Abstract
A well-established literature has identified different selective attentional orienting mechanisms underlying anxiety-related attentional bias, such as engagement and disengagement of attention. These mechanisms are thought to contribute to the onset and maintenance of anxiety disorders. However, conclusions to date have relied heavily on experimental work from subclinical samples. We therefore investigated individuals with diagnosed generalized anxiety disorder (GAD), healthy volunteers, and individuals with high trait anxiety (but not meeting GAD diagnostic criteria). Across two experiments we found faster disengagement from negative (angry and fearful) faces in GAD groups, an effect opposite to that expected on the basis of the subclinical literature. Together these data challenge current assumptions that we can generalize, to those with GAD, the pattern of selective attentional orienting to threat found in subclinical groups. We suggest a decisive two-stage experiment identifying stimuli of primary salience in GAD, then using these to reexamine orienting mechanisms across groups.
Keywords: anxiety, attention, cognition and emotion, emotional processing biases, selective attention
Experimental research suggests that dysfunctional forms of cognitive processing help to cause and maintain emotional disorders (Clark & Beck, 2010; Williams, Watts, MacLeod, & Mathews, 1997). Successful cognitive therapies involve identifying and challenging these dysfunctional cognitions. One example is biased attentional processing of emotional information, which is particularly implicated in the anxiety disorders (Yiend, 2010). Individuals with anxiety (clinically disordered and subclinically anxious) typically prioritize processing of threatening information in the visual environment in preference to benign or positive information (see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van Ijzendoorn, 2007, for meta-analysis of visual-spatial attentional “probe” tasks). This cognitive pattern is assumed to lead to exaggerated negative perceptions and evaluations, which helps maintain anxiety, establishing a vicious cycle of cause and effect (Mathews, 1990). Experimental findings to date have supported this view, demonstrating that attentional biases toward negative information are associated with clinical and subclinical anxiety using a range of stimuli including words, faces, and pictures (for reviews, see Bar-Haim et al., 2007; Mathews & MacLeod, 1994; Yiend, 2010).
This fundamental research in experimental psychopathology has emphasized that the direct targeting of dysfunctional biases in attention is an important strategy in the treatment of anxiety disorders. In particular, “attentional training” (sometimes called attention bias modification procedures or ABM) is aimed at reducing symptoms and behaviors associated with anxiety by systematically reducing negative attentional biases and training selective attention to orient away, or to disengage, from threat (Koster, Fox, & MacLeod, 2009; Woud & Becker, 2014). For example, in one study (Amir, Beard, Burns, & Bomyea, 2009), 14 patients with generalized anxiety disorder (GAD) were assigned to an active ABM procedure in which attention was systematically directed away from threat words, whereas 15 were assigned to a control training procedure in which attention was directed to threat-related and neutral stimuli equally often. Following eight sessions of training, there was a significant reduction in negative attention bias from pre- to post-ABM training in the active training group but not in the control group. Of importance, there was also a significant reduction in clinical symptoms in those who received the active training. It is remarkable that 50% of those who had received active training no longer met diagnostic criteria for GAD following the eight sessions compared with just 13% of those who had received placebo training. These results suggest that negative attentional biases may indeed play a critical role in the maintenance of GAD symptoms. Although subsequent studies have generally produced much smaller effect sizes, two meta-analyses support the view that ABM procedures show promise as a novel treatment for a variety of anxiety disorders (Hakamata et al., 2010; Hallion & Ruscio, 2011).
What has emerged in the recent literature is that the ability to manipulate attention biases is somewhat inconsistent and effect sizes on clinical outcome measures are generally lower than expected. In a useful overview, it has been noted that when a negative attention bias is successfully modified, a congruent impact on emotional reactivity occurs (Clarke, Notebaert, & MacLeod, 2014). However, the majority of studies of ABM in clinical groups have failed to shift attentional biases (7 out of 11), and therefore it is not surprising that the overall impact of ABM on clinical symptoms is inconsistent (Clarke et al., 2014). Several investigators, have suggested that there is now an urgent need to focus on maximizing the efficacy of bias modification procedures (Clarke et al., 2014; Fox, Mackintosh, & Holmes, 2014; Lester, Mathews, Davison, Burgess, & Yiend, 2011; Yiend, Lee, et al., 2014; Yiend, Parnes, Shepherd, Roche, & Cooper, 2014). To optimize such interventions, it is, however, vital to have a clearer understanding of the nature of the mechanism of change in specific anxiety disorders such as GAD.
To improve our understanding of attentional bias mechanisms, the field has borrowed from mainstream attentional research. There, an important conceptual distinction is that between selective attention (selection) and attentional orienting (orienting; Yiend, 2010). Bias in selective attention refers to certain material (threat, in the case of GAD) being prioritized over other material for further processing and is typically measured by traditional attentional bias tasks, such as the so-called attentional probe task. Attentional orienting, on the other hand, can be thought of as one possible mechanism by which attentional selection can be implemented. Orienting refers to the process of moving attention to a location, either in space (spatial orienting) or, less commonly, in time (temporal orienting).1 Orienting frequently uses Posner’s distinction among shifting, engagement, and disengagement of attention (Posner & Petersen, 1990). A long-standing concept in attention research, it has been of particular interest recently within psychopathology research as a means to further specify the cognitive mechanisms by which attentional biases operate.
Although selective attentional bias favoring threat in GAD is well evidenced, research on the components of orienting (disengage, engage) that might underlie this effect have, to date, been largely restricted to subclinical samples. A growing literature in subclinical anxiety has suggested that there are different components of anxiety-related attentional bias and that these may have different clinical implications. However, this has been assumed more often than tested in clinical populations. For example, it has been shown that participants reporting high levels of trait anxiety take longer to disengage their attention from threat-related words and faces (Fox, Russo, Bowles, & Dutton, 2001; Fox, Russo, & Dutton, 2002; Georgiou et al., 2005), affective pictures (Yiend & Mathews, 2001), and locations associated with negative outcomes (Derryberry & Reed, 2002). This suggests that anxiety-related attentional biases may be associated with problems in disengaging attention from negative material as well as enhanced engagement with threat (Fox, Mathews, Calder, & Yiend, 2007; Mathews, Fox, Yiend, & Calder, 2003). Attentional orienting mechanisms have already been investigated in social phobia (Amir, Elias, Klumpp, & Przeworski, 2003), but this disorder can show attentional effects at odds with those of other anxiety disorders (Staugaard, 2010), therefore results may not be generalizable to GAD.
Obtaining relevant empirical evidence about attentional orienting mechanisms underlying biased processing of threat in GAD patients is important for various reasons. First, cognitive theories of emotional disorders, including GAD, propose cognitive biases (including attentional bias) to be key factors in the etiology and maintenance of the psychopathology (Mathews & MacLeod, 2005). Biases are not seen as mere epiphenomena of altered mood states, but rather are considered to play an important role in increasing the risk of disorder onset, maintenance, and, if left unresolved after treatment, recurrence of a disorder. This position is supported by empirical evidence using longitudinal and manipulation designs, supporting a bidirectional causal model (Van Bockstaele et al., 2014) as well as pharmacological studies (e.g. Murphy et al 2008). Second, it is important to identify the appropriate cognitive mechanisms to target in the treatment of GAD. Biases in different components of attentional orienting could have different clinical implications. For instance, if individuals with clinical anxiety show speeded engagement toward threat, then detection and evaluation processes are implicated, suggesting that therapists might focus on reducing patients’ sensitivity to threat. If disengaging from threat is impaired, this suggests patients might derive more benefit from improving their ability to disregard negative information. Third, elucidating the involvement of specific orienting mechanisms (engagement, disengagement, or both) in GAD should more broadly enhance the development of translational research, such as the ABM training techniques described earlier. The primary aim of the present study was therefore to investigate the spatial attentional orienting mechanisms underlying threat-related selective attentional biases in GAD.
