Abstract
Background
How developmental mechanisms generate the phenotypic variation that is the raw material for evolution is largely unknown. Here we explore whether variation in a conserved signaling axis between the brain and face contributes to differences in morphogenesis of the avian upper jaw. In amniotes, including both mice and avians, signals from the brain establish a signaling center in the ectoderm (the Frontonasal ectodermal zone or “FEZ”) that directs outgrowth of the facial primordia.
Results
Here we show that the spatial organization of this signaling center differs among avians, and these correspond to Sonic hedgehog (Shh) expression in the basal forebrain and embryonic facial shape. In ducks this basal forebrain domain is present almost the entire width, while in chickens it is restricted to the midline. When the duck forebrain is unilaterally transplanted into stage matched chicken embryos the face on the treated side resembles that of the donor.
Conclusions
Combined with previous findings, these results demonstrate that variation in a highly conserved developmental pathway has the potential to contribute to evolutionary differences in avian upper jaw morphology.
Keywords: Forebrain, variation, Sonic hedgehog (Shh), Frontonasal Ectodermal Zone (FEZ), craniofacial development
INTRODUCTION
An important goal of evolutionary-developmental biology is to identify how developmental mechanisms generate phenotypic variation and contribute to evolutionary change (Hall, 1999; Carroll, 2008). Developmental mechanisms both structure the variation on which natural selection acts and themselves evolve due to the effects of selection. A key issue for unraveling this interaction is determining how change in complex and highly integrated systems is mediated through alterations of developmental mechanisms (Hendrikse et al., 2007). Most studies have used comparative approaches to determine what changes to developmental mechanisms have occurred, but because the same developmental processes can act at different times and in different locations, it can be exceedingly difficult to decipher what effect they have on variation (Mitteroecker and Bookstein, 2007; Hallgrímsson, 2009; Mitteroecker, 2009) Here, we address this issue in the avian upper jaw by focusing on how early signaling interactions between the developing brain and face contributes to variation in initial morphogenesis and differences in embryonic facial shape.
Morphogenesis of the amniote upper jaw is a highly regulated process that results from the signaling interactions among the forebrain, the neural crest cells, and the adjacent surface ectoderm (Marcucio et al., 2011). The Frontonasal Ectodermal Zone (FEZ) is a signaling center in the surface cephalic ectoderm that controls morphogenesis of the upper jaw (Hu et al, 2003). We initially identified the FEZ based on the boundary formed by the juxtaposition of Shh- and Fgf8-expressing cells in the surface cephalic ectoderm. Initially, we equated the expression boundary between these two genes with the FEZ, but subsequently it has become clear that the ectoderm flanking this boundary mediates FEZ function (Reviewed in: (Marcucio et al., 2011)) and that SHH signaling in the forebrain regulates Shh expression in the FEZ. (Marcucio, Cordero et al. 2005; Marcucio et al., 2005; Young et al., 2010; Chong et al., 2011). Perturbations to these signaling interactions can induce malformations such as holoprosencephaly (HPE), and cleft lip and palate (CL/P) (Ming and Muenke, 1998; Cordero et al., 2004; Marcucio et al., 2005, Krauss, 2007; Thomason et al., 2008; Lipinski, et al, 2010; Bae, et al, 2011; Kurosaka et al., 2014). Importantly, quantitative modulation of SHH signaling produces a continuous range of outcomes in the three dimensional shape of the mid-face (Young et al., 2010), suggesting that this axis may be a source of both normal and abnormal population-level variation. In addition, these signaling interactions are conserved in both mice and avians but differ in their spatial organization (Hu and Marcucio, 2009a; Hu and Marcucio, 2009b; Reid et al, 2011), suggesting that variations in timing, location, and strength of this axis may also play a role in interspecific variation, at least on a macroevolutionary scale.
Building on this body of work, we hypothesized that variation in SHH signals from the forebrain contribute to interspecific variation in morphology of the avian upper jaw by regulating the spatial expression domain of Shh in the FEZ. To test this idea we first examined Shh expression in the brain and FEZ of chicken and duck embryos, which we chose because they are readily available and amenable to experimental manipulation. We noted quantitative differences between different orders (Galliformes and Anseriformes), so we next transplanted the basal forebrain from embryos of one avian order into the other, and examined molecular and morphological outcomes. These experimental results strongly support that the idea that variation in the forebrain-facial ectoderm SHH-signaling axis influences morphogenesis, and if acted upon by natural selection, could contribute to evolutionary variation in the morphology of the face. These results suggest that early brain-face interaction acts as a developmental module (Wagner and Altenberg 1996) and illustrates how the organization of developmental processes can influence the evolution of a complex structure like the vertebrate face.
RESULTS
The FEZ expression pattern differs between chick and duck
To determine the extent to which FEZ spatial organization differs among the two bird species, we first incubated duck and chick embryos to HH 22, at which time Shh expression in the surface ectoderm is robust, and then performed whole mount in situ hybridization to examine Shh expression in the FEZ. In the chicken (n=20) the Shh expression domain was square and filled the entire stomodeal ectoderm, while in the duck (n=25) this domain appeared circular. Interestingly, the expression patterns are highly correlated with the shapes of the FNP anlagen at this time (Fig. 1), supporting our posited model that FEZ variation contributes to divergent facial morphologies (Hu and Marcucio, 2009a; Hu and Marcucio, 2009b), even among closely related organisms. Previously, we showed that the Shh expression in the FEZ is controlled by earlier signals from the forebrain and the neural crest cells. Our next step was to identify potential differences that may control these unique expression patterns of Shh in the FEZ.
Figure 1. 3D Morphometric analysis of FEZ and craniofacial shape based on optical projection tomography imaging.
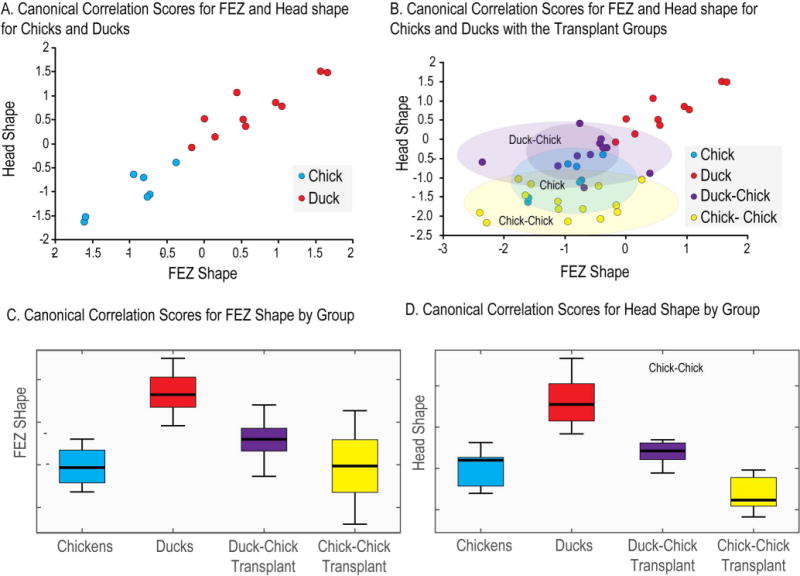
(A) Canonical correlation scores for chick and duck embryos for FEZ and Head shape. This plot shows the clear separation of FEZ and head shape in duck and chick embryos, as well as the correlation between Shh expression in the FEZ and facial shape. (B) Here the two hemi-forebrain transplant groups (duck to chick and chick to chick) are added to the data shown in graph A. Morphometric analysis for the transplants is performed only on the transplant side. The clear separation of facial shape among these groups is shown with the duck-chick transplant group shifted significantly towards the duck group. (C) and (D) show the means and dispersions of the canonical correlation scores for FEZ and head shape.
