SUMMARY
The reactivation of dormant alpha-human herpesvirus (αHHV) has been attributed to various causes often referred to as stressors. However, no clinical study investigating the relationship between stressors and reactivation exists in humans at this time. Herpes simplex virus type-1 (HSV-1), an important αHHV, was shown to have its gene expression and replication regulated by thyroid hormone (TH) using molecular biology approaches. Varicella zoster virus (VZV) is categorized in αHHV superfamily and shares similar homology with HSV-1. We hypothesize that a history of TH imbalance may be associated with the incidence of shingles (VZV reactivation). This current pilot study, based on a hospital medical claims database, was conducted as a retrospective case-controlled investigation to determine if a putative link between TH imbalance and incidence of shingles is present. An odds ratio of 2·95 with a χ2 value of 51·74 was calculated for the total population diagnosed with TH disruption and shingles. Further analyses indicated that African American males exhibited a much higher chance of simultaneous diagnoses. These results show that a TH imbalance history may affect VZV reactivation at different incidence rates in different races and age groups.
Key words: Chickenpox, odds ratio, P value, reactivation, shingles, thyroid hormone
INTRODUCTION
Thyroid hormones (TH), namely T3 and T4 based on the number of their iodine, assume crucial regulatory roles in the normal functioning of almost every organ of the human body [1]. These roles at the physiological level include proliferation, differentiation, and apoptosis, etc. Disruption of TH synthesis or actions cause widespread endocrine occurrences that may result from inadequate levels of iodine, thyroidectomy (partial or complete removal of the thyroid gland), or inherited genetic defects [2]. Consequently, TH deficiency may lead to serious disorders such as cretinism, dementia, arrhythmias, death, etc. Furthermore, the effects of TH are mediated through its nuclear receptor (TR), which is a transcriptional factor controlling gene expression. The overall gene regulation is tightly linked to TH levels [3–5]. While hormone imbalances have been hypothesized to affect viral pathogenesis, the recent literature suggests that TH plays an important role in herpes simplex virus-1 (HSV-1) gene silencing and replication and may also influence latency and reactivation processes of the virus [6, 7].
Alpha human herpesvirus (αHHV) refers to a superfamily of herpes viruses [HSV-1, HSV-2, varicella zoster virus (VZV)] infecting humans that is capable of establishing dormancy within the neurons of the nervous system after the primary infection phase [8]. Both HSV-1 and HSV-2 are widespread throughout the United States with documented respective seroprevalences of 57·7% and 17·0% between 1999 and 2004, respectively [8]. While most HSV-1 and HSV-2 infections are subclinical, complications such as encephalitis caused by HSV-1 reactivation account for nearly 10% of viral encephalitis cases [9]. HSV reactivation and its inherent mechanisms are still not fully understood [10]. Factors such as physical and emotional stress have been cited as triggers of HSV reactivation with one of them being concurrent decreased TH levels [11]. A recent pilot study using a retrospective cohort analysis of hospitalized patients suggested that TH dysfunction may be linked to αHHV reactivation [12].
VZV is a pervasive human neurotropic virus belonging to the αHHV superfamily. Similarly to HSV, it can establish latency in sensory neurons of ganglia after primary infection, characteristically varicella (chickenpox) during childhood. The latent VZV can reactivate decades later to cause zoster (commonly known as shingles) as well as a number of severe optical and neurological complications [13]. It is hypothesized that TH imbalance may participate in the reactivation of VZV as well. This study aims to test the hypothesis that a significant association exists between TH imbalance and VZV.
MATERIALS AND METHODS
Study design and data source
As mentioned, a retrospective case-control design was used to establish the relationship between TH imbalance and VZV. Patient-level data were acquired from the Peninsula Regional Medical Center (PRMC), a regional medical centre in Salisbury, MD, USA.
Inclusion and exclusion criteria
The disease states identified for inclusion were hypothyroidism (TH) and positive VZV-zoster diagnoses which correspond to ICD-9-CM codes of 244·0–244·9, and 053·0–053·9, respectively. The diagnosis of VZV-varicella (052) was examined and considered as a primary infection in comparison to reactivation (053, VZV-zoster).
The illnesses presenting confounding variable were identified, and all patients diagnosed with those disorders were excluded from the general population before further data sorting and statistical analyses. The excluded diagnoses and their corresponding ICD-9 codes were cancers (CA: 140–239), rheumatoid arthritis (RA: 714), systemic lupus erythematosus (SLE: 710), and asymptomatic/symptomatic human immunodeficiency virus (HIV: V08 and 042). These disease states constitute confounding factors as they are suspected to lead to thyroid gland irregularities or assumed to increase the risks of VZV reactivation.