The data reported here derive from two separate experiments, both involving patients meeting diagnostic criteria for GAD. Both studies attempted to identify the specific components of attentional orienting underlying naturally occurring selective attentional bias to threat. Although it is likely that GAD is characterized primarily by attention biases that are specific to personal concerns and worries, there is evidence that GAD patients, relative to matched controls, show attentional biases involving more general threat, for example involving angry facial expressions (Ashwin et al., 2012; Bradley, Mogg, White, Groom, & de Bono, 1999). Therefore, to facilitate comparisons with previous studies in subclinical anxiety (Fox et al., 2001; Fox et al., 2007; Georgiou et al., 2005), we used emotional and neutral facial expressions in the current investigations.
In the first of two experiments, we assessed the disengagement of attention from angry, happy, and neutral facial expressions in GAD patients and matched healthy volunteers using a task that we have previously used with individuals with subclinical anxiety (Georgiou et al., 2005). We assessed angry, rather than fearful, facial expressions because previous studies of disengage processes in trait anxiety (Fox et al., 2001) and biased attention in GAD (Ashwin et al., 2012; Bradley et al., 1999) have more typically used angry facial expressions. Experiment 1 also included a group of people reporting high levels of trait anxiety, but not meeting diagnostic criteria for GAD, for comparison with the previously reviewed findings in subclinical anxiety. GAD patients and high trait anxious groups were matched on self-reported trait anxiety (although not on depression), but differed on clinical status. This design allowed us to more clearly determine whether the pattern of attentional bias apparent in GAD would be similar to that found previously in subclinical anxiety.
The attention task used in Experiment 1 assessed the spatial orienting components of anxiety-related bias. The task involved the presentation of a face at the center of a computer screen for over half a second followed by a target letter that was flashed on the screen very briefly (50 ms) above, below, to the left, or to the right of the centrally located face. In a previous study with this task we reported that a subclinical group with high trait anxiety took longer to categorize the peripheral target letter when it was presented with a fearful face relative to when the centrally located face conveyed happiness, sadness, or a neutral expression (Georgiou et al., 2005), implying that high trait anxiety is linked with a delay in disengaging from fear-related material. In Experiment 1 the aim was to assess whether the GAD group would show a delay in disengaging from threatening (i.e., angry) facial expressions as was expected in the high trait anxious (subclinical) group. A delay was not expected in the matched control group.
Experiment 1
Method
Participants
A total of 14 GAD patients, 14 of their relatives (matched healthy volunteers), and 14 people with high levels of self-reported trait anxiety who did not pass criteria for GAD took part in the study. Patients were identified through clinician referrals for the North East Essex Mental Health Trust, and most were on a waiting list for an appointment with a clinical psychologist at the trust. Initial telephone screening was conducted using the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1996) by a researcher trained in clinical interviewing and the use of the SCID by an approved local trainer (M.L.). All patients were given the anxiety disorders modules, and additional relevant modules were completed as necessary. Inclusion criteria were likely diagnosis of GAD on the SCID, aged between 18 and 65 years, and native English speaking. Exclusion criteria (checked by telephone screening or at interview) were significant psychiatric comorbidity, addictions, or current major physical illness. Those in current receipt of psychological or pharmacological treatment were also excluded. Upon agreeing in principle to take part in the study, patients were asked to nominate a close relative who could also take part in the study as a matched healthy volunteer. A further group of 14 people who had reported high levels of trait anxiety (more than 45 on the State-Trait Anxiety Inventory trait anxiety scale) were also included in the study. These were recruited from the University of Essex campus and had responded to advertisements to partake in psychological studies. All healthy volunteers and high trait anxious participants were given a short form of the SCID either via telephone screening or at interview and were excluded if they reported any previous or current major psychopathology, major physical illness, or addictions.
Materials
Trait and state anxiety
The State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) is a well-validated self-report questionnaire. The trait anxiety form of the STAI consists of 20 items developed to measure the degree of dispositional trait anxiety. Participants score each item on a 4-point Likert-type scale, and the total score ranges from 20 (very low trait anxiety) to 80 (very high trait anxiety), with the population median being around 40. The state anxiety form of the STAI is similar but measures “how you feel now.”
Depression
The Beck Depression Inventory–II (BDI-II; Beck, Steer, & Brown, 1996) is a well-validated 21-item questionnaire that provides a measure of depression severity. Participants score each item on a 4-point Likert-type scale, and total scores of 0 to 13 are considered to be within the minimal range, scores of 14 to 19 reflect mild depression, scores of 20 to 28 reflect moderate depression, and scores from 29 to 63 are considered severe.
Mill Hill Vocabulary Scale
The Mill Hill Vocabulary Scale (MHVS; Raven, Court, & Raven, 1986) assesses verbal intelligence and consists of two lists of words divided into two sets (A and B) of 34 words, arranged in order of ascending difficulty, which those taking the test are asked to define. We used the multiple-choice version from Set B. Participants were asked to select the correct synonym from a list of six alternatives, and the maximum score was 33.
Attentional task
For this reaction time task, three different photographs were selected from the Ekman and Friesen (1975) set of emotional facial expressions. All were of the same individual but displayed different expressions: anger, happiness, and neutral. All photographs were presented in black and white, were matched for brightness, and measured 6.8 cm × 10.3 cm in size. In an earlier pilot study, 12 undergraduate students had rated the faces (among several other faces) in terms of whether they appeared to be “happy,” “sad,” “fearful,” “angry,” “surprised,” “disgusted,” or “neutral.” It was found that 100% categorized the angry face as “angry,” 100% categorized the happy face as “happy,” and 83.3% categorized the neutral face as “neutral,” whereas 16.7% categorized this face as “sad.” The target letters were P and X and were presented in Geneva size 24 font. They were presented 8 cm above, below, to the left, or to the right of the centrally presented face. At a viewing distance of 60 cm, this was 7.6 degrees of visual angle from the face stimulus.
Procedure
Testing took place either in a quiet room at the North East Essex Mental Health Trust in Clacton or at the University of Essex in Colchester. After consent procedures, participants completed the STAI–trait, BDI-II, and STAI–state forms, followed by the MHVS. Participants were shown the computer and button box, and the attentional task was explained in detail. It was explained that they would see an asterisk at the center of the screen and that they should keep their eyes focused on this location. It was explained that a face would shortly appear in this location followed by a letter (either X or P) above, below, to the left, or to the right of the face. They were instructed to keep their eyes on the face, but categorize the letter as quickly and accurately as possible by pressing either the red or the green button on the response box. Response mappings were counterbalanced across participants so that half pressed the red button for X whereas half pressed the green button for X and vice versa. Every trial began with an asterisk at the center of the computer screen for 1,000 ms. One of the three facial expressions was then presented, and after 600 ms one of the target letters was presented in one of the four locations for 50 ms. The face remained on the screen until the participant responded or, if there was no response, after 2,000 ms. There was a blank screen for 500 ms, and then the next trial began.
