Abstract
Tobacco use is a major economic and health problem. It is particularly concerning that women consume more tobacco products, have a more difficult time quitting smoking and are less likely to benefit from smoking cessation therapy than men. As a result, women are at higher risk of developing tobacco-related diseases. Clinical evidence suggests that women are more susceptible to anxiety disorders, and are more likely to smoke in order to cope with stress than men. During smoking abstinence, women experience more intense anxiety than men and report that the anxiety-reducing effects of smoking are the main reason for their continued tobacco use and relapse. Consistent with this, pre-clinical studies using rodent models suggest that females display more intense stress during nicotine withdrawal than males. This review posits that in women, stress is a principal factor that promotes the initiation of tobacco use and relapse behavior during abstinence. Studies are reviewed at both the clinical and pre-clinical levels to provide support for our hypothesis that stress plays a central role in promoting tobacco use vulnerability in females. The clinical implications of this work are also considered with regard to treatment approaches and the need for more research to help reduce health disparities produced by tobacco use in women.
1. The problem of tobacco use
Tobacco use is the number one cause of preventable deaths in the US, as it claims the lives of over 400,000 individuals each year (Center for Disease Control [CDC], 2008; 2011). Long-term tobacco use leads to deleterious health consequences such as lung cancer, emphysema, and a variety of cardiovascular diseases (D'Alessandro et al., 2012; Hecht, 2012; Milara and Cortijo, 2012). In addition, the consumption of tobacco products is reported to have a health-care cost of over 97 billion dollars per year (CDC, 2010). Given the magnitude of the problem, more research is needed to understand the underlying factors that promote tobacco use.
Several clinical reports have suggested that women are more susceptible to tobacco use than men (Pauly, 2008; Perkins and Scott, 2008; Perkins et al., 2012; Schnoll et al., 2007). For example, although the global incidence of smoking has declined, women consume more cigarettes relative to men (Hammond, 2009, Ng et al., 2014). Women also exhibit lower quit rates of smoking and are less likely to benefit from nicotine replacement therapy (NRT) than men (Cepeda-Benito et al., 2004; Perkins, 2001; Perkins and Scott, 2008; Piper et al., 2010). As a result, women who smoke are at a higher risk of developing tobacco-related diseases, including various types of cancer and chronic obstructive pulmonary disease (Dance, 2012; Kiyohara and Ohno, 2010; Langhammer et al., 2000 and 2003). Despite this evidence, in 2010, the USDHHS reported that mortality rates associated with smoking-related diseases are similar across women and men in the US. There is also empirical evidence suggesting that mortality rates are similar in women and men who smoke (Rostron et al., 2014; Thun et al., 2013). Although the problems associated with smoking in women are well recognized, there remains a critical knowledge gap regarding the factors that contribute to enhanced vulnerability to tobacco use in females.
Recent work in our laboratory has utilized rodent models to study the underlying factors that promote tobacco use in females. Based on this work, we presented a review suggesting that both the rewarding effects of nicotine and the aversive effects of withdrawal from this drug are enhanced in female versus male rodents (O'Dell and Torres, 2014). In the latter review paper, we also presented a mechanistic hypothesis suggesting that both the rewarding and aversive effects of withdrawal are modulated via a complex interaction between CRF, dopamine, and inhibitory gamma-aminobutyric acid (GABA) systems. Specifically, we posited that during withdrawal, CRF promotes a decrease in dopamine via an increase in GABAergic inhibition in the nucleus accumbens (NAcc). Indeed, a recent report illustrated that, within the VTA, CRF systems modulate dopamine via GABA transmission during nicotine withdrawal (Grieder et al., 2014). In support of our hypothesis, we recently demonstrated that the stress-associated genes corticotropin releasing factor (CRF) and urocortin (UCN) are elevated in the NAcc of female rats experiencing nicotine withdrawal (Torres et al., 2015). This work has led us to an overarching hypothesis, presented here, that stress is a central factor that promotes tobacco use in females. There is also recent emerging clinical evidence suggesting that stress and negative affect mood states play an important role in modulating tobacco use in women (Panagiotakopoulosa and Neigh, 2014; Perkins et al., 2012; Weinberger and McKee, 2012). Thus, the present review focuses primarily on the importance of stress in promoting tobacco use in females.
Although the terms anxiety and stress are often used interchangeably, they can have different connotations. For the purposes of this review, the term anxiety is reserved to describe a negative psychological mood state in humans that is induced by an ongoing or anticipated aversive event. The term stress is used to describe a negative psychological state that is accompanied by a biological response elicited by changing environmental demands.
2. The neuroendocrine stress response
The stress response is largely modulated within the hypothalamic-pituitary-adrenal (HPA) axis (Sawchenko et al., 1993; Vale et al., 1981). CRF is secreted from the hypothalamus when high levels of stress are experienced. CRF is 41-amino acid polypeptide that is primarily synthesized in the paraventricular nucleus (PVN) of the hypothalamus (Dunn and Berridge, 1990; Olschowka et al., 1982). The release of CRF stimulates adrenocorticotropic hormone (ACTH) release from the anterior pituitary gland (Semba et al., 2004). ACTH release stimulates the secretion of cortisol, a stress marker in humans, and other glucocorticoids from the adrenal cortex located above the kidneys (Semba et al., 2004). The release of cortisol by the adrenal cortex circulates in the bloodstream and serves as a major negative feedback signal for the HPA axis (Keller-Wood and Dallman, 1984). Although CRF in the hypothalamus plays a primary role in mediating the stress response, CRF is widely distributed throughout the brain (De Souza and Grigoriadis, 2002). For example, CRF is present in the neocortex, periaqueductal gray, olfactory bulb, hippocampus, pons, raphe nucleus, bed nucleus of the stria terminalis, and the shell of the NAcc (Hsu et al., 2009; Palkovits et al., 1992; Sarnyai et al., 2001). In addition, CRF producing neurons and CRF receptors have been found in the substantia nigra, ventral tegmental area, medulla oblongata, central nucleus of the amygdala, and locus coeruleus (Caberlotto et al., 2004; Ungless et al., 2003 and 2010).
3. Sex differences in baseline stress and anxiety levels
There is pre-clinical evidence showing that there are sex differences in HPA activation. For example, female rats display a more extended HPA activation following administration of a footshock stress than males (Heinsbroek et al., 1991). In addition, female rats display higher CRF neuronal activation in the PVN after restraint stress as compared to males (Babb et al., 2013). Previous reports have also proposed that there are sex differences within the molecular architecture of the CRF system. Specifically, the CRF receptor 1 subtype is internalized via beta-arrestin2 into the cell cytoplasm, which prevents CRF from binding to this receptor (Holmes et al., 2006). Bangasser et al. (2010) demonstrated that female rats display lower levels of beta-arrestin2 than males. Based on these findings, these authors have suggested that females are more responsive to CRF stimulation due to reduced internalization of the CRF-1 receptor than males (Bangasser and Valentino, 2012). Consistent with the notion that females display an enhanced stress response, Viau et al. (2005) showed that baseline levels of CRF mRNA are higher in the hypothalamus of female versus male rats. In addition, female rats display higher baseline plasma corticosterone levels than males (Babb et al., 2013). Taken together, these data suggest that females display a higher pre-disposition to more elevated stress responses than males.
