Abstract
As the leading cause of cancer death worldwide, lung cancer continues to impose a major burden on healthcare systems and cause significant challenges for clinicians and patients. Most patients present with advanced disease at the time of diagnosis and have a poor prognosis, with the vast majority surviving less than 5 years. Although new therapies have been introduced in recent years that target molecular disease drivers present in a subset of patients, there is a significant need for treatments able to improve response and extend survival while minimizing effects on quality of life. Recent evidence of clinical efficacy for immunotherapeutic approaches for lung cancer suggests that they will become the next major therapeutic advance for this disease. Non–small cell lung cancer (NSCLC), which accounts for around 85% of lung cancer cases, has historically been considered a nonimmunogenic disease; however, as with several other malignancies, recent data show that much of this lack of immune responsiveness is functional rather than structural (ie, possible to overcome therapeutically). This review explores the key elements of the immune system involved in NSCLC and briefly examines immunotherapeutic strategies in development to shift the balance of immune activity away from a tumor-induced immune-suppressive state toward an active antitumor immune response.
Keywords: Non–small cell lung cancer, Immune system, Immunotherapy, Checkpoint inhibitors, Cancer vaccines
INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide, claiming an estimated 1.59 million lives in 2012.1 Non–small cell lung cancer (NSCLC) is the predominant form of the disease, accounting for approximately 85% of cases.2 The majority of patients present with locally advanced or metastatic disease, and many do not survive more than 5 years beyond diagnosis.2,3 While targeted therapy has produced real benefit for specific molecular subtypes of NSCLC, traditional chemotherapy, which usually provides short-lived benefit, remains the only option for most patients. Consequently, there remains a major need for therapy that significantly extends patient survival without compromising quality of life.
In recent years, there has been an increasing recognition of the role of the immune system in cancer development and progression,4–6 with a corresponding focus on utilizing immunotherapy in the clinic and regulatory approvals of immunotherapy for renal cell cancer (interleukin [IL]-2 and interferon-α7), prostate cancer (sipuleucel-T8), and melanoma (ipilimumab,9 nivolumab,10 pembrolizumab11). Although NSCLC has historically been considered a nonimmunogenic disease, emerging evidence has demonstrated that the lack of an effective immune response is in fact often the result of specific, active immune-evasive mechanisms, which if understood can be overcome therapeutically with significant clinical efficacy. Harnessing this potential has therefore become a primary area of clinical interest.12–14 Given the increasing understanding of the role of immunology in oncology, this article examines the key elements of the immune system involved in cancer in general and in NSCLC specifically, and briefly outlines some of the immunotherapeutic strategies currently being developed to improve patient outcomes.
THE IMMUNE SYSTEM AND CANCER
The Antitumor Immune Response
The immune system is now recognized to have the potential to destroy cancer cells and inhibit tumor growth through responses elicited by its innate and adaptive arms.15 Innate immune responses are antigen nonspecific, develop quickly, and are mediated by various effector cells (natural killer [NK] cells, polymorphonuclear leukocytes, and mast cells, as well as antigen-presenting cells [APCs] such as macrophages and dendritic cells [DCs]), which lead to the secretion of interferon gamma (IFN-γ) and perforin, as well as inflammatory cytokines, that induce apoptosis of tumor cells.4 In contrast, adaptive immune responses are antigen specific, develop more slowly, offer immune memory, and comprise both humoral and cellular immunity mediated by B and T cells, respectively.15,16 In this respect, adaptive rather than innate immunity offers the greatest potential for durable, robust anticancer immune responses. Of note, some of the cells involved in innate immunity, such as DCs, macrophages, and NK cells, also play a role in adaptive immunity.4
The adaptive anticancer immune response is initiated by immature DCs, which are found in most human tumors and are capable of capturing antigens released from cancer cells (Fig. 1).17,18 After maturation (activation), DCs present tumor antigens within major histocompatibility complex (MHC) molecules to naive T cells in the tumor-draining lymph nodes, triggering a protective T-cell response composed of specific CD4+ helper T (Th) cells and CD8+ cytotoxic T cells. T-cell activation requires interaction not only between the antigen-MHC complex on DCs and T-cell receptors, but also among an array of co-stimulatory molecules, including CD80/86 on DCs and the CD28 receptor on T cells. After infiltrating the tumor, activated cytotoxic T cells are capable of recognizing and killing tumor cells directly in an MHC-restricted fashion. In addition, activated Th cells secrete cytokines that induce inflammation and recruit other immune cell populations to the tumor microenvironment to eliminate cancer cells. DCs may also induce B-cell–mediated antibody responses and NK cell activity.
FIGURE 1.
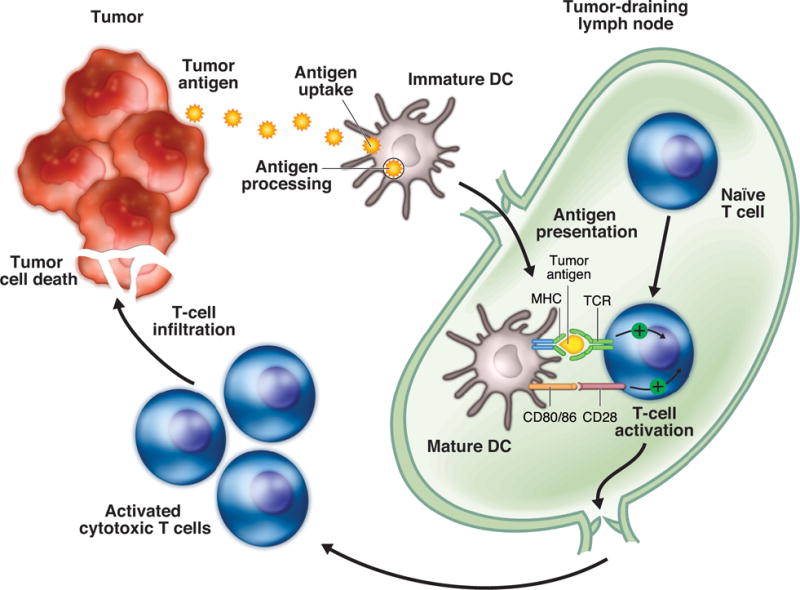
Adaptive anticancer immunity. The adaptive anticancer immune response is initiated by immature DCs, which capture and process tumor antigens. DCs subsequently undergo maturation and migrate to tumor-draining lymph nodes, where they present tumor antigens within MHC molecules to naïve T cells, triggering a protective T-cell response. T-cell activation requires interaction not only between the antigen-MHC complex on DCs and TCRs but also among an array of co-stimulatory molecules, including CD80/86 on DCs and the CD28 receptor on T cells. The adaptive anticancer immune response culminates with the infiltration of activated cytotoxic T cells into the tumor, killing cancer cells. DC, dendritic cell; MHC, major histocompatibility; TCR, T-cell receptor.
