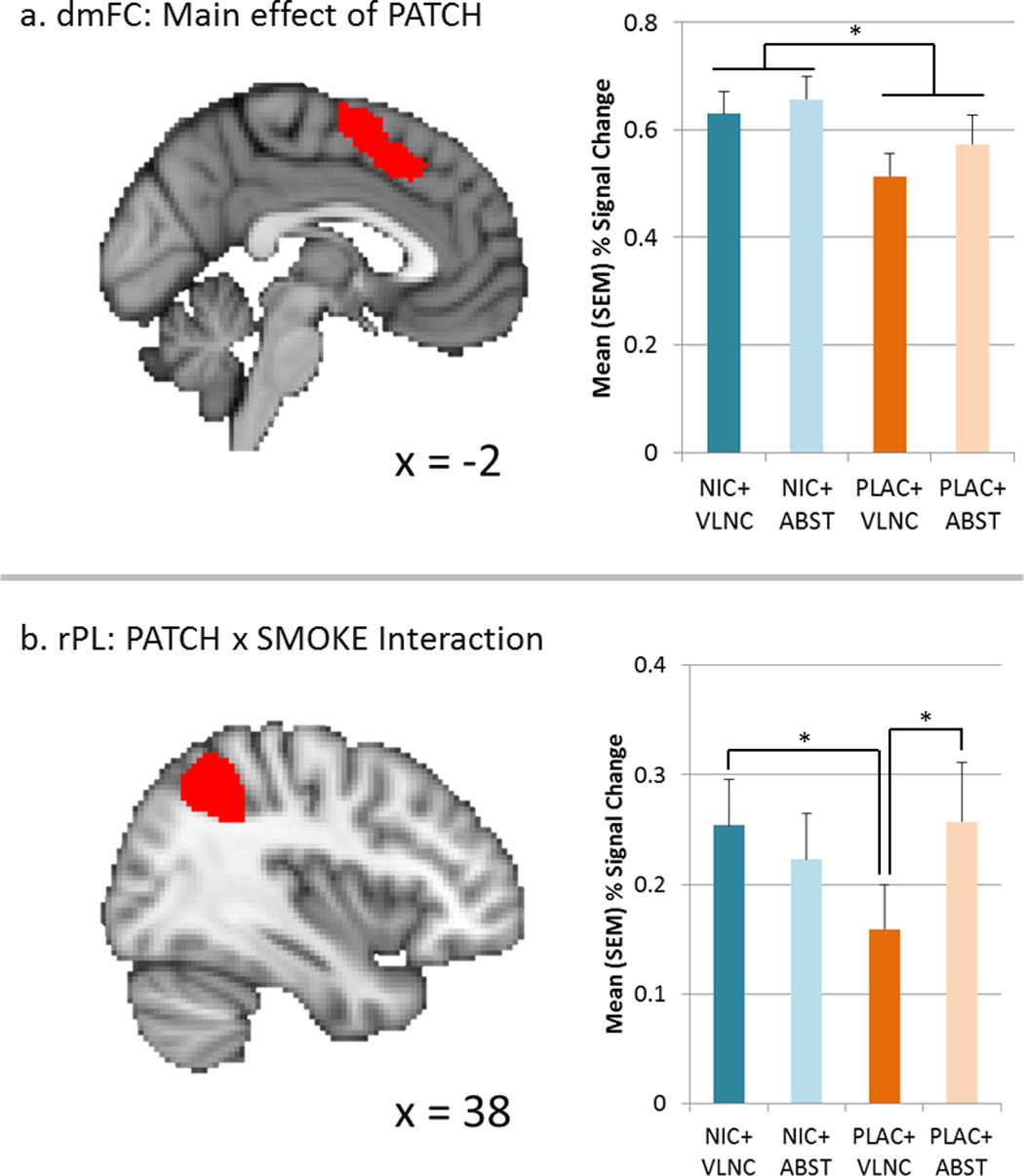
In dmFC (upper panel), 21 mg/d nicotine administration in the 24 h prior to scanning resulted in greater BOLD % signal change compared to placebo administration (main effect of PATCH, p=.026), irrespective of smoking behavior. In right PL, a PATCH × SMOKE interaction (p=.042) was characterized by lower % signal change following smoking VLNCs and wearing a placebo patch for 24 h prior to scanning relative to other conditions. * = p < .05; see text for additional statistical details.