Consistent with pre-clinical studies, clinical studies have shown that women display a stronger pre-disposition to stress responses. For example, women report higher rates of depression and generalized anxiety disorders than men (Hankin and Abramson, 2001; McLaughlin et al., 2011; Pigott, 2003; Somers et al., 2006). Women are also at greater risk of developing long-term anxiety disorders following a traumatic event as compared to men (Kobayashi and Mellan, 2012; Seedat and Stein, 2000). It is also reported that women take more days off from work due to anxiety-related causes (CDC, 2004) and are three times more likely to suffer from social anxiety than men (Bourke et al., 2012, Xu et al., 2008; Xu et al., 2012). Additionally, high levels of anxiety in adolescent females are correlated with a higher risk of developing depression and stress disorders later during adulthood, a relationship that has not been observed in adolescent males (Merikangas and Pine, 2002; Piccinelli and Wilkinson, 2000). It is important to note that there are some indices of anxiety that are more prevalent in men as compared to women. For example, chronic stress produces greater immune- and metabolic-associated health complications in men as compared to women (Kudielka and Kirschbaum, 2005; Penninx et al., 2003; Vogelzangs et al., 2012). Taken together, there is strong pre-clinical and clinical evidence to suggest that females have a greater pre-disposition to stress and anxiety than males.
4. Anxiety and tobacco use
Much work has shown that there is a strong link between anxiety and tobacco use. First, smoking is a common tool that is used to cope with anxiety (Park and Breland, 2007; Parrott and Murphy, 2012; Perkins et al., 2010; Slopen et al., 2012). Self-report studies have indicated that the main reason that people use tobacco is to reduce anxiety and induce a state of relaxation (Aronson et al., 2008; Fidler and West, 2009; McEwen et al., 2008). Furthermore, regular smokers report they do not quit because cigarettes help them cope with anxiety (Dupont et al., 2012; Perkins et al., 2012). A nation-wide survey also indicates that people primarily use cigarettes to manage their anxiety levels (American Psychological Association, 2012). College students also report smoking cigarettes to alleviate anxiety, but not to induce positive mood states such as happiness or satisfaction (Brown et al., 2011).
Second, research has shown that nicotine is the major habit forming compound in tobacco products (USDHHS, 2010). Long-term tobacco use produces dependence that is driven in large part by avoiding anxiety elicited by the removal of nicotine during abstinence (Aronson et al., 2008; Hughes and Callas, 2010; Perkins et al., 2010). The psychological effects elicited during smoking abstinence in humans include, but are not limited to, anxiety, depression, irritability, restlessness and agitation (Parrott, 2004; Parrott and Zeichner, 2001; Pauly, 2008). The physical signs of dependence include nausea, headache, sleep disturbances and hunger (Perkins et al., 2009 and 2012). Studies designed to assess the motivation for relapse to smoking have identified the avoidance of anxiety as the primary reason for relapse behavior (Battista et al., 2008; Fidler and West, 2009; Lawrence et al., 2010). During smoking abstinence, a physiological stress response is also elicited, including an increase in blood cortisol levels (Hogle and Curtin, 2006; Steptoe and Ussher, 2006). Studies by Mendelson et al. (2005 and 2008) have also shown that plasma cortisol levels increase 24 hours after smoking abstinence. The latter studies were conducted in a laboratory setting after smoking two or three cigarettes containing high levels of nicotine. These studies suggest that withdrawal from chronic tobacco use leads to an increase in anxiety and a biological stress response that may promote relapse to smoking.
5. Proposed model of stress and tobacco use in females
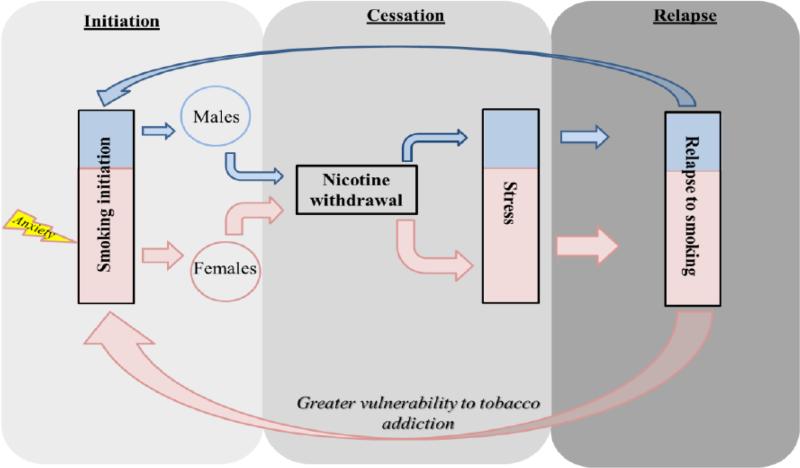
The diagram below depicts our proposed model of the contribution of stress to enhanced vulnerability to tobacco use in females. We postulate that anxiety plays a larger role in the initiation of tobacco use in females, and that a strong stress response during abstinence leads to relapse to a greater extent in females as compared to males. The color-coding reflects a larger relapse effect in women (pink) versus men (blue). First, our model posits that women may be more susceptible to initiate smoking behavior, because smoking is largely used to cope with anxiety in females. This may involve a pre-existing propensity to anxiety disorders or heredity factors, as reviewed below. Second, our model posits that stress produced by nicotine withdrawal is greater in females as compared to males. Because females experience a larger stress response during withdrawal, it is posited that they are more susceptible to relapse than males.
Below we present clinical and pre-clinical evidence to support our hypothesis that stress is a principal factor that promotes greater vulnerability to tobacco use in females. We consider the importance of stress in both the initiation and abstinence phases of tobacco use.
