Abstract
IMPORTANCE
For patients with limited prognosis, some medication risks may outweigh the benefits, particularly when benefits take years to accrue; statins are one example. Data are lacking regarding the risks and benefits of discontinuing statin therapy for patients with limited life expectancy.
OBJECTIVE
To evaluate the safety, clinical, and cost impact of discontinuing statin medications for patients in the palliative care setting.
DESIGN, SETTING, AND PARTICIPANTS
This was a multicenter, parallel-group, unblinded, pragmatic clinical trial. Eligibility included adults with an estimated life expectancy of between 1 month and 1 year, statin therapy for 3 months or more for primary or secondary prevention of cardiovascular disease, recent deterioration in functional status, and no recent active cardiovascular disease. Participants were randomized to either discontinue or continue statin therapy and were monitored monthly for up to 1 year. The study was conducted from June 3, 2011, to May 2, 2013. All analyses were performed using an intent-to-treat approach.
INTERVENTIONS
Statin therapy was withdrawn from eligible patients who were randomized to the discontinuation group. Patients in the continuation group continued to receive statins.
MAIN OUTCOMES AND MEASURES
Outcomes included death within 60 days (primary outcome), survival, cardiovascular events, performance status, quality of life (QOL), symptoms, number of nonstatin medications, and cost savings.
RESULTS
A total of 381 patients were enrolled; 189 of these were randomized to discontinue statins, and 192 were randomized to continue therapy. Mean (SD) age was 74.1 (11.6) years, 22.0% of the participants were cognitively impaired, and 48.8% had cancer. The proportion of participants in the discontinuation vs continuation groups who died within 60 days was not significantly different (23.8% vs 20.3%; 90% CI, −3.5% to 10.5%; P = .36) and did not meet the noninferiority end point. Total QOL was better for the group discontinuing statin therapy (mean McGill QOL score, 7.11 vs 6.85; P = .04). Few participants experienced cardiovascular events (13 in the discontinuation group vs 11 in the continuation group). Mean cost savings were $3.37 per day and $716 per patient.
CONCLUSIONS AND RELEVANCE
This pragmatic trial suggests that stopping statin medication therapy is safe and may be associated with benefits including improved QOL, use of fewer nonstatin medications, and a corresponding reduction in medication costs. Thoughtful patient-provider discussions regarding the uncertain benefit and potential decrement in QOL associated with statin continuation in this setting are warranted.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01415934
Safe and effective use of medicines includes medication selection and dosing for a targeted indication, monitoring for benefits and harms, and discontinuation when appropriate. Data from clinical trials guide the initiation of long-term medication therapy for primary or secondary prevention of cardiovascular disease but rarely define the timing, safety, or risks of discontinuing the agents. As a result, the number of medications often accumulates.1,2
This issue is particularly salient in the setting of advanced life-limiting illness, when patients face escalating numbers of medications prescribed for common comorbidities (eg, antihypertensives), disease-specific medications (eg, antineoplastics), and symptom palliation (eg, opioids).2,3 In the last year of life, the number of medicines increases by 50%.1 In addition, the effects of advanced disease may alter a patient’s metabolism of medications and increase the risk of adverse effects. Dysphagia and anorexia increase the burden of taking multiple pills.2
Many physicians advocate discontinuing unnecessary medicines in the setting of advanced life-limiting illness3 to reduce adverse effects, pill burden, and medication costs while potentially enhancing quality of life (QOL) and possibly survival.3–5 However, the choice of which medicines to discontinue, as well as timing and safety, is unclear.2,6,7
Statin therapy is commonly considered for discontinuation in the setting of advanced life-limiting illness.8 More than 25% of Medicare beneficiaries receive statin therapy.9 When this drug class is prescribed for primary prevention of cardiovascular disease, benefits accrue after 2 years.10,11 In the presence of cardiovascular disease (secondary prevention), benefits relate to both long-term lowering of lipid levels plus shorter-term effects on inflammation and endothelial function.12 The main adverse effects of statins are gastrointestinal symptoms (8%), myopathy and musculoskeletal pain (up to 7%), and rhabdomyolysis, which is rare (0.005%) but serious.13 Adverse effects are more problematic in older patients, especially those with metabolic disturbances, kidney or liver compromise, or polypharmacy.13–15 From a cost standpoint, value can be enhanced through thoughtful matching of treatments to patients who will benefit. Although an individual may accrue some financial benefit, the overall effect of discontinuing medicines on national health care spending is inherently a population-based and policy question.
Although there is compelling evidence for prescribing statins for primary or secondary prevention for people who are expected to live for many years, no evidence exists to guide decisions to discontinue statin therapy in patients with limited prognosis. We conducted this randomized trial to evaluate the safety and clinical impact of statin discontinuation in the palliative care setting. We hypothesized that there would be no significant difference in 60-day mortality (primary outcome), cardiovascular events, or performance status and that QOL, symptoms, number of medications, and satisfaction with care, as well as reduced cost, would be better among patients randomized to discontinue statin therapy.
Methods
Design
This study was a multicenter, parallel-group, unblinded, randomized, pragmatic clinical trial. Participants were randomized to either discontinue or continue statin therapy at the time of enrollment. The trial protocol is available in the Supplement.
Patients
Eligibility criteria were broad to maximize the generalizability of the findings. Eligible patients were English-speaking adults (aged ≥18 years) receiving a statin for 3 months or longer for primary or secondary prevention of cardiovascular disease. Eligible patients had a documented diagnosis of advanced, life-limiting illness determined by (1) at least 1 physician indicating he or she “would not be surprised if the patient died in the next year,”16–18 (2) life expectancy of more than 1 month, and (3) recent deterioration in functional status, with a reduction in the Australia-Modified Karnofsky Performance Status19 scale score to less than 80% in the previous 3 months. Study participants were either cognitively intact (Short Portable Mental Status Questionnaire20 score of ≤4 of 10) or represented by a legally authorized English-speaking person willing and able to provide proxy consent and study data. Exclusion criteria were treating physician’s opinion that the patient had active cardiovascular disease or sufficient risk of active cardiovascular disease to require ongoing therapy with statin medications, symptoms of myositis, liver function test (aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase) or creatine kinase levels of more than 2.5 times the upper limit of normal, or other contraindications to continuing statin therapy. The patient was also excluded if the patient or proxy was unwilling or unable to provide informed consent or if the treating physician was unwilling to have the patient enrolled. The study was conducted from June 3, 2011, to May 2, 2013.