All participants completed a practice block of 28 trials, and once they were happy with the procedure they started the main experiment. This consisted of 288 trials, which were divided equally into trials with targets above (72), below (72), to the left (72), or to the right (72) of the face. For each location, the centrally presented face was equally often angry (96), happy (96), or neutral (96). Likewise, the actual target letter (X or P) appeared equally often with each facial expression and in each location. Each participant received a different randomized order of trials.
All stimuli were presented on a Power Macintosh 7200/90 computer with a 29 cm × 21 cm Sony Trinitron Multiscan screen. Presentation of stimuli and data collection was controlled by PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993), and reaction times were recorded on a USB-based RB-834 response pad with a built-in timer that allowed data to be collected with a 1-ms accuracy.
Results
Participant characteristics
Characteristics of the participants in the three separate groups are shown in Table 1. A series of one-way ANOVAs showed that there were differences across the three groups on age, F(2, 39) = 9.10, p < .01, η2p = .32; trait anxiety, F(2, 39) = 75.58, p < .01; BDI-II, F(2, 39) = 33.81, p < .01, η2p = .80; and state anxiety, F(2, 39) = 32.00, p < .01, η2p = .62. As expected the GAD and control groups were matched on age and MHVS scores, Fs < 1, η2p < .02, whereas the GAD group reported higher levels of trait and state anxiety and depression on the BDI-II, Fs > 29.91, ps < .01, η2p > .53. The GAD and high trait anxiety groups were matched on trait and state anxiety, Fs < 1, η2p < .04, whereas the GAD group reported higher levels of depression on the BDI-II, F(1, 26) = 4.53, p < .05, η2p = .15. The high trait anxiety group also reported higher levels of trait anxiety, state anxiety, and depression on the BDI-II in comparison with the control group, Fs > 94.69, ps < .01, η2p > .78. Finally, the high trait anxiety group was significantly younger than both the GAD and control groups, Fs > 11.95, ps < .01, η2p > .78.
Table 1.
Participant Characteristics for Experiments 1 and 2
| Experiment 1 |
Experiment 2 |
||||
|---|---|---|---|---|---|
| Characteristic | GAD (n = 14) | Controls (n = 14) | HTA (n = 14) | GAD (n = 21) | Controls (n = 21) |
| Age | 43.1 (7.1)a | 45.0 (8.6)a | 32.8 (8.6)b | 40.48a | 38.62a |
| STAI–State | 53.8 (12.5)a | 33.3 (6.4)b | 57.2 (4.8)a | 42.58a | 32.67b |
| STAI–Trait | 63.0 (8.7)a | 36.4 (5.3)b | 61.4 (4.3)a | 57.76a | 35.19b |
| BDI-II | 23.5 (8.2)a | 7.4 (1.7)b | 18.4 (3.9)a | 19.52a | 3.76b |
| HADS–Anxiety | 12.71a | 4.10b | |||
| HADS–Depression | 8.71a | 1.62b | |||
| LSAS–Fear or Anxiety | 29.79a | 13.24b | |||
| LSAS–Avoidance | 26.29a | 11.48b | |||
| MHVS | 18.9 (3.4)a | 19.4 (3.0)a | |||
| GHQ | 20.05a | 9.90b | |||
Note: BDI-II = Beck Depression Inventory–II; GAD = generalized anxiety disorder; GHQ = General Health Questionnaire; HADS = Hospital Anxiety and Depression Scale; HTA = high trait anxiety; LSAS = Liebowitz Social Anxiety Scale; STAI = State-Trait Anxiety Inventory. Within each experiment, superscript letters that are the same indicate no differences between the groups, whereas different superscript letters indicate that the groups differed significantly. Values in parentheses are standard deviations.
Attentional task
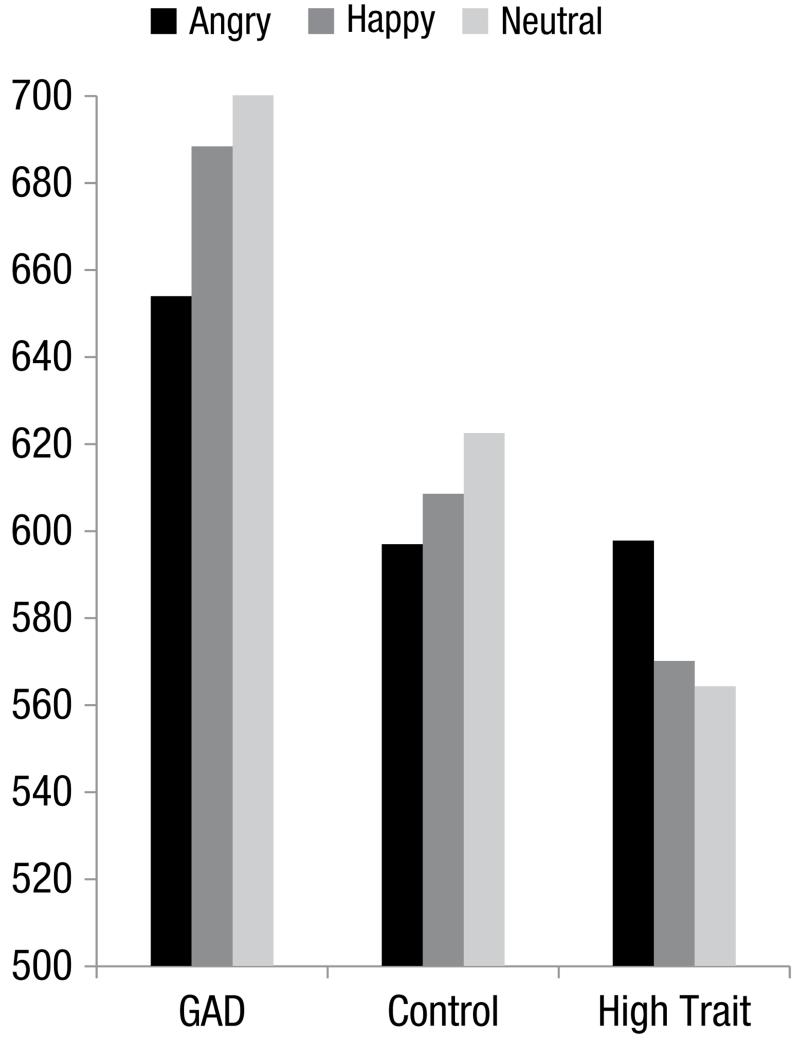
Reaction time data were prepared by removing trials on which errors occurred and eliminating high and low outliers (high outliers > 1500 ms, low outliers < 200 ms). In total, 3.5% of the data were excluded on this basis (errors were made on 2.4% of trials, and outliers represented 1.1% of trials).2 Mean reaction times by condition are shown in Figure 1. Data were analyzed by means of a 3 × 3 ANOVA, with factors Group (GAD, control, high trait anxiety) × Valence of Face (angry, happy, neutral). There were no main effects for either Group, F(2, 39) = 1.99, η2p = .09 or Valence of Face, F < 1, η2p = .02, whereas the Group × Valence of Face interaction did reach statistical significance, F(4, 78) = 3.56, MSE = 1828.83, p < .01, η2p = .16. Follow-up one-way ANOVAs were conducted on each Group separately for Valence of Face. For the GAD group, the main effect of Valence of Face was significant, F(2, 26) = 5.68, MSE = 1404.72, p < .01, η2p = .31. Further analysis using paired contrasts showed that reaction times for this group were faster when the central face was angry compared with neutral, F(1, 13) = 9.35, p < .01, η2p = .42. Reaction times for angry relative to happy face trials did not reach statistical significance, F(1, 13) = 4.03, p < .07, η2p = .24, and neither did those of happy compared with neutral face trials, F(1,13) = 1.65, η2p = .11. The main effect of Valence of Face did not reach significance for either the matched control group, F(2, 26) = 1.49, MSE = 1499.52, η2p = .10, p = .24, or the high trait anxious group, F(2, 26) = 1.72, MSE = 2582.33, η2p = .12, p = .20. Table 2 shows the relevant means and standard deviations for each group as a function of condition.
