Abstract
Adaptive behavior relies on the ability to effectively and efficiently ignore irrelevant information, an important component of attentional control. The current research found that fundamental difficulties in ignoring irrelevant material are related to dispositional differences in trait propensity to worry, suggesting a core deficit in attentional control in high worriers. The degree of deficit in attentional control correlated with the degree of difficulty in suppressing negative thought intrusions in a worry assessment task. A cognitive training procedure utilizing a flanker task was used in an attempt to improve attentional control. Although the cognitive training was largely ineffective, improvements in attentional control were associated with improvements in the ability to suppress worry-related thought intrusions. Across two studies, the findings indicate that the inability to control worry-related negative thought intrusions is associated with a general deficiency in attentional control.
Keywords: cognition and emotion, worry, cognitive training, rumination, attention
The type of repetitive thinking that occurs in worry and rumination is an important risk factor for anxiety and depression (Watkins, 2008). Worry has been defined as “a chain of thoughts and images, negatively affect-laden and relatively incontrollable” (Borkovec, Robinson, Pruzinsky, & DePree, 1983, p. 10), whereas rumination refers to repetitive and passive thinking about one’s mood and its consequences (Nolen-Hoeksema, 1991). Worry and rumination have much in common, both involving repetitive thinking about negative self-relevant topics, the main difference being the orientation of the negative repetitive thoughts. Worry typically has a primary focus on future threat, whereas rumination is oriented toward past negative events and failures.
The current study investigates worry, which is a salient symptom of high levels of trait anxiety and is a central diagnostic criterion of generalized anxiety disorder (GAD; Tyrer & Baldwin, 2006). Individuals with high levels of worry and those with GAD are characterized in particular by an inability to control worry once it has been initiated (Borkovec et al., 1983). For instance, when given instructions to actively worry about a personally relevant topic, individuals with high levels of self-reported worry report more negative thought intrusions during an attention focusing task compared with those with low levels of self-reported worry (Borkovec et al., 1983). The question remains which factors lead to the development of this pernicious and difficult to control type of repetitive thinking. A number of likely mechanisms have been identified. For instance, a recent cognitive model proposes that there are three fundamental building blocks of pathological worry (Hirsch & Mathews, 2012): biases in processing emotional information, depleted or misdirected executive control of attention, and the quasi-verbal form of worry itself. The authors point out that no one of these characteristics is unique to pathological worry, but they present evidence that these three building blocks combine in a particularly potent form in clinical conditions that are characterized by chronic worry such as GAD. Research taking an information processing approach to worry and anxiety has tended to focus on the first two building blocks—cognitive biases and deficits in attentional control.
The largest evidence base exists for biased information processing—the first building block—with a substantive literature demonstrating a variety of anxiety-related cognitive processing biases relating to threatening information. Common biases include shifts of attention, highly selective interpretation of ambiguity, as well as an elevated sense of personal risk (for comprehensive reviews, see Cisler & Koster, 2010; Mathews & MacLeod, 2005). These various different processing biases are likely to operate together, a combination that serves to maintain anxious mood states (Hirsch, Clark, & Mathews, 2006), are at least partially automatic, and may be better predictors of later physiological stress than self-report measures of trait anxiety and worry (Fox, Cahill, & Zougkou, 2010).
Deficits in attentional control and executive function—the second building block according to Hirsch and Mathews’s (2012) model—have not been investigated as comprehensively in relation to worry and anxiety. Nevertheless, the possibility is gaining ground that clinically relevant forms of worry (Eysenck & Derakshan, 2011) and rumination (Cohen, Mor, & Henik, 2014; Joormann, 2010) might be associated with fundamental problems in implementing attentional control. If people experience a specific difficulty in ignoring distracting thoughts and maintaining attentional focus, for instance, worry is likely to gain a stronger foothold in the cognitive system relative to those who have good attentional control.
The notion that deficits in attentional control are often a precursor to worry forms a cornerstone of the attentional control theory of anxiety (ACT; Eysenck, Derakshan, Santos, & Calvo, 2007). According to ACT, trait anxiety (and worry) disrupts the delicate balance between a stimulus-driven “bottom-up” attentional system and a goal-directed attentional system that is involved in the “top-down” control of attention (Yantis, 1993). Specifically, anxiety is assumed to increase the influence of the “bottom-up” system with a corresponding decreased influence of the goal-directed system (Eysenck et al., 2007). This imbalance leads to difficulties for the anxious person in controlling his or her natural tendency to selectively process threat-relevant material resulting in hypervigilance for threat and a consolidation of a variety of biased cognitive processes (cf. Hirsch & Mathews, 2012). ACT goes further than other models in predicting that this imbalance is also responsible for soaking up cognitive capacity, thus reducing efficiency and cognitive performance.
A number of gaps in our understanding of the etiology of worry remain. To a large extent this is because relatively few studies have examined whether attentional control is impaired in individuals prone to high worry using nonemotional stimuli. An early exception was the finding that when asked to name the color of a centrally presented colored bar, the degree of Stroop interference caused by spatially separate colored words was substantially larger in high trait-anxious relative to low trait-anxious participants (Fox, 1993). These results led Fox (1993) to propose that high trait anxiety—and by implication high worry—might be associated with a general deficit in inhibitory control, relating to a fundamental inability to maintain attentional focus even in the absence of threat-relevant stimuli (Fox, 1993). A more direct assessment of the inhibitory function in the absence of threat is gained by utilizing the antisaccade task, which has been identified as a relatively “process-pure” measure of inhibition (Miyake et al., 2000). This task involves presenting a visual cue to either the left or right of fixation and instructing the participant to make an eye movement to the opposite location to the cue as rapidly as possible. The main dependent variable is the latency of the first saccade to the correct side. In a series of experiments (Ansari & Derakshan, 2011; Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009), it was reported that high trait anxiety—and again, by implication, high worry—decreased the latency of the first saccade both when the cue was threat-related (an angry facial expression) and when it was neutral (an oval shape) indicating impaired inhibitory control in high trait-anxious participants.
Other studies have found evidence supportive of the notion that anxiety and worry are associated with impairments in executive control. For instance, when asked to engage in a random key pressing task while worrying, high worriers were found to have a restricted working memory capacity relative to low worriers (Hayes, Hirsch, & Mathews, 2008). Similarly, increasing cognitive load on experimental tasks is more disruptive for high trait-anxious relative to low trait-anxious individuals on performance (Berggren, Richards, Taylor, & Derakshan, 2013), and high trait-anxious individuals exhibit larger interference from flanking distractors, suggesting a greater difficulty in controlling interference (Pacheco-Ungietti, Acosta, Callejas, & Lupianez, 2010).