Study population and data processing
Phase 0: Collection of billable claims over study period
Following the medical centre's protocol review committee's approval, a Microsoft Excel dataset of patients admitted for at least one night between 1January 2006 and 30 June 2012 was created. For each admission, a maximum of 20 billable claim numbers also known as diagnosis codes (DX#) were recorded. In addition to the DX#, the dataset contained information such as last name, first name, date of birth, medical record number, gender, race, age at admission, and admission/discharge dates.
Phase 1: Creation of an anonymous dataset by removal of unique identifiers
Prior to the acquisition of the data, unique identifiers including names, medical record numbers, and date of birth, were removed. However, because a unique identifier was needed to track patients with multiple admissions, pseudo-medical record numbers (MR#) were created using an ‘If’ expression that assigned the same index number to matching medical record numbers.
Phase 2: Identification of subpopulations of interests and elimination of patients with confounding factors to create a confounding factor-free data table (CFF-DT)
Once the indexed dataset was received, it was copied to a new spreadsheet and information such as admission age, dates of admission and discharge, gender, race, and race description were deleted to display the MR# and the diagnosis codes only. The MR column was copied and pasted in the columns adjacent to the DX columns to generate a spreadsheet displaying 20 matched MR#-DX# tables. Because this modification of the original patient records involved a copy and paste process only, the MR# were expected to line up with the DX# as in the original table. Each set of the MR#-DX# table was selected and custom sorted based on an ascending order of the diagnosis codes. The diagnosis codes of interest were then located in the now ordered table and the MR# that received those diagnoses were identified, copied, and pasted in a new column designated the disease state of interest, for example VZV. Using this method, all the MR# of interest were identified and recorded. Existing duplicated MR# were removed from the disease state column to display a list of unique MR#. The newly identified subpopulations were VZV, TH, and confounding factors (CF). Schematic representation of the study design IS shown in Figure 1.
Fig. 1.
Characteristics of the study design. The dataset of the patients was obtained and the population with ICD-9 codes of confounding factors was removed. The numbers of included patients diagnosed with TH disruption (TH+) or incidence of shingles (VZV+) or both (TH+/VZV+) were calculated as well as patients with no such codes. Demographic data such as gender, age, and race were also sorted for studies described in Table 3a.
Phase 3: Transfer of dataset to Microsoft Access
In order to build relationships between the different subpopulations and run a variety of queries to assess interdependency among disease states, the original indexed dataset as well as the VZV, TH, and CF groups were transferred onto Microsoft Access tables.
Phase 4: Creation of queries to determine values needed for contingency tables
The first of the two main types of queries performed in this segment of the analysis was the ‘unmatched query’. The purpose of this query was to yield a list of unique MR# that would belong to one subpopulation and would not have a match in another. This meant that those patients only received one of the diagnoses of interest. For instance, to exclude the patients in the VZV and TH groups who received a confounding illness diagnosis, an unmatched query was performed to yield confounding factor-free VZV and TH groups.
The second type of query was the ‘matching query’ which was used primarily to isolate MR# that appeared in more than one subgroup. In the context of this analysis, the matching query helped identify patients who were diagnosed with both VZV and TH imbalance during the study period. Prior to conducting matching queries, a relationship link was created between the similar fields (MR#) of different tables. A secondary use of the matching query was to create demographic queries by linking MR# from specific disease groups to MR# from the original dataset. Demographic queries incorporated information such as gender, age and race, which permitted in-depth inquiries into the impact of demographic factors on the outcomes of the study.
Statistical analysis
The assessment of the study hypothesis which suggested that TH imbalance may lead to zoster diagnosis/reactivation encompassed three phases of statistical evaluations.
The first stage aimed to compare the chances of VZV-zoster diagnosis/reactivation in both the TH-positive and TH-negative patients by 2×2 contingency table analyses using SAS v. 9·3 software (SAS Institute Inc., USA) to evaluate the risk, risk ratio, odds, and odds ratio (OR). Theoretically, an OR > 1 would be significant in demonstrating the suspected effects of TH, but a much greater OR would considerably strengthen the hypothesis, all things being equal.