Fig. 1.
Mean reaction times (in milliseconds) for responses to peripheral target letters when centrally located faces conveyed an angry, happy, or neutral expression for individuals with generalized anxiety disorder (GAD), matched controls (Control), or those with high trait anxiety (High Trait).
Table 2.
Mean Reaction Times per Condition in Experiments 1 and 2
| Experiment 1 |
Experiment 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Peripheral Cuing Task |
||||||||
| Angry | Happy | Neutral | Angry | Fear | Happy | Neutral | ||
| GAD | 653.3 (152.9) | 687.6 (165.8) | 699.3 156.6) | |||||
| Healthy | 596.5 (129.7 | 608.0 (124.4) | 621.9 (136.0) | Invalid | 656.63 (15.5) | 654.50 (15.1) | 660.44 (16.7) | 668.47 (15.2) |
| High trait anxious | 597.3 (126.9) | 569.7 (150.7) | 563.9 (148.7) | Valid | 608.78 (15.2) | 616.14 (15.1) | 608.71 (14.6) | 600.14 (14.7) |
| Central Cuing Task |
||||||||
| Fear | Neutral | |||||||
|
|
||||||||
| 700 ms | 562.32 (13.8) | 555.05 (14.0) | ||||||
| 300 ms | 571.13 (13.7) | 573.50 (13.3) | ||||||
Note: Values are milliseconds, with standard errors in parentheses. GAD = generalized anxiety disorder.
Discussion
The findings of Experiment 1 imply that the pattern of impaired disengagement from threatening expressions, previously reported in high trait anxious subclinical samples, may not be directly generalizable to clinical anxiety. Although we did not replicate previous findings of impaired disengagement in individuals with subclinical high trait anxiety in the current study (Fox et al., 2001; Georgiou et al., 2005), we did find significant differences in the GAD group, in a direction opposite to that expected. Individuals with GAD were faster to respond to a peripheral target letter when a centrally presented face was angry relative to happy or neutral, a pattern not found in matched healthy volunteers. One explanation for these results is that GAD patients showed attentional avoidance of threatening facial expressions. Thus, rather than impaired disengagement of attention as expected, this patient sample appeared to show enhanced disengagement.
One problem with the task employed in Experiment 1 was that it is difficult to separate any effects of general interference from those specifically related to selective attention and attentional orienting. For instance, it is possible that patients were faster to respond on threat trials due to a generally increased arousal level in the presence of threat. Although randomly interspersing emotional and neutral trials might help protect against this, it remains possible that momentary fluctuations in physiological response to threat could account for a similar pattern of reaction times to that which we seek to attribute to attentional effects. We addressed this concern in Experiment 2 by using a methodology that provides a separate measure of general arousal, allowing us to more precisely isolate selective attentional effects. The paradigm chosen was the emotional adaptation of the so-called Posner peripheral cuing task (Fox et al., 2001; Yiend & Mathews, 2001). This task involves using emotional cues (here facial expressions) presented in the periphery of the visual field that capture attention at their location. The speed of identifying an arbitrary probe (such as a letter) at either the same or a different location from the cue acts as an indicator of the spatial orienting of attention and how orienting speed may vary according to the type of emotion depicted in the cue. On invalid trials attention must be disengaged from an emotional cue appearing in the periphery to detect a target occurring in a different location. By changing the emotion of the cue it is therefore possible to compare ease of disengaging attention from different types of emotional information.
Further limitations of Experiment 1 concerned the small sample size and the restricted range of facial expressions of emotion used in the study. With only 14 participants per group, power to detect small effects was low, and the possibility of false positives relatively high. Experiment 2 was therefore based on an a priori power calculation and tested groups of 21 GAD and 21 matched healthy volunteers. We also included a more comprehensive selection of facial expressions: fearful, angry, happy, and neutral.
Finally, Experiment 2 added a second task, an adaptation of a gaze direction cuing task, which has been used to assess the engagement component of spatial attentional orienting. Observing another person looking in a particular direction (eye gaze) has the effect of directing and engaging the observer’s attention to that same location (Driver et al., 1999; Langton & Bruce, 1999). Facial expression of emotion can therefore be used in combination with eye gaze to assess the effects of different emotional expressions on attentional engagement to a location cued by the direction of the gaze. On so-called congruent trials, if an emotional facial expression facilitates engagement to the location indicated by the averted eyes, that should lead to particularly fast (efficient) target identification compared with similar trials using neutral expressions with averted eye gaze. We have used this task in two previous studies with subclinical anxiety and found that those who reported high trait anxiety did show enhanced orienting toward a location (i.e., engagement) indicated by the eye gaze of a fearful facial expression relative to a neutral expression (Fox et al., 2007; Mathews et al., 2003). Of interest, on centrally cued trials in which the eye gaze does not move (very similar to the task used in Experiment 1 here), fearful expressions did not hold attention any more than neutral faces (Fox et al., 2007; Mathews et al., 2003), but angry facial expressions did hold the attention of high trait anxious participants to a disproportionate extent (Fox et al., 2007), indicating a difficulty in disengaging from angry facial expressions. We used just fearful and neutral expressions in the current investigation to determine whether a similar pattern of attentional orienting occurs in a group of patients diagnosed with GAD as we have observed in those reporting high levels of trait anxiety (Mathews et al., 2003). Once again, this is important to establish whether results found with subclinically anxious groups can be generalized to clinical groups.
Experiment 2
Method
Participants
Using 21 participants in each group this study had 80% power to detect a small effect size (f = 0.1) on the Trial Type (2) × Cue Type (2) within–between interaction, assuming six levels of repeated measurement (Erdfelder, Faul, & Buchner, 1996). A small effect on the task would equate to a difference of 20 ms on reaction times of around 500 ms, with a standard deviation of 100 ms.
A total of 21 GAD patients and 21 healthy volunteers participated in the study. Patients were identified through clinician referrals from Oxfordshire and Buckinghamshire Mental Healthcare Trust staff. These included consultant psychiatrists, psychologists, primary care counselors, and patient response to poster advertisements in a local psychiatric outpatient department. Initial telephone screening using the GAD-Q (Roemer, Borkovec, Posa, & Borkovec, 1995) was used to confirm likely GAD diagnosis. Exclusion criteria (checked by telephone screening or at interview) were significant psychiatric comorbidity, in current receipt of psychological or pharmacological treatment, current major physical illness, current addictions, and past serious head injury. Patients were not excluded if they had previously received an intervention for GAD but remained symptomatic at diagnostic level.
Healthy volunteers were recruited by responses to poster advertisement on local public notice boards, Internet advertisements, and local media publications. Exclusion criteria for healthy volunteers were checked during telephone screening and included past or present psychopathology as indicated by self-report, current major physical illness, current addictions, and past serious head injury. Inclusion criteria (in both groups) were age (18–65) and native English speaking. Despite screening procedures, 4 control participants reported levels of trait anxiety within the clinical range (50 or above on the STAI–trait; Spielberger et al., 1983). These participants were therefore ineligible to be included in the healthy control group and were replaced. This decision was made on a priori grounds, before any data analysis had been conducted, on the basis that all participants must meet the inclusion criteria for the relevant group to take part in the study. All participants had normal or corrected-to-normal vision.