6. The role of stress in tobacco use initiation in females
There is evidence to suggest that a pre-existing anxiety disorder may promote tobacco use. For example, a survey conducted by Lasser et al. (2000) suggests that 55 percent of people meeting the criteria for an anxiety disorder are also regular smokers. Furthermore, people who are nicotine-dependent and are also diagnosed with an anxiety disorder consume more than 30 percent of all cigarettes in the US (Grant et al., 2004). These findings are consistent with other reports showing that the rate of anxiety disorders is higher in individuals who smoke as compared to non-smokers (Lawrance et al., 2005; McClave et al., 2009). With regard to women, clinical studies have suggested that females use nicotine to cope with anxiety to a larger extent than men (Perkins, 2009; Perkins et al., 2012; Piper et al., 2010). In fact, nicotine has been shown to decrease anxiety elicited by a moderate stressor in women; however, nicotine increased anxiety and negative mood states in men (File et al., 2001). Furthermore, compared to males, female college students report more often that they initiate tobacco use to relieve negative mood states (Morrell et al., 2010). These findings corroborate with previous work showing that women report more often than men that the anxiety-reducing effects of cigarettes are the main reason for smoking (Nichter et al., 1997; Perkins et al., 1999).
There is also strong evidence that pre-existing anxiety disorders lead to tobacco use more often in women than men. For example, a large population survey showed that there is a stronger co-morbid association between anxiety disorders and smoking rates in women as compared to men (Mykletun et al., 2008). In addition, Stewart et al. (1997) showed that women with hypersensitivity to stressful stimuli are more likely to engage in cigarette use to cope with anxiety as compared to males. In a more recent study, Brook et al. (2012) showed that women with prior history of an anxiety disorder are more likely to develop tobacco dependence later in life relative to men. Women with posttraumatic stress disorder (PTSD) are also more likely to use cigarettes and report relapse to smoking more often than men with PTSD (Weinberger et al., 2009). Interestingly, John et al. (2004) also found that women have a greater likelihood of developing an anxiety disorder after a year of smoking than men. Taken together, these studies suggest that there is a strong relationship between anxiety and smoking, especially among women who are most susceptible to tobacco abuse.
Pre-clinical studies have examined the effects of stress on the rewarding effects of nicotine in female and male rodents. For example, female mice display more anxiety-like behavior compared to their male counterparts following chronic oral nicotine intake (Caldarone et al., 2008). Female rats also display a larger increase in nicotine intake following a pharmacological stressor as compared to males (Li et al., 2014). The authors of the latter report suggest that females are more sensitive to the effects of stress on nicotine intake as compared to males. Consistent with this, a recent report showed that chronic administration of nicotine in combination with immobilization stress produced a larger suppression of feeding behavior and body weight in females as compared to males (Faraday et al., 2005). However, we acknowledge a report showing that female and male rats display similar levels of nicotine intake and stress-induced reinstatement of nicotine-seeking behavior (Feltenstein et al., 2011). Future studies are needed to examine whether females are more sensitive to the effects of stress on the rewarding effects of nicotine.
Pre-clinical studies have compared the biological stress response produced by nicotine exposure in male and female rodents. Studies comparing the biological response to stress have revealed that plasma corticosterone levels are increased to a greater extent in female versus male rats following repeated nicotine injections (Gentile et al., 2011; Moidel et al., 2006; Rhodes et al., 2001 and 2004; Skwara et al., 2012) and continuous delivery (Faraday et al., 2005) of this drug. In an in vitro perfusion system, the presence of nicotine increased CRF and ACTH levels to a greater extent in hypothalamic tissue that was collected from female versus male rats (McKlveen et al., 2010; Moidel et al., 2006). Overall, these studies suggest that the behavioral and biological effects of nicotine on stress systems are greater in females as compared to males.
Another factor that is important to consider with regard to tobacco use initiation is the direct reinforcing effects of nicotine. Indeed, much work has shown that there is sex differences in the rewarding effects of nicotine that likely contribute to tobacco use initiation in females. For example, previous work has demonstrated that female rats display higher nicotine self-administration rates (Chaudhri et al., 2005; Lynch, 2009; Rezvani et al., 2008) and they acquire nicotine self-administration at lower doses of nicotine than males (Donny et al., 2000; Lanza et al., 2004). In addition, adult female rats display place preference following conditioning with a wider dose range of nicotine than males (Torres et al., 2009). These findings are consistent with other studies demonstrating that female rats (Edward et al., 2014; Lenoir et al., 2015) and mice (Kota et al., 2007; 2008) display a more robust place preference produced by nicotine than males. Female rats also display greater oral consumption of nicotine than males (Klein et al., 2004; Nesil et al., 2011). However, we also recognize one report showing that male rats display greater place preference produced by nicotine than females (Yararbas et al., 2010). Taken together, the majority of the literature suggests that the strong rewarding effects of nicotine likely promote tobacco use initiation in females. Thus, the possibility exists that the strong rewarding effects of nicotine and the greater contribution of stress are additive factors that together promote tobacco use vulnerability in women.
7. The contribution of stress in promoting withdrawal during abstinence in females
Clinical studies have shown that during smoking abstinence, women report more negative mood states, such as depression, anxiety and intense craving than men (al'Absi, 2006; Schnoll et al., 2007; Xu et al., 2008). Women smokers also report more often than men that the anxiety-reducing effects of cigarettes are the main reason for relapse (Perkins and Scott, 2008; Perkins et al., 2009 and 2012; Piper et al., 2010). Importantly, women also display higher levels of cortisol during smoking abstinence relative to men (Hogle and Curtin, 2006). These findings suggest that the strong aversive effects of withdrawal contribute to greater vulnerability to relapse behavior in women as compared to men.
In rodent studies, nicotine withdrawal has been widely studied using chronic nicotine administration via subcutaneous osmotic minipumps for 5-7 days (Kenny and Markou, 2001; Malin, 2001). Withdrawal from nicotine is produced via removal of the nicotine pump (spontaneous withdrawal) or administration of a nicotinic receptor antagonist (precipitated withdrawal). Using either spontaneous or precipitated methods, nicotine withdrawal produces a behavioral profile comprised of both physical and affective components. The negative affective properties of nicotine withdrawal have been studied in procedures that assess anxiety-like behavior in rodents (Bruijnzeel et al., 2012). Studies comparing sex differences produced by nicotine withdrawal have revealed that female adult rats display more physical signs of nicotine withdrawal relative to males (Hamilton et al., 2009). Also, female adult rats display elevated plasma ACTH and corticosterone levels during nicotine withdrawal relative to their male counterparts (Gentile et al., 2011). Consistent with these findings, Skwara et al. (2012) also demonstrated that plasma ACTH and corticosterone levels are increased in female rats following precipitated nicotine withdrawal as compared to males. Work in our laboratory revealed that females display greater anxiety-like behavior, plasma corticosterone and CRF gene expression in NAcc during nicotine withdrawal compared to male rats (Torres et al., 2013). There were no sex differences in CRF gene expression in the amygdala or hypothalamus, suggesting that sex differences during nicotine withdrawal are likely modulated within the local circuits of the NAcc. Taken together, these studies provide pre-clinical evidence suggesting that nicotine withdrawal elicits greater stress responses in females versus males.