Measures of trait anxiety are typically obtained in studies investigating deficits in attentional control, whereas specific measures of worry are less common. Thus, although previous research is suggestive, given that worry is highly correlated with trait anxiety (Hirsch & Mathews, 2012), the question remains of whether high worriers are particularly impaired in tasks that challenge attentional control. Addressing this question is a central aim of the current study. In previous research with an unselected sample of participants, we have shown that fear conditioning angry facial expressions and then utilizing these conditioned faces as distractors in a flanker task leads to increased difficulties in attentional control, as indexed by larger interference effects on a speeded letter classification task on trials with conditioned “angry” relative to unconditioned “angry” facial distractors (Yates, Ashwin, & Fox, 2010). We chose this fear conditioning procedure to investigate the hypothesis that high worriers would show larger deficits in induced attentional control. It has previously been shown that the degree of self-reported worry is a good predictor of the strength of fear conditioning (Joos, Vansteenwegen, & Hermans, 2012; Otto et al., 2007), which led us to predict stronger fear conditioning—indexed by a larger attentional deficit in high worriers relative to low worriers when required to ignore fear conditioned angry expressions. We assume that this result, if supported, is a reflection of a natural process of greater difficulty for high worriers in ignoring distracting negative events or thoughts.
In addition to inducing deficits in attentional control, we also wanted to assess the frequency of actual negative thought intrusions in a worry assessment task (Borkovec et al., 1983), rather than relying solely on self-report measures of worry. No studies to our knowledge have directly assessed the ability to suppress worry or intrusive thoughts in relation to deficits in attentional control, and this is an important gap in our current understanding. For theory development and for the development of more effective therapeutic interventions, it is critical to establish whether a deficit in attentional control correlates with a deficit in the ability to suppress negative and distressing thoughts. This is a central aim of the current study.
Our key hypothesis is that there will be a positive correlation between (a) deficits in attentional control and (b) difficulty in suppressing worry-related negative thoughts. To investigate this hypothesis, Study 1 examined the association between attentional control and the ability to suppress worry-related intrusive thoughts in groups of high and low worriers. Study 2 selected groups of high worriers and investigated the impact of a brief attentional control training intervention using a neutral flanker task. The efficacy of our attentional training intervention was examined in terms of (a) reducing the degree of attentional control deficit induced by the fear conditioning task and (b) improving the ability to inhibit negative intrusive thoughts. A key question concerns the degree of association between attentional control and degree of worrisome intrusive thoughts experienced in the worry assessment task.
Study 1
The aim of Study 1 was twofold. First, we asked whether a deficit in attentional control induced by fear conditioning (Yates et al., 2010) would be magnified by individual differences in trait worry. Specifically, we tested whether the tendency for CS+ angry face distractors to capture attention to a greater extent than CS− angry or neutral expression face distractors during a flanker task would be larger in high worriers compared with low worriers. A series of studies has shown that high worry specifically relates to enhanced fear conditioning leading to increased interference and reduced cognitive capacity when fear conditioned neutral faces are presented (Joos et al., 2012; Otto et al., 2007). Added together with the finding that high worriers are characterized by reduced working memory capacity (Hayes et al., 2008), we were led to predict increased distractibility (i.e., deficits in attentional control) in the current task for high relative to low worriers. Second, we investigated whether the degree of deficit shown on the fear conditioning task would relate to the ability to suppress negative intrusive thoughts in a direct assessment of worry. Our hypothesis is that regardless of the level of trait worry the degree of attentional control will correlate positively with the ability to control worrying intrusive thoughts.
Method
Design
The study consisted of four phases: a baseline screening phase, a fear conditioning phase, an attentional control assessment phase, and a worry assessment phase. Following baseline screening, 12 undergraduate students categorized as “high worriers” (>56 on the Penn State Worry Questionnaire; PSWQ) and 12 who were categorized as “low worriers” (<56 on the PSWQ) were invited to take part in the study.
Participants
A total of 24 student participants aged between 18 and 55 years of age with either high (8 females, 4 males) or low scores (4 females, 8 males) on the PSWQ (Meyer, Miller, Metzger, & Borkovec, 1990) took part in the study. We did not record ethnicity. Average age did not differ between the high worry (M = 39.17, SD = 7.68) and the low worry (M = 44.58, SD = 9.29) groups, t(22) = −1.56, p < .13, Cohen’s d = −0.63, and the gender difference was not significant statistically, χ2 = 2.67, p = .22.
Materials and tasks
Emotional assessment questionnaires
Trait worry was assessed by means of the PSWQ (Meyer et al., 1990), which consists of 16 items (e.g., “When I am under pressure, I worry a lot”), each with a 5-point answer scale ranging from 1 (not at all typical of me) to 5 (very typical of me), yielding a total score ranging from 16 to 80, with higher levels indicating higher worry levels. A score of more than 56 is generally considered to reflect a “high” degree of worry.
Trait and state anxiety was measured by means of the State–Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Each of the Trait (STAI-T) and the State (STAI-S) forms consists of 20 statements relating to anxiety statements that participants rate on a 4-point frequency scale, yielding a total score for state anxiety (“how I feel right now”) and trait anxiety (“how I feel generally”) ranging from 20 to 80. There are no clinical cutoff scores, but in our studies with student populations we typically find that the median is around 40, and therefore we consider that scores above 40 reflect relatively high degrees of anxiety.
Depression was measured by means of the Beck Depression Inventory–II (BDI-II; Beck, Steer, & Brown, 1996), which consists of 21 items that participants rate on a 4-point Likert-type scale and total scores range from 0 to 63. Scores from 0 to 13 are considered to be within the minimal range, 14 to 19 reflect mild depression, 20 to 28 reflect moderate depression, and 29 to 63 are considered severe.
Fear conditioning procedure
The target faces for the fear conditioning and attentional control phases were three black-and-white photographs selected from the Ekman Pictures of Facial Affect set (Ekman & Friesen, 1976). These included two angry faces (A) and (B) (codes WF3-01 and JJ3-12, respectively) and one neutral face (code EM2-04). All stimuli had the hair and nonfacial areas removed in Adobe Photoshop (Adobe Systems, San Jose, CA), so that only the central face area was visible. The location of each target face subtended a visual angle of 10.23° vertically and 8.17° horizontally (450 × 350 pixels) and was positioned in the center of the screen. Each face was presented on a black background.
There were two stages in the fear conditioning phase: habituation and acquisition. The intertrial intervals varied between 15 s and 25 s, with a mean of 20 s, and these were presented equiprobably to mitigate against anticipatory and habituation effects. Across both habituation and acquisition the target faces were presented in a randomized order with the restriction that only two successive presentations of each face were presented and all were presented at a viewing distance of 56 cm from the screen, which was positioned at eye level.