The forebrain contributes to morphological diversity of the face
Our goal was to test the intrinsic ability of the forebrain to direct unique expression patterns of Shh in the FEZ to control divergent facial morphology. To achieve this goal we developed a transplantation technique to exchange the basal forebrain among avian embryos prior to neural crest cell emigration. To develop this method, we initially removed the entire basal portion of the prosencephalon from donor quail embryos at HH 7 and transplanted these orthotopically into stage-matched chicken hosts. This approach led to substantial mortality within 24hrs of transplantation. However, if we transplanted only one side of the basal forebrain, the chimeras were viable (Fig. 2A). For this work we used unilateral transplants of quail prosencephalic tissue into chicken embryos to determine the distribution of donor-derived tissues in the chimeras. At 48 hrs after engraftment we observed that the graft had integrated and comprised the basal portion of the forebrain, and a few donor-derived endothelial and mesenchymal cells were apparent adjacent to the graft (Fig. 2C,D; n=5). At 72 hrs (~HH 22) after engraftment, the transplanted tissue was restricted to the ventral portion of one side of the telencephalon and the ventral part of the optic cup. Importantly, we examined sections throughout the embryo and at this time we found no evidence for the presence of large numbers of donor-derived neural crest cells (Fig. 2E; n=10). However, we found a very small number of angioblasts and endothelial cells that were derived from the donor tissue (Fig. 2F, n=10), likely due to adherent mesoderm on the graft. If there was a contaminating population of neural crest cells, they represented a very small proportion of the total cells that we did not detect, and likely did not contribute to the changes we observed. Others have shown that donor effects in chimeras are dose dependent, and changes are begun to be observed when the donor cells constitute approximately 50% of the neural crest population (Ealba and Schneider, 2013). Our initial goal was to create quail-duck chimeras, because our quantitative analyses placed the shape of quail embryos and the FEZ with the chick embryos (data not shown). However when we transplanted the basal forebrain of quail into duck embryos the chimeras were severely malformed at 72 hrs after engraftment, likely because intrinsic growth rate of the quail donor brain is accelerated compared to the duck host. Therefore, for the experiments we were forced to use duck embryos as donors and chicken embryos as hosts, because both of these species have more similar rates of brain growth. As controls, we created chick-chick chimeras.
Figure 2. Composition of forebrain chimeras.
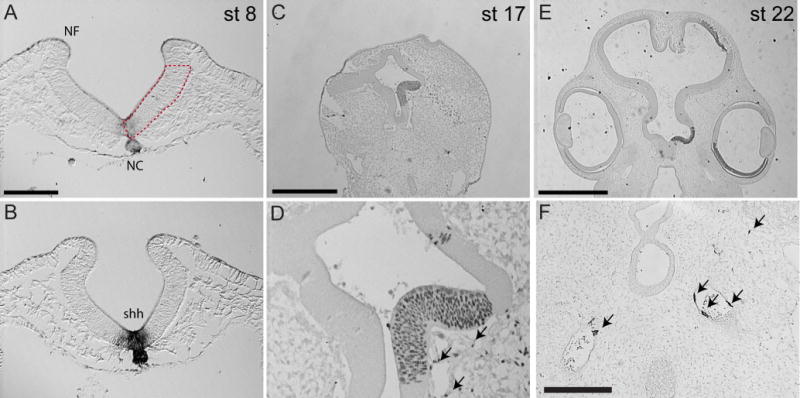
(A) A cross section of a chick embryo at the HH7 7 shows the region of the forebrain that was transplanted (boxed region). NF=neural fold, NC= Notochord. (B) In situ hybridization showing Shh expression in the basal neural tube at HH7. (C) A quail-chick chimera 48 hrs after transplantation showing that only the ventral portion of the neural tube was transplanted (n=5). (D) A high magnification of the grafted region in “C.” Arrows point to donor cells lining blood vessels (endothelial cells). (E) A chimera 72 hours after engrafting quail tissue into a chick host. The quail-derived cells were restricted to the ventral portion of the neural tube and optic cup on the grafted side of the embryo. (F) Immunohistochemistry using QH1 shows a small number of quail-derived endothelial cells and angioblasts (arrows) in HH 22 chimeras. Scale bars, A,BD, and F=100μm, B=0.5mm, E=1mm.
At HH22, chimeric embryos had facial asymmetry that was directly related to the species that the forebrain was derived from. In control embryos, (chick into chick, n=6) we observed no differences in morphology or the spatial pattern of expression of Shh (Fig. 1, and 3C). However, in chimeric animals morphology was different on the two sides of the face (Fig. 3D, n=15). At this stage, the nasal pits in chickens appear as narrow slits while in the duck they appear wider and more circular (Figs 3A,B). In the chimeric animals, the normal side appeared chicken-like: the nasal pits were narrow (Figs 3A,C,D), and the Shh expression domain appeared as it does in the normal and control (chick-chick) embryos (Figs 3A,C,D). In contrast, the chimeric side of the face appeared duck-like: the nasal pits were wider and appeared more circular (Fig. 3B,D), and the Shh expression domain was circular and reflected the changed shape of the stomodeal opening on this side of the head. These qualitative observations were confirmed by quantitative analyses of the shape of the nasal pit (Fig. 3E–G). Additionally, the eyes were developmentally more advanced on the control side compared to the chimeric side. On the control side, the epithelium of the retina was already pigmented (Fig. 3H), while on the chimeric side pigmentation was reduced, and the eye was smaller (Fig. 3I).
Figure 3. Effect of brain transplantation on morphology and Shh expression at stage 22.