8. Age differences in the contribution of stress to tobacco initiation and withdrawal in females
Our hypothesis that stress plays a central role in promoting tobacco use in females is supported by a recent pre-clinical study showing that administration of a pharmacological stressor (yohimbine) increased nicotine self-administration in adolescent female, but not male rats (Li et al., 2014). With regard to the direct anxiolytic effects of nicotine; however, there appears to be an important distinction between the pattern of results between female and male rats of different ages. As described above, nicotine administration produces direct anxiolytic effects that are greater in female versus male adult rodents. Based on this work, we suggest that the anxiolytic effects of nicotine likely promote tobacco use initiation in adult females. However, studies comparing the anxiolytic effects of nicotine during adolescence have produced a different pattern of results. Namely, the anxiolytic effects of nicotine are greater in male versus female adolescent rats (Cao et al., 2010) and mice (Damaj, 2001). Adolescent male mice also display a greater activation of the PVN following an injection of nicotine as compared to females (McCormick and Ibrahim, 2007). These findings suggest that nicotine may produce greater anxiolytic effects in adolescent males as compared to females. During adolescence, we suggest that there may be other factors such as the effects of nicotine on social behavior that may play a role in promoting tobacco use. This is based on the finding that nicotine increases social rewards in adolescent male rats (Theil et al., 2009). With regard to sex differences, female adolescent rats display a greater increase in social interaction behavior following administration of a low dose of nicotine as compared to males (Cheeta et al., 2001). This work suggests that nicotine may promote social interaction in a manner that enhances tobacco use in young females.
Much research has suggested that nicotine withdrawal is reduced during the adolescent period of development in rodents (for a review see O'Dell, 2009). With regard to sex differences, the majority of literature suggests that there are no differences in nicotine withdrawal in female and male rodents during adolescence. For example, the physical signs of nicotine withdrawal are similar in adolescent female and male mice (Kota et al., 2007 and 2008) and rats (Hamilton et al., 2010; Torres et al., 2013). There are also no sex differences in plasma corticosterone levels produced by nicotine withdrawal (Torres et al., 2013). It may not be surprising that there are no sex differences in nicotine withdrawal during adolescence, given the preponderance of evidence suggesting that nicotine withdrawal does not appear to play a major role in promoting tobacco use in adolescents.
9. Ovarian hormones modulate tobacco use
Clinical reports have shown that there is a relationship between tobacco use and ovarian hormones in women. With regard to nicotine reward, it is thought that high levels of estrogen are positively correlated with enhanced sensitivity to the rewarding effects of nicotine in women (Lynch and Sofuoglu, 2010). Also, during smoking abstinence, women display enhanced nicotine craving and increased relapse rates during the follicular-phase when estrogen levels are highest (Allen et al., 2009). There are also reports showing an accelerated metabolism of nicotine in women who use oral contraceptives that enhance estrogen levels (Benowitz et al., 2006; Dempsey et al., 2002). Thus, estrogen may promote tobacco use via a mechanism involving accelerated nicotine metabolism. Taken together, these studies suggest that estrogen likely promotes the various phases of tobacco use in women. However, the role of progesterone appears to be opposite to that of estrogen. For example, high levels of progesterone are correlated with a diminished urge to smoke in women (Schiller et al., 2012). Moreover, progesterone treatment attenuates the urge for tobacco use in female smokers (Sofuoglu et al., 2001 and 2009). Women also report lower subjective effects of nicotine in the luteal phase of the menstrual cycle when progesterone levels are higher than estrogen (DeVito et al., 2014). However, the literature on smoking rates during different phases of the menstrual cycle are not consistent, as there are reports showing that women smoke more cigarettes during the luteal phase (Sakai and Ohashi, 2013) and have less craving for cigarette during the follicular-phase (Franklin et al., 2004).
Pre-clinical studies in our laboratory have examined the role of ovarian hormones in modulating nicotine reward and withdrawal using ovariectomy (OVX) procedures in rats. With regard to nicotine reward, our initial studies revealed that OVX females displayed reduced place preference produced by nicotine as compared to intact females (Torres et al., 2009). Other laboratories have also found that ovarian hormones are necessary for the rewarding effects of other drugs of abuse. For example, OVX female rats are less likely to acquire cocaine self-administration (Lynch et al., 2001) and to reinstate cocaine-seeking behavior following extinction (Larson et al., 2005) relative to intact females. In addition, OVX female rats display longer acquisition of heroin self-administration (Roth et al., 2002) and diminished place preference produced by morphine (Mirbaha et al., 2009). With regard to nicotine withdrawal, we recently observed that intact females display higher levels of anxiety-like behavior and increased expression of several stress-associated neuropeptide genes including CRF and UCN in the NAcc (Torres et al., 2015). Interestingly, the latter effects were absent in OVX females. These studies imply that ovarian hormones are crucial for the manifestation of withdrawal produced by nicotine. Taken together, these studies suggest that ovarian hormones play an important role in modulating drug abuse, consistent with recent review papers summarizing pre-clinical work in this area of research (Bobzean et al., 2014).
Recently, we posited that estradiol is an important ovarian hormone that promotes the effects of stress on nicotine reward and withdrawal in females (O'Dell and Torres, 2014). Our hypothesis is based on studies demonstrating that estradiol potentiates stress in females. For example, activation of the beta estradiol receptor has been shown to increase anxiety-like behavior in female rats (Morgan and Pfaff, 2001; Walf and Frye, 2005). In addition, studies comparing CRF levels across the 4-day estrous cycle in female adult rats have found that the highest levels of CRF were observed during the proestrus phase, where estradiol levels are highest (Bohler et al., 1990; Nappi et al., 1997). Other work has also shown that direct activation of estradiol-beta receptors increase CRF mRNA expression in vitro (Chen et al., 2008; Lalmansingh and Uht, 2008; Zhu and Zhou, 2008). Estradiol has also been shown to promote gene expression of stress-associated neuropeptides (Kageyama et al., 2011; Sanchez et al., 2010; Vamvakopoulos and Chrousos, 1993). Collectively, these studies suggest that estradiol enhances stress responses via facilitating CRF systems. Thus, estradiol may be an important hormone that modulates sex differences produced by nicotine. A recent review has described the modulatory role of ovarian hormones on neurotransmission in different regions of the brain (Barth et al., 2015). However, there remains a knowledge gap with regard to the mechanisms by which estradiol promotes CRF mechanisms and anxiety-like behavior in a region-dependent manner. We also recognize that other ovarian hormones, such as progesterone may also play an important role in modulating tobacco use in females (Anker and Carroll, 2010; Carroll and Anker, 2010; Lynch and Sofuoglu, 2010).”