Promotion of Tumor Growth by the Immune System
Insights into cellular and molecular immunologic processes have revealed that the immune system is capable of not only inhibiting but also promoting tumor growth, through either the selection of tumor cells that are better able to survive in an immunocompetent host or the creation of conditions within the tumor microenvironment that facilitate tumor growth.5,6 It has been proposed that this dual host-protective and tumor-promoting role results from a dynamic relationship between cancer cells and the immune system termed “immunoediting,” which consists of three distinct phases: elimination, equilibrium, and escape (Fig. 2).15 In the elimination phase, acute immune responses, both innate and adaptive, recognize and destroy cancer cells (via a process termed “immunosurveillance”) before they develop into a clinically detectable tumor.5,6,15 Early evidence suggested that premalignant clones expressing novel somatic mutant epitopes (immunogenic portions of antigens) might be targeted by the immune system in the initial stages of tumor development.19 Tumor clones that escape the elimination phase remain dormant in the subsequent equilibrium phase, during which tumor growth does not occur but the immunogenicity of the tumor cells continues to be shaped by selective immune pressure from the adaptive immune response.6,15 In time, changes arising in the tumor cell population caused by this selective pressure and/or changes in the immune system as a result of prolonged tumor-mediated immunosuppression may lead to immune escape and tumor growth.6,15
FIGURE 2.
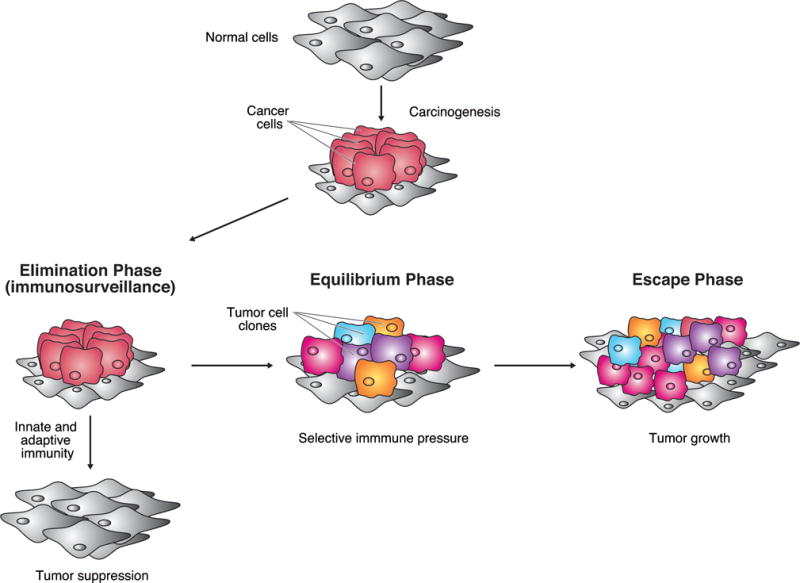
Cancer immunoediting. The proposed process of cancer immunoediting consists of three distinct phases: elimination, equilibrium, and escape. In the elimination phase, innate and adaptive immune responses recognize and destroy cancer cells (immunosurveillance), suppressing tumor development. In the equilibrium phase, tumor clones that escape the elimination phase remain dormant, during which tumor growth does not occur but the immunogenicity of the tumor cells continues to be shaped by selective immune pressure. In the escape phase, tumor cell clones that are resistant to the immune system proliferate unchecked. Adapted with permission from: Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–271.
Tumor cells entering the immune escape phase are able to create an immunosuppressive state within the tumor microenvironment by subverting the same mechanisms that under normal conditions help regulate the immune response and prevent damage to healthy tissue.6 Key immunosuppressive cell types found in the tumor microenvironment are regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages.6,17,20 Treg cells, which are positive for CD4, CD25, and the Foxp3 transcription factor, suppress the function and proliferation of tumor-specific CD4+ and CD8+ T cells and NK cells, while MDSCs induce Treg cells and limit effector T-cell proliferation via the production of various immunosuppressive molecules.6,17 Tumor-associated macrophages and stromal cells may also secrete cytokines that inhibit an adaptive immune response, such as IL-10 and transforming growth factor-β (TGF-β).16,20 In addition, both tumor cells and other cells present in the tumor microenvironment may express the immunosuppressive enzyme indoleamine-2,3-dioxygenase, which depletes the amino acid tryptophan (essential for T-cell function), increases local Treg populations, and induces tumor-specific T-cell deactivation.20
Even if T cells can otherwise be activated, specific physiologic regulatory mechanisms, or “checkpoints,” which play a key role in maintaining normal self-tolerance and limiting the extent of immune responses to infection, can be exploited by tumors as immune resistance mechanisms.21 Two of the most investigated checkpoint receptors in terms of immunotherapeutic targets for cancer are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) receptor, which down-regulate T-cell activation, proliferation, and function via different mechanisms.
CTLA-4 is expressed on the surface of T cells after activation and shares the same ligands (CD80/86 expressed by APCs) as the co-stimulatory T-cell CD28 receptor, which is required for T-cell activation (Fig. 3A).21 By virtue of its higher affinity for these ligands, CTLA-4 competes with the CD28 receptor in binding to CD80/86, thereby providing an inhibitory signal to the T cell and serving as a negative feedback loop for T-cell activation. In the cancer setting, inhibiting the T-cell response via the CTLA-4 pathway favors tumor survival over elimination. CTLA-4 is also constitutively expressed by Treg cells and has a critical effect on the ability of these cells to regulate antitumor immunity.22
FIGURE 3.