The habituation stage exposed the participants to two non-reinforced presentations of angry face A, angry face B and the neutral face stimuli. Each trial began with a fixation cross, presented for 500 ms, followed immediately by one non-reinforced face for 100 ms followed by a blank screen. Participants were instructed to press one of two response buttons to indicate whether they liked or disliked the face in the photograph. Each face stimuli was presented twice resulting in a total of 6 habituation trials. During the subsequent acquisition phase each of the three faces was presented 20 times, resulting in a total of 60 acquisition trials. The acquisition stage consisted of 3 types of trials: CS+, CS−, or N−. Each trial began with a fixation cross presented for 500 ms, followed by the CS+, CS−, or the N− for 100 ms, followed by either a blank screen for 500 ms on CS− and N− trials. For the CS+ trials, the presentation of the appropriate angry face was immediately followed by the delivery of an auditory unconditioned stimulus (US), which was a 500 ms burst of white noise. The conditioned stimulus (CS+) was counterbalanced across participants, with half of the participants conditioned to angry face (A) and the other half conditioned to angry face (B), to rule out any confounding artifacts in the faces.
Attentional control (flanker) task
Attentional control was assessed by means of a flanker task that followed immediately after the fear conditioning phase. Each trial in the flanker task began with a central fixation cross presented at the center of the computer screen for 500 ms that was followed immediately by a target display of six letters at the center of the screen. A distractor face was presented equally often either above or below the target letters and the entire display (letters and distractor face) was presented for 100 ms. Target displays always consisted of a single target letter (x or z) along with five flanking letters that were all o (e.g., o o x o o o). Each target letter appeared equally often in each of the six possible locations. Participants were required to categorize the target letter (x or z) by pressing one of two response buttons (counterbalanced across participants) as quickly as possible and a blank screen was then presented until response. After response a blank screen was maintained for 500 ms until the beginning of the next trial. The computer emitted a 500-Hz feedback tone anytime a participant made an error.
To ensure that the CS+ angry face did not lose its aversion during these flanker trials (that contained no US), a reinforcement acquisition phase consisting of 12 (4 CS+, 4 CS−, and 4 N−) trials was added before each experimental block, using exactly the same design as the initial conditioning phase (see Yates et al., 2010). On each trial, the distractor face was equally likely to be the CS+, the CS−, or the neutral expression, either above or below the central letter display. The three distractor faces were presented 24 times in each block, creating a total of 72 trials in each block. There were four blocks in the experiment, making 288 trials in total.
Worry assessment task
This task was adapted from that developed by Borkovec et al. (1983) with elements from Wegner, Schneider, Carter, and White’s (1987) thought suppression “white-bear” task. The task consisted of three phases with each phase lasting 5 min. In the first phase (pretest), participants were asked to close their eyes and focus all their attention on their breathing. They were told that if their mind wandered they should just gently bring their attention back to their breathing. Following the pretest, participants engaged in the second phase of the procedure (worry induction). All participants were asked to identify the topic about which they were currently most worried. This was discussed briefly with the experimenter to ensure that it related to a potentially negative future situation. Participants were then asked to worry intensely about this topic in their usual fashion and continue until they were asked to stop. They were told that if their mind did wander to other topics during this period they should gently bring their attention back to their worry topic. After 5 min of worrying, participants immediately entered the third phase of the procedure (posttest). Participants were asked to now try to suppress their worry topic and not to think about this topic but rather to focus on their breathing just as in the pretest phase. However, participants were instructed to press a handheld counter if they did have a thought about their worry topic. The experimenter signaled the end of the 5-min posttest period and recorded the total number of intrusive thoughts that had occurred from the total score recorded on the handheld counter. They also interviewed each participant briefly to verify that each counter press related to a negative intrusive thought that related to their main “worry” topic.
Materials and stimuli
All stimuli were presented on a Macintosh iMac4 computer with the screen set at a resolution of 1,680 × 1,050 pixels. Stimulus presentation and data collection were controlled by SuperLab Version 4 software (Cedrus Corporation, San Pedro, CA) and response times (RTs) were collected by means of a USB-based RB-834 response pad with a built in timer that allowed data to be collected at 1 ms resolution (Cedrus Corporation). The aversive auditory stimulus of ~90db was delivered binaurally through Sennheiser HD 495 digital headphones connected to a sound card in the iMac computer.
Procedure
Participants initially completed the PSWQ and the STAI-T at a general departmental screening session early in the academic year and were asked to consent to being contacted for future studies. A total of 12 of those scoring more than 56 on the PSWQ and 12 scoring less than 56 on the PSWQ were subsequently contacted and asked to take part in a study designed to assess “worry and negative thought intrusions.” When they came to the lab the nature of the study was explained to them, and once they signed a consent form they were asked to complete the PSWQ once again. This was scored quickly by the experimenter in another room and if the score remained above 56 the participant was invited to a separate cubicle to undergo the fear conditioning procedure and the flanker task. Following this, each participant then moved to a different room that contained a comfortable armchair and low-level lighting. The procedure for the “suppression” version of the worry assessment task was explained and participants had the opportunity to ask any questions and practice with the handheld counter before they began. Finally, participants were debriefed and paid £6 or given course credit.
Results
As shown in Table 1, there were clear differences in the expected direction between the worry groups on self-reported worry, trait anxiety and depression at both baseline and at test.
Table 1.
Means and Standard Deviations (in Parentheses) for “High” (PSWQ > 56) and “Low” (PSWQ < 56) Worriers on Subjective Measures as Well as the Degree of “Response Interference” (i.e., RT Difference Between CS+ and N− Trials) and the Number of Thought Intrusions on the Worry Assessment Task
| Measure | High worry | Low worry | t(22) | Cohen’s d |
|---|---|---|---|---|
| PSWQ (baseline) | 61.75 (4.94) | 19.33 (3.42) | 24.46* | 9.98 |
| STAI-T (baseline) | 64.06 (7.37) | 36.08 (5.47) | 10.57* | 4.31 |
| BDI-II (baseline) | 22.92 (6.68) | 7.42 (1.88) | 8.97* | 3.16 |
| PSWQ (test) | 64.42 (6.24) | 20.67 (3.20) | 21.60* | 8.82 |
| Response interference | 53.58 (31.01) | 24.19 (25.71) | 2.53*** | 1.03 |
| Worry intrusions | 18.08 (6.40) | 10.00 (6.41) | 3.09** | 1.26 |
Note: BDI-II = Beck Depression Inventory–II; PSWQ = Penn State Worry Questionnaire; RT = response time; STAI-T = State–Trait Anxiety Inventory–trait.
p < .001.
p < .01.
p < .05.