Patients were enrolled from 15 Palliative Care Research Cooperative Group member sites21 after relevant institutional review board approval. The patients provided written informed consent and received no financial compensation. The full study protocol can be found in the trial protocol in the Supplement.
Randomization
Block randomization was used to allocate participants to study arms in a 1:1 ratio stratified by study site and cardiovascular disease history (yes or no). Block sizes of 2, 4, and 6 were randomly generated using SAS, version 9.2 (SAS Institute Inc), and data were maintained in a secure central server. Participants were randomized immediately after providing informed consent and completing baseline data collection. A secure web-site was used to communicate randomization allocation to study site personnel.
End Points
The primary end point of the study was the proportion of deaths within 60 days of trial enrollment. In the original protocol, the primary end point was survival; the sample size estimate of 1200 participants (600 per group) was based on a projected median overall survival of 13 weeks and a 2-week difference in survival. The study was designed with 2 interim analyses (total of 3 analyses, including the end of the study) using an O’Brien-Fleming design22 with analysis intervals spaced by equal information time. Partway through the trial, the pooled median survival was approximately 9 months (approximately 3 times the original survival projections); a resizing calculation estimated a new sample size requirement of more than 30 000. Upon recommendation from the study data and safety monitoring board, the primary end point was changed to the proportion of deaths within 60 days of trial enrollment. The consequent revised sample size target was 360 participants; plans for further interim analyses were dropped. Although we focused on 60-day mortality as the primary outcome, we captured longer-term mortality and other important clinical outcomes that provide critical contextual information once the effect on mortality is understood.
Secondary end points addressed 2 safety concerns: survival and time to first cardiovascular-related event, defined as a new cardiovascular event or invasive cardiovascular procedure with hospital or emergency department admission. Additional secondary end points addressed patient-centered outcomes important in the setting of advanced life-limiting illness: performance status, QOL, symptoms, number of nonstatin medications, statin-related adverse effects, and satisfaction with health care (assessed by likelihood to recommend current health care). With data and safety monitoring board approval, we enrolled more participants than the revised sample size target to increase information about secondary end points.
Study Procedures and Assessments
Baseline assessment, which was conducted in person by a trained research assistant, included demographics, primary diagnosis, comorbid illnesses, Charlson Comorbidity Index score,23 the results of the most recent laboratory studies, statin medication history, cognition (as measured by the Short Portable Mental Status Questionnaire20), and insurance status. Survival, performance status, and health resource utilization data were collected weekly during the first month and then monthly until death or 1 year. Patient-reported outcomes (eg, QOL, symptoms, and satisfaction) were collected in person or by telephone at weeks 2, 4, 8, 12, 16, 20, and 24.
Quality of life was measured with the McGill Quality of Life Questionnaire, reflected by a single-item overall QOL score and selected subscales (physical symptom, psychological symptom, existential well-being, and support).24,25 A total score was computed as the mean of the 4 subscales. If at least half of the items in a subscale were answered, missing values were imputed using the mean of the completed items. Scores ranged from 0 to 10, with higher scores indicating better QOL.
Symptoms were measured using the Edmonton Symptom Assessment System scale.26 The 9 standard items on the scale (pain, fatigue, nausea, depression, anxiousness, drowsiness, appetite, well-being, and breathing) were supplemented with 4 additional items specific to statin use (muscle-related pain, weakness, headache, and fever). Scores were summed from the 9 standard items, the 4 supplemental items, and for all 13 items. The same imputation rule used for determination of QOL was applied to missing responses. Performance status was measured using the Australia-Modified Karnofsky Performance Status scale,19 with scores ranging from 0 (death) to 100 (no symptoms, no evidence of disease).
We documented the number of nonstatin medications that were (1) regularly scheduled, (2) administered as needed on at least 50% of the days in the prior week, and (3) administered as needed on fewer than 50% of days in the prior week. All 3 measures were combined into a variable quantifying the total number of nonstatin medications. Satisfaction with care was quantified through a question that asked about the likelihood of recommending the current health care to others and used a 5-point Likert scale (1, very unlikely to 5, very likely).
Prespecified adverse events monitored at each assessment included hospital admissions, emergency department visits, new cardiovascular events, invasive procedures for cardiac events, venous thromboembolism, and pneumonia. Ad hoc adverse events were documented and monitored by site investigators.
Participants remained in the study until death, 1 year after enrollment, or study closure. If a participant wished to withdraw, he or she was given the option for passive data collection via medical record review to document survival and health services utilization. If proxy response was used, only the following objective data were collected: participant survival status, functional status, use of hospice or palliative care, likelihood to recommend the care received, prespecified adverse events, adherence to randomization assignment, and, if applicable, reason for study withdrawal.
Cost Savings
We estimated the patient-specific monthly cost of the baseline statin therapy using a national average retail price (February 2012) compiled by Consumer Reports.27 We measured neither out-of-pocket cost nor the amount paid by third-party insurance; an average retail price approximates a societal cost. Two authors (T.W.L., S.Y.Z.) adjudicated ambiguous information. We estimated the cost savings resulting from statin discontinuation by first converting monthly to daily costs and then tracking the avoided costs from the time each patient was randomized until death or censorship.
Statistical Analysis
End points were summarized using routine descriptive statistics. All analyses were performed using an intent-to-treat approach. Safety analyses included the primary end point (death within 60 days) and time-to-event analyses for secondary end points (time to death and time to first cardiovascular-related event); these end points were tested with a noninferiority hypothesis, with each end point using a 1-sided α = .05–level test. For these 3 safety analyses, the established differences to exclude in the noninferiority hypotheses were 5%, 3 weeks, and 2 weeks, respectively, as determined a priori to be clinically meaningful by the study investigators.