The second stage was designed to show the dependence between the VZV-zoster and TH groups. To do so, the experimental hypothesis stating that the groups are related was formulated, and the null hypothesis claiming otherwise, meaning no relationship between groups was tested using a χ2 test of independence. Values of χ2 greater than the critical value corresponding to the predetermined P value of 0·05 would be significant, and would allow the rejection of the null hypothesis, hence proving the existence of a relationship between the VZV and TH groups.
The third stage aimed to confirm the theory that the VZV patients, as identified using ICD-9 code 053, were suffering from the reactivation of the zoster virus and were not displaying symptoms of a primary chickenpox (CKP) infection (ICD-9 code 052). To do so, both OR and χ2 were calculated based on the Statistical Analysis System (SAS). The data processing method that yielded the values needed for this analysis was similar to the one used for the VZV-TH assessment. The chances of VZV-varicella diagnosis in both the TH-positive and TH-negative patients were used as a control.
RESULTS
Characteristics of the patient population
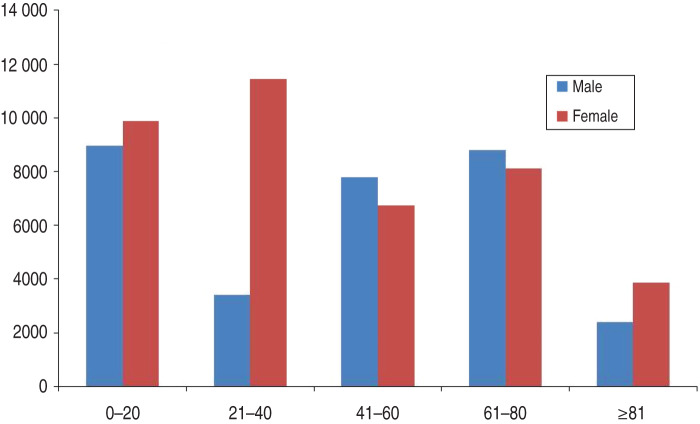
The hospital data listed 149 734 admissions for the time interval between 1 January 2006 and 30 June 2012. These admissions were represented by a total of 82 426 unique patients (the exclusion group consisted of 11 067 individuals). It was found that 5303 and 210 patients had TH imbalance and VZV only, respectively, while 50 patients were positively diagnosed with both disorders (Fig 1). Demographic data were analysed and used for further investigation. Gender distribution of inclusion population and patients with different age groups were demonstrated (Fig. 2).
Fig. 2.
Analysis of the total study population. The age and gender distribution between genders was analysed and showed similar numbers of hospitalizations except for the 21–40 years age group in which the number of females were approximately three times higher than their male counterparts, probably due to procedures related to childbirth.
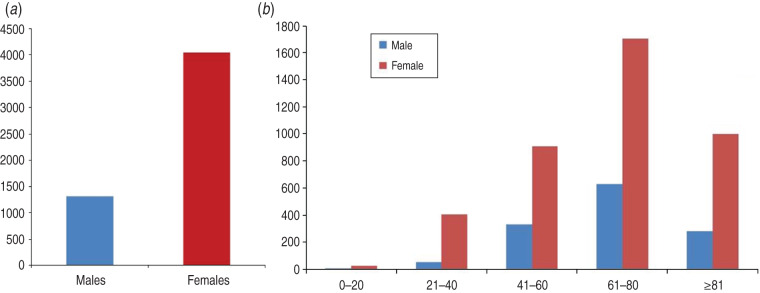
Females represented about 75% of included patients diagnosed with TH imbalances (Fig. 3a). Furthermore, while no major gender differences existed in the 0–20 years age group, female patients aged >21 years displayed a more pronounced surge in the number of TH diagnoses (Fig. 3b) compared to males.
Fig. 3.
Assessment of included patients with thyroid hormone (TH) dysfunction. (a) Gender distribution of patients with TH diagnoses. Total number of female patients exhibiting TH codes is about 3·2-fold higher than their male counterparts. (b) Age distribution of TH diagnoses. The number of patients with TH dysfunction increased as people aged. Females had more TH problems than males throughout the study groups.