DISCUSSION
This behavioral and fMRI study provides novel evidence that nicotine and non-nicotine factors associated with smoking have differential effects on working memory performance and underlying brain function. This was achieved by systematically evaluating the effects of administering nicotine (vs. placebo) while smoking very low nicotine content cigarettes (or maintaining abstinence) in the 24 h prior to scanning. As hypothesized, placebo, compared to nicotine, was associated with worse working memory performance and lower BOLD signal in dmFC, irrespective of smoking condition. Less expectedly, smoking and nicotine interacted to modulate parietal BOLD signal. For both behavioral and brain outcomes, effects were observed across working memory load levels. Collectively, our data confirm that cognitive impairments associated with smoking abstinence are due in large part to abstinence from nicotine and the loss of signaling in a brain region associated with distraction resistance (see below). These findings have important implications for treating smoking abstinence-induced cognitive performance deficits and for policies aimed at reducing cigarette nicotine levels.
Whereas the current study is the first to systematically evaluate the effects of nicotine and non-nicotine factors on functional neuroanatomical correlates of working memory, our hypotheses were informed by prior studies investigating the effects of nicotine and non-nicotine factors on cognitive performance. Previous studies have shown that nicotine, when administered to overnight deprived smokers improves working memory performance (Atzori et al., 2008; Beaver et al., 2011; Foulds et al., 1996; Kleykamp et al., 2011) whereas smoking VLNC cigarettes induces or perpetuates working memory deficits (Gilbert et al., 1997; Wesnes and Warburton, 1983). The results of the present study, which systematically evaluated the effects of nicotine and smoking in a single sample of smokers, are consistent with these prior studies in that we observed better accuracy and decreased RT variability following 24 h nicotine administration, irrespective of smoking condition.
The observation of greater BOLD signal during nicotine (compared to placebo) administration in dmFC—a region comprised of the dorsal anterior cingulate (dACC) and supplementary motor area (SMA) and pre-SMA—is consistent with prior neuroimaging studies and the known role of dorsal midline structures in executive function. In a study of 63 smokers who were scanned during a visual n-back task, greater sustained activation was observed in frontal ROIs including the dmFC under smoking compared to a 24 hr abstinence condition (Falcone et al., 2013b). These effects were not observed to vary across working memory load levels (discussed below). Activation in dmFC is nearly ubiquitous across studies of working memory (Nee et al., 2013; Owen et al., 2005; Wager and Smith, 2003). In a recent meta-analysis of fMRI studies (Nee et al., 2013) in which various components of executive function during working memory were investigated, the dmFC was implicated in decreasing or mitigating interference of working memory processes from external distractors (i.e. distractor resistance). This proposed role for the dmFC and our data are consistent with previous literature suggesting that nicotine enhances attention to task-relevant targets by decreasing distraction (Gilbert et al., 2005; Gilbert et al., 2007; Kassel, 1997; Kassel and Shiffman, 1997; Tsiora et al., 2013). Likewise, research with rodents and non-human primates suggests that nicotine decreases the influence of distractors on task performance (Bain et al., 2003; Hahn et al., 2002) and that this effect is mediated via the α4β2 nicotine receptor system (Howe et al., 2010; Prendergast et al., 1998). Future neuroimaging studies in which distraction is manipulated will be needed to test the hypothesis that the dmFC is specifically involved in this effect of nicotine/nicotine withdrawal.
It is noteworthy that the observed effects of nicotine and smoking on behavior and BOLD signal did not interact with level of difficulty on the N-back task. This finding is consistent with previous studies of the effects of smoking abstinence on tasks requiring sustained attention and with varying working memory loads. In a previous study in which we examined the effects of smoking abstinence on a sustained attention task that required working memory (Kozink et al., 2010), we observed disruption of performance regardless of working memory load. Similarly, in a prior neuroimaging study employing the N-back task, memory load level did not interact as a function of smoking (Falcone et al., 2013b). Given that nicotine × memory load interactions have been more pronounced in human laboratory studies (Kleykamp et al., 2011; Mendrek et al., 2006), the lack of load effects in our and other imaging studies may be due to methodological factors.
Related to this, it is also noteworthy that we did not observe effects in dlPFC given that prior research has observed modulation of working memory activation in this region by smoking abstinence (Falcone et al., 2013b; Kozink et al., 2010; Xu et al., 2006). This may have been due to a lack of a 3-back condition which might have further taxed dlPFC-dependent processes and revealed an effect. Future studies that include a wider ranges of task difficulties and also assess the effects of acute nicotine and/or smoke administration following abstinence (as in Xu et al., 2006) might lead to a more precise understanding of the role of dlPFC in nicotine abstinence-induced changes in cognitive performance.