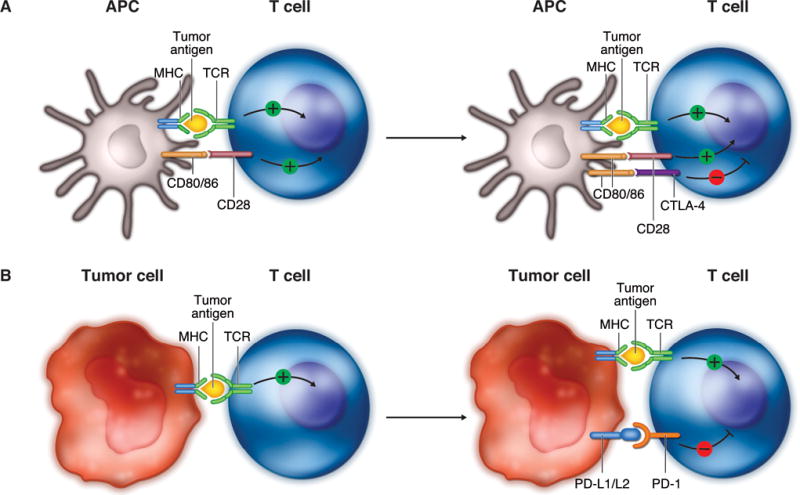
Immune checkpoints. A, Cytotoxic CTLA-4 is expressed on T cells after activation and competes with the co-stimulatory T-cell CD28 receptor for CD80/86 expressed by APCs, providing an inhibitory signal to the T cell. B, PD-1 receptor is up-regulated on activated T cells and subsequently binds to one of its ligands, PD-L1 or PD-L2, which are commonly expressed on tumor cells, providing an inhibitory signal to the T cell. APC, antigen-presenting cell; MHC, major histocompatibility; TCR, T-cell receptor; CTLA-4, cytotoxic T-lymphocyte antigen-4; PD-L1/L2, programmed death ligand-1/ligand-2; PD-1, programmed death-1. Adapted with permission from: Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264.
The PD-1 pathway is also an important mechanism by which tumors develop immune resistance (Fig. 3B).15,21 Upregulation of the PD-1 receptor on activated T cells and subsequent binding to one of its ligands, programmed death ligand-1 (PD-L1) or programmed death ligand-2 (PD-L2), provide an inhibitory signal during the effector phase of the T-cell response, reducing cytokine production, cell proliferation, and cell survival signaling. PD-1 is also expressed at high levels on Treg cells, enhancing their proliferation in the presence of a PD-1 ligand. In addition, PD-1 may be induced on activated NK cells, thereby limiting their lytic activity. Present on a wide variety of hematopoietic and nonhematopoietic cells, PD-L1 and PD-L2 are also commonly expressed on tumor cells.21,23 Although the clinical significance of PD-L1 expression on tumor cells is yet to be fully characterized, it is thought to confer a survival advantage to the tumor via the PD-1 pathway.17 PD-L1 tumor cell expression is induced via IFN-γ secreted by infiltrating Th cells as part of an adaptive immune resistance mechanism.20 Recent evidence shows that the induction of tumor PD-L1 expression can also be up-regulated by oncogenic signaling intrinsic to the tumor cells themselves.24
In addition to immunosuppressive mechanisms that undermine antitumor immunity, chronic inflammation can paradoxically promote tumor growth.25 In fact, chronically activated leukocytes produce a range of molecules that can directly stimulate tumor growth, including epidermal growth factor, TGF-β, and TNF-α. The development of this chronic inflammatory environment also confers a survival advantage to tumor cells by increasing the chance of DNA damage and accumulation of oncogenic mutations.
ROLE OF THE IMMUNE SYSTEM IN NSCLC
The Immunosuppressive NSCLC Tumor Microenvironment
Like other tumor types, NSCLC can establish an immunosuppressive tumor microenvironment conducive to tumor growth.12–14 For instance, NSCLC tumors have been shown to contain large numbers of Treg cells that constitutively express high levels of CTLA-4 on their surface and directly inhibit T-cell proliferation.26,27 In addition, in NSCLC, tumor-infiltrating CD8+ T cells have shown increased PD-1 expression that was associated with impaired immune function.28 PD-L1 expression has also been found to be up-regulated on NSCLC tumor cells29 and shown to correlate with the suppression of maturation of tumor infiltrating DCs30 and reduced tumor T-cell infiltration.31 Furthermore, dysfunction of the antigen-presentation apparatus appears to impair immunologic activity in the tumor microenvironment, as lung tumor cells can down-regulate surface expression of MHC class I/tumor antigen expression, thereby helping these cells to evade the immune system.32 Lung tumor cells may also release immune suppressive cytokines, including IL-10 and TGF-β.33
Immune Correlates of Clinical Outcome in NSCLC
Further underscoring the involvement of the immune system in NSCLC, a number of immune correlates of clinical outcome in patients with NSCLC have been identified. One of the most remarkable pieces of clinical evidence for immune system involvement in NSCLC is the presence of “preformed” antitumor T cells and antibodies in the blood of patients with NSCLC.34,35 Moreover, tumor-infiltrating lymphocytes (TILs), composed mainly of CD8+ T cells, were significantly associated with improved survival and correlated with tumor grade, size, vascular invasion, and poor levels of differentiation among patients with NSCLC.36 The presence of TILs has also been linked with a better survival outcome in NSCLC at an early stage as well as a reduced risk of systemic recurrence.37 In separate studies, high levels of infiltrating CD8+ T cells, both CD8+ and CD4+ T cells, and T cells expressing the pan T-cell marker CD3,38–40 as well as higher densities of mature DCs in tertiary lymphoid structures,41 have been associated with improved survival. Conversely, the number of Treg cells42,43 and higher numbers of macrophages with pro-tumor functions44 in NSCLC tumors have been shown to be independent predictors of reduced survival. PD-L1 expression on tumor cells also correlates with an unfavorable prognosis in patients with NSCLC.23,29,30 Finally, high activity levels of nuclear factor kappa-light-chain-enhancer of activated B cells, a transcription factor constitutively activated in many tumor types, have been associated with the recruitment and infiltration of antitumor T cells into tumor tissue and extended survival in patients with NSCLC.45
DEVELOPMENT OF IMMUNOTHERAPY FOR NSCLC
Given the clear role of the immune system in NSCLC, research efforts are being intensively directed toward the development of various immunotherapies for the disease, particularly those promoting adaptive immune responses.12–14 Cancer immunotherapy can be broadly divided into antigen-specific and antigen-nonspecific therapies, with the respective aims of stimulating specific antitumor immunity and influencing steps after the immune system has been previously stimulated. Examples of antigen-specific and antigen-nonspecific immunotherapies include cancer vaccines and immune checkpoint inhibitors, respectively. A number of immunotherapeutic strategies for NSCLC, including those that stimulate immune processes and counteract tumor immune evasion, are being investigated in clinical trials (Table 1).46–59
TABLE 1.