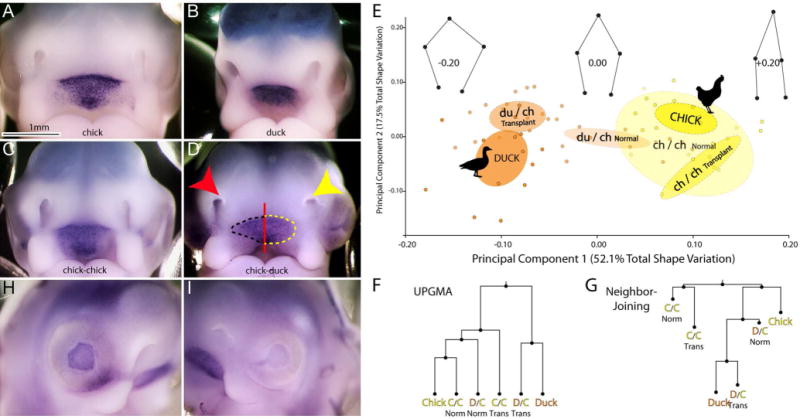
(A) Frontal view of a chick and (B) duck embryo at HH 22 after in situ hybridization to illustrate Shh expression at this stage. (C) In a chick-chick chimera no obvious difference in Shh expression is observed on the control (right side of embryo) and chimeric (left side of the embryo) sides of the embryo. (D) In a duck-chick chimera the nasal pit (yellow arrow) on the chimeric side (left side of embryo) is rounded and appears more duck like at this stage. On the control side (right side of embryo) the nasal pit (red arrow) appears as a slit, which is similar to the chick. Similarly, Shh expression on the operated side resembles the rounded duck-like expression pattern (outlined in yellow), while on the control side, the expression domain remains host-like (outlined in black). (E) Principal Components Analysis (PCA) of nasal pit morphology demonstrates that chicken (yellow, n=7 embryos, n=14 sides) and duck (orange, n=7, embryos, n=14 sides) separate along PC1 (51.5% total shape variation). Chimeric side nasal pits of experimental embryos (du/ch transplant n=15 embryos, n=30 sides) overlap ducks, while the normal side of experimental embryos (du/ch normal) tends to be more chicken-like, matching predictions and qualitative observations. Control animals comprised of chick-chick transplants (ch/ch transplant) overlap with normal chicks (Chick). Wireframes show landmark displacements along PC1. Ellipses show 95% confidence intervals of group means. (F) Both UPGMA and (G) Neighbor-joining trees of Procrustes distances between group means links Duck-Chimera to the exclusion of a Chicken-Normal. In F and G, the distances between all groups were significantly different (p<0.05) except for normal chickens and chick-chick chimeras (p>0.05). (H) On the control side of the embryo eye development is more advanced as evidenced by the dark pigmentation of the pigmented retina compared to (I) the lighter pigmentation in the pigmented retina on the chimeric side of the embryo.
Scale bar=1mm.
To support the 2D morphometric analysis of the nasal pits, we also obtained 3D data for head and FEZ shape using optical projection tomography and analyzed shape using canonical correlation analysis. This revealed clear separation of head shape for the two transplant groups (t-test, df=24 p=0.008). Procrustes permutation tests in MorphoJ confirmed this result with head shape differing significantly between the two transplant groups (p<0.001). The duck-chick transplant group is moved in the direction of the duck group although it was significantly different from both the duck and chick group. The chick-chick is closer to the chick group (Procrustes distance = 0.12) than to the duck group (Procrustes distance = 0.21). Both transplant groups, though, appear shifted towards the lower end of the head shape range in Fig. 1B,D such that both overlap the range of variation in the chick group. The chick-chick group likely differs from the chick group due to the perturbing effect of the transplant surgery. While FEZ shape was not significantly different between the transplant groups, the shape of the FEZ in the duck-chick chimeras was shifted in the direction of the duck shape (Fig. 1B,C). The 3D data thus confirm the results of the 2D morphometric analysis and suggest that transplanting duck forebrain into a chick embryo moves facial morphology significantly in the direction of the duck.
In a subset of embryos (n=7/23), the Shh expression domain on the chimeric side appeared reduced compared to the untreated side (Fig. 4A,B,C), suggesting it was developing slower. Our previous research revealed that neural crest cells are required for the induction of Shh in the facial ectoderm (Hu and Marcucio, 2012). Here, we wanted to determine if the duck and chicken forebrain induced changes in the rate of neural crest cell migration into the FNP. During migration, expression of HNK1 is high, but is reduced once neural crest cells arrive at their destination(s). We analyzed chimeras at 24 and 48 hours after transplantation, because by HH22 HNK1 expression is nearly absent from the FNP crest cells. At 24 hours we observed widespread HNK1 expression and no difference between control and chimeric sides (not shown). However, at HH18, we observed more HNK1 expression in neural crest cells on the chimeric side of the embryo compared to the untreated side, suggesting that it was less mature and that the neural crest cells were still migrating into the FNP region of this side (Fig. 4D,E,F; chick-chick n=6, duck-chick, n=5). Further, at day 6, the facial primordia were fused in control embryos (Fig. 5A, n=6), and on the control side of the chimeras (Fig. 5B). However, on the side receiving the duck graft the facial primordia were not yet fused (Fig. 5B, n=4/6).
Figure 4. Effect of the forebrain on neural crest migration into the FNP.
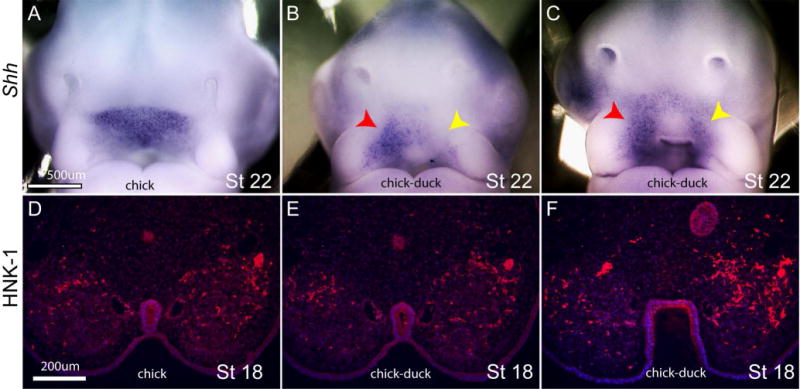
(A) Normal Shh expression at stage 22. (B,C) In these chimeras the Shh expression domain on the donor side (yellow arrow) is weaker than on the control side. Chick-duck is used to illustrate which side is the chick and duck side of the chimera. (D–F) These are progressively more proximal sections through a chimera at stage 18 (~24hrs prior to Stage 22 shown in A–C) that consistently show more HNK1 expression (red) on the chimeric side compared to the control side of the embryo. Rp=Rathke’s Pouch. Scale bar A–C=500μm, D–F = 200 μm.
Figure 5. Effect of forebrain on the timing of fusion of the facial primordia.
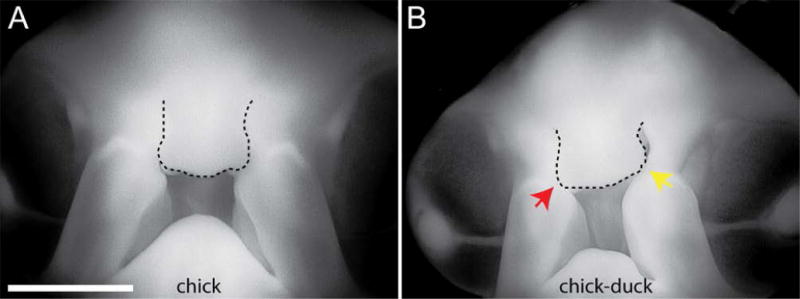
(A) At day 6 of development, the facial primordia forming the upper jaw have fused. The FNP is outlined. (B) In the chimeric embryos growth of the FNP is slower on the chimeric side and fusion has not occurred on that side of the embryo by this time. Scale bar=2mm.