Materials
All stimuli for the experimental tasks were taken from standardized sets. For the peripheral cuing task, Caucasian stimuli were selected from the JACFEE/JACNeuF sets of facial expressions (Matsumoto & Ekman, 1988). Eight identities of each emotion (happy, neutral, angry, and fearful) were chosen based on the normative data provided, each being presented a total of 12 times during the task. For the central cuing task, stimuli were those used previously by Mathews et al. (2003). Eight identities of each emotion (neutral and fearful) were used from the Ekman series on the basis of the normative ratings provided (Ekman & Friesen, 1976). Each identity had previously been digitally manipulated to produce eye gaze shift (left and right) for use on relevant trials. Stimuli were assigned to trial condition within each type of emotion according to a fixed random order.
Procedure
After completing consent procedures, healthy volunteers were asked to complete the General Health Questionnaire (Goldberg & Williams, 1988). Patients were given the SCID (First et al., 1996) by a researcher experienced in clinical interviewing and specifically trained in its use by an approved local trainer. All patients were given the anxiety disorders modules, and the SCID screen was used to identify additional relevant modules that were completed as necessary. Participants then received the following two computerized experimental tasks in counterbalanced order.
Peripheral cuing task
This task used the method employed by Yiend and Mathews (2001) and Fox et al. (2001) to compare attentional disengagement from faces of different emotional expressions. Participants fixated a central cross while a face cue appeared either on the left or right. Their task was to identify a subsequent target letter (E or F) as quickly as possible but without making errors. Targets either appeared opposite (an invalid trial) or in the same location (a valid trial) as the face cue. A total of 384 trials were presented, using a valid to invalid ratio of 2 to 1. Cues were presented for either 200 ms or 500 ms, and four different emotional facial expressions were used as cues: happy, angry, fearful, and neutral. The factors Cue Duration (2), Facial Expression (4), and Validity (2: valid, invalid) were used in a fully crossed design with 16 trials in each invalid condition and 32 in each valid condition. Trials were presented in a randomized order generated automatically by the computer software with optional rest breaks. The task lasted around 20 minutes.
Central cuing task
This task used the method employed previously by Mathews et al. (2003). Participants fixated a central cross, after which a face cue appeared in the center, replacing fixation. The eyes then shifted to the left or right, cueing attention to that location. The task was to identify a subsequent target letter (E or F) appearing in either the cued (congruent trials) or uncued (incongruent trials) location as quickly as possible but without making errors. A total of 384 trials were presented, using a congruent to incongruent ratio of 1 to 1. Cues were presented for two durations, 300 ms or 700 ms, and depicted either fearful or neutral facial expressions. The factors Cue Duration (2), Facial Expression (2), and Congruency (3: valid, invalid, central—eyes do not move) were used in a fully crossed design with 32 trials per condition, presented in a randomized order. The task lasted around 20 minutes, with optional rest breaks.
At the end of the experimental tasks all participants completed the following questionnaire measures in an individually allocated random order: the BDI (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), the Liebowitz Social Anxiety Scale (Liebowitz, 1987), the Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983), and the state and trait versions of the STAI (Spielberger et al., 1983).
Results
Participants
Table 1 shows participant characteristics. Patients and healthy volunteers differed significantly on all measures of mood state, trait, and symptoms, but not on age.
Peripheral cueing task
Error trials totaled 2.9% of the data and outliers 0.8% (high outliers > 1,370 ms; low outliers < 200 ms).3 Mean reaction times to identify the target in the peripheral cuing task were subjected to a mixed model ANOVA with one between-subjects factor, Group (patient, control), and three within-subjects factors, Cue Duration (200, 500 ms), Facial Expression (anger, fear, happy, neutral), and Validity (invalid, valid). There was a main effect of Validity, F(1, 40) = 30.67, p < .01, η2p = .43, reflecting faster reaction times on valid than invalid trials (608 ms, MSE = 14.73 vs. 660 ms, MSE = 15.58, respectively). A main effect of Cue Duration, F(1, 40) = 29.79, p < .01, η2p = .43, revealed that reaction times were faster when cues were presented for longer (624 ms, MSE = 14.51, vs. 645 ms, MSE = 14.60). No interactions involving Group approached significance, (all Fs < 2.5, largest η2p = .06), nor was there a main effect of Group (F < 1, η2p = .02). There was one significant interaction, Validity × Facial Expression, F(1,40) = 6.61, p < .01, η2p = .15. Table 2 shows the relevant means.
To interpret this interaction according to our hypotheses about the effects of emotional compared with neutral expressions, we used reaction times to neutral trials as a baseline against which to subtract the effects of emotion cuing for valid and invalid trials separately, using the following equation:
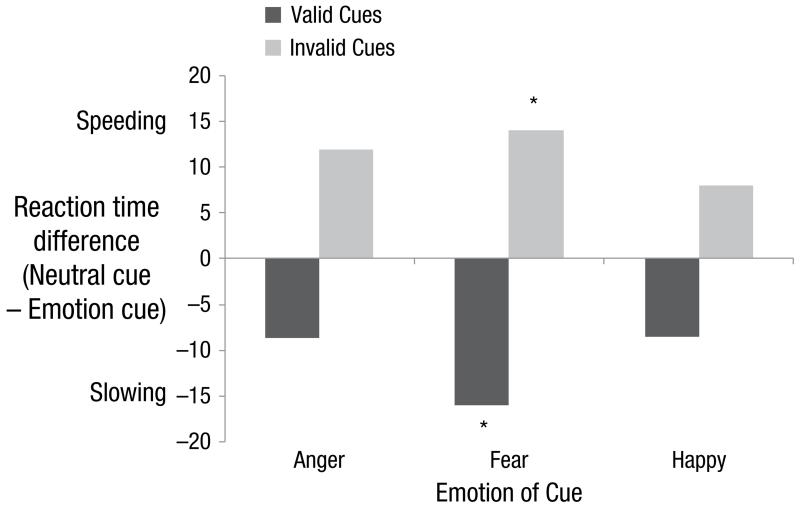
Thus a negative index indicates that emotion cues slowed reaction times, whereas a positive index indicates that emotion cues speeded reaction times, compared with neutral. Slowing on valid trials can therefore be interpreted as slower engagement to emotion, while speeding on invalid trials can be interpreted as faster disengagement from emotion. Subsequent analyses were carried out on these index scores. Figure 2 illustrates these data. For completeness, hypothesis-driven follow-up t tests were conducted comparing each index score to zero (no effect of emotion). After correcting for multiple comparisons, two effects remained significant. Fear cues significantly slowed reaction times on valid trials, t(41) = 4.47, p < .01, d = 0.69, and significantly speeded reaction times on invalid trials, t(41) = 2.77, p < .01, d = 0.43.
Fig. 2.
The effects of emotional expression cues on spatial attentional orienting in the peripheral cuing task. Positive values reflect a reaction time speeding effect, and negative values a reaction time slowing effect, of emotion compared with neutral cues. *Contrast with zero survives correction for multiple comparisons.