In addition to the predicted effects of nicotine, we also observed an unanticipated interaction between nicotine administration and smoking on BOLD signal in the superior part of the right parietal cortex. Like the dmFC, the SPL is a core component of sustained attention and working memory networks (Owen et al., 2005; Wager and Smith, 2003). However, the observed interaction in right SPL is difficult to interpret, especially in the absence of an interactive effect of these factors on task performance measures. As such, this finding suggests that non-nicotine factors may have only subtle effects on working memory and/or sustained attention.
Our findings have implications for clinical practice and policy. Initial data reports suggest that the magnitude of abstinence-induced cognitive deficits including attention and working memory may be predictive of cessation outcomes (Patterson et al., 2010) and remediation of cognitive deficits has been proposed as an intervention to improve cessation outcomes (Ashare and Schmidt, 2014). Our data suggest that these deficits are due in large part to cessation of nicotine and thus support the use of nicotine replacement therapies in order to remediate these effects; they also provide evidence that the dmFC is modulated by nicotine abstinence and as such, abstinence-induced deficits may be reversed by targeting this region (e.g. with rTMS). It is also worth noting that in this same sample, we observed effects of smoking (vesus abstinence) on withdrawal symptoms that were substantially more robust than the effects of nicotine (versus placebo)(Addicott et al., 2014). This, and previous literature, strongly support the notion that nicotine and non-nicotine factors have differential influence on a range of clinically relevant outcomes and as such, clinical intervention should be designed to target both of these factors.
At a policy level, passage of the Family Smoking Prevention and Tobacco Control Act in 2009 provided the FDA with regulatory authority to reduce the nicotine content of cigarettes to levels that would decrease the addictive potential of cigarettes (Congress, 2009; Hatsukami et al., 2010). Our results suggest that enacting such a policy, particularly if nicotine levels were reduced substantially and rapidly, could negatively impact neurocognition of a substantial number of smokers. While the impact of such a policy on real-world cognitive function and outcomes is unknown, further research on this topic is warranted.
Strengths of our study include a fully factorial, within-subjects design in which smoking behavior was crossed with nicotine administration over 24 h periods. One potential limitation of our study was the lack of a smoking as usual condition. Although we attempted to replicate smoking as usual by providing smokers with their preferred cigarette type (menthol vs. nonmenthol) and nicotine replacement, sensory and pharmacological differences between our manipulation and smoking as usual may have accounted for some effects. To address this question we compared withdrawal symptoms between the training (when participants could smoke freely) and NRT+DENIC sessions. No significant differences were observed suggesting that, while not a perfect analogue, the nicotine patch + VLNC condition replicated important aspects of smoking as usual.
In summary, the results of our study provide novel evidence that abstinence from nicotine factors associated with smoking result in worse cognitive performance and decreased BOLD signal in dmFC, a brain region involved in distractor resistance. Our finding of differential effects of nicotine and non-nicotine factors on neurocognition suggest that treatment of abstinence-induced deficits will require targeting abstinence from nicotine (e.g. via nicotine replacement therapy) and to a lesser extent abstinence from non-nicotine factors. Future research will be required to evaluate the effects of these factors on other forms of cognition, correlates of everyday cognitive outcomes and relations with clinical outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant R01 DA023516-01 to Dr. McClernon.
Footnotes
FINANCIAL DISCLOSURES
BF is the recipient of an unrestricted donation from the Kohlheim Family Foundation. JER received grant funding from Philip Morris USA, is a consultant Philip Morris International (PMI), and has a patent purchase agreement with PMI for nicotine inhalation technology. FJM is a Site PI on an investigator initiated grant (GRAND) from Pfizer, Inc. The other authors declare no conflicts of interest.