To examine the consequences of the transplanted forebrain on Shh expression and facial development, chimeras were further incubated to day 10. At this time the upper beak of chick embryos comes to a distinct point (Fig. 6A, n=4), while the duck bill is wider and more rounded (Fig. 6B, n=4). Survival of chimeras to later stages was very low, but in animals that survived to day 10, we observed morphological changes that qualitatively resembled these differences in upper jaw shape (Fig. 6C,D, n=3). In addition, the eye on the chimeric side exhibited a similar transformation. At this age the chicken eye is large and has many “conjunctival” papillae present, while the stage-matched duck eye is smaller and does not harbor as many conjunctival papillae. In the chimera, the eye on the normal side appeared chicken-like (Fig. 6G), but on the chimeric side, the eye was smaller and contained fewer conjunctival papillae (Fig. 6H).
Figure 6. Effect of the forebrain on morphology of the upper jaw.
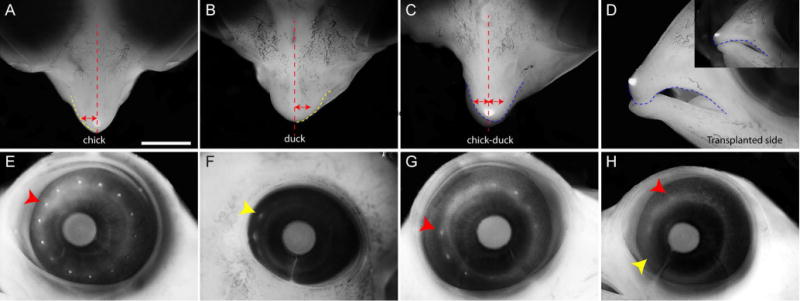
(A) Dorsal view of the upper jaw of a chick embryo at day 10. The red dotted line marks the midline and the yellow dotted line outlines the shape of the beak. The red arrow spans from the midline to the edge of the upper beak in the chick. (B) Dorsal view of the upper jaw of a duck at day 10. The midline is marked by the red dotted line and the yellow dotted line outlines the upper bill. Note that the bill is more broad than the beak of the chick. The red arrow is the same size as in A and does not span from the midline to the edge of the bill. (C) In the duck-chick chimera the beak on the grafted side (left side of the embryo) is wider than on the control side (red arrows re the same size). The shape is also more rounded as in B and is shown by the shape of the dotted orange line on either side of the midline. (D) Lateral view of the chimera in C showing the effect of the broadening of the upper beak on the curvature of the upper jaw. The inset is the control side showing the flatness on this side. (compare dotted blue lines) (E) At day 10 the chick have more conjunctivial papillae (red arrowhead) than (F) the duck. The ducks eye is also smaller than the chick eye. (G). The control side of the chimera shown in C has more conjunctivial papillae than on the (H) chimeric side (red and yellow arrowheads). Scale bar = 2mm.
Shh expression patterns in the ventral brain are species-specific
In these transplants, we replaced the basal portion of the chick forebrain with that from the duck. In this design, many differences in gene expression patterns may lead to the outcomes we observed. To begin to define the molecular signals from the brain that operated in our experiments, we examined expression of Shh. Shh expression in the ventral forebrain of chicken and duck embryos is very different. We performed in situ hybridization on serial sections through the entire forebrain of duck (n=5) and chick (n=5) embryos at HH 10 and determined that at the most anterior region of the chicken forebrain Shh is expressed in a broad domain in the ventral part of the neural tube, but does not extend all the way to the lateral edge of the brain (Fig. 7A). In the duck this domain occupies almost the entire ventral neural tube (Fig. 7D). In both species, the prechordal plate mesoderm also expresses Shh and is directly apposed to the forebrain, but this expression domain does not correspond to the width of Shh expression in the forebrain in either species (Fig. 7A, B, D, E). This basic pattern is maintained in more caudal regions of the forebrain, e.g., the Shh expression domain in the duck is much wider than that in the chicken (Figs. 7B,E). In the midbrain, the Shh expression domains appear to be similar in each organism, but the duck domain is still wider (Fig. 7C,F).
Figure 7. Unique expression patterns of Shh in the basal forebrain of avian species.
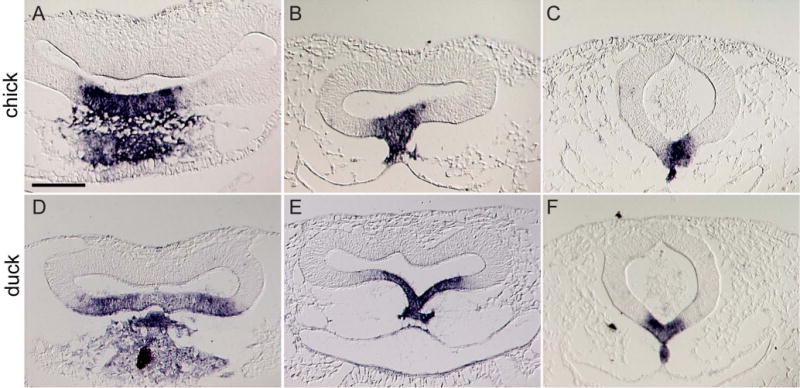
(A) In the region of the anterior and (B) posterior part of the chick forebrain (labeled “a fb” and “p fb” respectively) Shh expression is restricted to the midline of the basal part of the neural tube. The prechordal mesoderm (pm) also expresses Shh. (C) In the mid-brain Shh is expressed in the chick floor plate (fp) and notochord (nc). (D) In the anterior region of the duck forebrain, the Shh expression is wider than in the chick and encompasses nearly the entire ventral portion of the brain. (E) Similarly, in the posterior region of the duck forebrain, the Shh domain extends more laterally than in the chick. (F) In the floorplate of the duck midbrain, the Shh domain remains wider than in the chick, but this is less dramatic compared to the changes in the forebrain. Scale bar = 100μm.
Expanding Shh expression in the brain alters the organization of the FEZ
To test if the difference in the expression pattern of Shh in the forebrain could have produced the changes we observed in the face, we used electroporation to misexpress Shh in the neuroepithelium of the ventral forebrain. As a control, a plasmid encoding beta-galactosidase was electroporated into the neuroepithelium. The morphology and Shh expression domain in the FEZ in control embryos were indistinguishable from normal embryos (Fig. 8C; n=9), and the beta-galactosidase expression was restricted to the neuroepithelium (Fig. 8A,B; n=11). However, after inducing expression of Shh in the neuroepithelium, the Shh expression domain in the FEZ was expanded (Fig. 8E; n=9) and this was accompanied by gross morphological changes in the face. After expression of Shh the middle part of the face was wider in embryos at HH22 (Fig. 8C (n=20) compared to 8E (n=9)), and at day 10, these embryos had wider and shorter upper jaws (Fig. 8D (n=6) compared to 8F (n=6)) suggesting a direct effect on facial growth due to misexpression of Shh in the neuroepithelium.
Figure 8. Effect of the expansion of Shh expression on the morphology of the upper jaw.