For the primary end point, discontinuing statin therapy was considered to be noninferior to continuing therapy if the 90% CI for the difference in proportion who died (θ = pdiscontinue − pcontinue) ruled out a 0.05 increase in the proportion of deaths for patients who discontinued statin therapy compared with those who continued the therapy (ie, the upper limit of the 90% CI for the difference in proportions is <0.05). A nonparametric log-rank test was used to compare time-to-event differences between the 2 study groups.
Patient-centered secondary end points (QOL, symptoms, performance status, number of nonstatin medications, and likelihood to recommend the care being given) were measured longitudinally at multiple time points. For each analysis, a growth-curve model was fit to the data using a piecewise-linear function with knots at 4, 8, and 12 weeks. Each outcome was summarized using an area-under-the-curve summary calculated from baseline through week 20. This 20-week cut point was chosen to maximize use of data while accounting for the fact that the amount of data diminished as the study progressed, thereby increasing variability and uncertainty of area-under-the-curve estimates when data beyond 20 weeks were included. The area under the curve was rescaled so that scores could be interpreted as the mean across 20 weeks. Group differences were assessed using a 2-sided α = .05–level test.
Repeated-measures outcomes were analyzed using a mixed-effects model performed with maximum-likelihood estimation for incomplete repeated measures. This approach allowed all available data to be used in the estimation of model variables and assumed that missing data were missing at random. Dropout rates and reasons for dropout were similar between study arms.
Results
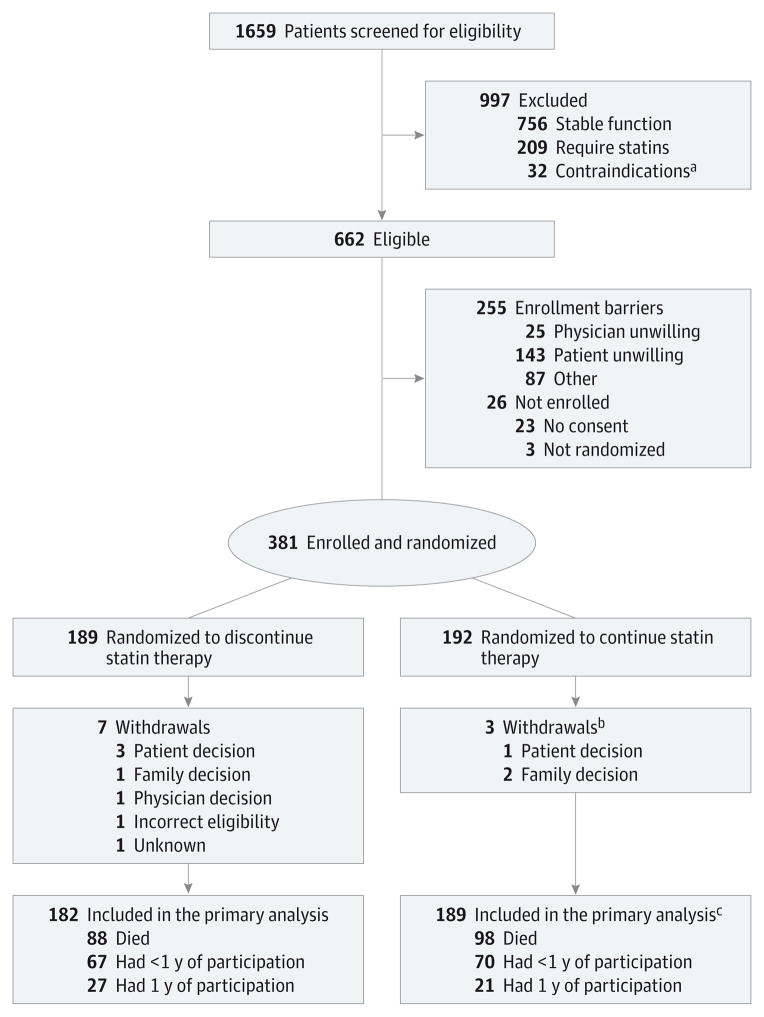
Of 381 patients enrolled, 189 were randomized to discontinue statin therapy and 192 to continue therapy (Figure 1). Median follow-up time was 18 weeks (quartile [Q]1 = 8, Q3 = 36) for all participants. Follow-up time for participants who died during the study was a median of 10 (Q1 = 5, Q3 = 23) weeks.
Figure 1. CONSORT Flow Diagram.
A total of 189 patients were randomized to discontinue statin therapy and 192 were randomized to continue therapy.
aContraindications to continuing or discontinuing statin therapy.
bDistribution of withdrawals between study arms; P= .85.
cDistribution of outcomes between study arms; P= .58.
Participants were generally older, white, and receiving Medicare and had declining performance status (Table 1). Approximately half of the participants (48.8%) had cancer as their primary diagnosis, 58.0% had cardiovascular disease, and 69.0% had received statins for more than 5 years; 36.0% of the patients were enrolled in hospice at study initiation. The intervention groups were similar at baseline except for cognitive impairment, with a larger proportion of people who were cognitively impaired randomized to discontinue statin therapy (27.0% vs 17.2%; P = .02).
Table 1.