Authors Contribution
FJM and JER were responsible for the study concept and design. FJM, BF and RK oversaw data collection and analysis. BF and RK conducted the data analysis. BF, RK, MAA, and MMS assisted with interpretation of findings. ECW served as study physician. DMVW collected study data and managed the study database. FJM drafted the manuscript. BF, RK, MAA and MMS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Addicott MA, Froeliger B, Kozink RV, Van Wert DM, Westman EC, Rose JE, McClernon FJ. Nicotine and non-nicotine smoking factors differentially modulate craving, withdrawal and cerebral blood flow as measured with arterial spin labeling. Neuropsychopharmacology. 2014;39:2750–2759. doi: 10.1038/npp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin Drug Discov. 2014;9:579–594. doi: 10.1517/17460441.2014.908180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori G, Lemmonds CA, Kotler ML, Durcan MJ, Boyle J. Efficacy of a nicotine (4 mg)-containing lozenge on the cognitive impairment of nicotine withdrawal. Journal of clinical psychopharmacology. 2008;28:667–674. doi: 10.1097/JCP.0b013e31818c9bb8. [DOI] [PubMed] [Google Scholar]
- Bain JN, Prendergast MA, Terry AV, Jr, Arneric SP, Smith MA, Buccafusco JJ. Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology (Berl) 2003;169:150–160. doi: 10.1007/s00213-003-1483-1. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, Matthews PM. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1792–1800. doi: 10.1038/npp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. NeuroImage; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Development of deactivation of the default-mode network during episodic memory formation. Neuroimage. 2014;84:932–938. doi: 10.1016/j.neuroimage.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congress. Family Smoking Prevention and Tobacco Control Act. 2009 [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. P Natl Acad Sci USA. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Smith RM, Chenoweth MJ, Bhattacharjee AK, Kelsoe JR, Tyndale RF, Lerman C, Cent PRN. Neuroimaging in Psychiatric Pharmacogenetics Research: The Promise and Pitfalls. Neuropsychopharmacol. 2013a;38:2327–2337. doi: 10.1038/npp.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, Laprate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addiction biology. 2013b doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Human Brain Mapping. 1994;1:153–171. [Google Scholar]
- Froeliger B, Gilbert DG, McClernon FJ. Effects of nicotine on novelty detection and memory recognition performance: double-blind, placebo-controlled studies of smokers and nonsmokers. Psychopharmacology. 2009;205:625–633. doi: 10.1007/s00213-009-1571-y. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Estes SL, Welser R. Does noise stress modulate effects of smoking/nicotine? Mood, vigilance, and EEG responses. Psychopharmacology (Berl) 1997;129:382–389. doi: 10.1007/s002130050204. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Izetelny A, Radtke R, Hammersley J, Rabinovich NE, Jameson TR, Huggenvik JI. Dopamine receptor (DRD2) genotype-dependent effects of nicotine on attention and distraction during rapid visual information processing. Nicotine Tob Res. 2005;7:361–379. doi: 10.1080/14622200500125245. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Sugai C, Plath LC, Asgaard G, Zuo YT, Huggenvik J, Botros N. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine Tob Res. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Sugai C, Zuo Y, Rabinovich NE, McClernon FJ, Froeliger B. Brain indices of nicotine's effects on attentional bias to smoking and emotional pictures and to task-relevant targets. Nicotine Tob Res. 2007;9:351–363. doi: 10.1080/14622200701188810. [DOI] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19:e1–e10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345–395. [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacol. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD. Smoking and attention: a review and reformulation of the stimulus-filter hypothesis. Clin Psychol Rev. 1997;17:451–478. doi: 10.1016/s0272-7358(97)00032-9. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. Attentional mediation of cigarette smoking's effect on anxiety. Health Psychol. 1997;16:359–368. doi: 10.1037//0278-6133.16.4.359. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Eissenberg T. Effects of transdermal nicotine and concurrent smoking on cognitive performance in tobacco-abstinent smokers. Experimental and clinical psychopharmacology. 2011;19:75–84. doi: 10.1037/a0022417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozink RV, Lutz AM, Rose JE, Froeliger B, McClernon FJ. Smoking withdrawal shifts the spatiotemporal dynamics of neurocognition. Addiction biology. 2010;15:480–490. doi: 10.1111/j.1369-1600.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Molecular psychiatry. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A meta-analysis of executive components of working memory. Cereb Cortex. 2013;23:264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Jr, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (-)-nicotine reduce distractibility in adult monkeys. Psychopharmacology (Berl) 1998;136:50–58. doi: 10.1007/s002130050538. [DOI] [PubMed] [Google Scholar]
- Redcay E, Moran JM, Mavros PL, Tager-Flusberg H, Gabrieli JD, Whitfield-Gabrieli S. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Joel DL, McGurrin P, Denlinger RL, Forbes EE, Donny EC. Dissociated Effects of Anticipating Smoking versus Monetary Reward in the Caudate as a Function of Smoking Abstinence. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiora S, Potter DD, Kyle JS, Maxwell AM. The effect of withdrawal and intake of nicotine on smokers' ability to ignore distractors in a number parity decision task. Psychiatry journal. 2013;2013:823158. doi: 10.1155/2013/823158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back task: a preliminary study. Psychiatry Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