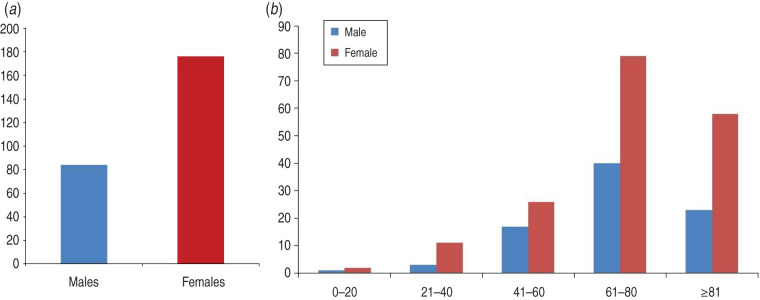
Concerning the VZV group, the analyses found that the ratio of females to males was approximately 2:1 (Fig. 4a), and the ratio was maintained once the age factor was introduced in the analysis (Fig. 4b).
Fig. 4.
Evaluation of included patients with incidence of shingles. (a) Gender distribution of patients hospitalized due to complication of shingles exhibited a ratio of 2:1 (females to males). (b) Age distribution and such a ratio were maintained once age factor was introduced in the analysis.
Statistical analyses of correlation and association between TH dysfunction and VZV reactivation
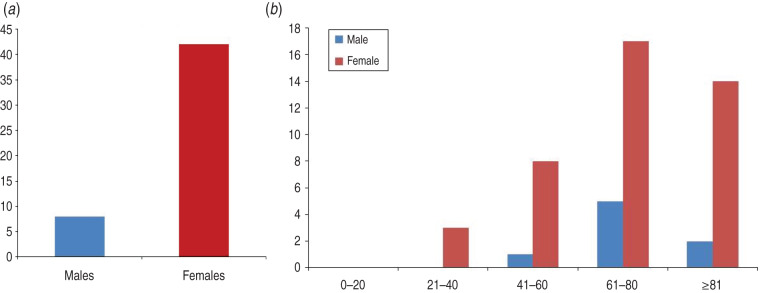
The group of 50 patients, who received a dual diagnosis of VZV and TH, was made up of 42 females and eight males. The female:male gender ratio in this group was determined as 5:1 (Fig. 5a). In terms of age distribution, patients in the 61–80 years age group had the highest incidence of both TH and VZV diagnoses (Fig. 5b).
Fig. 5.
Investigation of patients with both thyroid hormone (TH) disruption and shingles outbreak. (a) Gender distribution of patient cohorts with both diagnoses of shingles (VZV) and TH. (b) Gender distribution in different age groups with both TH and VZV diagnoses.
The odds of a patient reactivating a dormant VZV with TH imbalance compared to a patient with normal TH function was found to be 2·95 (Pearson χ2 = 51·74, P < 0·0001), an approximate threefold increase in the odds of VZV reactivation for patients suffering from TH imbalance (Table 1a).
Table 1.
Analyses of patients with diagnoses of thyroid hormone (TH) complications and VZV infections
| (a) Diagnoses of TH complications and zoster (VZV-053). An odds ratio was calculated as 2·95 with P < 0·0001. |
 |
| (b) Diagnoses of TH complications and chickenpox (VZV-052). No patient was found with both diagnoses. |
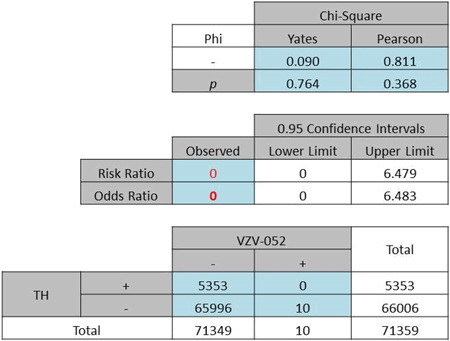 |
To confirm that patients were hospitalized due to the reactivation of the virus instead of a primary infection, statistical analyses through the use of chickenpox diagnosis code 052.** were used for comparison. The examination of chickenpox codes showed that while 10 patients were diagnosed with the illness, no patient was identified with both chickenpox and TH. This finding resulted in an OR of 0 (Pearson χ2 = 0·811, P = 0·368, Table 1b). Together these results indicated that TH disruption appeared to have influence on VZV and this effect was specific to reactivation instead of primary infection.
Impact of the gender factor in the evaluation of the hypothesis
A gender-specific analysis for the female population returned an OR of 2·81 (Pearson χ2 = 36·93, P < 0·0001, Table 2a). The male population generated a slightly lower OR of 2·42 (Pearson χ2 = 5·99, P = 0·014, Table 2b). No significant difference was found in this category.
Table 2.