Participant Characteristics
| Variable | No. (%) | P Value | ||
|---|---|---|---|---|
| Discontinued Statin (n = 189) | Continued Statin (n = 192) | Total (N = 381) | ||
| Age, mean (SD), y | 74.8 (11.7) | 73.5 (11.5) | 74.1 (11.6) | .29 |
| Sex | ||||
| Male | 98 (51.9) | 112 (58.3) | 210 (55.1) | .20 |
| Female | 91 (48.1) | 80 (41.7) | 171 (44.9) | |
| Race | ||||
| White | 153 (81.0) | 162 (84.4) | 315 (82.7) | .30 |
| Black | 32 (16.9) | 22 (11.5) | 54 (14.2) | |
| Other | 3 (1.6) | 7 (3.6) | 10 (2.6) | |
| Multiple | 1 (0.5) | 1 (0.5) | 2 (0.5) | |
| Ethnicity | ||||
| Hispanic | 6 (3.2) | 10 (5.2) | 16 (4.2) | .32 |
| Non-Hispanic | 182 (96.3) | 181 (94.3) | 363 (95.3) | |
| Unknown | 1 (0.5) | 1 (0.5) | 2 (0.5) | |
| Educational level | ||||
| <High school | 27 (14.3) | 24 (12.5) | 51 (13.4) | .63 |
| High school graduate | 100 (52.9) | 95 (49.5) | 195 (51.2) | |
| College graduate | 61 (32.3) | 70 (36.5) | 131 (34.4) | |
| Unknown | 1 (0.5) | 3 (1.6) | 4 (1.0) | |
| Insurance | ||||
| Medicare | 140 (74.1) | 140 (72.9) | 280 (73.5) | .34 |
| Medicaid | 18 (9.5) | 16 (8.3) | 34 (8.9) | |
| Private | 23 (12.2) | 20 (1.4) | 43 (11.3) | |
| Other | 8 (4.2) | 13 (6.8) | 21 (5.5) | |
| Uninsured | 0 | 3 (1.6) | 3 (0.8) | |
| History of cardiovascular disease | ||||
| Yes | 111 (58.7) | 110 (57.3) | 221 (58.0) | .78 |
| No | 78 (41.3) | 82 (42.7) | 160 (42.0) | |
| Statin use, y | ||||
| <1 | 4 (2.1) | 2 (1.0) | 6 (1.6) | .69 |
| 1–5 | 50 (26.5) | 51 (26.6) | 101 (26.5) | |
| >5 | 129 (68.3) | 134 (69.8) | 263 (69.0) | |
| Unknown | 6 (3.2) | 5 (2.6) | 11 (2.9) | |
| Primary diagnosis | ||||
| Malignant tumor | 84 (44.4) | 102 (53.1) | 186 (48.8) | .09 |
| Other | 105 (55.6) | 90 (46.9) | 195 (51.2) | |
| Charlson Comorbidity Index score, mean (SD) | 4.8 (2.9) | 4.9 (2.7) | 4.9 (2.8) | .67 |
| AKPS score, mean (SD) | 52.4 (13.2) | 54.5 (12.8) | 53.5 (13.0) | .13 |
| Cognitively impaired | ||||
| Yes | 51 (27.0) | 33 (17.2) | 84 (22.0) | .02 |
| No | 138 (73.0) | 159 (82.8) | 297 (78.0) | |
| Enrolled in hospice | ||||
| Yes | 63 (33.3) | 74 (38.5) | 137 (36.0) | .27 |
| No | 124 (65.6) | 115 (59.9) | 239 (62.7) | |
| Unknown | 2 (1.1) | 3 (1.6) | 5 (1.3) | |
| Nonstatin medications, mean (SD) | 11.6 (5.1) | 11.5 (4.9) | 11.6 (5.0) | .84 |
Abbreviation: AKPS, Australia-Modified Karnofsky Performance Status.
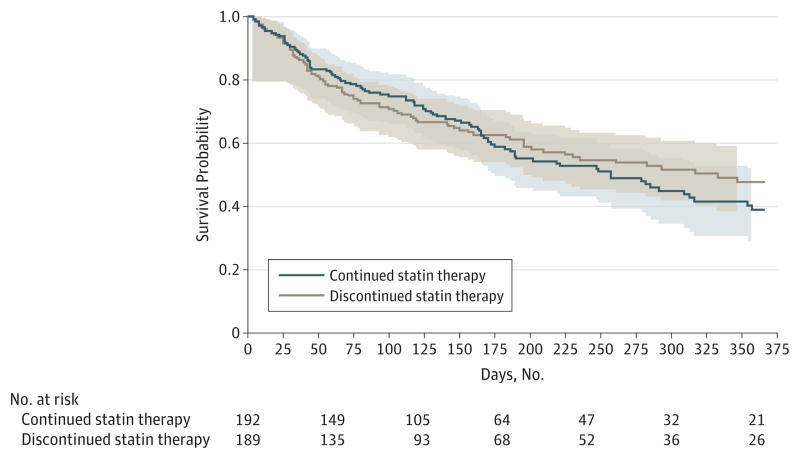
Median and mean survival for the entire study population was 219 and 213 days (31 and 30 weeks), respectively. The proportion of participants who died within 60 days was not significantly different between groups (discontinuation vs continuation, 45 [23.8%] vs 39 [20.3%]; 90% CI, −3.5% to 10.5%; P = .36). Noninferiority was not achieved because the upper confidence limit for the difference in proportion of participants who died within 60 days (10.5%) exceeded the noninferiority margin of 5%. Survival was similar between the groups, with a median time to death for the discontinuation vs continuation groups of 229 days (90% CI, 186–332) vs 190 days (90% CI, 170–257), respectively (P = .60) (Figure 2). There was no significant difference in time to first cardiovascular-related event (P = .64); only 24 of the participants (6.3%) experienced a cardiovascular-related event (discontinuation, 13; continuation, 11).
Figure 2. Product-Limit Survival Estimates.
The 90% confidence bands are indicated. Light gray shading indicates the 90% confidence bands for the continuation arm of the study; light brown shading, the 90% confidence bands for the discontinuation arm of the study.