In the preceding analyses there were no significant group interactions, suggesting that the pattern of orienting applied to GAD patients and healthy volunteers alike. However, given the purpose of the experiment and the previous literature, a further hypothesis-driven analysis was conducted as a stringent test of whether the pattern of findings held true in the patient sample alone. The main analysis was repeated on the patient sample only, namely a repeated measures ANOVA of design Cue Duration (200, 500 ms) × Facial Expression (anger, fear, happy, neutral) × Validity (invalid, valid). This revealed a significant Validity × Facial Expression interaction, F(3, 60) = 2.98, p = .04, η2p = .13, as previously, with means following the same pattern as the main findings, reported earlier.
Central cueing task
Error trials totaled 1.8% of the data and outliers 2.1% (high outliers > 1,160 ms; low outliers < 100 ms).4 A mixed model ANOVA was conducted on mean reaction times to identify the target, with one between-subjects factor, Group (patient, control), and three within-subjects factors, Cue Duration (300, 700 ms), Facial Expression (fear, neutral), and Cue Congruency (central, congruent, incongruent). There was a main effect of Cue Congruency, F(2, 80) = 20.11, p < .01, η2p = .34, reflecting faster reaction times on congruent than central trials (556 ms, SE = 13.30 vs. 568 ms, SE = 13.70) and on central than incongruent trials (568 ms, SE = 13.70 vs. 572 ms, SE = 13.94). Thus the general effect of spatial attentional cuing on this task was as expected. A main effect of Cue Duration, F(2, 80) = 18.54, p < .01, η2p = .32, revealed that reaction times were faster when cues were presented for longer (559 ms, SE = 13.85 vs. 572 ms, SE = 13.45). No interactions involving Group approached significance (all Fs < 1.5, largest η2p = .03), nor was there a main effect of Group (F < 0.5, η2p < .01). There was one significant interaction, Cue Duration × Facial Expression, F(1, 40) = 6.98, p = .01, η2p = .15. Table 2 shows the relevant data. Follow-up pairwise comparisons showed that at cue durations of 700 ms (but not 300) participants were significantly slowed by fearful compared with neutral cues, t(41) = 2.55, p = .02, d = 0.40.
Discussion
Despite patients and healthy volunteers being highly differentiated in their levels of psychopathology, the two groups did not differ significantly in their attentional processing of emotional expressions on either of the two tasks administered. Instead, on the peripheral cuing task both anxious patients and healthy volunteers showed relative speeding on invalid trials with emotional cues, especially when fear-related compared with neutral cues were used. As in Experiment 1, this unexpected finding suggested faster, not slower, disengagement of attention from emotional expressions, a pattern that was especially unexpected for the GAD group based on previous results in subclinical anxiety. In addition, reaction times on valid trials suggested slower, not faster, engagement of attention to emotional expressions, especially fear, which again was particularly unexpected for the GAD group on the basis of previous nonclinical research. On both valid and invalid trials, fear cues were particularly effective at eliciting this pattern of spatial attentional avoidance, as illustrated in Figure 2. Of importance, there was no evidence of a general slowing effect of emotion, which can compromise the interpretation of cuing data (see Yiend, 2010, p. 29, for details).
On the second task, an emotional adaptation of an eye gaze cuing task, there was no evidence that spatial attentional orienting was influenced by the valence of the central cue. There was, as expected, a congruency or cue validity effect, but this did not interact with the emotional expression of the facial cue or participant group. In addition, irrespective of how attention was directed, participants showed a general slowing when fearful compared with neutral information was presented at the longer duration (700 ms). These results are in marked contrast to our previous findings in subclinical anxiety where the facial expression of the cue did influence the allocation of attention and this enhancement was influenced by the degree of self-reported trait anxiety. Specifically, fearful faces were more effective at eliciting a shift of attention to the gazed at location in individuals with high relative to low trait anxiety (Fox et al., 2007; Mathews et al., 2003). The absence of this pattern in the current sample of GAD patients further emphasizes the difficulty of generalizing from subclinical studies to clinical populations.
General Discussion
Experiments 1 and 2 produced conceptually similar patterns of results. Experiment 1 found that individuals meeting diagnostic criteria for GAD showed faster disengagement from angry than from neutral facial expressions, a pattern that was quite different from that found in a group of people who did not meet diagnostic criteria for GAD but who were matched with the clinical group on the level of self-reported trait anxiety. Experiment 2 showed spatial attentional orienting effects indicating avoidance of fearful facial cues that, once again, did not differ between GAD and healthy volunteers. Both groups showed avoidance of fearful expressions, being faster to disengage from, and slower to engage to, fearful compared with neutral or happy facial cues. Moreover, this pattern held up in a stringent hypothesis-driven test of the GAD patient sample alone. Using a gaze cueing task, the pattern of results found for our GAD sample was, once again, different from that previously found with the same task in people with subclinical levels of high trait anxiety (Fox et al., 2007; Mathews et al., 2003). The general implication of these findings, across the two experiments, is that previously reported anxiety-specific effects of impaired disengagement from, and speeded engagement toward, threatening information in subclinical samples may not be as relevant for clinical populations as has been widely assumed.
These results have important implications for experimental psychopathology. It is widely assumed that studies in subclinical analogue samples can be generalized to the corresponding clinical disorder, and this has particularly been the case for the phenomenon of impaired disengagement of attention in anxiety. However, there are insufficient published studies in clinical anxiety groups to validate this assumption. The present data with two samples of GAD patients underline the need for caution in generalizing previous findings from subclinical samples. Rather than delayed disengagement and faster engagement with fear-relevant stimuli as expected, we found faster disengagement and slower engagement with threat, a pattern indicative of attentional avoidance of threat-relevant material. Attentional avoidance of relatively mild levels of threat-relevant material has been reported elsewhere (Mogg et al., 2000; Wilson & MacLeod, 2003) and is integral to two current models of attentional orienting toward fear-relevant stimuli (Mathews & Mackintosh, 1998; Mogg & Bradley, 1998). It is proposed that this avoidance is an evolutionarily adaptive response allowing current goals to be pursued, unimpeded by relatively minor and insignificant environmental challenges. Of importance, however, the absence of this attentional avoidance of mild threat is considered to be an important cognitive component associated with high levels of trait anxiety (e.g., Fox et al., 2001; Yiend & Mathews, 2001). Although the present data are broadly consistent with this suggestion, the observation of this same pattern of avoidance in the clinical anxiety groups runs counter to expectations. Taken together, these studies could be taken to indicate that impaired disengagement from threatening information is a specific attentional marker of vulnerable, subclinical samples, but does not extend to, or may even be reversed in, clinical anxiety.
The implications of the findings we report here are especially pertinent for translational research. For example, new experimentally based treatments are being developed for various disorders based on manipulations of cognitive biases (e.g., Amir et al., 2009; Hayes, Hirsch, & Mathews, 2010; Lester et al., 2011; Yiend, Savulich, Coughtrey, & Shafran, 2011). Researchers applying these manipulations to clinical anxiety have generally assumed that it is necessary to correct the impaired disengagement of attention from threat, given the findings in subclinical samples. However, the pattern of data we report suggests that this assumption may not be warranted. The present data challenge this assumption and suggest that generalizing conclusions from subclinical to clinical anxiety may be premature. A more nuanced understanding of the pattern of spatial orienting to threat across clinical and subclinical anxiety may be required. Certainly, further work on the underlying mechanisms of spatial attention processing in GAD is now warranted.