Investigation of correlation of thyroid hormone (TH) disruption and shingles in different genders
| (a) Odds ratio of total female patients included in the study: 2·81, P < 0·0001 |
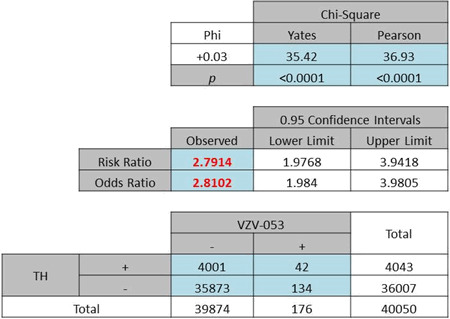 |
| (b) Odds ratio of total male patients included in the study: 2·42, P < 0·05 |
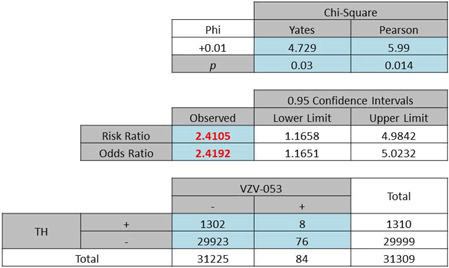 |
Impact of race and gender factors in the evaluation of the hypothesis
An examination revealed that the post-exclusion population encompassed different races including African Americans (Blacks), Caucasians (Whites), Hispanics, Asians, Indians, and a racially undetermined (Others) group. Whites were the largest group (69·6%) with 49 649 ( 27 191 females, 22 458 males) patients. African Americans represented 21·6% of all patients with 15 447 individuals (9118 females, 6329 males). The remaining number of patients was accounted for as described in Table 3a.
Table 3.
Race-gender distribution of post-exclusion population
| (a) Patient counts with various ethnic backgrounds listed. Gender differences are included. |
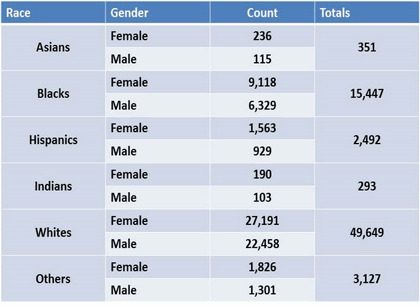 |
| (b) Odds ratio of total Caucasians: 2·65, P < 0·0001 |
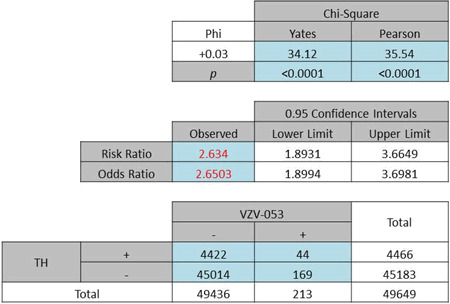 |
| (c) Odds ratio of total African Americans: 2·99, P = 0·082 (Yates) or 0·031 (Pearson) |
 |
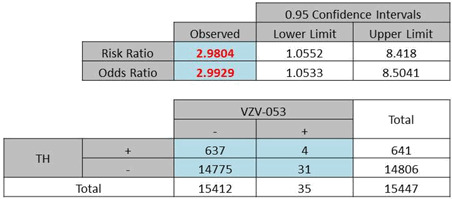 |
The analysis of the race factor in this study was limited to Whites and Blacks which are the predominant races in this area of the Delmarva Peninsula. The computations yielded ORs of 2·65 (Pearson χ2 = 35·54, P < 0·0001) and 2·99 (Pearson χ2 = 4·673, P = 0·031) for Whites (Table 3b) and Blacks (Table 3c), respectively.
A further exploration of the hypothesis using both the race and gender factors yielded ORs of 2·49 (Pearson χ2 = 24·89, P < 0·0001) and 1·67 (Pearson χ2 = 0·489, P = 0·485) for White females (Table 4a) and Black females (Table 4b), respectively. By contrast with the female subgroup, a difference in OR was noted for the male subgroup (White males: OR 1·94, Pearson χ2 = 2·949, P = 0·114, Table 5a; Black males: OR 8·19, Pearson χ2 = 10·204, P < 0·001, Table 5b).
Table 4.