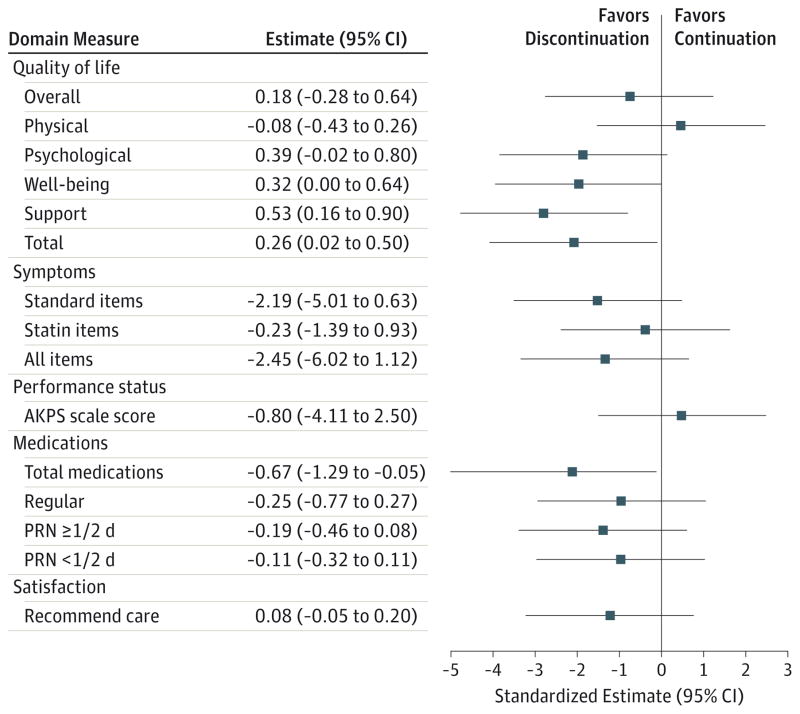
Total McGill QOL was significantly higher among the group discontinuing statin therapy (mean area under the curve, 7.11 vs 6.85; P = .04) (Table 2, Table 3, and Figure 3 for all QOL results). Small differences in QOL subscales were observed, with significant differences in the support (P = .005) and well-being (P = .05) domains but not in the psychological (P = .06) and physical (P = .64) domains. The single question measuring overall QOL demonstrated no significant difference (6.53 vs 6.35; P = .44).
Table 2.
Patient-Reported Outcomesa
| Variable | Baseline | Mean AUC
|
AUC Differenceb
|
||
|---|---|---|---|---|---|
| Discontinued Statin (n = 189) | Continued Statin (n = 192) | Estimated (95% CI) | P Value | ||
| Quality of life | |||||
|
| |||||
| Overall | 6.12 | 6.53 | 6.35 | 0.18 (−0.28 to 0.64) | .44 |
|
| |||||
| Physical | 5.19 | 5.43 | 5.51 | −0.08 (−0.43 to 0.26) | .64 |
|
| |||||
| Psychological | 7.21 | 7.38 | 6.99 | 0.39 (−0.02 to 0.80) | .06 |
|
| |||||
| Well-being | 7.30 | 7.37 | 7.05 | 0.32 (0.00 to 0.64) | .05 |
|
| |||||
| Support | 8.31 | 8.38 | 7.86 | 0.53 (0.16 to 0.90) | .005 |
|
| |||||
| Total | 6.98 | 7.11 | 6.85 | 0.26 (0.02 to 0.50) | .04 |
|
| |||||
| Symptoms | |||||
|
| |||||
| Standard | 27.2 | 25.2 | 27.4 | −2.2 (−5.0 to 0.6) | .13 |
|
| |||||
| Statin items | 7.1 | 7.0 | 7.2 | −0.2 (−1.4 to 0.9) | .71 |
|
| |||||
| All items | 34.6 | 32.4 | 34.8 | −2.5 (−6.0 to 1.1) | .18 |
|
| |||||
| AKPS score | 54.3 | 47.7 | 48.5 | −0.8 (−4.1 to 2.5) | .63 |
|
| |||||
| Nonstatin medications | |||||
|
| |||||
| Total | 10.9 | 10.1 | 10.8 | −0.7 (−1.3 to −0.1) | .03 |
|
| |||||
| Regular | 8.9 | 8.4 | 8.7 | −0.3 (−0.8 to 0.3) | .34 |
|
| |||||
| PRN, days | |||||
|
| |||||
| <½c | 0.9 | 0.9 | 1.1 | −0.2 (−0.5 to 0.1) | .16 |
|
| |||||
| ≥½d | 1.0 | 0.9 | 1.0 | −0.1 (−0.3 to 0.1) | .33 |
|
| |||||
| Satisfaction with care (willing to recommend) | 4.55 | 4.63 | 4.55 | 0.08 (−0.05 to 0.20) | .22 |
Abbreviations: AKPS, Australia-Modified Karnofsky Performance Status; AUC, area under the curve; PRN, administered as needed.
Patient-reported outcome results at baseline (week 0), group estimates at week 20, and AUC mean during 20 weeks modeled using all study data.
Discontinued - continued.
Number of PRN nonstatin medications that were administered on more than half of the days during the study.
Number of PRN nonstatin medications that were administered on less than half of the days during the study.
Table 3.
Cost Savings Associated With Statin Therapy Discontinuationa
| Variable | Cost Savings, $ | |
|---|---|---|
| Prescribed | Generic Formulation Only | |
| Mean survival, d | 212.6 | 212.6 |
| Mean saved per patient | ||
| Days | 3.37 | 2.96 |
| During mean lifespan in this trial | 716.46 | 629.30 |
| Projected annual US savings | ||
| 2014 Population, million | 603 | 529 |
| 2040 Population, billion | 1 | 879 |
Cost calculated using 2012 US dollars.
Figure 3. Summary of Patient-Reported Outcomes.
In this visual summary of Table 3, the estimates and 95% CIs are presented using standardized units so that the CI widths are comparable; results favoring discontinuation of statin therapy are aligned on the left side of zero. The numeric estimates and 95% CIs are presented in the units of the actual analyses, thereby aligning with Table 3. AKPS indicates Australia-Modified Karnofsky Performance Status; PRN, administered as needed.