10. Stress promotes poly drug use in females
Converging lines of evidence suggest that anxiety is a key factor that promotes addiction to a variety of drugs in women. For example, more women with a prior anxiety-related disorders report alcohol abuse than men (Landheim et al., 2003). Other clinical reports have shown that women with chronic anxiety produced by a traumatic event, are more likely than men to use alcohol and have polydrug use problems (Jaquier et al., 2014; Peters et al., 2012; Weiss et al., 2014). Consistent with this, women veterans diagnosed with PTSD have a higher risk for poly drug use (Dobie et al., 2004). Furthermore, women report greater anxiety and guilt after cocaine use than men (Kennedy et al., 2013). Clinical findings also suggest that anxiety management techniques are beneficial in reducing drug use in women (Wu et al., 2014). These studies suggest that anxiety contributes to addiction to a variety of abused substances in women.
A review of the pre-clinical literature suggests that stress has profound effects on the rewarding effects of drugs of abuse other than nicotine (Aguilar et al., 2013). Work focused on other drugs of abuse is consistent with pre-clinical studies that have compared sex differences with nicotine. For example, female rats also show greater reinstatement of cocaine-seeking behavior as compared to males after intracerebroventricular infusions of CRF (Buffalari et al., 2012) or intraperitoneal injections of yohimbine (Anker and Carroll, 2010; Feltenstein et al., 2011). Additionally, female rats self-administer more heroin on a food restriction paradigm that induces stress than males (Carroll et al., 2001). Stressed female rats display a longer-lasting enhancement of cocaine-induced increases in dopamine levels in the NAcc as compared to stressed males (Holly et al., 2012). However, we do acknowledge other reports showing that chronic stressors may not induce sex-differences in cocaine intake (Haney et al., 1995) and cocaine-induced increases in NAcc dopamine levels (Shimamoto et al., 2011).
11. Other factors that promote tobacco use in females
There are a variety of factors other than stress that may also promote tobacco use in females. First, women use tobacco products as a tool to control appetite and decrease weight more often than men (Austin and Gortmaker, 2001; Kaufman and Augustson, 2008; Pomerleau and Snedecor, 2008). Second, genetic factors may play an important role in promoting tobacco use susceptibility in women. For example, Colamussi et al. (2007) showed that women with first-degree relatives who smoke display higher levels of stress-induced cigarette craving as compared to their male counterparts. In addition, a meta-analysis found that in comparison to males, females with a twin who smokes are more likely to engage in smoking behavior (Li et al., 2003). Although sociocultural factors also play a role in smoking initiation among female twins (Hamilton et al., 2006), nicotine dependence has been mostly closely attributed to hereditary and genetic factors among female twins (Kendler et al., 1999). Clinical studies have also highlighted the importance of other external factors such as having a friend who smokes (Oh et al., 2011; Holahan et al., 2012), lower socioeconomic status (Wewers et al., 2012) and educational level (Kandel et al., 2009) as strong predictors of nicotine use in women. Taken together, these studies imply that there are important factors other than stress that may also contribute to tobacco use in women.
12. Clinical implications
The literature suggests that as compared to males, females experience stronger rewarding effects of nicotine and greater negative effects during withdrawal from this drug. Given that both rewarding effects and withdrawal processes contribute to tobacco use, we suggest that stronger effects in both domains contribute to greater vulnerability to tobacco use in females. The present review posits that a major factor that promotes tobacco use in females is stress produced by withdrawal. These observations suggest that female smokers may require specialized medications that help to alleviate intense stress experienced during abstinence. Future work is needed to help guide the development of novel therapeutic agents that may be more useful in smoking cessation in women. Given that most smoking cessation medications focus on alleviating withdrawal, one approach might include CRF antagonists in combination with other treatments, such as NRT or partial nicotinic agonists. In fact, one review suggested that CRF receptor antagonists might be useful medications for alleviating negative mood states produced by drug withdrawal (Logrip et al., 2011). Future studies are needed to understand the complex interactions in the brain that modulate sex differences to tobacco use. Recent research has made important advances in our understanding of the underlying circuitry that promotes tobacco use. The recent literature suggests that CRF systems within key structures of the mesolimbic pathway, including the VTA (Grieber et al., 2014), NAcc (O'Dell and Torres, 2014), and amygdala (Bruijnzeel, 2012; George et al., 2012) play an important role in orchestrating the behavioral effects of nicotine and withdrawal from this drug. Future studies are needed to elucidate the underlying neurochemical systems within these structures that promote tobacco use. Ultimately, this work is important towards elucidating the role of stress in promoting smoking behavior and reducing health disparities produced by chronic tobacco use in women.
Figure 1.
Stress is a principal factor that promotes tobacco use in females
Acknowledgements
The authors would like to thank Dr. Luis M. Carcoba, Joseph A. Pipkin and Rodolfo J. Flores for their helpful comments in the preparation of this review paper. The authors also appreciate the support that was provided from The National Institute on Drug Abuse (R01-DA021274, R24-DA029989 and R25-DA033613) and the National Institute of Minority Health Disparities (G12MD007592) as part of the UTEP Border Biomedical Research Center. This work was also partially supported by funds of the Intramural Research Program of the NIDA Scientific Director's Fellowship for Diversity in Research (OVT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Aguilar MA, García-Pardo MP, Montagud-Romero S, Miñarro J, Do Couto BR. Impact of social stress in addiction to psychostimulants: what we know from animal models. Curr Pharm Des. 2013;19:7009–25. doi: 10.2174/138161281940131209124708. [DOI] [PubMed] [Google Scholar]
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;593:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Lunos S, Hatsukami DK. Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav. 2009;3411:928–31. doi: 10.1016/j.addbeh.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association Stress in America: Our health at risk. 2012. [Oct. 2014].
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;1072-3:264–7. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson KR, Almeida DM, Stawski RS, Klein LC, Kozlowski LT. Smoking is associated with worse mood on stressful days: results from a national diary study. Ann Behav Med. 2008;363:259–69. doi: 10.1007/s12160-008-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32(5):709–23. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. doi: 10.3389/fnins.2015.00037. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, Stewart SH, Fulton HG, Steeves D, Darredeau C, Gavric D. A further investigation of the relations of anxiety sensitivity to smoking motives. Addict Behav. 2008;3311:1402–8. doi: 10.1016/j.addbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;795:480–8. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;83:259–62. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62(3):210–8. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Brook DW, Koppel J, Whiteman M. Psychosocial predictors of nicotine dependence among women during their mid-sixties. The American Journal on Addiction. 2012;21:302–12. doi: 10.1111/j.1521-0391.2012.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AE, Carpenter MJ, Sutfin EL. Occasional smoking in college: who, what, when and why? Addict Behav. 2011;3612:1199–204. doi: 10.1016/j.addbeh.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;365:1418–41. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE. Corticotrophin releasing factor CRF induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;1052:209–14. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology. 2004;29(1):15–22. doi: 10.1038/sj.npp.1300296. (2004) [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, Picciotto MR. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;4392:187–91. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(1):82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;581:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001;93:307–16. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. Morbidity and Mortality Weekly Report 2008. [2014 Sep. 7]. [PubMed]
- Centers for Disease Control and Prevention Vital Signs: Current Cigarette Smoking Among Adults Aged ≥ 18 Years—United States, 2005–2010. Morbidity and Mortality Weekly Report 2011. [2014 Oct. 7].