Immunotherapeutic Agents in Clinical Development for the Treatment of Advanced Non–Small Cell Lung Cancer
| Agent | Description |
|---|---|
| Checkpoint Inhibitors | |
| Nivolumab | Fully human IgG4 monoclonal antibody directed against PD-1 on T cells |
| Pembrolizumab (MK-3475) | Humanized IgG4 monoclonal antibody directed against PD-1 on T cells |
| BMS-936559 | Fully human IgG4 monoclonal antibody directed against PD-L1 on tumor cells |
| MPDL3280A | Human IgG1 monoclonal antibody directed against PD-L1 on tumor cells |
| MEDI4736 | Fully human IgG1 monoclonal antibody directed against PD-L1 on tumor cells |
| Ipilimumab | Fully human IgG1 monoclonal antibody directed against CTLA-4 on T cells |
| Lirilumab (IPH2102) | Fully human monoclonal antibody directed against the killer-cell immunoglobulin-like receptor on NK cells |
| BMS-986016 | Monoclonal antibody directed against the lymphocyte-activation gene 3 on tumor infiltrating lymphocytes |
| Vaccines | |
| Tecemotide (liposomal BLP25) | Vaccine composed of the exposed core peptide of MUC-1 |
| Racotumomab | Patient idiotype-specific vaccine against NGg GM3 |
| TG4010 | Vaccine that uses a recombinant vaccinia virus (modified virus of Ankara) that encodes for human MUC-1 and IL-2 |
| Nonspecific Immune Stimulator | |
| Talactoferrin alfa | Recombinant human lactoferrin |
CTLA-4, cytotoxic T lymphocyte antigen-4; IgG, immunoglobulin G; IL-2, interleukin-2; MUC-1, mucin 1; NGg, N-glycolil; NK, natural killer; NSCLC, non–small cell lung cancer; PD-1, programmed death-1; PD-L1, programmed death ligand-1
Immune Checkpoint Inhibitors
Perhaps the most significant advances in NSCLC immunotherapy have been made by targeting immune checkpoint pathways to prevent or reduce tumor-mediated immune suppression. In particular, several monoclonal antibodies that block immune checkpoint pathways, such as those involving PD-1 and CTLA-4, are being investigated in clinical trials with NSCLC (Table 2).47–50,52–55 These immune checkpoint inhibitors offer the advantage of enhancing the host’s own antitumor immune response without regard to the specific tumor antigen, thus conferring broader clinical application than antigen-specific immunotherapies such as vaccines.12
TABLE 2.
Results of Trials of Immune Checkpoint Inhibitors in Clinical Development for the Treatment of Patients with Advanced Non–Small Cell Lung Cancer
| Agent (reference) |
Study design (study name) |
Number of patients |
Median follow- up |
Objective response rate, % (n/N) |
Median (range) response duration, weeks |
Median progression- free survival |
Median overall survival, months |
1-year survival rate, % |
2-year survival rate, % |
Incidence of adverse events, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Nivolumab (Brahmer et al47) | Phase I dose-ranging study of nivolumab (1, 3, and 10 mg/kg IV Q2W) in previously treated patients with advanced solid tumors, including advanced NSCLC | 129 (NSCLC cohort) | 27 months | Across all doses: 17 (22/129)a 3 mg/kg Q2W doseb: 24 (9/37)a |
Across all doses: 74.0 (6.1+ to 133.9+) 3 mg/kg Q2W dose: 74.0 (16.1+ to 133.9+) |
Across all doses: 2.3 months (95% CI, 1.8–3.7)a 3 mg/kg Q2W dose: 1.9 months (95% CI, 1.7–7.3)a |
Across all doses: 9.9 (95% CI, 7.8–12.4) 3 mg/kg Q2W dose: 14.9 (95% CI, 7.3–NE) |
Across all doses: 42 (95% CI, 34–51) 3 mg/kg Q2W dose: 56 (95% CI, 38–71) |
Across all doses: 24 (95% CI, 16–32) 3 mg/kg Q2W dose: 45 (95% CI, 27–61) |
Treatment-related any grade: fatigue, 24; decreased appetite, 12; diarrhea, 10 Treatment-related grade 3–4: 14 |
| Nivolumab (Gettinger et al48) | Phase I multi-cohort study of nivolumab as monotherapy or combined with chemotherapy, targeted therapy, or ipilimumab in chemotherapy-naïve patients with advanced NSCLC (CheckMate 012) | 20 (cohort treated with nivolumab 3 mg/kg IV Q2W) | 66.1 weeks | 30 (6/20)a | NR | 36.1 weeksa | NR | 75 (95% CI, 50–89) | NA | Treatment-related any grade: fatigue, 40; nausea, 20; rash, 20; diarrhea, 15 Treatment-related grade 3–4: 20 |
| Nivolumab (Rizvi et al49) | 21 (non-squamous EGFR MT cohort treated with nivolumab 3 mg/kg IV Q2W plus erlotinib 150 mg/day PO) | 71.9 weeks | 19 (4/21)a | NR | 29.4 weeksa | NR | 73 (95% CI, 46–88) | NA | Treatment-related any grade: rash, 48; fatigue, 29; paronychia, 29; diarrhea, 24; skin fissures, 24 Treatment-related grade 3–4: 24 |
|
| Nivolumab (Rizvi et al50) | Phase II single-arm study of nivolumab 3 mg/kg IV Q2W in patients with advanced, refractory squamous NSCLC (CheckMate 063) | 117 | 8.0 months | 15 (17/117)a | NR | 1.9 months (95% CI, 1.8–3.2)a | 8.2 (95% CI, 6.1–10.9) | 40.8 (95% CI, 31.6–49.7) | NA | Treatment-related any grade: fatigue, 33; nausea, 15; asthenia, 12; diarrhea, 10 Treatment-related grade 3–4: 17 |
| Pembrolizumab (Garon et al52) | Phase I study of pembrolizumab (2 mg/kg IV Q3W, 10 mg/kg IV Q3W, and 10 mg/kg IV Q2W) with treatment-naïve and previously-treated patients with advanced NSCLC (KEYNOTE-001) | 262 | NA | 21a (treatment-naïve patients: 26; previously-treated patients: 20) | NA | Treatment-naïve patients: 27 weeks (95% CI, 14–45)a Previously-treated patients: 10 weeks (95% CI, 9.1–15.3)a |
Treatment-naïve patients: NR (95% CI, NE–NE) Previously-treated patients: 8.2 (95% CI, 7.3–NR) |
NA | NA | Treatment-related any grade: fatigue, 20; pruritus, 9; arthralgia, 8; decreased appetite, 8; diarrhea, 7 Treatment-related grade 3–4: 9 |
| MPDL3280A (Soria et al53) | Phase I study of MPDL3280A IV Q3W in patients with squamous or non-squamous NSCLC | 53 | NA | 24 (9/37)a | Median was not reported (range: 1+ to 214+ days) | NA | NA | NA | NA | All-cause grade 3–4: 34 (pericardial effusion, 6; dehydration, 4; dyspnea, 4; fatigue, 4) |
| MEDI4736 (Brahmer et al54) | Phase I dose-escalation dose-expansion study of MEDI4736 (0.1–10 mg/kg IV Q2W; 15 mg/kg IV Q3W) in patients with advanced solid tumors, including advanced NSCLC | 155 (NSCLC cohort) | 6 weeks | Across all doses: 16 (9/58)a | NA | NA | NA | NA | NA | Treatment-related any grade: 29 Treatment-related grade 3–4: 3 |
| Ipilimumab (Lynch et al55) | Phase II study of ipilimumab (10 mg/kg IV Q3W) plus paclitaxel/carboplatin (concurrent or phased administration) versus paclitaxel/carboplatin (control) in chemotherapy-naïve patients with advanced NSCLC | 204 | NA | Control: 18 (12/66)c Concurrent: 21 (15/70)c Phased: 32 (22/68)c |
NA | Control: 4.6c Concurrent: 5.5c (HR, 0.81; p = 0.13 versus control) Phased: 5.7c (HR, 0.72; p = 0.05 versus control) |
Control: 8.3 Concurrent: 9.7 (HR, 0.99; p = 0.48 versus control) Phased: 12.2 (HR, 0.87; p = 0.23 versus control) |
Control: 39 Concurrent: 50 Phased: 42 |
Control: 18 Concurrent: 18 Phased: 16 |
Treatment-related grade 3–4: Control: 37 Concurrent: 41 Phased: 39 |
Abbreviations: CI, confidence interval; EGFR MT, epidermal growth factor receptor mutant; HR, hazard ratio; irPFS, immune-related progression-free survival; NA, not available; NE, not estimable; NR, not reached; NSCLC, non–small cell lung cancer; PO, oral administration; Q2W, every 2 weeks; Q3W, every 3 weeks.