Discontinuing statin therapy had no significant effect on physical symptoms or performance status (Table 2). Participants whose therapy was discontinued trended toward lower summary 9-item Edmonton Symptom Assessment System scores (25.2 vs 27.4; P = .13). There were no significant differences in statin-specific symptoms (muscle-related pain, weakness, headache, and fever) (7.0 vs 7.2; P = .71). Longitudinal performance status assessment (Australia-Modified Karnofsky Performance Status score) also was not significantly different between the groups (47.7 vs 48.5; P = .63).
Although participants in both study arms received many medications, the total number of nonstatin agents was significantly lower in the group discontinuing statin therapy by 0.7 medications (10.1 vs 10.8; P = .03). Cost savings (Table 3) attributable to statin therapy discontinuation were $3.37 per day (95% CI, 2.83–3.91) for a mean savings of $716.46 for participants with a mean follow-up time of 212.6 days. If all patients had been receiving a generic statin formulation at randomization (75% were), daily savings would have been $2.96 per day ($629.30 per patient) at the mean follow-up time, representing potential savings in the United States of $603 million in 2014.
Most study participants had high satisfaction with their current health care, with 5 as the highest possible score (discontinuation, 4.63; continuation, 4.55; P = .22). Adverse events were rare, with only 33 experienced by 19 of the participants (5.0%). No serious adverse events were determined to be study related.
Discussion
In a study population with a median survival of approximately 7 months and primary diseases evenly divided between cancer and noncancer diagnoses, it appears that stopping statin therapy is safe and potentially associated with benefit, including improved QOL and fewer other nonstatin medications combined with a corresponding reduction in medication costs.
Scientific and Clinical Context of the Results
More than 80% of Americans are expected to die of chronic illnesses,9 primarily cardiovascular disease, cancer, dementia, and chronic lung disease. Clinical trial28 evidence supports the use of statins in patients with hyperlipidemia and ischemic heart disease to reduce the risk of cardiovascular events and mortality, as well as to reduce the risk of cardiovascular events in patients with multiple cardiac risk factors. Beneficial outcomes are generally evident after at least 2 years of treatment.29 Given their positive effect on morbidity and death, statins are among the most prescribed medications, and this number is expanding30; 40% of statins are prescribed for primary prevention of cardiovascular disease, and therapy is frequently continued until the end of life.7 The risks and burdens vs benefits of statins for patients with a limited prognosis has been a clinical uncertainty.31 Based on our study findings, it is reasonable for providers to discuss with patients and their caregivers whether to discontinue statins prescribed for primary or secondary prevention of cardiovascular disease when advanced illness is consistent with a high risk for death within the next 6 to 12 months.
The inability to discern a difference in survival between patients who continue and discontinue statin therapy may be related to any of several mechanisms. The effect of statins on reducing plaque growth may be more important early in the disease course.32 For patients with advanced illness, underlying organ failure (kidney, liver, and heart) potentially offsets the beneficial effects of statins even when reductions in low-density lipoprotein levels are achieved.28 Indeed, the finding of a decreased survival benefit in sicker patients and a greater benefit in healthier patients has been demonstrated in many clinical trials, especially among those with heart failure33 or those undergoing dialysis.34 Altered metabolism of medications may also partially explain the lack of benefit with statins.35
Recent trials confirm increased average creatine kinase levels and muscle symptoms36 as well as reduced strength and exercise tolerance37,38 in patients receiving statins, which can worsen in the setting of advancing life-limiting illness. Patients randomized to the discontinuation arm showed a significant reduction in the mean number of nonstatin medications; it is possible that discontinuing statin therapy reduces the number of adverse effects and decreases the need for medications taken to treat those effects.1 Certainly, simplification of medicine regimens has important benefits in terms of health, patient and caregiver burden, and cost.
Previous observational studies have investigated statin discontinuation with variable results. In the systematic review by Gomez Sandoval et al,39 most observational studies did not demonstrate an increase in mortality with statin therapy discontinuation; studies that did tended to be in younger populations who appeared less sick than the population we studied.
We included participants in the present study with an advanced life-limiting illness and declining functional status; these were patients who would not routinely be expected to live longer than 1 year. We chose this population because, based on several large clinical trials, benefits from statins are seen at the earliest after 2 years of therapy.29 Given well-documented evidence40 of benefit over time for primary and secondary prevention of cardiovascular disease, our findings should not be generalized beyond the population with life-limiting illness and limited prognosis that we studied.
Application: The Case for Patient-Centered Decision Making
Given the uncertain benefit and possible harm of continuing statin therapy among people with life-limiting illness and functional decline, patient-centered decision making regarding therapy discontinuation is warranted. Patient-centered decision making entails informing patients or their proxies about treatment options, including the trade-offs between risks and benefits, and incorporating patient preferences when implementing a decision.41,42 This approach is appropriate when there is no clearly superior choice and patients’ preferences are a key element of making the best choice.43–45 For people with advanced illness, the present study provides critical information to inform discussions between physicians and patients: “How can we make a decision together about the management of your statin medication based on your personal wishes, circumstances, and the evidence?”
For patients with shorter life expectancy, greater concern about pill burden, and more comfort-oriented goals of care, physicians may endorse discontinuing statins as a means to reduce the number of medications without apparent harmful effects on survival or QOL. With symptoms and QOL as the concern, people whose statin therapy was discontinued had trends toward improvement in these outcomes (Table 2). For patients who do not want to discontinue statin therapy, the data suggest that continuing the medicine is not likely to be harmful.
There is an increasing evidence base that discontinuation of some therapies may be beneficial for selected patient populations. If the results we report—improved QOL, no significant differences in mortality, and modest cost savings—had been produced by a randomized clinical trial of a new drug in patients with advanced life-limiting illness, the trial would be heralded as a breakthrough and there would be discussion of how to speed access to this new drug. The same energy needs to be applied to determining when it is appropriate for physicians to discuss discontinuing statin therapy with their patients.