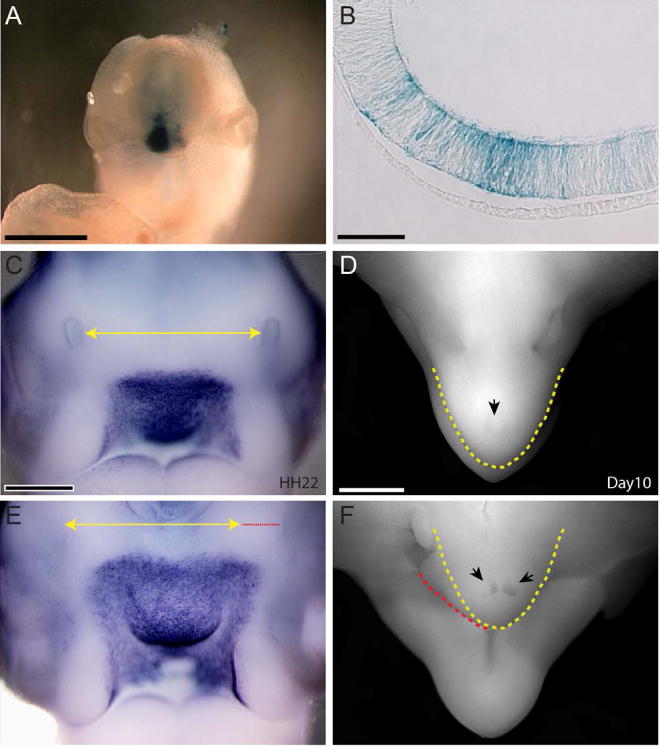
(A) Frontal view of an embryo electroporated with HSP-68-LacZ showing beta-galactosidase activity is restricted to the forebrain region. (B) Section through embryo in A shows transgene expression is restricted to the neuroepithelium and is not in the surface ectoderm confirming that changes in morphology of the jaw are due to changes in the pattern of Shh expression in the forebrain. (C) Whole mount Shh in situ hybridization at Stage 22 showing the normal expression domain. The Yellow line marks the width of the face between the middle part of the nasal pits at this stage. (D) Dorsal view of a chick embryo at day 10 showing the normal morphology of the upper jaw. The shape of the beak is outlined in yellow, and the black arrow indicates the egg tooth. (E) Shh was misexpressed in the brain of chick embryos by electroporation pcDNA3-Shh into the basal forebrain at Stages 9 and 10. Embryos were analyzed at Stage 22. The Shh expression domain appears wider and so does the FNP. The yellow line is the same as in A and the red line indicates the lateral expansion caused by ectopic Shh expression. (F) At day 10 embryos with expanded Shh expression in the basal forebrain have a beak that is more broad, and there are two egg teeth (black arrows). The yellow line shows the normal shape of the beak, and the red line illustrates the deviation from the normal shape. Scale bar A,C,E = 500μm, B=50μm, D,F = 1mm.
DISCUSSION
The brain and face interact in multiple ways during facial morphogenesis. For example, molecular signals from the brain to the facial ectoderm establish a signaling center that regulates morphogenesis of the jaw (Marcucio et al., 2011), while physical or epigenetic interactions between these tissues affect size and shape of the facial primordia (Parsons et al., 2011). Here, we examined whether variation in the signaling axis from the forebrain to the surface cephalic ectoderm and the adjacent neural crest contributes to evolutionary variation in the upper jaw. We transplanted the basal forebrain between avian species prior to the generation of neural crest cells, and we examined molecular and morphological outcomes in the resulting chimeras. Our results revealed that variation in this signaling axis contributes to order-level differences in jaw shape. Our data after mis-expressing Shh indicate a contribution of Shh to the dialogue occurring among these tissues. Our results also suggest that other factors from the brain are likely to be involved and need to be explored in detail. Collectively, these results strongly support the idea that developmental interactions between the forebrain and FEZ can produce variation in facial shape. Variation that results from such interactions can be acted upon by natural selection to produce evolutionary change (Atchley and Hall, 1991; Lieberman et al, 2004) and may also contribute to the generation or modulation of structural birth defects of the face.
The outcomes of these transplant experiments have significant implications for understanding the developmental determinants of variation in facial shape, and they add to findings from earlier experiments that establish the forebrain-FEZ signaling axis as a conserved module that plays a key role in facial development in amniotes (Marcucio et al., 2005). The modular organization of development constrains and enables evolutionary change, and modularity is very likely a necessary condition for the evolution of complex organisms (Wagner and Altenberg, 1996; Wagner et al., 2007). Modules can be thought of as divisions of structure that are, to a degree, autonomous from other such divisions (Schlosser and Thieffry, 2000; Wagner et al, 2007; Klingenberg, 2008). They can also be defined as a set of processes that result in a pattern of phenotypic covariation (Schlosser and Thieffry, 2000; Mitteroecker and Bookstein, 2007; Klingenberg, 2008). The forebrain-FEZ interaction represents a module in this latter sense. As such, it exists during a window of developmental time just prior to and during the fusion of the facial prominences but results in a pattern of covariation that persists across later developmental stages. How this module can be modulated by altering SHH signaling in the forebrain (Marcucio et al., 2005; Young et al., 2010; Chong et al., 2011) or SHH signal reception in the mesenchyme (Jeong et al., 2004) in ways that produce a continuous range of variation in three-dimensional facial shape (Young et al., 2010).
We show here that variation in the brain-FEZ axis contributes to phenotypic differences in facial shape between two orders of birds. This is important for understanding the evolution of complex phenotypes. These results illustrate how variation in a central developmental process can contribute to variation along an integrated axis of phenotypic variation within species but also over large regions of morphospace and among species with facial morphology as different as duck and chick. The results also illustrate mechanistically how the modular organization of development can facilitate the generation of phenotypic variation at the scale of different orders of birds (Anseriformes vs Galliformes): slight changes in the brain lead to alterations in the adjacent tissues and produce morphological changes.
Importantly, this mechanism does not necessarily imply a specific role for Shh mutations in the diversification of upper jaw shape in birds. It does, however, strongly suggest that forebrain-face signaling interactions mediated by the SHH pathway may be important for both enabling and constraining the evolutionary diversification of the midface and beak in birds. For example, we speculate that enhancers regulating Shh expression in the brain may differ among birds, leading to changes in the pattern of Shh expression and subsequent facial shape, but such variants have not been identified. In addition, mutations in many different genes can influence the Shh pathway both directly and indirectly. Our results along with related work on this system suggest that the potential genetic changes converge on the brain-face interaction module as one of the mechanistic drivers for the generation of evolutionary variation in the avian upper jaw and face.
This work highlights the relative significance of early-acting developmental mechanisms as a significant source of evolutionary variation, and this agrees with interpretations of other investigators (Abramyan, et al 2014). In a common view of development, variation is highly constrained around a temporal waist known as the phylotypic stage (Slack et al., 1993). The existence and quantitative verification of such a stage has proven problematic (Hall, 1997; Bininda-Emonds et al., 2003). We have recently determined that among a wide variety of amniotes, a phylotypic stage during facial development exists at the time of fusion of the facial primordia (Young et al., 2014). Here, our data show that ordinal-level variation among avians, and quite possibly more closely related species, can arise in the initial set-up of the face, suggesting that broad similarities among amniotes occur during fusion, while specific differences between individual organisms are observed very early during development. This evidence for early origins of evolutionary variation suggests that not all phylogenetic differences arise from differential growth, timing, and allometric effects as has been previously suggested (McKinney and MacNamara, 1991).