Exposure-outcome correlation of female patients from diverse races
| (a) Odds ratio of Caucasian females: 2·49, P < 0·0001 |
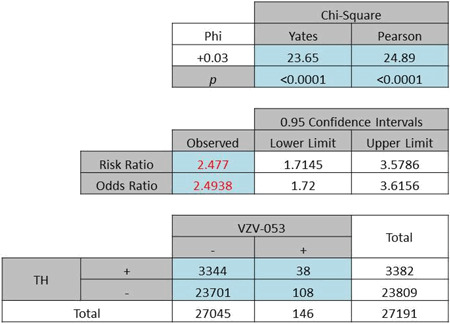 |
| (b) Odds ratio of African American females: 1·66, P = 0·812 (Yates) or 0·485 (Pearson) |
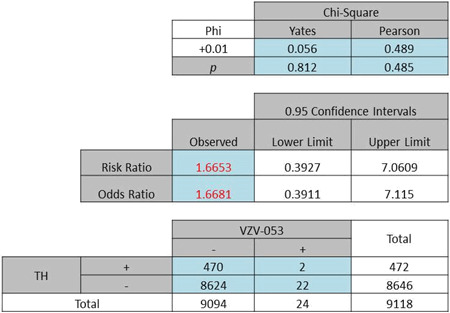 |
Table 5.
Exposure-outcome correlation of male patients from diverse races
| (a) Odds ratio of Caucasian males: 1·94, P = 0·196 (Yates) or 0·114 (Pearson) |
 |
| (b) Odds ratio of African American males: 8·18, P < 0·03 |
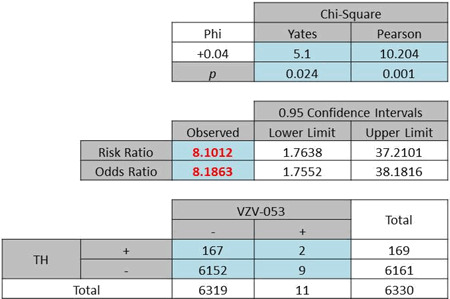 |
Impact of the age factor in the evaluation of the hypothesis
The age-specific investigation of the data resulted in relevant numbers for only two age groups. The 21–40 years age group had an OR of 8·61 (Pearson χ2 = 15·75. P < 0·0001, Table 6a). The 41–60 years age group had an OR of 2·842 (Pearson χ2 = 8·45, P = 0·004, Table 6b).
Table 6.
Exposure-outcome correlation of patients in different age cohorts
| (a) The 21–40 years age group exhibited an odds ratio of 8·61, P < 0·005 |
 |
| (b) The 41–60 years age group exhibited an odds ratio of 2·84, P < 0·01 |
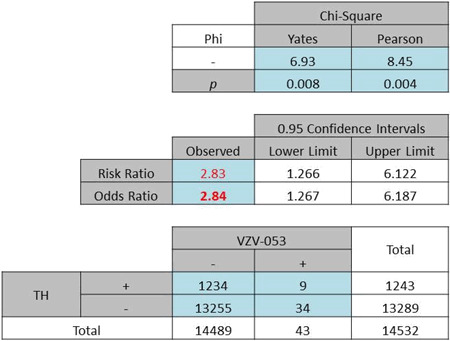 |
DISCUSSION
Rationales
Although there is no molecular evidence suggesting that the TH exhibited regulatory effects on VZV gene expression and replication, we hypothesize that TH disruption may participate in the VZV reactivation, similar to what we observed in HSV-1, based on the facts of similarities between these two viruses. First, they belong to the α herpesvirus family with high degree of genome homology. Second, they are both human neurotropic viruses and can maintain latency in sensory neurons after primary infection. Third, both viruses can result in complications in eyes after reactivation. Fourth, both HSV-1 and VZV can be effectively treated by acyclovir, indicating the importance of viral thymidine kinase (TK). Previously HSV-1 TK thyroid hormone response element (TREs) were compared to consensus TREs and TSHα palindrome TREs, the most studied negative TREs. The analyses showed that HSV-1 TK TREs exhibited a pair of palindrome TREs with 6-nucleotide spacing located between the TATA box and transcription initiation site, very similar context compared to TSHα palindrome TREs [14]. Our data-mining studies examining the VZV TK promoter revealed a couple of putative palindrome TREs within the TATA box and the transcription initiation site. The first one is a palindrome with 5-nucleotide spacing (Pal5) similar to HSV-1 TK TREs and the second is a palindrome with no nucleotide in between (Pal0) similar to TSHα TREs (Fig. 6). Additional studies using reporter plasmids to characterize the VZV TREs are underway.
Fig. 6.