Attentional control
RTs (ms) on error trials and RTs more than three standard deviations from the mean were removed from data before analysis and accounted for 2.4% of the data. A 2 (Worry Group: high, low) × 3 (Condition: CS+, CS−, N−) ANOVA revealed a main effect for Condition, Pillai’s F(2, 21) = 24.50, p < .001, partial η2 = .70, with responses for the CS+ condition (RT M = 640.38 ms, SD = 64.89 ms) being slower than the CS− condition (RT M = 616 ms, SD = 103.56 ms) and the N− condition (RT M = 601.47 ms, SD = 88.94 ms), confirming that the fear conditioning procedure was successful in increasing response interference (i.e., a deficit in attentional control). The Worry Group × Condition interaction failed to reach significance, Pillai’s F(2, 21) = 3.05, p < .07, partial η2 = .22. However, a planned t test on the magnitude of response interference (mean RT for CS+ minus mean RT for N−) supported the a priori hypothesis that high worriers showed greater response interference following fear conditioning relative to low worriers, t(22) = 2.53, p < .05, Cohen’s d = 1.03.
Worry thought intrusion
The number of worry thought intrusions recorded during Phase 3 of the worry assessment task are also shown in Table 1 for each of the worry groups. The a priori hypothesis that high worriers would exhibit more worry intrusions than low worriers was confirmed, t(22) = 3.09, p < .01, Cohen’s d = 1.26.
Correlation between attentional control and worry intrusions
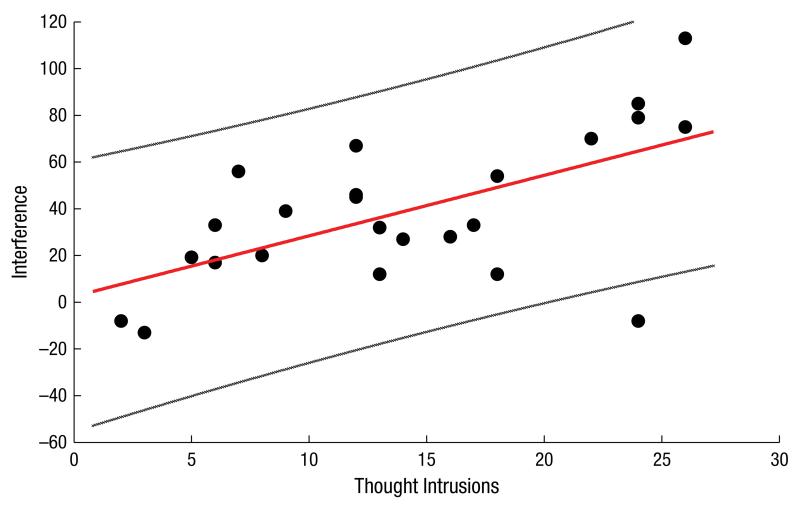
Figure 1 shows a scatterplot of the mean response interference scores and the mean number of thought intrusions for all participants. The zero-order correlation between response interference and number of worry thought intrusions was r = .61, p < .001, and this remained significant when controlling for PSWQ scores at test, r = .54, p < .01.
Fig. 1.
Scatterplot showing positive correlation between the magnitude of response interference and the number of negative thought intrusions reported for each participant in Study 1. The dotted lines represent 95% confidence intervals.
Discussion
We replicated previous findings that a fear conditioning procedure led to deficits in attentional control as measured by increased response interference in a flanker task in which participants had to ignore distracting photographs of facial expressions (Yates et al., 2010). Distracting angry facial expressions were more difficult to ignore when they had been conditioned by means of bursts of aversive white noise (CS+). As predicted, we found that this effect was greater in those who reported high relative to low levels of trait worry. These results are consistent with research showing that worry is associated with increased fear conditioning effects with neutral stimuli (Joos et al., 2012) and also supports research demonstrating that both high worry (Hayes et al., 2008) and high trait anxiety (Berggren et al., 2013; Pacheco-Ungietti et al., 2010) are related to deficits in attentional control.
Our second hypothesis that deficits in attentional control would be associated with greater difficulty in controlling negative thought intrusions was also supported. A positive correlation was found between the magnitude of response interference and the number of worry thought intrusions occurring during the worry assessment task. Even when controlling for self-reported trait worry, this relationship still held: Those with less attentional control on the flanker task experienced more negative thought intrusions on the worry assessment task.
Study 2
Study 1 found that a deficit in attentional control was associated with increased difficulty in suppressing negative intrusive thoughts. We do not know the nature of causation from these results. It may be the case that impaired attentional control leads to greater difficulties in suppressing worry, but it may equally be true that increased worry induces a high cognitive load thus impairing attentional and executive control (cf. Cohen et al., 2014). Study 2 was therefore conducted to address this question of causation and sought to establish whether training attentional control would reduce the number of negative thought intrusions occurring in high worriers. Specifically, we asked whether an induced improvement in attentional control would lead to an improvement in the ability to suppress worry.
To improve attentional control, participants were required to complete four training sessions across a 2-day period each lasting about 15 min (two training sessions per day). The active training task consisted of a modified low-load letter-classification flanker task (Lavie, 1995) in which participants had to categorize a centrally located target letter (z or x) by pressing one of two response keys while ignoring larger distractor letters that were either congruent with the target (e.g., x flanked by X) or incongruent with the target (e.g., x flanked by Z). Distracting letters were incongruent to the target on 80% of the trials and congruent on 20% of the trials to ensure that the task was difficult but with some congruent trials. A control training condition consisted of the same centrally located target letters but this, time there were no distracting letters presented.
Method
Design
The study consisted of four phases: a baseline screening phase, a baseline assessment phase, a training phase, and a posttraining assessment phase. Following baseline screening, 28 undergraduate students categorized as “high worriers” (>56 on the PSWQ; Meyer et al., 1990) were invited to take part in the study.
Participants
A total of 28 student participants who were part of a second-year research methods course (taught by E.F.) reporting scores of 56 or above on the PSWQ (Meyer et al., 1990) at both baseline screening and at test took part in the study. All participants were between 18 and 47 years of age, and we did not record ethnicity. In all, 14 participants were randomly assigned to “active” training, and 14 were randomly assigned to “control” training groups. The gender balance was similar across active (6 female, 8 male) and control (8 female, 6 male) training groups, as was age (M = 38.36, SD = 11.44 and 38.43, SD = 8.88, for active and control training groups, respectively), t(26) < 1, Cohen’s d = −0.01.