This is not to imply that later acting developmental mechanisms do not contribute to the generation of phenotypic variation. Indeed, transplantation of the neural crest primordia between duck and quail results in dramatic transformations of beak morphology as the transplanted cells differentiate (Schneider and Helms, 2003). The cells differentiate on an autonomous time frame and into intrinsic patterns indicating that regulating the timing of growth and other morphogenetic events impact morphological variation (Merrill et al., 2008). As with other complex phenotypes, variation can be generated at multiple times through multiple developmental mechanisms. The relative importance of individual mechanisms that generate phenotypic variation on the overall phenotypic variance structure will depend, at least in part, on the genetic variances underlying those mechanisms in natural populations (Hallgrímsson et al., 2009; Jamniczky and Hallgrimsson, 2009). This is the central idea behind our proposed Palimpsest model for the developmental basis for phenotypic variance-covariance structure (Hallgrimsson et al, 2007, 2009).
In addition to the changes in morphology that we observe, we also discovered that the brain contributes to the rate of facial development. In each chimera, the experimental side developed slower than the control side of the face. HNK1 is normally down-regulated once the neural crest cells arrive in the facial primordia. Here, more HNK1 expression is observed on the transplanted side than the control side indicating that migration into this region is occurring over a longer period of time (Fig. 4). Likewise, fusion of the primary palate is delayed on the transplanted side (Fig. 5). Finally, the slower developing duck brain delays formation of the conjunctival papillae even in dorsal regions of the eye, which is derived exclusively from host tissues (Fig. 6). The exact mechanisms that regulate timing of facial development are unclear at the moment. However, the conjunctivial papillae form in a stereotyped order within the surface ectoderm and appear to signal to the mesenchyme to induce formation of the scleral ossicles (Coulombre and Coulombre, 1962; Pinto and Hall, 1991; Franz-Odendaal, 2008; Duench and Franz-Odendaal, 2012). While the exact mechanism through which the brain regulates timing of facial development is unknown, presumably the developmental rate of the duck brain is slower which leads to a delay in the signaling interactions that are being controlled by the brain. However, future work is required to elucidate the exact mechanisms.
In conclusion, we have shown here that Shh expression patterns in the forebrain and FEZ differ between duck and chicken embryos. Transplanting the basal forebrain from one species to another both alters FEZ expression patterns and the shape of the face in the direction of the donor species, and this appears to be due to differences in Shh expression in the basal forebrain, although this was not directly examined in the chimeras. These results highlight the significance of forebrain-face interaction as an early-acting module in facial development. This module is critical not only for face formation but also for the generation of variation in the shape of the face both within and among species. This finding suggests also that at least some of the mechanistic basis for morphological divergence in the avian face involves developmental processes that are active early in facial development, prior to even the formation of the facial primordia and neural crest cell generation. This surprising result provides insight into the developmental basis of the evolution of the avian face, and may have implications for the roles of analogous processes in other complex phenotypes.
EXPERIMENTAL PROCEDURES
Forebrain Transplantation
Fertilized eggs from white Leghorn chicken (Gallus gallus), White Pekin duck (Anas platyrhynchos), and Japanese quail (Cortunix coturnix japonica) were incubated at 37°C in a humidified chamber until stage-matched at HH7/8 (Hamburger, 1951). Eggs were prepared for surgery as described in Hu and Marcucio, 2011. Tissue grafts of the basal forebrain were harvested from stage 7/8 embryos using sharpened tungsten needles. The grafts, measuring 0.3 mm in height by 0.2 mm in width, were transferred into DMEM containing Neutral Red (0.01% in PBS, 23°C, 2 minutes), which was added to facilitate visualization when transferred to the host. Stage 7/8 embryos served as hosts. Embryos were positioned in the egg to gain access to the forebrain, and the graft site (left side of basal forebrain) was prepared by removing the basal portion of the forebrain with tungsten needles; care was taken to avoid excessive disruption of underlying endoderm. The donor grafts were positioned to replace the extirpated tissue. Chimeric embryos were incubated for 48 and 72hours, and to day 6 or 10 for molecular, cellular, histological, and morphological analyses.
In situ hybridization
In situ hybridization was performed on paraffin sections and whole embryos as described (Albrecht et al., 1997). Subclones of chick Shh and Pax6 were linearized to transcribe digoxygenin-labeled antisense riboprobes. Tissues were hybridized with 0.5–1 μg/ml digoxygenin-labeled RNA, and after washing were incubated with an alkaline phosphatase-conjugated anti-digoxygenin antibody (Boehringer). NBT/BCIP substrate (Roche) was used for color detection. The in situ hybridization signal was visualized as described previously (Albrecht et al., 1997).
Immunohistochemistry
To detect quail cells, chimeric embryos were removed 48 hours after engraftment, fixed in Serra’s fixative, embedded in paraffin wax, and cut into 10 μm coronal sections. Quail cells were detected using immunohistochemistry with QCPN, and quail angioblasts/endothelial cells were detected with QH1 (Developmental Studies Hybridoma Bank), followed by incubation with a second antibody conjugated to Horseradish peroxidase (HRP). Diaminobenzidine (DAB, Sigma) was used to detect HRP. Sections were counterstained with Fast Green FCF (Fisher) and imaged using brightfield optics.
For HNK-1 immunofluorescence, embryos were fixed in 4% paraformaldehyde/PBS and processed as above. The sections were incubated in HNK-1 monoclonal antibody (Developmental Studies Hybridoma Bank, Iowa City; diluted 1:300). HNK-1 binding was visualized using a donkey-anti-mouse IgM Rhodamine-TRITC antibody (Molecular Probes), and sections were counterstained using Bis-benzimide. Multiple color channels were merged into a single image in Adobe Photoshop (Adobe Systems).
Electroporation
Electroporation was performed with the embryo facing ventral side down. DNA (1mg/ml) was mixed with bromophenol blue for visualization and injected into the neural tube using a picospritzer (PV830 Puenmatic Pico Pump form WPI). Electrodes, made from 1 mm diameter platinum wire, placed above the area to be electroporated and five 25 milli seconds pulses of 10 V were delivered using an Intracel TSS20 Ovodyne electroporator. The polarity of the current was reversed and the series of pulses was repeated. Embryos were then incubated at 38°C until analysis. For delivery of Shh we used pcDNA3 vector (Life Technologies) encoding full length Shh, and for delivery of beta-galactosidase we used the HSP68-LacZ vector (Addgene).