Limitations
Our trial has several important limitations. First, the primary endpoint and target sample size were modified midway through the study in collaboration with the data and safety monitoring board. Despite these revisions, noninferiority for the primary end point (the proportion of participants who died within 60 days) was not achieved. Second, enrolling more patients would have increased statistical power for the assessment of the important secondary end points; nonetheless, secondary end points trended together with a general pattern in favor of discontinuing statins. Third, this study was a pragmatic trial without blinding. Study participants and their physicians knew whether statin therapy was being continued or discontinued. In theory, the absence of blinding could bias toward identification of more adverse consequences of discontinuing therapy, but this bias was not noted. Because the primary treating physician also had to be willing for the patient to be randomized, this factor may have biased the findings toward those in whom it may have been safer to discontinue statins. Fourth, patients who enrolled were those willing to be randomized to statin therapy discontinuation. Not all patients with limited prognosis would be willing to consider discontinuing therapy. Fifth, application of the trial results requires prognostication, which is difficult, as evidenced by the difference between the anticipated survival at the outset of the study and the observed survival. Nonetheless, the application of routine criteria helped to define a population of seriously ill individuals with a median survival of 7 months. Sixth, we do not know how much a patient paid for a prescription vs what their insurance company paid or what proportion of patients nationwide who have a limited prognosis and are taking a statin could clinically discontinue their statin therapy. Finally, the participants discontinuing statin therapy were more likely to be cognitively impaired, which most likely dampened positive findings in favor of discontinuing the medication by biasing the study toward more deaths and fewer available patient-reported data.
Conclusions
To the extent possible, evidence should inform decisions to initiate, continue, and discontinue medication therapy. This study provides evidence that suggests that survival is not affected when statins prescribed for primary or secondary prevention of cardiovascular disease are discontinued in this population. Although the cost savings identified were modest, the data suggest that statin therapy discontinuation in selected patients may improve QOL at reduced aggregate health care cost. The strengths of this study are its pragmatic design and conduct in multiple clinical settings with recruitment of a population that is representative of the broad range of life-limiting diagnoses encountered in clinical practice. These aspects of the study enhance the generalizability and applicability of its findings to real-world clinical practice. Given the value and symbolism that patients may ascribe to preventive chronic medications and the importance of prognosis in timing this decision, the choice to continue or stop therapy with statin medications merits patient-centered decision making between the physician and the patient. Additional research exploring the use of other medications (eg, anticoagulants, antihypertensives, or oral hypoglycemics) in populations with limited life expectancies is needed.
Acknowledgments
Funding/Support: This study received funding from the National Institute of Nursing Research (grants UC4-NR012584 and U24-NR014637). Furthermore, this work included the support of resources and facilities within the Veterans Affairs Health Care System (eg, Phoenix, Arizona, and Birmingham, Alabama).
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The National Institute of Nursing Research had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The contents of this article do not represent the views of the Department of Veterans Affairs or the US government.
Additional Contributions: The Palliative Care Research Cooperative Group is indebted to the patients who participated in this research and their families as well as to the clinical research coordinators who were important to all aspects of conducting the study. The following senior clinical and research leaders at their institutions assisted in conducting this project: Elizabeth Bayliss, MD, MSPH (Kaiser Permanente, Denver), Doug Conner, PhD (Kaiser Permanente, Denver), Stephen Connor, PhD, and J. Cameron Muir, MD (Capital Caring), Linda Lloyd, DrPH (San Diego, California), R. Sean Morrison, MD (Icahn School of Medicine at Mount Sinai), and Anna Roshal, MD (Washington University–St. Louis). There was no financial compensation.
Author Contributions: Drs Kutner and Abernethy had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kutner, Taylor, Ritchie, Bull, Fairclough, Hanson, LeBlanc, Aziz, Currow, Ferrell, Cleary, Dev, Pantilat, Portenoy, Sloan, Von Gunten, Abernethy.
Acquisition, analysis, or interpretation of data: Kutner, Blatchford, Taylor, Ritchie, Fairclough, Hanson, LeBlanc, Samsa, Wolf, Aziz, Currow, Wagner-Johnston, Zafar, Cleary, Goode, Kamal, Kassner, Kvale, McCallum, Ogunseitan, Pantilat, Portenoy, Prince-Paul, Sloan, Swetz.
Drafting of the manuscript: Kutner, Blatchford, Taylor, Ritchie, Fairclough, LeBlanc, Wolf, Ferrell, Dev, Kamal, Kassner, Portenoy, Abernethy.
Critical revision of the manuscript for important intellectual content: Kutner, Blatchford, Taylor, Ritchie, Bull, Fairclough, Hanson, LeBlanc, Samsa, Aziz, Currow, Wagner-Johnston, Zafar, Cleary, Dev, Goode, Kamal, Kvale, McCallum, Ogunseitan, Pantilat, Portenoy, Prince-Paul, Sloan, Swetz, Von Gunten, Abernethy.
Statistical analysis: Blatchford, Fairclough, Samsa, Wolf, Kassner, Sloan.
Obtained funding: Kutner, Abernethy.
Administrative, technical, or material support: Kutner, Taylor, Ritchie, Bull, Hanson, LeBlanc, Aziz, Currow, Goode, McCallum, Ogunseitan, Pantilat, Portenoy, Abernethy.
Study supervision: Kutner, Taylor, Aziz, Dev, Goode, Kvale, McCallum, Ogunseitan, Pantilat, Swetz, Abernethy.