As with all research, the studies reported here suffer from a number of limitations. For instance, our sample sizes were relatively small and may have been insufficient to detect between-group differences in the orienting of spatial attention. In the first experiment with only 14 participants per group, power to detect small effects was low. However the second experiment was specifically powered to detect the necessary interaction effect, assuming a small effect size (see methods section for power analyses). Moreover, the fact that both experiments found a broadly similar pattern of rapid disengagement, or avoidance, of threat-relevant cues that was opposite to that found in subclinical samples mitigates against issues of power being a parsimonious explanation of our findings. In addition, demonstration of the expected general-emotion-related spatial orienting effects found in Experiment 2 suggests that those tasks were appropriately sensitive, but that the pattern of attentional processing on these tasks previously observed in subclinical anxiety (Fox et al., 2001; Fox et al., 2007; Mathews et al., 2003) was not observed in a GAD sample.
A more nuanced possibility, and one which the present data cannot speak to, is that attentional orienting effects (engagement and disengagement) may operate over different timescales in clinical and subclinical samples. The results of Ellenbogen and Schwartzman (2009) raise this possibility. They tested 36 patients with a variety of anxiety disorders (11 had GAD as a primary diagnosis) and reported that they were fast to disengage from supraliminal threatening pictures (similar to the effects seen in the present data) and that impaired disengagement was limited to pictures presented subliminally. They concluded that this pattern of results reflected early vigilance followed by effortful avoidance. However, as their investigation included other conditions and clinical groups, the impact of their findings for GAD is difficult to assess. Nevertheless, our finding of attentional avoidance in a GAD sample with supraliminal stimuli is consistent with this notion. It is possible that we would have found the anticipated pattern of enhanced engagement and impaired disengagement had we also examined subliminal effects.
Arguably, the most important limitation of the present research concerns the nature of the stimuli used to test for attentional effects. Early findings, reviewed by Mathews and MacLeod (1994, p. 36), indicated that attentional effects may depend critically on relevance of the stimuli to the individual participant’s current emotional concerns. For example, socially phobic patients are particularly likely to attend to socially threatening words, whereas panic disorder patients are more likely to attend to physically threatening words. Even in nonanxious groups, words matching current emotional concerns are differentially attended, whether negative or positive in valence (Reimann & McNally, 1995). Facial stimuli may not therefore have tapped the most appropriate content for GAD, because the major symptom, worry, is thought to be primarily verbal. Indeed the original finding of attentional bias in GAD (MacLeod et al., 1986) used word stimuli, as have some successful GAD attentional training studies (Amir et al., 2009). Against this, some studies have used facial expressions as stimuli and found attentional differences between GAD and healthy participants (Ashwin et al., 2012; Bradley et al., 1999; Mogg, Millar, & Bradley, 2000; Waters, Mogg, Bradley, & Pine, 2008). This literature is somewhat difficult to interpret, however, as the studies are few and they use different measures of attention (e.g., eye movements; Mogg et al., 2000) or different samples (e.g., children; Waters et al., 2008). Another possible explanation for the mixed pattern of findings is that some GAD patients worry about social threats, and therefore biased attention to threat-related facial expressions would be a reasonable expectation. However, for many GAD patients, whose worries relate to other dimensions, these stimuli would not necessarily trigger attentional bias. It seems clear that more information is required, not only on attentional mechanisms in GAD, but also on exactly what type of stimuli are associated with triggering these mechanisms.
In light of the findings we present here, it is useful to consider how the field of attentional bias in GAD should seek to move forward to decisively resolve the questions raised by our data. In our view, the previously discussed link between the nature of the stimuli presented and the emotional concerns of the individual is the issue most in need of being addressed in future studies. We therefore advocate more precise specification of both the form and content of emotional cues that best match emotional concerns in clinical groups such as GAD, as a necessary precursor to revisiting the questions raised in the present experiments, such as the role of attentional engagement and disengagement, as well as of possible differences in this respect between clinical and high trait anxious groups.
The first necessary experimental step therefore involves establishing the type of stimuli that best evoke the primary emotional concerns of the target group (e.g., GAD patients), and determining how these stimuli differ from those in nonclinical groups, including those with high trait anxiety. This would determine whether specific concerns (e.g., about social disapproval) are less frequent or central in GAD patients than in high trait anxious groups, as discussed earlier. A related question concerns the way in which emotional concerns are typically represented: For example, in patients with social phobias, threats are typically represented in the form of images of oneself performing poorly in social situations, whereas in GAD patients, future threats are more often represented in quasi-verbal form (Hirsch, Hayes, Mathews, Perman, & Borkovec, 2012).
The second step would be to use this information to revisit the questions addressed in the current experiments: namely, to test whether stimuli known to evoke relevant emotional concerns in clinical groups elicit differential attentional engagement, slowed disengagement, or both, in comparison with nonclinical high trait anxiety and healthy volunteer groups. An ideal study would include analysis of a wide time scale of stimulus processing. Given the results of Ellenbogen and Schwartzman (2009), it would be important to assess attentional mechanisms with very fast (subliminal) presentation times up to much longer presentation times. The ideal study would be powered, a priori, to detect small effects, using either these or other similar relevant data. The sample should compare GAD patients with sociodemographically matched healthy volunteers and with a subclinical participant group also matched to patients for their levels of trait anxiety and depression. A study of this design would be well placed to provide essential new empirical data to guide future developments, not only in the field of attentional processing in anxiety per se, but also in the rapidly growing field concerned with the translational applications of this research.
Perhaps it will transpire that—given stimuli similarly evocative of individual emotional concerns—attentional (and other) effects are actually quite similar across clinical and subclinical anxiety, even if the form or content of the evocative stimuli differ. This finding would suggest that rather than the nature of attentional responding per se, it may be the type, intensity, or range of emotional concerns that is particularly characteristic of clinical conditions. Alternatively, it may be that the underlying pattern and direction of attentional processing does indeed differ across groups. Thus it may be the case that cues that are related to central emotional concerns do indeed elicit different degrees (or directions) of attentional engagement, or disengagement, in clinical than in comparison subclinical groups. Answering these questions is likely to be difficult, but addressing them is essential to throw light on the cognitive mechanisms that play a causal role in emotional disorders, as well as having obvious implications for identifying profitable targets for treatment.
In summary the two studies presented here suggest GAD patients may show a pattern of attentional biases opposite to that observed using similar methods in subclinical samples to date. Specifically, instead of impaired disengagement from threatening expressions, the data suggest selective attentional avoidance of threat-related facial expressions in GAD. These results pose a challenge to assumptions made to date about the generic nature of attentional biases in GAD, indicating that it may be premature to generalize from existing subclinical studies of attentional biases. Further work is undoubtedly needed to resolve the questions the present studies raise, and we have attempted to make some concrete suggestions for how best the field can be further advanced. This is particularly important in the context of developing cognitive manipulations (Hertel & Mathews, 2011) designed to modify specific biases that may be used in future treatments or treatment adjuncts. It will be important to have a deeper understanding of the nature and type of attentional biases that occur in GAD before we can be confident that reducing specific biases is likely to have clinical benefits. Our data point to a need for further basic research into patterns of attentional orienting in clinical anxiety.
Acknowledgments
The authors thank Katie Sheehan for assistance with the data collection in Experiment 2 and Sophie Lovejoy for assistance in experimental programming in Experiment 1.
Funding
This research was supported by Research Grant 782 awarded to Jenny Yiend by Oxfordshire Health Services Research Committee and by Project Grant 064290/Z/01/Z from the Wellcome Trust awarded to Elaine Fox and Riccardo Russo. Elaine Fox is currently supported by an Advanced Investigator Award from the European Research Council (Grant 324176).
Footnotes
Attention also may be oriented to particular stimulus dimensions that co-occur in the same spatial location at the same time (e.g., to the color or content of a word).