Geometric Morphometrics
Embryos were photographed in anterior view, as previously described (Chong et al., 2012). Coordinates (x,y) for five landmarks describing the shape of the nasal pit were identified and recorded for each side of chimeric and normal side transplants (N=15), as well as for stage-matched normal chickens (N=7) and ducks (N=7). Each side was treated as a separate individual. After superimposition to remove the effects of scale and orientation, Procrustes data were regressed against centroid size and the residuals used as the input for a Principal Components Analysis (PCA). Procrustes distances among group means were calculated, and the significance derived from bootstrap resampling (10,000 replicates). All analyses were performed in MorphoJ (Klingenberg, 2011).
Optical Projection Tomography
Following in situ hybridization, embryos were prepared for and imaged using Optical Projection Tomography (OPT). Embryos were embedded in 1% low melt agarose (Invitrogen), and mounted on specially designed magnetic chucks using LocTite blue (Hensel North America). Blocks were then dehydrated through three changes of 100% Methanol over 2 days and then cleared over night in a 2:1 mixture of Benzyl benzoate: Benzyl Alcohol. Cleared embryos were then imaged on the Bioptonics 3000 system (Sky Scan, Germany) in both the visual light channel and the GFP channel. Scans were reconstructed using the NRrecon program (Sky Scan, Germany).
Morphometric Analysis of OPT data
Data from all channels were then imported into Amira to create a full 3D reconstruction (Version 5.0, FEI, Hillsboro OR, USA) and thresholded to remove ring and other imaging artifacts. After eliminating individuals obviously damaged during preparation for OPT, the sample for 3D morphometric analysis consisted of 10 duck embryos, 7 chick embryos, 11 duck-chick transplants and 14 chick-chick transplants. To quantify the 3D shape of the FEZ, the data from the optical channel was used and the region of the FEZ was extracted from other optically dark regions of the embryo such as the eyes. The resulting segmented expression domains were then subjected to morphological analysis as described in Xu et al. (in press). To focus analysis of the transplant embryos only on the transplant side, we cut each FEZ and head down the midline and reflected the transplant side on itself to create a symmetrical object that could be compared to the chick, duck and quail images. After shape regularization, shape topography vectors were extracted from the 3D FEZ shapes.
Head shape was quantified using our standard set of 67 craniofacial landmarks (Young et al, 2010) and geometric morphometrics. An analysis of a random subset of 20 these 67 landmarks yielded similar results to those outlined in this paper (Fig. 9). An analysis of a random subset of 20 these 67 landmarks yielded similar results to those reported in this paper. We quantified the relationship between FEZ and head shape using canonical correlation analysis (CCA) (Krzanowski 1988; Seber 1984). Here, the FEZ morphospace was determined by the first two PC scores of the shape topography vectors (Xu et al, in press). Head shape space was based on the first six PC scores. CCA works by finding the pair of directions in the FEZ and head morphospaces that maximize the correlation coefficient between them.
Figure 9. Landmarks used for head shape analysis.
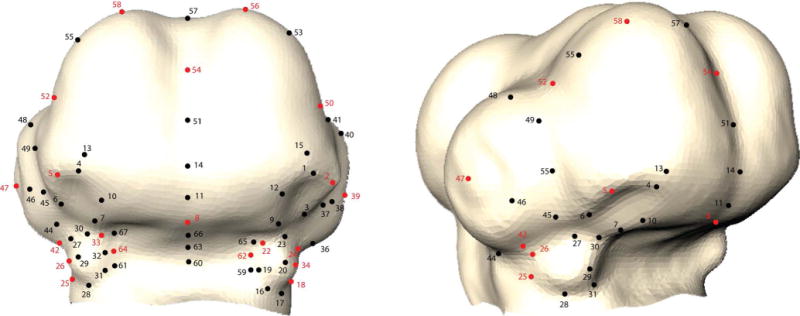
Frontal and partial lateral view of avian embryo head showing the 67 landmarks (black and red). Of those, 20 random landmarks were chosen for confirmational analyses. These are shown in red.
Acknowledgments
This research was funded by NIH/NIDCR (R01-DE019648 to R.M and B.H, R01-DE018234 to R.M., F32DE018596 to N.M.Y., F32DE02214 to. R.M.G.), and by the NSF (DBI-1052942) to W.M. The QCPN antibody developed by Carlson, B and Carlson, J and the QH1 antibody were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We also would like to thank Gina Baldoza for support in operation of the laboratories.
Funding: NIH/NIDCR: R01-DE019648 to R.M and B.H, R01-DE018234 to R.M., F32DE018596 to N.M.Y., F32DE02214 to. R.M.G., and N.S.F.: (DBI-1052942) to W.M.
Footnotes
All authors have no conflicts.
References
- Abramyan J, Leung KJ, Richman JM. Divergent palate morphology in turtles and birds correlates with differences in proliferation and BMP2 expression during embryonic development. J Exp Zool B Mol Dev Evol. 2014;322(2):73–85. doi: 10.1002/jez.b.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht UEG, Helms JA, Lin H. Visualization of gene expression patterns by in situ hybridization. In: Daston GP, editor. Molecular and cellular methods in developmental toxicology. Boca Raton, FL: CRC Press; 1997. pp. 23–48. [Google Scholar]
- Atchley WR, Hall BK. A Model for Development and Evolution of Complex Morphological Structures. Biol Rev Camb Philos Soc. 1991;66(2):101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Bae GU, Domené S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 2011 Aug 12;89(2):231–40. doi: 10.1016/j.ajhg.2011.07.001. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bininda-Emonds OR, Jeffery JE, Richardson MK. Inverting the hourglass: quantitative evidence against the phylotypic stage in vertebrate development. Proceedings Biological sciences / The Royal Society. 2003;270:341–346. doi: 10.1098/rspb.2002.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chong HJ, Young NM, Hu D, Jeong J, McMahon AP, Hallgrimsson B, Marcucio RS. Signaling by SHH rescues facial defects following blockade in the brain. Developmental dynamics : an official publication of the American Association of Anatomists. 2011 doi: 10.1002/dvdy.23726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. The skeleton of the eye. I. Conjunctival papillae and scleral ossicles. Developmental Biology. 1962;5:382–401. doi: 10.1016/0012-1606(62)90020-9. [DOI] [PubMed] [Google Scholar]
- Duench K, Franz-Odendaal TA. BMP and Hedgehog signaling during the development of scleral ossicles. Developmental Biology. 2012;365:251–258. doi: 10.1016/j.ydbio.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Ealba E, Schneider RS. A simple PCR-based strategy for estimating species-specific contributions in chimeras and xenografts. Development. 2013 Jul;140(14):3062–8. doi: 10.1242/dev.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz-Odendaal TA. Toward understanding the development of scleral ossicles in the chicken, Gallus gallus. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:3240–3251. doi: 10.1002/dvdy.21754. [DOI] [PubMed] [Google Scholar]
- Hall BK. Phylotypic stage or phantom: is there a highly conserved embryonic stage in vertebrates? Trends in ecology & evolution. 1997;12:461–463. doi: 10.1016/s0169-5347(97)01222-6. [DOI] [PubMed] [Google Scholar]
- Hall BK. Evolutionary Developmental Biology. Dordrecht: Kluwer; 1999. [Google Scholar]