Based on Response Evaluation Criteria In Solid Tumors (RECIST).
The nivolumab dose selected for phase 3 studies.
Based on immune-related response criteria (irRC)
Nivolumab (BMS-936558; ONO-4538), a fully human immunoglobulin G4 (IgG4) monoclonal antibody directed against PD-146,60 that was approved in 2014 in the United States61 and Japan62 for treating patients with advanced melanoma, is being investigated for advanced NSCLC.47 Nivolumab was approved in the United States in 2015 for treating patients with metastatic squamous NSCLC with progression on or after platinum-based chemotherapy.63 In a phase I study (Table 2) in previously treated patients with advanced NSCLC (n=129), nivolumab demonstrated an objective response rate (ORR) based on Response Evaluation Criteria In Solid Tumors (RECIST) v1.0 of 17% across all doses evaluated and 24% with 3 mg/kg dose given every 2 weeks (the dose selected for phase 3 studies), with an estimated median response duration of 74 weeks both across all doses and at the 3 mg/kg dose.47 Across all doses, responses occurred early, with 50% of patients demonstrating a response at 8 weeks, and were ongoing in 45% of patients. Responses occurred in various NSCLC patient subpopulations, including those with squamous and nonsquamous cell histology (17% and 18%), who received <3 and ≥3 prior therapies (12% and 21%), who were <70 and ≥70 years of age (17% and 18%), and with and without tumors driven by epidermal growth factor receptor (EGFR) (17% and 20%) or Kristen rat sarcoma oncogene homolog (KRAS) mutations (14% and 25%). Across all doses, median OS was 9.9 months, with 1- and 2-year survival rates of 42% and 24%, respectively. With the 3 mg/kg dose, median OS was 14.9 months, with 1- and 2-year survival rates of 56% and 45%, respectively.47 Although it is difficult to compare findings between trials, the survival results with nivolumab are promising relative to previous experience with approved therapies in treatment-refractory, advanced NSCLC populations (median OS, 6–8 months; 1-year survival rate, approximately 30%).64–67 Nivolumab had a manageable safety profile, with the most common treatment-related adverse events (any grade) being fatigue (24%), decreased appetite (12%), and diarrhea (10%).47 Grade 3–4 treatment-related adverse events occurred in 14% of patients. In another phase I study (CheckMate 012; Table 2), nivolumab showed clinical activity, with a RECIST v1.1-based ORR of 30%, in chemotherapy-naïve patients with advanced NSCLC.48 Additionally, in the same phase I study (CheckMate 012; Table 2), chemotherapy-naive patients with EGFR-mutant advanced NSCLC achieved a RECIST v1.1-based ORR of 19% with nivolumab plus erlotinib, an EGFR tyrosine kinase inhibitor.49 Nivolumab also demonstrated clinical meaningful activity (RECIST v1.1-based ORR: 15%) in a phase II, single-arm study (CheckMate 063; Table 2) with patients having advanced, refractory squamous NSCLC (n = 177).50 Phase III trials are evaluating nivolumab monotherapy versus current standard of care as first-line therapy for squamous and non-squamous NSCLC (NCT02041533 [CheckMate 026]) or subsequent line of therapy for non-squamous NSCLC (NCT01673867 [CheckMate 057]). A phase III study (NCT01642004 [CheckMate 017]) evaluating nivolumab versus docetaxel in patients with stage IIIB/IV squamous NSCLC with disease recurrence or progression during or after one prior platinum doublet-based chemotherapy regimen was stopped early because an assessment conducted by the independent Data Monitoring Committee found that the study met its primary endpoint of superior OS with nivolumab.51 It should be noted that some of these phase III trials did not select patients for PD-L1 expression and may thus provide an opportunity for its validation as a potential predictive biomarker.
Pembrolizumab (MK-3475), a humanized IgG4 monoclonal antibody directed against PD-1 that was approved in 2014 in the United States for treating patients with advanced or unresectable melanoma who are no longer responding to other agents,68 is being assessed for NSCLC.52 In a phase I study (KEYNOTE-001; Table 2) with patients with treatment-naïve and previously treated NSCLC (n = 262), pembrolizumab demonstrated a RECIST v1.1-based ORR of 21%.52 ORR was higher for patients who were treatment naïve versus previously treated (26% and 20%, respectively), had non-squamous versus squamous histology (23% versus 18%, respectively), were current or former versus never smokers (27% versus 9%, respectively), and had tumor with KRAS versus EGFR mutations (39% versus 36%, respectively). Median OS in the treatment-naïve and previously-treated cohorts was not reached and 8.2 months, respectively. The most common treatment-related adverse events (any grade) were fatigue (20%), pruritus (9%), arthralgia (8%), decreased appetite (8%), and diarrhea (7%). Grade 3–4 treatment-related adverse events occurred in 9% of patients. A phase II/III study is ongoing comparing two dose levels of pembrolizumab with docetaxel in pretreated patients with advanced NSCLC (NCT01905657 [KEYNOTE-010]). Phase III studies comparing first-line pembrolizumab monotherapy with platinum-based doublet chemotherapy in PD-L1–positive, advanced NSCLC are recruiting patients (NCT02142738 [KEYNOTE-024] and NCT02220894 [KEYNOTE-042]).