Characterization of tentative VZV TRE. Consensus thyroid hormone response element (TRE) was provided and compared to tentative VZV TK TRE, HSV-1 TK TRE and TSHα palindrome TRE, a model negative TRE. The pairs of palindrome repeats with different numbers of nucleotide spacing located between the TATA box and transcription initiation site were noted and are underlined. TRE repeats with homology to consensus sequence are denoted by an asterisk (*). Speculative VZV TRE with palindrome repeats and 5-nucleotide spacing (Pal5) as well as no nucleotide in between (Pal0) were observed and are presented.
Clinical significance
There is no well-controlled clinical report investigating the relationship between TH imbalance and VZV reactivation although there have been a number of conversations noted in the public domain discussing the episodes of hypothyroidism followed by shingles. The current study is an attempt to connect the TH alteration and VZV outbreak. We examined the database (2006–2012) from a rural regional hospital using a historical cohort analyses. Our results showed that for those patients suffering TH disruption, their chance to have a shingles diagnoses exhibited an approximate threefold increase (Table 1a). Interestingly, our data found no patient simultaneously possessed both CKP (VZV 052) and TH diagnoses codes (Table 1b). These observations suggested a link between TH dysfunction and shingles (VZV reactivation) but not chickenpox (VZV primary infection). We understand the population pool in this study was relatively small. However, this study, in our opinion, represented a good example of the health condition of the general public in a rural area and served as a pilot investigation to characterize a complex interplay regarding hormone status and virus reactivation.
Differences in age and gender
Our examination concurred with the general perception that females display more TH disruption than males (Fig. 3a). Our records revealed that more TH dysfunctions were identified in patients in the 61–80 years age group (Fig. 3b). This is slightly different from our previous report where patients in 41–60 years age group exhibited most cases of TH illness [12]. In addition, the number of female patients diagnosed with both shingles and TH dysfunction was five times as much as their male counterparts (Fig. 5a), we did not observe a significant gender difference in terms of the OR (Tables 2a and 2b). The exact mechanisms of which are yet to be determined.
Disparity of races and genders
Our analyses indicated that there was no significant difference in different races (Tables 3b, 3c). Further analyses assessing gender and race indicated that African American males displayed a much higher OR at 8·18 (Table 5b), an approximate fourfold increase compared to their White counterparts. This is the first report presenting this disparity and requires more in-depth investigation to understand the reasons.
Benefits and drawbacks
In this study, we used a retrospective approach to disclose a hypothetical connection between TH illness and VZV reactivation manifested as shingles. An obvious benefit over the prospective method is that this surveying technique typically requires a shorter period of time to finish the study since patients' records have already been documented appropriately. Furthermore, it is less costly to continue the research and the efforts would be preserved to concentrate on the data collection and analyses. However, historical studies have several marked shortcomings such as their selection bias of data and statistical hitches that may markedly affect authenticity. It is thus critical to have precise record maintenance to avoid errors of connecting exposure and consequence during data assessments. Another major limitation of this study is the utilization of a hospital claims database. More precisely, it stems from the fact that the hospital claims database is not a comprehensive one as it only provided the diagnosis codes of diseases treated at that hospital. For instance, if a patient was diagnosed with TH dysfunction at one hospital and with VZV at another, the subject will be erroneously classified as belonging to one or the other of the disorder groups instead of being categorized as positive for both disorders. A better approach to this defect of data gathering would require the use of an insurance claims database such as Medicare, which would encompass all the diagnosis codes for an individual patient regardless of the hospital or location from where the claims originated. Moreover, the retrospective aspect of this study, as well as the limitation of available data, namely laboratory test results for thyroid-stimulating hormone (TSH) and free-T4 (thyroxine), eliminated the possibility of specifying when patients experienced TH fluctuations, and whether those fluctuations took place during admissions and hospital stays. The question of how the patients' TH levels were disrupted could not be clearly answered either, even though according to an expert endocrinologist opinion, the majority of the patients suffered from Hashimoto's disease, as their medical records listed a diagnosis code of 244·9 ‘unspecified acquired hypothyroidism’ which often turns out to be Hashimoto's disease.
Questions of interest
Though this study did not seek to find a link between corticosteroid levels and TH disruption, it is undoubtedly crucial to mention that steroids can induce HSV-1 and that dexamethasone, a corticosteroid used for the management of multiple disease states, was shown to reduce TH levels (reviewed in [11]). We are, however, unsure about the effect of steroids on VZV reactivation specifically. We checked our data and did find several patients treated with steroid and synthroid at 100 μg by mouth daily. The steroid may have played a role in triggering the VZV reactivation by, at least in part, decreasing the TH level. This possible cause-and-effect link between steroids and TH levels constitutes a testable hypothesis that can be investigated in a prospective study, but the present study did not attempt to answer this.