Materials and procedure
Baseline pretraining assessment
The nature of the study was explained to participants, and they were asked to sign an informed consent form before proceeding. Each participant then completed the PSWQ again, followed by the STAI-T and the BDI-II. They were then brought into an experimental cubicle to complete the fear conditioning and the attentional control (flanker) tasks exactly as in Study 1. Fear conditioning consisted of the habituation and acquisition trials with the same equipment, procedure, and stimuli as before. The flanker task followed immediately after the acquisition trials and again was identical to Study 1. Following this, each participant then moved to a different room that contained a comfortable armchair and low-level lighting. The procedure for the worry assessment task was explained and participants had the opportunity to ask any questions and practice with the handheld counter before they began. This task was identical to that used in Study 1. At the conclusion of the worry assessment task, participants had a short break. They then returned to the cubicle in which they had completed the fear conditioning and flanker tasks and the nature of the training trials were explained. Participants then completed a block of practice training trials to ensure that they understood the procedure, and a timetable for their four subsequent sessions was agreed with the experimenter. An appointment was then made for the posttraining assessment 3 days later.
Training phase
Each participant returned to the laboratory on four separate occasions for 2 days following baseline (pretraining) assessment. Training consisted of a modified form of a letter cancelation task developed by Lavie (1995) that was originally designed to measure the degree of response interference from to-be-ignored distracting letters. The targets were the lower-case letters z and x—each requiring a different keyed response—and these were presented in a light gray color on a black background, and at a viewing distance of about 56 cm they each subtended a visual angle of about 0.61° vertically and 0.51° horizontally. The target letter appeared equally often in one of six central locations and each of the other locations was occupied by o on every trial. For the active training group, distractor letters were also presented on every trial. These were presented in uppercase (X or Z) and appeared randomly and equiprobably either above or below the central display. Distractor letters (in uppercase) subtended a visual angle of 1.03° vertically and 0.51° horizontally and the distractor edge (either above or below) was about 1.75° of visual angle from the central fixation. For the control training group no distractors appeared on any trial. Before each trial, a light gray fixation cross was presented at the center of the screen for 1,000 ms. This was immediately replaced by the letter display, which appeared for 100 ms. Participants used the same response pad as they had used for the fear conditioning procedure and response mapping was counter-balanced across participants. Thus, half of the participants pressed the left-hand response key for x and the right-hand key for z, whereas this was reversed for the other participants. Response mapping was always kept consistent for each participant between the preassessment flanker task and the training task. Following the response to the target letter, there was an intertribal interval of 1,000 ms before the next trial began.
Each training block consisted of 80 trials and each training session consisted of three blocks of trials (i.e., 240 trials per session). Target displays were identical for the active and control training groups. For the active training group, 80% of trials contained incongruent distractor letters (e.g., z flanked by X), whereas 20% of the trials were congruent (e.g., z flanked by Z). No distractors appeared for the control training group. Each participant completed four training sessions of three blocks so that the total number of trials was 960 for each participant. Participants completed two 10- to 15-min training sessions a day (morning and afternoon) in the laboratory.
Posttraining assessment
Participants returned to the laboratory the day following their last training session or on the afternoon following their last morning session. Thus, the posttraining assessment occurred either 2 or 3 days following the pretraining baseline assessment. Following a brief discussion to ensure that they had completed all of their training sessions, each participant was brought into the lab and underwent the fear conditioning and flanker task exactly as in the pretraining session. The mapping of faces in the conditioning task (i.e., either Face A or Face B was selected as the CS+) was kept the same as in the pretraining session. However, for the flanker task the response mapping of the target letter x and z was reversed from that which the participants had used in the training sessions. This was to increase the difficulty of the flanker task to provide a better index of the participants’ ability to ignore the distracting facial stimuli.
Following this, each participant then moved to a different room that contained a comfortable armchair and low-level lighting. The procedure for the suppression version of the worry assessment task was explained—this was identical to the pretraining assessment, although participants were informed that they did not have to think about the same worry if this had changed. Again, the instruction was to “worry about the topic that causes you most worry at the moment.” Finally, participants were debriefed and given course credit for their participation.
Results
As shown in Table 2, there were no differences prior to training between the active and control training groups on self-reported trait anxiety, depression, or worry. The degree of response interference and number of thought intrusions was also equivalent between the groups.
Table 2.
Means and Standard Deviations (in Parentheses) for “Active” (With Distractors) and “Control” (No Distractors) Training Groups on Subjective Measures as Well as the Degree of “Response Interference” (i.e., RT Difference Between CS+ and N− Trials) Prior to Training (Response Interference—Pre) and Following Training (Response Interference—Post) and the Number of Thought Intrusions on the Worry Assessment Task Both Before (Worry Intrusions—Pre) and After (Worry Intrusions—Post) Training
| Measure | Active | Control | t(22) | Cohen’s d |
|---|---|---|---|---|
| PSWQ (baseline) | 65.00 (5.13) | 61.43 (4.03) | 2.05 | 0.77 |
| STAI-T (baseline) | 55.21 (16.18) | 49.00 (13.63) | 1.10 | 0.41 |
| BDI-II (baseline) | 15.36 (8.83) | 14.50 (8.06) | <1 | 0.10 |
| PSWQ (test) | 65.36 (6.04) | 61.07 (5.85) | 1.91 | 0.72 |
| Response interference—pre | 43.21 (19.19) | 28.73 (27.28) | 1.62 | 0.61 |
| Response interference—post | 25.43 (11.43) | 25.57 (39.52) | <1 | −0.00 |
| Worry intrusions—pre | 15.50 (5.16) | 15.14 (8.76) | <1 | 0.02 |
| Worry intrusions—post | 12.14 (4.54) | 14.43 (6.71) | −1.06 | −0.40 |
Note: BDI-II = Beck Depression Inventory–II; PSWQ = Penn State Worry Questionnaire; RT = response time; STAI-T = State–Trait Anxiety Inventory–Trait.
Attentional control
RTs (ms) on error trials and RTs more than three standard deviations from the mean were removed before analysis and accounted for 1.8% of the data. For ease of analysis interference scores were calculated by subtracting RTs on N− trials from RTs on CS+ trials for both pretraining and posttraining RTs on the flanker task. Thus, high scores indicate greater difficulty in ignoring a fear conditioned face. Interference scores were entered into a 2 (Training Group: active, control) × 2 (Assessment Period: pretraining, posttraining) ANOVA. There was no main effect for either Training Group, F(1, 26) < 1, partial η2 = .00, or Assessment Period, Pillai’s F(1, 26) = 3.92, p < .06, partial η2 = .13, and no significant interaction between these two factors, Pillai’s F(1, 26) = 1.91, p < .18, partial η2 = .07. We conducted further a priori planned comparisons, which showed that although there were no differences in the magnitude of response interference either before or after training between the two training groups the magnitude of response interference did decrease significantly from pre- to posttraining for the active training group (M = 43.21 versus 25.43), t(13) = 2.57, p < .05, Cohen’s d = 1.16. The degree of nonoverlap between the two assessment periods for this group was 59.1% (Cohen, 1988). There was no change from before to after training for the control training group, t(13) < 1, Cohen’s d = 0.10, with a nonoverlap between the two assessment periods of 7.7%. Note that Cohen’s d was calculated using the pooled variance as the denominator due to repeated measures for these comparisons.