- Hallgrímsson B, Hall BK. Variation: A Central Concept in Biology. New York: Elsevier/Academic Press; 2005. [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young N, Rolian C, Parsons T, Boughner J, Marcucio R. Deciphering the Palimpsest: Studying the Relationship Between Morphological Integration and Phenotypic Covariation. Evolutionary Biology. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Jamniczky HA, Young NM, Rolian C, Schmidt-Ott U, Marcucio RS. The generation of variation and the developmental basis for evolutionary novelty. Journal of experimental zoology, Part B, Molecular and developmental evolution. 2012;318:501–517. doi: 10.1002/jez.b.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Jamniczky H, Young NM, Rolian C, Parsons TE, Boughner JC, Marcucio RS. Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation. Evolutionary Biology. 2009;36:355–376. doi: 10.1007/s11692-009-9076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrimsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Am J Phys Anthropol Suppl. 2002;35:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of developmental stages in development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hendrikse JL, Parsons TE, Hallgrimsson B. Evolvability as the proper focus of evolutionary developmental biology. Evol Dev. 2007;9:393–401. doi: 10.1111/j.1525-142X.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- Heyne GW, Melberg CG, Doroodchi P, Parins KF, Kietzman HW, Everson JL, Ansen-Wilson LJ, Lipinski RJ. Definition of critical periods for hedgehog pathway antagonist-induced holoprosencephaly, cleft lip, and cleft palate. PLoS One. 2015 Mar 20;10(3):e0120517. doi: 10.1371/journal.pone.0120517. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009a;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. Unique organization of the frontonasal ectodermal zone in birds and mammals. Dev Biol. 2009b;325:200–210. doi: 10.1016/j.ydbio.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. Neural crest cells pattern the surface cephalic ectoderm during FEZ formation. Developmental dynamics : an official publication of the American Association of Anatomists. 2012;241:732–740. doi: 10.1002/dvdy.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamniczky HA, Hallgrimsson B. A comparison of covariance structure in wild and laboratory muroid crania. Evolution; international journal of organic evolution. 2009;63:1540–1556. doi: 10.1111/j.1558-5646.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP. Morphological Integration and Developmental Modularity. Annu Rev Ecol Syst. 2008;39:115–132. [Google Scholar]
- Krauss RS. Holoprosencephaly: new models, new insights. Expert Rev Mol Med. 2007 Sep 24;9(26):1–17. doi: 10.1017/S1462399407000440. Review. [DOI] [PubMed] [Google Scholar]
- Krzanowski WJ. Principles of Multivariate Analysis: A User’s Perspective. New York, NY: Oxford University Press; 1984. [Google Scholar]
- Kurosaka H, Iulianella A, Williams T, Trainor PA. Disrupting hedgehog and Wnt signaling interacations promotes cleft lip pathogenesis. J Clin Invest. 2014 Apr;124(4):1660–71. doi: 10.1172/JCI72688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Krovitz GE, McBratney-Owen B. Testing hypotheses about tinkering in the fossil record: the case of the human skull. Journal of experimental zoology Part B, Molecular and developmental evolution. 2004;302(3):284–301. doi: 10.1002/jez.b.21004. [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Song C, Sulik KK, Everson JL, Gipp JJ, Yan D, Bushman W, Rowland IJ. Cleft lip and palate results from Hedgehog signaling antagonism in the mouse: Phenotypic characterization and clinical implications. Birth Defects Res A Clin Mol Teratol. 2010 Apr;88(4):232–40. doi: 10.1002/bdra.20656. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Young NM, Hu D, Hallgrimsson B. Mechanisms that underlie co-variation of the brain and face. Genesis. 2011;49:177–189. doi: 10.1002/dvg.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney ML, MacNamara KJ. Heterochrony: The Evolution of Ontogeny. New York and London: Plenum Press; 1991. [Google Scholar]
- Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development. 2008;135:1223–1234. doi: 10.1242/dev.015933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming JE, Muenke M. Holoprosencephaly: from Homer to Hedgehog. Clin Genet. 1998 Mar;53(3):155–63. doi: 10.1111/j.1399-0004.1998.tb02666.x. 1998. Review. [DOI] [PubMed] [Google Scholar]
- Mitteroecker P. The Developmental Basis of Variational Modularity: Insights from Quantitative Genetics, Morphometrics, and Developmental Biology. Evolutionary Biology. 2009;36(4):377–385. [Google Scholar]
- Mitteroecker P, Bookstein F. The conceptual and statistical relationship between modularity and morphological integration. Syst Biol. 2007;56(5):818–836. doi: 10.1080/10635150701648029. [DOI] [PubMed] [Google Scholar]
- Parsons TE, Schmidt EJ, Boughner JC, Jamniczky HA, Marcucio RS, Hallgrimsson B. Epigenetic integration of the developing brain and face. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:2233–2244. doi: 10.1002/dvdy.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto CB, Hall BK. Toward an understanding of the epithelial requirement for osteogenesis in scleral mesenchyme of the embryonic chick. Journal of Experimental Zoology. 1991;259:92–108. doi: 10.1002/jez.1402590112. [DOI] [PubMed] [Google Scholar]
- Reid BS, Yang H, Melvin VS, Taketo MM, Williams T. Ectodermal Wnt/β-catenin signaling shapes the mouse face. Dev Biol. 2011 Jan 15;349(2):261–9. doi: 10.1016/j.ydbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G, Thieffry D. Modularity in development and evolution. Bioessays. 2000;22(11):1043–1045. doi: 10.1002/1521-1878(200011)22:11<1043::AID-BIES11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Seber G. Multivariate observations. Hoboken, NJ: John Wiley and Sons, Inc; 1984. [Google Scholar]
- Slack JM, Holland PW, Graham CF. The zootype and the phylotypic stage. Nature. 1993;361:490–492. doi: 10.1038/361490a0. [DOI] [PubMed] [Google Scholar]
- Thomason HA1, Dixon MJ, Dixon J. Facial clefting in Tp63 deficient mice results from altered Bmp4, Fgf8 and Shh signaling. Dev Biol. 2008 Sep 1;321(1):273–82. doi: 10.1016/j.ydbio.2008.06.030. 2008. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Altenberg L. Complex adaptations and the evolution of evolvability. Evolution. 1996;50:967–976. doi: 10.1111/j.1558-5646.1996.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Pavlicev M, Cheverud J. The Road to Modularity. Nat Genet. 2007;8:921–931. doi: 10.1038/nrg2267. [DOI] [PubMed] [Google Scholar]
- Xu Q, Jamniczky HE, Hu D, Green RM, Marcucio RS, Hallgrimsson B, Mio W. Correlations between the Morphology of Sonic Hedgehog Expression Domains and Embryonic Craniofacial Shape. Evolutionary Biology. doi: 10.1007/s11692-015-9321-z. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young NM, Hu D, Lainoff AJ, Smith FJ, Diaz R, Tucker AS, Trainor PA, Schneider RA, Hallgrímsson B, Marcucio RS. Embryonic bauplans and the developmental origins of facial diversity and constraint. Development. 2014 Mar;141(5):1059–63. doi: 10.1242/dev.099994. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