Targeting the PD-1 ligand PD-L1 may provide an alternative strategy for NSCLC. In a phase I study (Table 2) with patients with squamous or nonsquamous NSCLC (n=37), MPDL3280A, a human IgG1 monoclonal antibody directed against with an engineered fragment crystallizable (Fc) domain to prevent antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cell-mediated cytotoxicity (CDCC) of tumor-infiltrating T cells meant to be activated,69 achieved an RECIST v1.1-based ORR of 24%.53 ORR was greater in the former or current smokers (25%) than in never smokers (16%). Grade 3–4 adverse events, regardless of attribution, occurred in 34% of patients and included pericardial effusion (6%), dehydration (4%), dyspnea (4%), and fatigue (4%). A phase II study is currently examining the use of MPDL3280A in patients with PDL1-positive advanced NSCLC (FIR study [NCT01846416]). Phase II and III studies are also underway comparing MPDL3280A with docetaxel among patients with advanced NSCLC who failed previous platinum therapy (POPLAR study [NCT01903993] and OAK study [NCT02008227], respectively).
MEDI4736, a fully human IgG1 monoclonal antibody directed against PD-L1 containing an engineered IgG1 Fc domain to prevent ADCC and CDCC of tumor-infiltrating T cells,69 produced a RECIST v1.1-based ORR of 16% in a phase I study (Table 2) with patients with NSCLC (n=155) who were mostly pretreated54 and is being further studied in phase II/III studies with patients with NSCLC (Lung-MAP study [NCT02154490] and ATLANTIC study [NCT02087423]).
Research is underway to identify biomarkers that might predict which patients respond best to PD-1 pathway inhibitors, the most promising of which may be the level of PD-L1 expression in the tumor microenvironment. Considering the role of PD-L1 in tumor immune evasion, it is logical to assume that PD-L1 expression would be a viable biomarker.21 In preliminary analyses with various PD-1 pathway inhibitors, expression of PD-L1 on tumor cells at baseline appeared to correlate with increased efficacy.46–48,50,52,53 For example, in the phase II CheckMate 063 study with nivolumab in patients having advanced, refractory squamous NSCLC, RECIST-based ORR was greater in patients with PD-L1–positive tumors (≥5% tumor cells expressing PD-L1 by immunohistochemistry [IHC]) versus PD-L1–negative tumors (24% [6/25] versus 14% [7/51], respectively).50 Similarly, among treatment-naïve and previously-treated patients with advanced NSCLC receiving pembrolizumab in the phase I KEYNOTE-001 trial, ORR by RECIST was greater in patients with PD-L1–positive tumors (≥1% tumor cells expressing PD-L1 by IHC) than in those with PD-L1–negative tumors (23% versus 9%).52 Patients in that study who were PD-L1 strong-positive (≥50% membranous staining in tumor cells) versus PD-L1 weak-positive or negative demonstrated longer median OS (hazard ratio [HR], 0.59; 95% confidence interval [CI], 0.35–0.99) and median progression-free survival (HR, 0.52; 95% CI, 0.33–0.80). Expression of PD-L1 on tumor-infiltrating immune cells may also be predictive of response with anti-PD-L1 agents, as suggested in a phase 1 study of MPDL3280A with NSCLC patients showing that RECIST-based ORRs were significantly associated with tumor-infiltrating immune cell PD-L1 expression (p = 0.015).70 PD-L1–positive tumor-infiltrating immune cells included macrophages, DCs and T cells. In that study, responses occurred in 83% of patients (5/6) with an IHC score of 3 (≥10% of cells per area expressing PD-L1) compared with 20% (4/20), 15% (2/13), and 14% (1/7) of patients with IHC scores of 0 (<1% of cells), 1 (≥1% but <5% of cells), and 2 (≥5% but <10% of cells), respectively. The conclusions that can be drawn from these analyses, however, are limited by several factors, including small patient numbers due to low rate of tissue sample ascertainment, the use of archival (versus fresh) tumor samples, use of ORR (which may not be the optimal endpoint to assess the predictive role of biomarkers for immune-based therapies), the dynamic nature of and intra-tumor variations in PD-L1 expression, the effect of prior treatment on PD-L1 status, lack of standardization of IHC assays, and undefined cut-off values for PD-L1 positivity.23,47,50 The use of PD-L1 as a biomarker is therefore being further explored in larger NSCLC trials.
The fully human IgG1 monoclonal antibody ipilimumab, which is directed against CTLA-4, has shown antitumor activity and a survival advantage with advanced melanoma9 and may have potential in treating patients with advanced NSCLC.55 A phase II study (Table 2) compared ipilimumab plus paclitaxel and carboplatin (concurrent or phased administration) with paclitaxel and carboplatin alone (control) in chemotherapy-naïve patients with stage IIIB/IV NSCLC (n = 204).55 In that study, phased administration (pacliatxel and carboplatin followed by ipilimumab plus pacliatxel and carboplatin) demonstrated significantly improved median immune-related progression-free survival (irPFS), the primary study end point, compared with paclitaxel and carboplatin alone (5.7 versus 4.6 months; HR, 0.72; p = 0.05), with greater improvements in irPFS occurring with squamous versus non-squamous histology. However, concurrent administration (ipilimumab plus paclitaxel and carboplatin followed by paclitaxel and carboplatin) did not significantly improve irPFS versus control. There was a non-statistical trend toward improved median OS with phased administration compared with control, but not with concurrent administration. Ipilimumab did not appear to impact toxicities associated with paclitaxel and carboplatin. Immune-mediated adverse events (eg, rash, pruritus, diarrhea) occurred more frequently in the ipilimumab arms than in the control arm. The combination of ipilimumab with paclitaxel and carboplatin is being further investigated in a phase III study in patients with stage III/IV recurrent squamous NSCLC (NCT01285609).