Worry thought intrusion
The number of worry thought intrusions recorded during Phase 3 of the worry assessment task both before and after training are also shown in Table 2. These mean worry intrusions were subjected to a 2 (Training Group: active, control) × 2 (Assessment Period: pretraining, posttraining) ANOVA. This showed no main effects for either Training Group, F(1, 26) = 3.02, p < .10, partial η2 = .1, or Assessment Period, Pillai’s F(1, 26) < 1, and the interaction term was not significant, Pillai’s F(1, 26) = 1.27, p < .27, partial η2 = .5. As shown in Table 2, there were no differences in number of mean worry intrusions prior to training as expected, but against expectations there were also no differences following four sessions of training, t(26) = −1.06, p < .15, Cohen’s d = −0.40. The a priori hypothesis that those in the active training group would show a reduced number of worry thought intrusions from before to after training was not confirmed (M = 15.50 versus 12.14), t(13) = 1.73, p < .054. The effect size was medium (Cohen’s d = 0.71), showing a nonoverlap between the two assessment periods of 43.2%. There was no reduction in worry thought intrusions in the control training group, t(13) < 1, and the effect size was low (Cohen’s d = 0.09), with a nonoverlap of 7.4%. Note that Cohen’s d was calculated using the pooled variance as the denominator due to repeated measures for these comparisons.
Correlations between attentional control and worry intrusions
As found in Study 1, there were positive correlations between response interference and number of worry thought intrusions (when controlling for PSWQ scores at test) for measures made both during the pretraining session (r = .56, p < .01) and the posttraining assessment (r = .62, p < .001). The magnitude of response interference at pre- and posttraining sessions also correlated (r = .42, p < .05), as did the number of worry thought intrusions at pre- and posttraining sessions (r = .54, p < .01), again controlling for PSWQ scores at pretraining.
It is important that the degree of change in response interference (response interference posttraining minus response interference prior to training) correlated with the degree of change in the number of worry thought intrusions (intrusions at posttraining minus intrusions prior to training), r(25) = .50, p < .001, even when controlling for PSWQ scores at baseline, r(25) = .54, p < .001. These correlations between the degree of change in attentional control and degree of change in number of worry thought intrusions (controlling for baseline PSWQ) were significant only for the active training group, r(11) = .65, p < .05, and not for the control training group, r(11) = .39, although sample size here is very small.
Discussion
The results show that four sessions of training on an attentional control task did not reduce the magnitude of interference induced by fear conditioned angry faces. Moreover, the critical interaction that we expected between training group (active versus control) and time of assessment (pre- versus posttraining) also did not reach statistical significance. Nevertheless, a planned comparison revealed that attentional control (as measured by decreases in response interference) did improve following four sessions of the active training intervention with a relatively large effect size (Cohen’s d = 1.16), whereas no improvement was observed following four sessions of the control training intervention (Cohen’s d = 0.10). The number of worry thought intrusions, however, did not decrease significantly following four sessions of active cognitive training, in spite of the significant increase in attentional control in this group. Although the decline in negative intrusions from before to after the attentional control training intervention in this active training group was not significant, the effect size was medium: Cohen’s d = 0.71. In contrast, the effect size of the nonsignificant decrease in the control training group was low: Cohen’s d = 0.10. In support of our primary hypothesis, however, the results did show that the magnitude of improvement in attentional control from before to after training was correlated with the magnitude of decrease in the frequency of negative thought intrusions occurring on the worry assessment task.
General Discussion
Across two experiments strong positive correlations between deficits in attentional control and difficulty in suppressing worry-related negative thought intrusions were found. Individuals reporting higher levels of self-reported worry demonstrated (a) increased distractibility from fear conditioned angry facial expressions when presented as to-be-ignored distractors on a flanker task and (b) greater difficulty in suppressing worry relative to low worriers. In a second experiment with a sample of high worriers, strong positive correlations between attentional control and ability to suppress negative thought intrusions were again apparent. Following four sessions of attentional control training (by means of a letter flanker task) there was some evidence that as attentional control improved, so did the ability to suppress worry-related intrusive thoughts when compared with a control training condition.
These results add to a growing body of evidence that high worry is characterized by deficits in attentional control (Hayes et al., 2008), which is a central prediction of current cognitive models of pathological worry and anxiety (Eysenck et al., 2007; Hirsch & Mathews, 2012). The current studies used fear conditioning to induce deficits in attentional control and found support for previous demonstrations that repetitive worrying thoughts about negative life events is associated with higher levels of conditionability (Joos et al., 2012; Otto et al., 2007). Joos et al. (2012) suggested that there are likely to be two reasons for stronger fear conditioning in high worriers. First, repetitive thought as measured by the PSWQ might lead to more conditioned responding to the CS+ because of an inflation of the aversive value of the US representation (US inflation). Alternatively, it might be the case that the repetitive nature of worry itself means that people high in trait worry are more prone to a behavioral style of mental rehearsal leading to an enhanced learning of the CS-US association. In other words, high worriers who constantly reflect on their experiences could rehearse the CS-US contingencies resulting in a strengthening of the memory trace of this association (Joos et al., 2012). We cannot separate these potential mechanisms in the current study, but our results do indicate that the heightened conditionability of high worriers results in deficits in attentional control as measured by a flanker task.
The current results go considerably further than previous studies in showing that enhanced distractibility—induced by fear conditioning—is directly associated with difficulties in suppressing intrusive thoughts in a worry assessment task rather than just in correlations with questionnaire indices of worry. Finally, we found some evidence that improving attentional control by means of training on a letter classification flanker task was correlated with improvements in the ability to control negative thought intrusions in a worry assessment task. Although the overall effects of our training task were not significant the correlation observed between the degree of improvement in attentional control and the decline in the number of negative thought intrusions occurring in a worry assessment task suggests that attentional control training on a neutral cognitive training task may produce benefits that transfer to an enhanced ability to control the frequency of worry. This hypothesis needs to be tested and confirmed in a larger study.
