Abstract
The NADPH oxidase of professional phagocytes has an important role in host defense against certain microbes, including tuberculous mycobacteria. The identification of patients with rare inherited hypomorphic mutations in genes encoding components of this enzyme complex could produce new mechanistic insights.
In the later part of the 19th century, Ilya Metchnikoff began to develop his ideas on phagocytosis (derived from the Greek word phagein, meaning ‘to eat’) as a mechanism of host defense using translucent star fish larvae and Daphnia water fleas to directly observe white corpuscle function in vivo. Some 50 years later, Baldridge and Gerard noted that this activity is accompanied by a massive oxygen consumption that is biologically distinct from mitochondrial respiration1. It is now known that the ‘respiratory burst’ characteristic of professional phagocytic cells is accomplished by NOX2, a specialized NADPH oxidase enzyme complex2. In this issue of Nature Immunology, Bustamante and colleagues describe a ‘macrophage-specific’ mutation in CYBB (which encodes the gp91phox component of NOX2) in two families with mycobacterial disease and suggest that this is a susceptibility gene to add to those already shown to compromise interferon-γ-mediated immunity3.
NOX2 consists of the membrane-bound flavocytochrome b gp91phox, which incorporates two heme moieties of low potential positioned on opposite sides of the membrane bilayer and transfers electrons from a cytosolic substrate NADPH to oxygen in the phagocytic vacuole, generating enormous quantities of superoxide (O2−), when activated during phagocytosis. Other components of the system include the similarly membrane-bound p22phox, cytosolic factors p40phox, p47phox and p67phox, and the Rho family GTPase p21rac, which all translocate to the membrane in a tightly regulated way (Fig. 1).
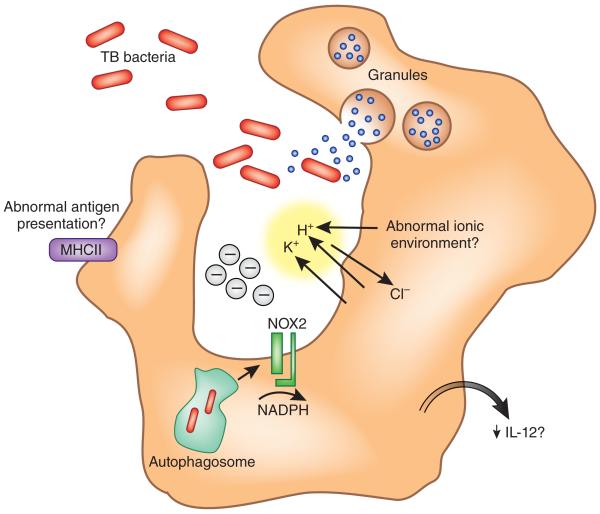
Figure 1.
Professional phagocytic cells, including macrophages and dendritic cells, depend on a functional NADPH oxidase for normal clearance of microbes. Deficiency in NOX2 leads to dysregulation of the ionic content of the phagocytic or autophagocytic vacuole and abnormal proteolysis, with possible downstream consequences for antigen presentation and production of inflammatory mediators important for elimination of intracellular organisms such as Mycobacterium tuberculosis (TB bacteria). MHCII, major histocompatibility complex class II.
The importance of the aerobic respiratory burst for the killing and digestion of pathogens has been recognized for many years, but the mechanisms of this process have remained controversial. Although the generation of toxic free radicals and halogenated derivatives of oxygen is an accepted explanation for this, closer inspection of physiologically relevant evidence suggests that the model is simplistic. More likely, a charge flux created by electron transport directly regulates the pH and ionic composition of the phagocytic vacuole. In this way, the largely cationic microbicidal enzymes released into the phagosome are made soluble by dissociation from the sulfated negatively charged granule matrix in an environment with a pH favorable for optimal activity.
The most impressive witness to the importance of NOX2 for microbial killing is the inherited immunodeficiency chronic granulomatous disease (CGD). Patients with CGD develop life-threatening infections with bacterial organisms such as Staphylococcus aureus and fungi such as Candida and Aspergillus. Less well known perhaps is the fact that they are more susceptible to tuberculous mycobacterial infections, including infection from immunization with the Mycobacterium bovis bacillus Calmette-Guérin vaccine (although apparently not infection by environmental nontuberculosis mycobacteria)4.
CGD is caused by mutations in genes encoding various components of the NOX2 enzyme complex, most commonly the gene encoding gp91phox (CYBB). Although a good proportion of these are effectively null and produce very little biochemical activity, a significant number (usually missense mutations) result in molecules that retain some superoxide production in isolated polymorphonuclear leukocytes (mainly neutrophils)5. The so-called ‘variant’ patients with these mutations may have less severe disease but even so can develop phenotypic manifestations of CGD. Does the proposal by Bustamante and colleagues that the ‘macrophage-specific’ CYBB mutation predisposes patients to susceptibility to mycobacterial disease3 stand up to close scrutiny? Well, yes and no. First, evidence for susceptibility to mycobacterial disease in the families would seem to be clear, as it segregates with the newly identified missense mutations defined in CYBB. What is less persuasive is the conclusion that macrophages (necessarily derived from monocytes in vitro) are severely functionally compromised, whereas freshly derived neutrophils and monocytes from the patients are entirely normal. This may be stretching a point, as the data shown could also be interpreted as indicating that there is defective expression of gp91phox in all phagocytic cells and that their superoxide-generating capacity is correspondingly suboptimal. In the absence of relevant surrogate killing assays, it is not really possible to say whether this is clinically or physiologically important. So, many would conclude that the patients described by Bustamante and colleagues fall into the usual category of CGD variants.