In addition to discussions expanding on the possible effects of hyperthyroidism, hypothyroidism, or various degrees of fluctuations on the reactivation of VZV, a question that arose during the discussions as a counterpart to the working hypothesis was: ‘If hypothyroidism increased VZV reactivation possibly due to sub-therapeutic treatment of the condition, could sub-therapeutically treated hyperthyroidism protect against reactivation? Our data indicated that there were 570 patients with hyperthyroidism diagnostic codes, none of whom showed evidence of VZV reactivation even while using steroids (data not shown). It seems that under-treated hyperthyroidism may have a protective effect against VZV reactivation, but further investigation is required to verify this assumption.
CONCLUSION
The present study suggested, for the first time, that TH level may play a critical role in the pathogenesis of shingles resulting from VZV reactivation. Therefore, TH levels should be considered a regular biomarker for recurrent VZV diagnoses. In addition, physicians should counsel their patients on the future relative risk of TH illness on αHHV recurrence and suitable medical treatment for TH disturbance may be considered while treating patients of HSV-1, HSV-2, and VZV. The critical nature of the possible ramifications stemming from proving this working hypothesis warrants first, more studies using other larger and more comprehensive databases, and second, the initiation of prospective clinical and laboratory investigations that aim to decipher the complex interactive pathophysiology of these illnesses.
ACKNOWLEDGEMENTS
We acknowledge the support from the University of Maryland Eastern Shore, School of Pharmacy and Health Professions and the Peninsula Regional Medical Center in Salisbury Maryland. S.V.H. is further supported by NINDS/NIH R01NS081109.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lazar MA. Thyroid hormone action: a binding contract. Journal of Clinical Investigation 2003; 112: 497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi YB, et al. Thyroid hormone regulation of apoptotic tissue remodeling during anuran metamorphosis. Cell Research 2001; 11: 245–252. [DOI] [PubMed] [Google Scholar]
- 3.Hsia SC, Shi YB. Chromatin disruption and histone acetylation in regulation of the human immunodeficiency virus type 1 long terminal repeat by thyroid hormone receptor. Molecular and Cellular Biology 2002; 22: 4043–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsia SC, et al. Role of chromatin disruption and histone acetylation in thyroid hormone receptor action: implications in the regulation of HIV-1 LTR. Histology and Histopathology 2003; 18: 323–331. [DOI] [PubMed] [Google Scholar]
- 5.Hsia SC, Wang H, Shi YB. Involvement of chromatin and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Cell Research 2001; 11: 8–16. [DOI] [PubMed] [Google Scholar]
- 6.Bedadala GR, et al. Thyroid hormone controls the gene expression of HSV-1 LAT and ICP0 in neuronal cells. Cell Research 2010; 20: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsia SC, et al. Regulation of herpes simplex virus type 1 thymidine kinase gene expression by thyroid hormone receptor in cultured neuronal cells. Journal of Neurovirology 2010; 16: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields BN, et al. (eds). Fields' Virology, 5th edn. Philadelphia, Pennsylvania: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 9.Martinez PA, et al. The effect of highly active antiretroviral therapy on outcome of central nervous system herpesviruses infection in Cuban human immunodeficiency virus-infected individuals. Journal of Neurovirology 2007; 13: 446–451. [DOI] [PubMed] [Google Scholar]
- 10.Goel N, et al. The ability of an HSV strain to initiate zosteriform spread correlates with its neuroinvasive disease potential. Archives of Virology 2002; 147: 763–773. [DOI] [PubMed] [Google Scholar]
- 11.Hsia SC, Bedadala GR, Balish MD. Effects of thyroid hormone on HSV-1 gene regulation: implications in the control of viral latency and reactivation. Cell & Bioscience 2011; 1: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsia SH, Hsia SCV. A cohort historical analysis of the relationship between thyroid hormone malady and alpha-human herpesvirus activation. Journal of Steroids & Hormonal Science 2014; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilden D, Nagel MA, Cohrs RJ. Varicella-zoster. Handbook of Clinical Neurology 2014; 123: 265–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figliozzi RW, et al. Thyroid hormone-dependent epigenetic suppression of herpes simplex virus-1 gene expression and viral replication in differentiated neuroendocrine cells. Journal of the Neurological Sciences 2014; 346: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]