The current results present a first step in supporting the hypothesis that pathological worry and the ability to control negative repetitive thinking may be associated with general deficits in attentional control. The implication is that these cognitive deficits are likely to play a causal role in the development of negative repetitive thinking that, in turn, is an important cognitive marker of psychopathology (Eysenck et al., 2007; Hirsch & Mathews, 2012). There are of course a number of clear limitations to the current studies. First, the sample sizes were small and some critical statistical effects were nonsignificant. There is therefore a need to replicate some of the core findings from the current two studies with larger samples. Although some effects were nonsignificant we are, however, encouraged by the fact that the critical effect sizes ranged from medium to large suggesting that this line of work is worth pursuing.
Another limitation of the current study relates to the fact that that we presented just four short sessions of training across 2 to 3 days. It is likely that this level of training was too brief to have strong and enduring effects on attentional control. To illustrate, recent studies investigating the impact of working memory training on improving executive control have used considerably longer and more frequent sessions of training (Owens, Koster, & Derakshan, 2013; Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, 2013). Owens et al. (2013) demonstrated that eight 30-min sessions of general working memory training resulted in significant gains in working memory capacity and improved inhibitory function for dysphoric participants, whereas an emotional version of working memory training presented over 20 days of 20- to 30-min training sessions resulted in significant gains in the ability to regulate emotions (Schweizer et al., 2013). Against this, another recent study has reported that a single session of cognitive training designed to improve executive control was successful in reducing self-reported state rumination (Cohen et al., 2014). Cohen et al. (2014) modified the flanker task by pairing emotional and neutral pictures with incongruent trials in such a way that those in the active training condition were required to recruit attentional control when processing emotional stimuli, whereas the opposite was true for those in the control training condition. This training task successfully reduced the degree of rumination reported on a self-assessment questionnaire.
The worry assessment task itself is another limitation of the current study. We combined aspects of Borkovec et al.’s (1983) worry assessment task with elements of Wegner et al.’s (1987) “white bear” paradigm. Participants were asked to press a handheld counter whenever they experienced an intrusion of a worry-related thought that they were trying to suppress. Although this is an ecologically acceptable measure of thought intrusion it does, of course, orient people to the very thought that they are trying to suppress. Even when trying to block out specific thoughts it is necessary to constantly monitor thought processes for precisely those forbidden thoughts to comply with the task. Colette Hirsch and her colleagues have developed a better modification of the Borkovec et al. (1983) worry assessment task that consists of a 5-min phase in which participants focus their attention on their breathing followed by a 5-min worry period just as in the current study. The second phase is then followed by a second 5-min breathing period. During each of the 5-min breathing phases, participants are prompted at 12 random intervals and asked to report what they were thinking of at that moment (Hirsch, Hayes, & Mathews, 2009). The outcome measure is the frequency and nature of thoughts that are reported at these moments and extensive ratings are obtained from participants regarding the nature of the thoughts occurring, which are then assessed by at least two experimenters for worry-related content. The frequency of negative intrusive thoughts on this task has been reduced by cognitive bias modification (CBM) interventions designed to shift negative cognitive biases to process threat (Hayes, Hirsch, & Mathews, 2010; Hirsch et al., 2009). The current studies use a similar worry assessment task, albeit with the disadvantage that the worry-related thoughts are primed to some extent because of the counter-pressing task, but nevertheless provide some evidence that boosting attentional control with a neutral flanker can also lead to a reduction in intrusive negative thinking.
Although there are clear limitations of the current study, the results nevertheless provide interesting new data on the association between attentional control and the ability to suppress negative thought intrusions. When attentional control was improved following training there was evidence that the ability to control the incidence of worry was reduced. It would be useful for future research to build on these results by utilizing a better measure of worry—perhaps that developed by Hirsch et al. (2009)—as well as developing a more effective attentional control training procedure.
Several questions remain that point to the potential shape of a worthwhile research agenda. First, it is important to develop effective cognitive training interventions to boost attentional control. At the moment, it is not clear whether such training needs to include affective stimuli (Cohen et al., 2014; Schweizer et al., 2013) or whether training methods with nonaffective stimuli might be just as effective (Owens et al., 2013; current studies). Second, we do not know whether it is best to target working memory capacity (Owens et al., 2013; Schweizer et al., 2013) or ability to ignore distracting stimuli (Cohen et al., 2014; current studies), or indeed some other aspect of cognitive control, to improve the ability to control worry. Third, we do not understand why training is effective in some individuals but not in others as was observed in the current study and in more general studies of cognitive training (Jaeggi, Buschkuehl, Jonides, & Shah, 2010). Therefore, a useful focus for future research would be to determine what training interventions are most likely to lead to enduring improvements in attentional control and transfer to control of worry. This would require a systematic comparison of different active training interventions (e.g., training that targets working memory versus distractibility) with adequate sample sizes (based on a power analysis). The active training interventions should be compared with a plausible control training condition that engages participants to the same extent as the active training. Ideally, assessments of attentional control as well as direct assessments of the ability to control worry would be taken at varying time-intervals to determine whether the effects of training are long-lasting, or as is more likely, several booster sessions are required. To illustrate, an intense 2-week session of physical exercise will boost physical fitness but this improvement will decline over time if no further activity is undertaken. It is highly likely that cognitive training methods designed to boost attentional control will also require on-going follow-up sessions to maintain the benefits.
In addition, based on Hirsch and Mathews (2012) cognitive model, the optimal cognitive training methods in terms of improving the ability to control worry are likely to combine elements of shifting negative cognitive biases while simultaneously targeting attentional control or executive function. However, there is still little empirical evidence for this prediction. CBM interventions designed to shift negative biases have been highly inconsistent in terms of their impact on clinical outcome measures, and the field is characterized by low-quality randomized controlled trials (RCTs) with small sample sizes (Cristea, Kok, & Cuijpers, 2014). However, when good quality RCTs are selected and training and clinical outcomes are assessed under controlled conditions (e.g., in the lab versus at home), there is some evidence that CBM is effective in clinically anxious populations (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, in press).