Even so, there are some interesting observations in this paper3 that deserve further attention, both in terms of NOX2 assembly and also the mechanisms of mycobacterial elimination. The expression of gp91phox in neutrophil and monocyte membranes is well above that necessary to produce normal NOX2 activity6. In contrast, macrophages cultured in vitro down-regulate expression of p47phox and, to a lesser extent, gp91phox, which results in functional deficiency7. This can be ‘rescued’ by priming agents, but that indicates that the process of tissue culture produces limiting conditions. Similarly, B cells immortalized by Epstein Barr virus generate much less O2− than phagocytic cells do as a result of the deficiency in both p67phox and gp91phox in the immortalized cells relative to that in professional phagocytic cells8. One possibility is that the incorporation of heme may be limiting, as this is necessary for the maturation of a 65-kilodalton gp91phox precursor and assembly of the membrane-bound flavocytochrome b9. Overall it would seem that defects that manifest in relatively minor ways in uncultured phagocytes are exaggerated in macrophages and B cell lines grown in the laboratory. How well this reflects the in vivo tissue macrophage situation therefore becomes a crucial consideration but is very difficult to assess in patients. The development of a targeted mouse model representative of the mutations described by Bustamante et al. would be of genuine assistance.
A more important question perhaps is how NOX2 contributes to immunity to tuberculous mycobacteria, and here there is no genuine mechanistic insight. Both NOX2-dependent phagocytic and autophagocytic protective mechanisms may be disturbed and could potentially contribute to impaired killing by macrophages10. Detrimental changes in the pH of the phagocytic vacuole would intuitively not seem to be important, as these organisms are susceptible to an acidic environment. In fact, they actively promote alkalinity as a survival strategy in part through inhibited accumulation of a vacuolar proton ATPase11. In CGD or when NOX2 activity is chemically suppressed, a phase of relative alkalinization of neutrophil vacuole contents is abrogated, leading to exaggerated acidification. However, there seem to be considerable differences between neutrophil and macrophage phagosomes in their maturation, in addition to their inventory of antimicrobial enzymes, and any disturbance in the ionic and pH environment may be operationally important12. In addition to macrophages, dendritic cells have NOX2 activity, which has been shown to be important for antigen processing and presumably, therefore, for the effective generation of an adaptive response13. Furthermore, there may be differences in cytokine output from cells deficient in NOX2, and specifically in those relevant to interferon-γ-mediated immunity14. Once again the contribution of these mechanisms to susceptibility to mycobacteria would need to be explored in more detail.
So what is the take-home message? With increasing clinical knowledge, there is no question that NOX2 has a role in protection against tuberculous mycobacteria. The cell type responsible for this deficiency would most logically be the macrophage, as these have been linked to the pathophysiology of many other mycobacterial susceptibility disorders. However, some dendritic cell subsets are closely related to macrophages and may well be important. At the biochemical level, the assembly of the NOX2 complex seems to differ among various cell types, and these mechanisms or restrictions are worthy of further study. Whatever else holds true, CGD patients are susceptible to tuberculous infection. Even for those with residual NOX2 activity who may be relatively protected against more typical bacterial and fungal infections, there is good reason to be cautious in terms of intracellular macrophage-resident organisms, including some mycobacteria. Therapeutically there are important considerations. Reconstitution of NOX2 expression in neutrophils in an amount sufficient to correct antimicrobial deficiency in this cell lineage by gene therapy may not necessarily predict protection against mycobacteria. Conversely, agents that enhance macrophage function in vivo may be particularly beneficial for patients with variant CGD who have intracellular microbial infection. As George Bernard Shaw wrote, “There is at bottom only one genuinely scientific treatment for all diseases, and that is to stimulate the phagocytes.”15
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Adrian J Thrasher, Centre for Immunodeficiency, University College London Institute of Child Health, London, UK.
Anthony W Segal, Department of Molecular Medicine, University College London, London, UK. a.thrasher@ich.ucl.ac.uk.
References
- 1.Baldridge LW, Gerard RW. Am. J. Physiol. 1932;103:235–236. [Google Scholar]
- 2.Segal AW. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustamante J, et al. Nat. Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee PP, et al. Pediatr. Infect. Dis. J. 2008;27:224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- 5.Kuhns DB, et al. N. Engl. J. Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal AW, Garcia R, Goldstone H, Cross AR, Jones OT. Biochem. J. 1981;196:363–367. doi: 10.1042/bj1960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy R, Malech HL. J. Immunol. 1991;147:3066–3071. [PubMed] [Google Scholar]
- 8.Chetty M, Thrasher AJ, Abo A, Casimir CM. Biochem. J. 1995;306:141–145. doi: 10.1042/bj3060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu L, et al. J. Biol. Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, et al. Proc. Natl. Acad. Sci. USA. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flannagan RS, Cosio G, Grinstein S. Nat. Rev. Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 12.Rybicka JM, Balce DR, Khan MF, Krohn RM, Yates RM. Proc. Natl. Acad. Sci. USA. 2010;107:10496–10501. doi: 10.1073/pnas.0914867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantegazza AR, et al. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman FZ, Hayee B, Chee R, Segal AW, Smith AM. Immunology. 2009;128:253–259. doi: 10.1111/j.1365-2567.2009.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw GB. The Doctor’s Dilemma: A Tragedy. Vol. 28. Brentano’s; New York: 1911. [Google Scholar]