Interestingly, early results of ipilimumab in combination with nivolumab in melanoma suggest that a two-pronged approach may provide clinical benefit based on the apparently complementary roles of CTLA-4 and PD-1 in negative immune regulation.71 A phase I study is also ongoing to evaluate this combination in treatment-naive advanced patients with NSCLC (NCT01454102). In addition, MEDI4736 combined with tremelimumab, an IgG2 anti-CTLA-4 antibody, is being investigated in patients with previously-treated NSCLC in a phase I study (NCT02000947).72
Overall, immune checkpoint inhibitors that target the PD-1 or CTLA-4 pathways have manageable safety profiles (Table 2).46–50,52–55 These agents are characteristically associated with immune-related adverse events (eg, rash, pruritus, diarrhea, hypothyroidism, hepatitis), which are consistent with the their mechanism of action and can often be managed with protocol-specified guidelines (eg, close patient follow-up and early administration of systemic corticosteroids and/or other immunosuppressive agents).23,47,55,69
Agents that inhibit other immune checkpoint pathways may also have potential in treating patients with advanced NSCLC.73 For example, lirilumab (IPH2102), a fully human IgG4 monoclonal antibody that blocks the interaction between killer-cell immunoglobulin-like receptors on natural killer (NK) cells with their ligands,73,74 is being assessed in combination with nivolumab (NCT01714739) or ipilimumab (NCT01750580) in phase I studies with patients with NSCLC. In addition, BMS-986016, a monoclonal antibody that binds to lymphocyte-activation gene 3, a CD4-related immune checkpoint receptor co-expressed with PD-1 on tolerant tumor-infiltrating lymphocytes,74 is being evaluated in combination with nivolumab in patients with advanced solid tumors in a phase 1 study (NCT01968109).
Although durable clinical responses have been documented in patients with various tumor types treated with immune checkpoint inhibitors, the genetic basis for these benefits is only beginning to be understood. Recent research suggests that patients more likely to achieve meaningful responses to CTLA-4 inhibition have tumors displaying neoantigens, which result from specific somatic mutations harbored by the tumor and elicit an antitumor response augmented by CTLA-4 inhibition.75 Such tumor antigens could be immunogenic and potentially function as targets of T cells activated by immune checkpoint inhibition.76,77 Therefore, methods are being developed for predicting immunogenic tumor mutations, thereby identifying patients who would best benefit from immune checkpoint inhibitors and allowing personalized treatment.77
Cancer Vaccines
Cancer vaccines aim to stimulate the immune system to recognize and respond to one or more tumor antigens, which ideally show exclusive or elevated expression on cancer cells.12 However, as most tumor antigens are closely related or identical to self-antigens and therefore weakly antigenic, cancer vaccines usually incorporate strong adjuvants to stimulate efficient DC presentation of these proteins.17 A number of cancer vaccines are currently in clinical trials in NSCLC, including tecemotide (liposomal BLP25),56 racotumomab,57 and TG4010,58 which have shown a range of responses and survival outcomes (reviewed in detail elsewhere).12–14 Current evidence suggests that, despite the potential for inducing long-lasting immune memory, vaccine therapy may be most effective in patients with a lower disease burden.13
Nonspecific Immune Stimulation
Nonspecific immune stimulation has been investigated therapeutically in different cancers, including NSCLC.12 One agent recently evaluated in NSCLC is talactoferrin alfa, a recombinant form of human lactoferrin and an oral DC-mediated immunotherapy that stimulates cytokine release in the intestine, with subsequent recruitment and activation of DCs. Although talactoferrin alfa did not lead to improved OS versus placebo in a phase III study in patients with advanced, pretreated NSCLC,59 it is now undergoing additional evaluation in treatment-naive patients with advanced or metastatic NSCLC.
CONCLUSIONS
Although traditionally considered a nonimmunogenic disease, NSCLC is now recognized to elicit an endogenous immune response. Emerging results with a range of immunotherapeutic agents such as immune checkpoint inhibitors indicate that this therapeutic modality could eventually have a significant impact upon the survival and quality of life of patients with NSCLC, for whom the outlook is currently bleak. Further investigation into the dysregulation of the immune system induced by tumor cells during development of NSCLC and additional results from ongoing studies will provide insight on how immunotherapy can be used to shift the balance of immune control away from a tumor-induced immune suppressive state to an active antitumor immune response.
Acknowledgments
Professional medical writing assistance was provided by Mark Palangio at StemScientific and was funded by Bristol-Myers Squibb.
Conflicts of Interest and Sources of Funding: David P. Carbone was a paid consultant for Boehringer-Ingelheim, Bristol-Myers Squibb, Clovis, Genetech, Novartis, and Pfizer, and is currently receiving research grants from Bristol-Myer Squibb. David R. Gandara is currently receiving research grants from Bristol-Myers Squibb and Merck. Scott J. Antonia is currently a paid consultant for Bristol-Myers Squibb and is currently receiving research grants from MedImmune. Luis Paz-Ares is currently a paid consultant for Bristol-Myers Squibb. Christoph Zielinski is an ongoing paid consultant for Bristol-Myers Squibb. No financial support or compensation was received by the authors for this publication.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed 23 January, 2014. [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell cancer guidelines. V4.2015. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 19 February, 2014.