References
- 1.Currow DC, Stevenson JP, Abernethy AP, Plummer J, Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc. 2007;55(4):590–595. doi: 10.1111/j.1532-5415.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ. 2004;329(7471):909–912. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 4.Bain KT, Holmes HM, Beers MH, Maio V, Handler SM, Pauker SG. Discontinuing medications: a novel approach for revising the prescribing stage of the medication-use process. J Am Geriatr Soc. 2008;56(10):1946–1952. doi: 10.1111/j.1532-5415.2008.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vollrath AM, Sinclair C, Hallenbeck J. Discontinuing cardiovascular medications at the end of life: lipid-lowering agents. J Palliat Med. 2005;8(4):876–881. doi: 10.1089/jpm.2005.8.876. [DOI] [PubMed] [Google Scholar]
- 6.Holmes HM, Min LC, Yee M, et al. Rationalizing prescribing for older patients with multimorbidity: considering time to benefit. Drugs Aging. 2013;30(9):655–666. doi: 10.1007/s40266-013-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell BJ, Rowett D, Abernethy AP, Currow DC. Prescribing for comorbid disease in a palliative population: focus on the use of lipid-lowering medications. Intern Med J. 2014;44(2):177–184. doi: 10.1111/imj.12340. [DOI] [PubMed] [Google Scholar]
- 8.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 9.Miller GE, Stagnitti MN. Trends in Statin Use in the Civilian Noninstitutionalized Medicare Population, 1997 and 2002. Washington, DC: Agency for Healthcare Research and Quality; Sep, 2005. [Google Scholar]
- 10.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 11.Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166(21):2307–2313. doi: 10.1001/archinte.166.21.2307. [DOI] [PubMed] [Google Scholar]
- 12.Spencer FA, Allegrone J, Goldberg RJ, et al. GRACE Investigators. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Ann Intern Med. 2004;140(11):857–866. doi: 10.7326/0003-4819-140-11-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 14.Mansi I, Frei CR, Pugh MJ, Makris U, Mortensen EM. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173(14):1–10. doi: 10.1001/jamainternmed.2013.6184. [DOI] [PubMed] [Google Scholar]
- 15.Silveira MJ, Kazanis AS, Shevrin MP. Statins in the last six months of life: a recognizable, life-limiting condition does not decrease their use. J Palliat Med. 2008;11(5):685–693. doi: 10.1089/jpm.2007.0215. [DOI] [PubMed] [Google Scholar]
- 16.Gishen F, Eades J, Tookman A. Utility of the “surprise question” in a day therapy palliative care practice: should specialist palliative care be focusing on total symptom burden and complexity rather than prognostication? BMJ Support Palliat Care. 2014;4:117. [Google Scholar]
- 17.Moroni M, Zocchi D, Bolognesi D, et al. SUQ-P Group. The “surprise question” question in advanced cancer patients: a prospective study among general practitioners. Palliat Med. 2014;28(7):959–964. doi: 10.1177/0269216314526273. [DOI] [PubMed] [Google Scholar]
- 18.Murray S, Boyd K. Using the “surprise question” can identify people with advanced heart failure and COPD who would benefit from a palliative care approach. Palliat Med. 2011;25(4):382. doi: 10.1177/0269216311401949. [DOI] [PubMed] [Google Scholar]
- 19.Abernethy AP, Shelby-James T, Fazekas BS, Woods D, Currow DC. The Australia-Modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481] BMC Palliat Care. 2005;4:7. doi: 10.1186/1472-684X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 21.Abernethy AP, Aziz NM, Basch E, et al. A strategy to advance the evidence base in palliative medicine: formation of a palliative care research cooperative group. J Palliat Med. 2010;13(12):1407–1413. doi: 10.1089/jpm.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 23.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson Comorbidity Index. Methods Inf Med. 1993;32(5):382–387. [PubMed] [Google Scholar]
- 24.Cohen SR, Mount BM, Bruera E, Provost M, Rowe J, Tong K. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med. 1997;11(1):3–20. doi: 10.1177/026921639701100102. [DOI] [PubMed] [Google Scholar]
- 25.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease: a preliminary study of validity and acceptability. Palliat Med. 1995;9(3):207–219. doi: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- 26.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 27.Consumers Union of the United States. [Accessed December 1, 2014];Evaluating statin drugs to treat high cholesterol and heart disease: comparing effectiveness, safety, and price. http://consumerhealthchoices.org/wp-content/uploads/2012/08/BBD-Statins-Full.pdf. Published April 2012.
- 28.Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Bulbulia R, Bowman L, Wallendszus K, et al. Heart Protection Study Collaborative Group. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378(9808):2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 31.Ko D, Kutner J, Blatchford P, Abernethy A. Management of medications for co-morbidities: a survey of current practice in hospice and palliative care using statins as a test case. Paper presented at: National Institute of Nursing Research State of the Science Conference; August 11, 2011; Bethesda, MD.. [Google Scholar]
- 32.Ridker PM, Wilson PW. A trial-based approach to statin guidelines. JAMA. 2013;310(11):1123–1124. doi: 10.1001/jama.2013.276529. [DOI] [PubMed] [Google Scholar]
- 33.Kjekshus J, Apetrei E, Barrios V, et al. CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 34.Wanner C, Krane V, März W, et al. German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 35.Thompson PD, Clarkson PM, Rosenson RS National Lipid Association Statin Safety Task Force Muscle Safety Expert Panel. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97(8A):69C–76C. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–1182. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan GM, Thompson PD. The effects of statins on skeletal muscle strength and exercise performance. Curr Opin Lipidol. 2010;21(4):324–328. doi: 10.1097/MOL.0b013e32833c1edf. [DOI] [PubMed] [Google Scholar]
- 39.Gomez Sandoval YH, Braganza MV, Daskalopoulou SS. Statin discontinuation in high-risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17(33):3669–3689. doi: 10.2174/138161211798220891. [DOI] [PubMed] [Google Scholar]
- 40.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 42.Katz SJ, Hawley S. The value of sharing treatment decision making with patients: expecting too much? JAMA. 2013;310(15):1559–1560. doi: 10.1001/jama.2013.278944. [DOI] [PubMed] [Google Scholar]
- 43.Bakitas M, Kryworuchko J, Matlock DD, Volandes AE. Palliative medicine and decision science: the critical need for a shared agenda to foster informed patient choice in serious illness. J Palliat Med. 2011;14(10):1109–1116. doi: 10.1089/jpm.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman RM, McNaughton-Collins M. The superiority of patient engagement and shared decision-making in noninferiority trials. J Gen Intern Med. 2014;29(1):16–17. doi: 10.1007/s11606-013-2593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(1):6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]