A pertinent point that has been argued is that one reason why clinical outcomes are often not observed following CBM is because the training intervention has not successfully modified the negative bias in the expected direction (Clarke, Notebaert, & MacLeod, 2014). If the magnitude of negative bias has not been reduced, then the information processing model would not predict significant change in emotional reactivity or clinical outcome, which is of course what is often found (Clarke et al., 2014). This has led some commentators to conclude that a great deal of basic science work is required before CBM interventions can be presented as therapeutic interventions for clinical populations (Fox, Mackintosh, & Holmes, 2014). An important research focus is therefore the development of optimal methods to modify negative cognitive biases. As an aside, we note that meta-analysis that combine across CBM studies that were designed to modify biases in interpretation and biases in attention (e.g., Cristea et al., 2014) miss the point that these different cognitive biases reflect different cognitive mechanisms that theoretically are pathways to quite different clinical outcomes (Williams, Watts, Mathews, & MacLeod, 1997). We would suggest that it is important for future research to assess the impact of modifying biases in attention, interpretation and memory separately (as well as together) in terms of impact on ability to control worry. Once this has been established, it is then important to systematically assess whether cognitive training methods designed to enhance the ability to control worry need to target (a) specific cognitive biases (interpretation, attention, memory), (b) specific combinations of cognitive biases (e.g., interpretation and attention), (c) specific aspects of attentional control (working memory capacity, distractibility etc.), and (d) whether combining various training approaches (e.g., CBM and working memory training) leads to greater benefits and transfer to the control of negative repetitive thinking than cognitive training that targets a single mechanism (e.g., distractibility). There is clearly a lot to do in this important field of clinical psychological science.
In summary, there is a need for basic science research to establish the optimal type, frequency, and duration of cognitive training interventions to translate these interventions into treatment strategies to help people improve their ability to control the persistent negative repetitive thinking that is characteristic of both worry and rumination. More generally, such prospective studies should aim to determine (a) what training interventions are most likely to lead to long-term improvements in the ability to control negative intrusive thoughts, (b) what cognitive and neural mechanisms are responsible for these improvements when they do occur, and (c) what individual characteristics influence whether a cognitive training intervention will be successful or not.
Acknowledgments
Funding
This work was conducted while all of the authors were at the Department of Psychology, University of Essex and was supported by a project grant from the Wellcome Trust to E.F. (Grant 07670/Z/05/Z). The writing of the article was supported by a European Research Council Advanced Investigator award to E.F. (CogBIAS Project; Grant 324176), who is now at the University of Oxford.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- Ansari TL, Derakshan N. Anxiety impairs inhibitory control but not volitional action control. Cognition & Emotion. 2010;24:241–254. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berggren N, Richards A, Taylor J, Derakshan N. Affective attention under cognitive load: Reduced emotional biases but emergent anxiety-related costs to inhibitory control. Frontiers in Human Neuroscience. 2013;7:188. doi: 10.3389/fnhum.2013.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: Some characteristics and processes. Behaviour Research and Therapy. 1983;21:9–16. doi: 10.1016/0005-7967(83)90121-3. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJF, Notebaert L, MacLeod C. Absence of evidence or evidence of absence: Reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry. 2014;14:8. doi: 10.1186/1471-244X-14-8. doi:10.1186/1471-244X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen N, Mor N, Henik A. Linking executive control and emotional response: A training procedure to reduce rumination. Clinical Psychological Science. 2014;3:15–25. doi:10.1177/2167702614530114. [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: Meta-analysis. British Journal of Psychiatry. 2014;206:7–16. doi: 10.1192/bjp.bp.114.146761. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. Experimental Psychology. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fox E. Attentional bias in anxiety: Selective or not? Behavior Research and Therapy. 1993;31:487–493. doi: 10.1016/0005-7967(93)90129-i. [DOI] [PubMed] [Google Scholar]
- Fox E, Cahill S, Zougkou K. Preconscious processing biases predict emotional reactivity to stress. Biological Psychiatry. 2010;67:371–377. doi: 10.1016/j.biopsych.2009.11.018. doi:10.1016/j.biopsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Mackintosh B, Holmes EA. Travellers’ tales in cognitive bias modification research: A commentary on the special issue. Cognitive Therapy and Research. 2014;38:239–247. [Google Scholar]
- Hayes S, Hirsch CR, Mathews A. Restriction of working memory capacity during worry. Journal of Abnormal Psychology. 2008;117:712–717. doi: 10.1037/a0012908. [DOI] [PubMed] [Google Scholar]
- Hayes S, Hirsch CR, Mathews A. Facilitating a benign attentional bias reduces negative thought intrusions. Journal of Abnormal Psychology. 2010;119:235–240. doi: 10.1037/a0018264. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Clark DM, Mathews A. Imagery and interpretations in social phobia: Support for the combined cognitive biases hypothesis. Behavior Therapy. 2006;37:223–236. doi: 10.1016/j.beth.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Hayes S, Mathews A. Looking on the bright side: Accessing benign meanings reduces worry. Journal of Abnormal Psychology. 2009;118:44–54. doi: 10.1037/a0013473. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Mathews A. A cognitive model of pathological worry. Behaviour Research and Therapy. 2012;50:636–646. doi: 10.1016/j.brat.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences. 2010;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J. Inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19:161–166. [Google Scholar]
- Joos E, Vansteenwegen D, Hermans D. Worry as a predictor of fear acquisition in a nonclinical sample. Behavior Modification. 2012;36:723–750. doi: 10.1177/0145445512446477. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Linetzky MM, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. doi: 10.1002/da.22344. in press. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Otto MW, Leyro TM, Christian K, Deveney CM, Reese H, Pollack MH, Orr SP. Prediction of “fear” acquisition in healthy control participants in a de novo fear-conditioning paradigm. Behavior Modification. 2007;31:32–51. doi: 10.1177/0145445506295054. doi:10.1177/0145445506295054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Koster EHW, Derakshan N. Improving attention control in dysphoria through cognitive training: Transfer effects on working memory capacity and filtering efficiency. Psychophysiology. 2013;50:297–307. doi: 10.1111/psyp.12010. [DOI] [PubMed] [Google Scholar]
- Pacheco-Ungietti AP, Acosta A, Callejas A, Lupianez J. Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science. 2010;21:298–304. doi: 10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the emotional brain: Improving affective control through emotional working memory training. Journal of Neuroscience. 2013;33:5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory, STAI (Form Y): Self-evaluation questionnaire. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Tyrer P, Baldwin D. Generalized anxiety disorder. Lancet. 2006;368:2156–2166. doi: 10.1016/S0140-6736(06)69865-6. [DOI] [PubMed] [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner DM, Schneider DJ, Carter S, White T. Paradoxical effects of thought suppression. Journal of Personality and Social Psychology. 1987;53:5–13. doi: 10.1037//0022-3514.53.1.5. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod CM, Mathews A. Cognitive psychology of emotional disorders. 2nd ed. Wiley; Hoboken, NJ: 1997. [Google Scholar]
- Yates AJ, Ashwin C, Fox E. Does emotion processing require attention? The effects of fear conditioning and perceptual load. Emotion. 2010;10:822–830. doi: 10.1037/a0020325. doi:10.1037/a0020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. Stimulus-driven attention capture and attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1993;19:676–681. doi: 10.1037//0096-1523.19.3.676. [DOI] [PubMed] [Google Scholar]