- 3.Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851. doi: 10.1016/j.ccm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012;35:299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrand-Rosenberg S. Immune surveillance: a balance between pro- and anti-tumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012;23(Suppl 8):viii35–40. doi: 10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd FA, Douillard JY, Blumenschein GR., Jr Immunotherapy for non-small cell lung cancer: novel approaches to improve patient outcome. J Thorac Oncol. 2011;6:1763–1773. doi: 10.1097/JTO.0b013e31822e28fc. [DOI] [PubMed] [Google Scholar]
- 13.Brahmer JR. Harnessing the immune system for the treatment of non–small-cell lung cancer. J Clin Oncol. 2013;31:1021–1028. doi: 10.1200/JCO.2012.45.8703. [DOI] [PubMed] [Google Scholar]
- 14.Forde PM, Reiss KA, Zeidan AM, et al. What lies within: novel strategies in immunotherapy for non-small cell lung cancer. Oncologist. 2013;18:1203–1213. doi: 10.1634/theoncologist.2013-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 16.Mortellaro A, Ricciardi-Castagnoli P. From vaccine practice to vaccine science: the contribution of human immunology to the prevention of infectious disease. Immunol Cell Biol. 2011;89:332–339. doi: 10.1038/icb.2010.152. [DOI] [PubMed] [Google Scholar]
- 17.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiedenfeld EA, Fernandez-Viña M, Berzofsky JA, et al. Evidence for selection against human lung cancers bearing p53 missense mutations which occur within the HLA A*0201 peptide consensus motif. Cancer Res. 1994;54:1175–1177. [PubMed] [Google Scholar]
- 20.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 21.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 23.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rech J, Vonderheide RH. Dynamic interplay of oncogenes and T cells induces PD-L1 tumor microenvironment. Cancer Discov. 2013;3:1330–1332. doi: 10.1158/2159-8290.CD-13-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 27.Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. doi: 10.1177/030089161209800612. [DOI] [PubMed] [Google Scholar]
- 30.Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 31.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 32.Korkolopoulou P, Kaklamanis L, Pezzella F, et al. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer. 1996;73:148–153. doi: 10.1038/bjc.1996.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res. 2014;3:15–22. doi: 10.3978/j.issn.2218-6751.2013.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter SF, Minna JD, Johnson BE, et al. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–174. [PubMed] [Google Scholar]
- 35.Carbone DP, Ciernik IF, Kelley MJ, et al. Immunization with mutant p53- and K-ras-derived peptides in cancer patients: immune response and clinical outcome. J Clin Oncol. 2005;23:5099–5107. doi: 10.1200/JCO.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 36.Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. discussion 371–372. [DOI] [PubMed] [Google Scholar]
- 37.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 38.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuang X, Xia X, Wang C, et al. A high number of CD8+ T cells infiltrated in NSCLC tissues is associated with a favorable prognosis. Appl Immunohistochem Mol Morphol. 2010;18:24–28. doi: 10.1097/PAI.0b013e3181b6a741. [DOI] [PubMed] [Google Scholar]
- 40.Al-Shibli K, Al-Saad S, Andersen S, et al. The prognostic value of intraepithelial and stromal CD3-, CD117- and CD138-positive cells in non-small cell lung carcinoma. APMIS. 2010;118:371–382. doi: 10.1111/j.1600-0463.2010.02609.x. [DOI] [PubMed] [Google Scholar]
- 41.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 42.Petersen RP, Campa MJ, Sperlazza J, et al. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu K, Nakata M, Hirami Y, et al. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 44.Dai F, Liu L, Che G, et al. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopewell EL, Zhao W, Fulp WJ, et al. Lung tumor NF-κB signaling promotes T cell-mediated immune surveillance. J Clin Invest. 2013;123:2509–2522. doi: 10.1172/JCI67250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brahmer JR, Horn L, Gandhi L, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in patients with advanced non-small cell lung cancer (NSCLC); survival and clinical activity by subgroup analysis: results from a phase 1b study; Poster presented at the American Society of Clinical Oncology 2014 Annual Meeting; May 30–June 3, 2014; Chicago, IL, USA. poster 293. [Google Scholar]
- 48.Gettinger SN, Shepherd FA, Antonia SJ, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status; Poster presented at the American Society of Clinical Oncology 2014 Annual Meeting; May 30–June 3, 2014; Chicago, IL, USA. poster 38. [Google Scholar]
- 49.Rizvi NA, Chow LQM, Borghaei H, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC; Poster presented at the American Society of Clinical Oncology 2014 Annual Meeting; May 30–June 3, 2014; Chicago, IL, USA. poster 36. [Google Scholar]
- 50.Rizvi NA, Mazières M, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncology. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bristol-Myers Squibb Press Release (issued, 11 January 2015) CheckMate-017, A Phase 3 Study of Opdivo (Nivolumab) Compared to Docetaxel in Patients with Second-Line Squamous Cell Non-small Cell Lung Cancer, Stopped Early. http://news.bms.com/press-release/checkmate-017-phase-3-study-opdivo-nivolumab-compared-docetaxel-patients-second-line-s. Accessed 7 March 2015.
- 52.Garon EB, Gandhi L, Rizvi N, et al. Antitumor activity of pembrolizumab (pembro; MK-3475) and correlation with programmed death ligand 1 (PD-L1) expression in a pooled analysis of patients with advanced NSCLC; Presented at the European Society of Medical Oncology Congress; September 26–30, 2014; Madrid, Spain. (abstract LBA43) [Google Scholar]
- 53.Soria JC, Cruz C, Bahleda R, et al. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): Additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1) Eur J Cancer. 2013;49(Suppl 2) abstract 3408. [Google Scholar]
- 54.Brahmer JR, Rizvi NA, Lutzky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC; Poster presented at the American Society of Clinical Oncology 2014 Annual Meeting; May 30–June 3, 2014; Chicago, IL, USA. (abstract 8021) [Google Scholar]
- 55.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 56.Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137:1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alfonso S, Diaz RM, de la Torre A, et al. 1E10 anti-idiotype vaccine in non-small cell lung cancer: experience in stage IIIb/IV patients. Cancer Biol Ther. 2007;6:1847–1852. doi: 10.4161/cbt.6.12.5000. [DOI] [PubMed] [Google Scholar]
- 58.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 59.Ramalingam S, Crawford J, Chang A, et al. Talactoferrin alfa versus placebo in patients with refractory advanced non-small-cell lung cancer (FORTIS-M trial) Ann Oncol. 2013;24:2875–2880. doi: 10.1093/annonc/mdt371. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2:846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 61.FDA News Release (issued, 22 December 2014) FDA approves Opdivo for advanced melanoma. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427716.htm. Accessed 23 February 2015.
- 62.Ono Pharmaceutical Co., Ltd. Press Release (issued, 4 July 2014) Human anti-human pd-1 monoclonal antibody “OPDIVO® intravenous infusion 20 mg/100 mg” receives manufacturing and marketing approval in japan for the treatment of unresectable melanoma. Available at http://www.ono.co.jp/eng/news/pdf/sm_cn140704.pdf. Accessed 8 September 2014.
- 63.FDA News Release (issued, 4 March 2015) Nivolumab (Opdivo) Available at: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm436566.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery.
- 64.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 65.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 66.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 67.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 68.FDA News Release (issued, 4 September 2014) FDA approves Keytruda for advanced melanoma. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm412802.htm. Accessed 8 September 8 2014.
- 69.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13:847–861. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonia S, Goldberg S, Balmanoukian A, et al. Combination with tremelimumab in patients with advanced non-small cell lung cancer (NSCLC); Poster presented at the European Society of Medical Oncology Congress; September 26–30, 2014; Madrid, Spain. (poster 1327P) [Google Scholar]
- 73.Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control. 2014;21:80–89. doi: 10.1177/107327481402100112. [DOI] [PubMed] [Google Scholar]
- 74.Aranda F, Vacchelli E, Eggermont A, et al. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology. 2014;3(1):e27297. doi: 10.4161/onci.27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]