- Centers for Disease Control and Prevention Vital Signs: Youth risk behavior surveillance-United Sates, 2009. Morbidity and Mortality Weekly Report 2010. [2014 Oct 12]. [PubMed]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;724:712–22. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180(2):258–66. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Tucci S, Sandhu J, File SE. In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology. 2001;25(4):601–7. doi: 10.1016/S0893-133X(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Chen XN, Zhu H, Meng QY, Zhou JN. Estrogen receptor-alpha and -beta regulate the human corticotropin-releasing hormone gene through similar pathways. Brain Res. 2008;1223:1–10. doi: 10.1016/j.brainres.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;882-3:251–8. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol. 2012;193:297–305. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- Damaj MI. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Ther. 2001;296(1):132–40. [PubMed] [Google Scholar]
- Dance A. Health impact: Breathless. Nature. 2012;489(7417):S2–3. doi: 10.1038/489S2a. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Grigoriadis DE. Corticotropin-releasing factor: Physiology, pharmacology, and role in central nervous system disorders. Neuropsychopharmacology: The fifth generation of progress. 2002:91–106. [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;3012:594–8. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;396:1431–40. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med. 2004;1644:394–400. doi: 10.1001/archinte.164.4.394. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell P, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Research Reviews. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dupont P, Reynaud M, Aubin HJ. Stress and smoking in treatment-seeking smokers. Rev Med Liege. 2012;674:195–201. [PubMed] [Google Scholar]
- Edwards AW, Konz N, Hirsch Z, Weedon J, Dow-Edwards DL. Single trial nicotine conditioned place preference in pre-adolescent male and female rats. Pharmacol Biochem Behav. 2014;125:1–7. doi: 10.1016/j.pbb.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Blakeman KH, Grunberg NE. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav. 2005;804:577–89. doi: 10.1016/j.pbb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology Berl. 2011;2161:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler JA, West R. Self-perceived smoking motives and their correlates in a general population sample. Nicotine. Tob. Res. 2009;11:1182–88. doi: 10.1093/ntr/ntp120. [DOI] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. Int J Neuropsychopharmacol. 2001;44:371–6. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O'Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;61:171–5. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Gentile NE, Andrekanic JD, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME. Sexually diergic hypothalamic-pituitary-adrenal HPA responses to single-dose nicotine, continuous nicotine infusion, and nicotine withdrawal by mecamylamine in rats. Brain Res Bull. 2011;853-4:145–52. doi: 10.1016/j.brainresbull.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106(1):58–64. doi: 10.1016/j.physbeh.2011.11.004. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan J, Pickering RP. Co-occurrence of 12-month alcohol and drug use disorders and personality disorders in the United States: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:362–68. doi: 10.1001/archpsyc.61.4.361. [DOI] [PubMed] [Google Scholar]
- Grieder TE, Herman MA, Contet C, Tan LA, Vargas-Perez H, Cohen A, Chwalek M, Maal-Bared G, Freiling J, Schlosburg JE, Clarke L, Crawford E, Koebel P, Repunte-Canonigo V, Sanna PP, Tapper AR, Roberto M, Kieffer BL, Sawchenko PE, Koob GF, van der Kooy D, George O. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci. 2014;17(12):1751–8. doi: 10.1038/nn.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AS, Lessov-Schlaggar CN, Cockburn MG, Unger JB, Cozen W, Mack TM. Gender differences in determinants of smoking initiation and persistence in California twins. Cancer Epidemiol Biomarkers Prev. 2006;156:1189–97. doi: 10.1158/1055-9965.EPI-05-0675. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Berger SS, Perry ME, Grunberg NE. Behavioral effects of nicotine withdrawal in adult male and female rats. Pharmacol Biochem Behav. 2009;921:51–9. doi: 10.1016/j.pbb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Perry ME, Berger SS, Grunberg NE. Behavioral effects of nicotine withdrawal differ by genetic strain in male and female adolescent rats. Nicotine Tob Res. 2010;12(12):1236–45. doi: 10.1093/ntr/ntq179. [DOI] [PubMed] [Google Scholar]
- Hammond SK. Global patterns of nicotine and tobacco consumption. Handb Exp Pharmacol. 2009;192:3–28. doi: 10.1007/978-3-540-69248-5_1. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;6981-2:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: an elaborated cognitive vulnerability-transactional stress theory. Psychol Bull. 2001;127(6):773–96. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;13112:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RP, Van Haaren F, Feenstra MG, Endert E, Van de Poll NE. Sex- and time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock Physiol. Behav. 1991;49:1251–1256. doi: 10.1016/0031-9384(91)90359-v. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;434:344–56. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, North RJ, Holahan CK, Hayes RB, Powers DA, Ockene JK. Social influences on smoking in middle-aged and older women. Psychol Addict Behav. 2012;263:519–26. doi: 10.1037/a0025843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Shimamoto A, Debold JF, Miczek KA. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology Berl. 2012;2241:179–88. doi: 10.1007/s00213-012-2846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96(4):934–49. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol. 2009;512(6):825–48. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Callas PW. Definition of a quit attempt: a replication test. Nicotine Tob Res. 2010;1211:1176–9. doi: 10.1093/ntr/ntq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquier V, Flanagan JC, Sullivan TP. Anxiety and posttraumatic stress symptom pathways to substance use problems among community women experiencing intimate partner violence. Anxiety Stress Coping. 2014:1–11. doi: 10.1080/10615806.2014.968562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity--a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;763:287–95. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Tamasawa N, Suda T. Signal transduction in the hypothalamic corticotropin-releasing factor system and its clinical implications. Stress. 2011;144:357–67. doi: 10.3109/10253890.2010.536279. crH. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend. 2009;104(Suppl 1):S24–33. doi: 10.1016/j.drugalcdep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AR, Augustson EM. Predictors of regular cigarette smoking among adolescent females: does body image matter? Nicotine Tob Res. 2008;108:1301–9. doi: 10.1080/14622200802238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5(1):1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;292:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Epstein DH, Phillips KA, Preston KL. Sex differences in cocaine/heroin users: drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013;132:29–37. doi: 10.1016/j.drugalcdep.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;704:531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. 2010;75:381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;781:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Mellman TA. Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behav Sleep Med. 2012;10(3):180–90. doi: 10.1080/15402002.2011.654296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj M. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology. 2008;198:201–10. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. The Journal of Pharmacology and Experimental Therapeutics. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene crh expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3',5'-cyclic adenosine 5'-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;1491:346–57. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landheim AS, Bakken K, Vaglum P. Gender differences in the prevalence of symptom disorders and personality disorders among poly-substance abusers and pure alcoholics. Substance abusers treated in two counties in Norway. Eur Addict Res. 2003;91:8–17. doi: 10.1159/000067732. [DOI] [PubMed] [Google Scholar]
- Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;216:1017–23. doi: 10.1183/09031936.03.00053202. [DOI] [PubMed] [Google Scholar]
- Langhammer A, Johnsen R, Holmen J, Gulsvik A, Bjermer L. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study HUNT. J Epidemiol Community Health. 2000;5412:917–22. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza ST, Donny EC, Collins LM, Balster RL. Analyzing the acquisition of drug self-administration using growth curve models. Drug Alcohol Depend. 2004;75(1):11–21. doi: 10.1016/j.drugalcdep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;821:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;28420:2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Sawyer MG, Zubrick SR. Smoking status, mental disorders and emotional and behavioural problems in young people: child and adolescent component of the National Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry. 2010;449:805–14. doi: 10.3109/00048674.2010.482921. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Mitrou F, Zubrick SR. Non-specific psychological distress, smoking status and smoking cessation: United States National Health Interview Survey. BMC Public Health. 2005;2011;11:256. doi: 10.1186/1471-2458-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D, Vignoli B, Izenwasser S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol Biochem Behav. 2015;132:56–62. doi: 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;981:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Li S, Zou S, Coen K, Funk D, Shram MJ, Le A D. Sex differences in yohimbine-induced increases in the reinforcing efficacy of nicotine in adolescent rats. Addiction biology. 2014;19:156–64. doi: 10.1111/j.1369-1600.2012.00473.x. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Koob GF, Zorrilla EP. Role of corticotropin-releasing factor in drug addiction: potential for pharmacological intervention. CNS Drugs. 2011;254:271–87. doi: 10.2165/11587790-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;684:641–6. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;186:451–61. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Lin A, Saldaña M, Balch L, Irvin ML, Chandrasekara H, Alvarado CL, Hieda Y, Keyler DE, Pentel PR, Ennifar S, Basham LE, Naso R, Fattom A. Passive immunization against nicotine prevents nicotine alleviation of nicotine abstinence syndrome. Pharmacol Biochem Behav. 2001;681:87–92. doi: 10.1016/s0091-3057(00)00436-6. [DOI] [PubMed] [Google Scholar]
- McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among U.S. adults. Addict Behav. 2009;346-7:491–7. doi: 10.1016/j.addbeh.2009.01.005. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Ibrahim FN. Locomotor activity to nicotine and Fos immunoreactivity in the paraventricular nucleus of the hypothalamus in adolescent socially-stressed rats. Pharmacol Biochem Behav. 2007;86(1):92–102. doi: 10.1016/j.pbb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- McEwen A, West R, McRobbie H. Motives for smoking and their correlates in clients attending Stop Smoking treatment services. Nicotine Tob Res. 2008;105:843–50. doi: 10.1080/14622200802027248. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Wilson JM, Rubin RT, Rhodes ME. Sexually diergic, dose-dependent hypothalamic-pituitary-adrenal axis responses to nicotine in a dynamic in vitro perfusion system. J Pharmacol Toxicol Methods. 2010;613:311–8. doi: 10.1016/j.vascn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Xuan Z, Subramanian SV, Koenen KC. State-level women's status and psychiatric disorders among US women. Soc Psychiatry Psychiatr Epidemiol. 2011;46(11):1161–71. doi: 10.1007/s00127-010-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;334:749–60. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;309:1751–63. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Pine D. Genetic and other vulnerability factors for anxiety and stress disorders. Neuropsychopharmacology: The Fifth Generation of Progress. 2002:868–82. [Google Scholar]
- Milara J, Cortijo J. Tobacco, inflammation, and respiratory tract cancer. Curr Pharm Des. 2012;1826:3901–38. doi: 10.2174/138161212802083743. [DOI] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR. Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacol Biochem Behav. 2009;923:399–403. doi: 10.1016/j.pbb.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Moidel MA, Belz EE, Czambel RK, Rubin RT, Rhodes ME. Novel in vitro perfusion system for the determination of hypothalamic-pituitary-adrenal axis responses. J Pharmacol Toxicol Methods. 2006;533:264–71. doi: 10.1016/j.vascn.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40(4):472–82. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morrell HE, Cohen LM, McChargue DE. Depression vulnerability predicts cigarette smoking among college students: Gender and negative reinforcement expectancies as contributing factors. Addict Behav. 2010;35(6):607–11. doi: 10.1016/j.addbeh.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykletun A, Overland S, Aarø LE, Liabø HM, Stewart R. Smoking in relation to anxiety and depression: evidence from a large population survey: the HUNT study. Eur Psychiatry. 2008;232:77–84. doi: 10.1016/j.eurpsy.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Bonneau MJ, Rivest S. Influence of the estrous cycle on c-fos and CRH gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology. 1997;651:29–46. doi: 10.1159/000127162. [DOI] [PubMed] [Google Scholar]
- Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1-2):189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311(2):183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Vuckovic N, Quintero G, Ritenbaugh C. Smoking experimentation and initiation among adolescent girls. Tob. Control. 1997;6:185–95. doi: 10.1136/tc.6.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. 2014;76:566–80. doi: 10.1016/j.neuropharm.2013.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DL, Heck JE, Dresler C, Allwright S, Haglund M, Del Mazo SS. Determinants of smoking initiation among women in five European countries: a cross-sectional survey. BMC Public Health. 2010;10:74. doi: 10.1186/1471-2458-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, O'Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3(6):995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Csiffary A, Antoni FA, Vale W, Eskay RL. Chemical neuroanatomy of brain structures involved in the stress structures involved in the stress response with special references to corticotropin releasing factor. Stress: Neuroendocrine and Molecular Approaches. 1992;1:3–12. [Google Scholar]
- Panagiotakopoulosa L, Neigh GN. Sex differences in neurological and psychiatric disorders development of the HPA axis: Where and when do sex differences manifest? Frontiers in Neuroendocrinology. 2014;35(3):285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Park MJ, Breland D. Alcohol and cigarette use among adolescent and young adult males. American J. of Men's Health. 2007;14:339–46. doi: 10.1177/1557988307306753. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Murphy RS. Explaining the stress-induced effects of nicotine to cigarette smokers. Hum. Psychopharmacol Clin Exp. 2012;27:150–55. doi: 10.1002/hup.1247. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Heightened stress and depression follow cigarette smoking. Psychol Rep. 2004;941:33–4. doi: 10.2466/pr0.94.1.33-34. [DOI] [PubMed] [Google Scholar]
- Parrott DJ, Zeichner A. Effects of nicotine deprivation and irritability on physical aggression in male smokers. Psychol Addict Behav. 2001;152:133–9. [PubMed] [Google Scholar]
- Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–16. doi: 10.2741/2696. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Coddington SB, Karelitz JL, Jetton C, Scott JA, Wilson AS, Lerman C. Variability in initial nicotine sensitivity due to sex, history of other drug use, and parental smoking. Drug and Alcohol Dependence. 2009;99:47–57. doi: 10.1016/j.drugalcdep.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–15. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Lerman C. Smoking in response to negative mood in men versus women as a function of distress tolerance. Nicotine Tob Res. 2012;14(12):1418–25. doi: 10.1093/ntr/nts075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA. Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology. 2010;210:25–34. doi: 10.1007/s00213-010-1811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2012;382:1527–31. doi: 10.1016/j.addbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;107:1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;155:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Peters EN, Khondkaryan E, Sullivan TP. Associations between expectancies of alcohol and drug use, severity of partner violence, and posttraumatic stress among women. J Interpers Violence. 2012;2711:2108–27. doi: 10.1177/0886260511432151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26(3):621–72. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;126:647–57. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Snedecor SM. Validity and reliability of the Weight Control Smoking Scale. Eat Behav. 2008;93:376–80. doi: 10.1016/j.eatbeh.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Kholdebarin E, Dawson E, Levin ED. Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. Int J Neuropsychopharmacol. 2008;11(1):63–70. doi: 10.1017/S1461145706007528. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Kennell JS, Belz EE, Czambel RK, Rubin RT. Rat estrous cycle influences the sexual diergism of HPA axis stimulation by nicotine. Brain Res Bull. 2004;643:205–13. doi: 10.1016/j.brainresbull.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, O'Toole SM, Czambel RK, Rubin RT. Male-female differences in rat hypothalamic-pituitary-adrenal axis responses to nicotine stimulation. Brain Res Bull. 2001;546:681–8. doi: 10.1016/s0361-9230(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Rostron BL, Chang CM, Pechacek TF. Estimation of Cigarette Smoking–Attributable Morbidity in the United States. JAMA Intern Med. 2014;174(12):1922–1928. doi: 10.1001/jamainternmed.2014.5219. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–8. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- Sakai H, Ohashi K. Association of menstrual phase with smoking behavior, mood and menstrual phase-associated symptoms among young Japanese women smokers. BMC Womens Health. 2013;13:10. doi: 10.1186/1472-6874-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;1713:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological Rev. 2001;53(2):209–43. [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovács K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Exp Clin Psychopharmacol. 2012;204:251–7. doi: 10.1037/a0027759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. Womens Health. 2007;16:1211–18. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- Seedat S, Stein DJ. Trauma and post-traumatic stress disorder in women: a review. Int Clin Psychopharmacol. 2000;3:S25–33. [PubMed] [Google Scholar]
- Semba J, Wakuta M, Maeda J, Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29(2):215–26. doi: 10.1016/s0306-4530(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology Berl. 2011;2181:271–9. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwara AJ, Karwosk TE, Czambel RK, Rubin RT, Klein ME. Influence of environmental enrichment on hypothalamic-pituitary-adrenal HPA responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav. Brai. Res. 2012;234:1–10. doi: 10.1016/j.bbr.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Dutra LM, Williams DR, Mujahid MS, Lewis TT, Bennett GG, Ryff CD, Albert MA. Psychosocial stressors and cigarette smoking among African American adults in midlife. Nicotine and Tobacco Research. 2012;1410:1161–69. doi: 10.1093/ntr/nts011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;691-2:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol. 2009;247:559–64. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JM, Goldner EM, Waraich P, Hsu L. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry. 2006;51(2):100–13. doi: 10.1177/070674370605100206. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;593:228–35. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. Journal of Substance Abuse. 1997;9:223–40. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Lopez AD, Hartge P. Smoking-related mortality in the United States. N Engl J Med. 2013;368(18):1753. doi: 10.1056/NEJMc1302783. [DOI] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, O'Dell LE. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry. 2013;4:38. doi: 10.3389/fpsyt.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;2062:303–12. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Pipkin JA, Ferree P, Carcoba LM, O'Dell LE. Nicotine withdrawal increases stress-associated genes in the nucleus accumbens of female rats in a hormone-dependent manner. Nicotine Tob Res. 2015;17(4):422–30. doi: 10.1093/ntr/ntu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [Google Scholar]
- Ungless MA, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci Biobehav Rev. 2010;35(2):151–6. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–7. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;924:1896–902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–46. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, Smit JH, de Jonge P, Penninx BW. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;21:2–9. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30(9):1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Maciejewski PK, McKee SA, Reutenauer EL, Mazure CM. Gender differences in associations between lifetime alcohol, depression, panic disorder, and posttraumatic stress disorder and tobacco withdrawal. Am J Addict. 2009;182:140–7. doi: 10.1080/10550490802544888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, McKee SA. Mood and smoking behavior: the role of expectancy accessibility and gender. Addict Behav. 2012;37(12):1349–52. doi: 10.1016/j.addbeh.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NH, Duke AA, Sullivan TP. Evidence for a curvilinear dose-response relationship between avoidance coping and drug use problems among women who experience intimate partner violence. Anxiety Stress Coping. 2014;276:722–32. doi: 10.1080/10615806.2014.899586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers ME, Salsberry PJ, Ferketich AK, Ahijevych KL, Hood NE, Paskett ED. Risk factors for smoking in rural women. J Womens Health Larchmt. 2012;215:548–56. doi: 10.1089/jwh.2011.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZH, Tennen H, Hosain GM, Coman E, Cullum J, Berenson AB. Stress mediates the relationship between past drug addiction and current risky sexual behaviour among low-income women. Stress Health. 2014 doi: 10.1002/smi.2587. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, London ED. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–61. doi: 10.1080/14622200802412929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Schneier F, Heimberg RG, Princisvalle K, Liebowitz MR, Wang S, Blanco C. Gender differences in social anxiety disorder: results from the national epidemiologic sample on alcohol and related conditions. J Anxiety Disord. 2012;26(1):12–9. doi: 10.1016/j.janxdis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Yararbas G, Keser A, Kanit L, Pogun S. Nicotine-induced conditioned place preference in rats: sex differences and the role of mGluR5 receptors. Neuropharmacol. 2010;58(2):374–82. doi: 10.1016/j.neuropharm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhou JN. SUMO1 enhances 17-beta estradiol's effect on CRH promoter activation through estrogen receptors. Neuro Endocrinol Lett. 2008;292:230–4. [PubMed] [Google Scholar]