Analysis without the use of reaction time outlier cutoffs renders results uninterpretable. This is due to the resulting inclusion of (a) anticipatory responses and (b) responses involving temporary lapses of attention or inattention. This practice is in line with methodological guidelines given in Yiend and Mathews (2004).
See Note 2.
See Note 2.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: Facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Holas P, Broadhurst S, Kokoszka A, Georgiou GA, Fox E. Enhanced anger superiority effect in generalized anxiety disorder and panic disorder. Journal of Anxiety Disorders. 2012;26:329–336. doi: 10.1016/j.janxdis.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI–II, Beck Depression Inventory: Manual. 2nd ed. Harcourt Brace; Boston, MA: 1996. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT. Cognitive therapy of anxiety disorders. Guilford; New York, NY: 2010. [Google Scholar]
- Clarke PJF, Notebaert L, MacLeod C. Absence of evidence or evidence of absence: reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry. 2014;14 doi: 10.1186/1471-244X-14-8. doi:10.1186/1471-244X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, & Computers. 1993;25:257–271. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face: A guide to recognizing emotions from facial clues. Prentice Hall; Englewood Cliffs, NJ: 1975. [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Ellenbogen MA, Schwartzman AE. Selective attention and avoidance on a pictorial cueing task during stress in clinically anxious and depressed participants. Behaviour Research and Therapy. 2009;47:128–138. doi: 10.1016/j.brat.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Erdfelder E, Faul F, Buchner A. G*Power: A general power analysis program. Behavior Research Methods, Instruments, & Computers. 1996;28:1–11. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis 1 Disorders–Research Version. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fox E, Mackintosh B, Holmes EA. Travellers’ tales in cognitive bias modification research: A commentary on the special issue. Cognitive Therapy and Research. 2014;38:239–247. [Google Scholar]
- Fox E, Mathews A, Calder AJ, Yiend J. Anxiety and sensitivity to gaze direction in emotionally expressive faces. Emotion. 2007;7:478–486. doi: 10.1037/1528-3542.7.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: Evidence for delayed disengagement from emotional faces. Cognition & Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou GA, Bleakley C, Hayward J, Russo R, Dutton K, Eltiti S, Fox E. Focusing on fear: Attentional disengagement from emotional faces. Visual Cognition. 2005;12:145–158. doi: 10.1080/13506280444000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, Williams P. A user’s guide to the GHQ. NFER-Nelson; Windsor, England: 1988. [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Pine DS. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effects of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hayes S, Hirsch CR, Mathews A. Facilitating a benign attentional bias reduces negative thought intrusions. Journal of Abnormal Psychology. 2010;119:235–240. doi: 10.1037/a0018264. [DOI] [PubMed] [Google Scholar]
- Hertel PT, Mathews A. Cognitive bias modification: Past perspectives, current findings, and future applications. Perspectives on Psychological Science. 2011;6:521–536. doi: 10.1177/1745691611421205. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Hayes S, Mathews A, Perman G, Borkovec T. The extent and nature of imagery during worry and positive thinking in generalized anxiety disorder. Journal of Abnormal Psychology. 2012;121:238–243. doi: 10.1037/a0024947. doi:10.1037/a0024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Fox E, MacLeod C. Introduction to the special section on cognitive bias modification in emotional disorders. Journal of Abnormal Psychology. 2009;118:1–4. doi: 10.1037/a0014379. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6:541–567. [Google Scholar]
- Lester KJ, Mathews A, Davison PS, Burgess JL, Yiend J. Modifying cognitive errors promotes cognitive well being: A new approach to bias modification. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:298–308. doi: 10.1016/j.jbtep.2011.01.001. doi:10.1016/j.jbtep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Mathews A. Why worry? The cognitive function of anxiety. Behaviour Research and Therapy. 1990;28:455–468. doi: 10.1016/0005-7967(90)90132-3. [DOI] [PubMed] [Google Scholar]
- Mathews A, Fox E, Yiend J, Calder A. The face of fear: Effects of eye gaze and emotion on attentional engagement. Visual Cognition. 2003;10:823–835. doi: 10.1080/13506280344000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion (JACFEE) San Francisco State University, Department of Psychology, Intercultural and Emotion Research Laboratory; San Francisco, CA: 1988. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, McNamara J, Powys M, Rawlinson H, Seiffer A, Bradley BP. Selective attention to threat: A test of two cognitive models of anxiety. Cognition & Emotion. 2000;14:375–399. [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in EMs to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Yiend J, Lester K, Cowen PJ, Harmer CJ. Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. International Journal of Neuropsychopharmacology. 2008;12:169–179. doi: 10.1017/S1461145708009164. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford Psychologists Press; Oxford, England: 1986. [Google Scholar]
- Reimann BC, McNally RJ. Cognitive processing of personally relevant information. Cognition & Emotion. 1995;9:325–340. [Google Scholar]
- Roemer L, Borkovec M, Posa S, Borkovec TD. A self-report diagnostic measure of generalized anxiety disorder. Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:345–350. doi: 10.1016/0005-7916(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Staugaard SR. Threatening faces and social anxiety: A literature review. Clinical Psychology Review. 2010;30:669–690. doi: 10.1016/j.cpr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele BB, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EHW. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140:682–721. doi: 10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- Waters AM, Mogg K, Bradley BP, Pine DS. Attentional bias for emotional faces in children with generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:435–442. doi: 10.1097/CHI.0b013e3181642992. [DOI] [PubMed] [Google Scholar]
- Williams JM, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorder. 2nd ed. Wiley; Chichester, England: 1997. [Google Scholar]
- Wilson E, MacLeod C. Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology. 2003;112:212–218. doi: 10.1037/0021-843x.112.2.212. [DOI] [PubMed] [Google Scholar]
- Woud ML, Becker ES. Editorial for the Special Issue on Cognitive Bias Modification Techniques: An Introduction to a Time Traveller’s Tale. Cognitive Therapy and Research. 2014;38:83–88. [Google Scholar]
- Yiend J. Invited review. The effects of emotion on attention: A review of attentional processing of emotional information. Cognition & Emotion. 2010;24:3–47. [Google Scholar]
- Yiend J, Lee JS, Tekes S, Atkins L, Mathews A, Vrinten M, Shergill S. Modifying interpretation in a clinically depressed sample using “cognitive bias modification-errors”: A double blind randomised controlled trial. Cognitive Therapy and Research. 2014b Advance online publication. doi:10.1007/s10608-013-9571-y. [Google Scholar]
- Yiend J, Mathews A. Anxiety and attention to threatening pictures. Quarterly Journal of Experimental Psychology Section A—Human Experimental Psychology. 2001;54:665–681. doi: 10.1080/713755991. doi:10.1080/02724980042000462. [DOI] [PubMed] [Google Scholar]
- Yiend J, Mathews A. Biases in attention: Methods, mechanisms and meaning. In: Wenzel A, Rubin DC, editors. Cognitive methods in clinical research. APA Books; Washington, DC: 2004. pp. 97–117. [Google Scholar]
- Yiend J, Parnes C, Shepherd K, Roche M-K, Cooper M. Negative self-beliefs in eating disorders: A cognitive-bias-modification study. Clinical Psychological Science. 2014a Advance online publication. doi:10.1177/2167702614528163. [Google Scholar]
- Yiend J, Savulich G, Coughtrey A, Shafran R. Biased interpretations in perfectionism and their modification. Behaviour Research and Therapy. 2011;49:892–900. doi: 10.1016/j.brat.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. doi:10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]