Abstract
A cluster of 3 facial Mycobacterium chelonae infections occurred after cosmetic dermal filler injections at a plastic surgery clinic. Pulsed-field gel electrophoresis showed that M chelonae isolated from the clinic tap water were identical to the patient wound isolates. Review of injection procedures identified application of nonsterile ice to the skin prior to injection as a possible source of M chelonae. Surveys of regional laboratories and a national plastic surgery listserv identified no other cases related to the injection of this brand of dermal filler. This is the first report of cutaneous M chelonae infections following the injection of dermal fillers. It adds to a growing body of literature on postinjection M chelonae infections and reinforces the importance of optimal skin disinfection steps prior to percutaneous procedures.
Keywords: cosmetic medicine, dermal filler, mycobacterium, infection, chelonae, hyaluronic acid, tap water
The injection of filler materials such as hyaluronic acid into the subcutaneous tissue of the face has become one of the most commonly performed cosmetic procedures. Unlike injections for medications or vaccinations, however, the filler material is intended to remain at the injection site for an extended period of time. In this respect, filler materials resemble implants, and these injections may deserve strict adherence to sterile technique.
Nontuberculous mycobacteria (NTM) are ubiquitous in the environment and are known to exist in municipal and hospital water systems.1–4 They can cause skin and soft tissue infections in both health care and community settings.5–9 Nontuberculous mycobacteria are classified into 4 groups based on their colony morphology, growth rate, and pigment. One major pathogenic group is the rapidly growing mycobacteria, of which the main clinically relevant species include Mycobacterium chelonae, Mycobacterium abscessus, and Mycobacterium fortuitum.3,9 Infection presents as skin nodules, with or without drainage or discoloration, usually 3 to 6 weeks after inoculation.10 Outbreaks of M chelonae cutaneous infections associated with surgical or percutaneous procedures are well described and generally occur when tap water somehow contaminates the procedure.3,4,11–19 Although injection-related NTM infections are also described, no such infections have been reported in the context of commercially prepared dermal filler products.
After 2 cases of M chelonae facial infection following dermal filler injection were confirmed (and another case presumed) from a single aesthetic medicine clinic, we undertook an investigation to identify the source of infection and to determine whether there were other unreported cases.
CASE REPORTS
For the purposes of this investigation, we defined a confirmed case as a patient with a skin/soft-tissue infection and a positive skin-aspirate culture for M chelonae following dermal filler injections between October 2008 and January 2009. We defined a presumptive case as an individual with a clinical presentation consistent with NTM skin infection and response to empiric treatment for mycobacterium following dermal filler injection during the same time period.
Confirmed case 1 was a 48-year-old, otherwise healthy woman who underwent multiple facial injections in October 2008 from a syringe of Juvederm Ultra Plus (Allergan, Inc, Irvine, California) that had previously been split into 2 portions, as was common at the facility where she was treated. She sought medical attention approximately 24 hours later at a local emergency department, complaining of redness at the injection site. She was treated symptomatically and did not have further medical follow-up until 3 weeks later, when she sought treatment for facial redness, swelling, and drainage. Rapidly growing Mycobacterium species, later identified as M chelonae, was isolated from an aspirate of the left cheek. Under the care of an infectious disease consultant, she was treated with clarithromycin and linezolid by mouth and intravenous amikacin. Because of progressive leukopenia, the linezolid was discontinued after 4 weeks; therapy was completed with amikacin for 12 weeks and clarithromycin for 6 months. At the conclusion of therapy, the lesion had resolved without scarring.
Confirmed case 2 was a 46-year-old, otherwise healthy woman who underwent facial injections with an unopened syringe of Juvederm Ultra Plus in November 2008. Eighteen days later, she reported the onset of facial redness and itching. An aspirate of the right cheek grew M chelonae. The same infectious disease consultant treated this case in the first month with clarithromycin and moxifloxacin; subsequently, the patient received 2 months of linezolid plus 3 months of azithromycin. Her lesions resolved by the conclusion of antibiotic therapy.
Presumptive case 1 was a 54-year-old, otherwise healthy woman who underwent injections of Juvederm Ultra from an unopened syringe in November 2008. Two weeks after the injections, she developed redness, warmth, swelling, and changes in skin texture at the injection site. A facial aspirate was obtained; mycobacterial cultures were sterile and routine culture demonstrated only Propionibacterium species. Signs and symptoms resolved after 4 months of treatment with azithromycin plus moxifloxacin.
DISCUSSION
To determine whether additional cases had occurred at the clinic where the first 2 cases were confirmed, we reviewed appointment logs for all visits coded for dermal filler or other facial injections. Using a standardized questionnaire, phone interviews were conducted with all patients who received a dermal injection at this facility between October 1 and December 31, 2008. Twenty-eight unique individuals, including the original 3 patients described above, received Juvederm Ultra or Juvederm Ultra Plus at the clinic in October and November 2008. We interviewed 13 (52%) of the 25 other patients but identified no additional cases. We also interviewed 8 (50%) of the 16 additional patients who received an injection of other products within 1 day of the injection date in the confirmed cases. Among these 8, 1 patient who received a Restylane (Medicis, Inc, Scottsdale, Arizona) injection was suspected of having an NTM infection but to our knowledge did not seek medical attention.
To address our concern that a commercially distributed dermal filler product might be contaminated with M chelonae, we contacted 30 local laboratories in the region that perform mycobacterial culture. Each lab was asked to review its records to identify cultures growing M chelonae from June through December 2008; no isolates from wound sources were identified.
To assess whether other clusters had been noted nationally, we posted notices on listservs likely to be seen by plastic surgeons, dermatologists, and infectious disease providers. We asked readers to report confirmed or presumptive cases of soft tissue infection with M chelonae following dermal filler injection; we received no replies of other infections associated with these products. We also contacted the manufacturer of Juvederm products, but it reported no other clusters of infections.
Environmental Investigation
Public health staff visited the clinic site to assess procedures, review lot numbers, and collect specimens for laboratory testing. Environmental specimens collected on December 30, 2008, included tap water from the faucet of the primary exam treatment room; water from the ice-machine intake, as well as swabs of the intake and interior of the ice machine; samples of anesthetic creams stored in bulk containers; samples of topical disinfectants, including isopropyl alcohol and an ammonium/phenolic compound; samples of household disinfectant for nonhuman surfaces; and open bottles of saline solution from the exam room.
Our chart review of the 2 confirmed cases found that those patients were injected with Juvederm Ultra Plus from different lot numbers. The procedure records, however, did not detail the use of specific antiseptics, anesthetics, or the sequence in which topical products were used at each visit. A typical injection procedure was described as beginning with the application of EMLA (App Pharmaceuticals, Schaumberg, Illinois) or a similar topical anesthetic for 20 to 40 minutes. Subsequently, 70% isopropyl alcohol from multiuse containers was applied to intended injection sites for disinfection. In response to the first case, the disinfectant was changed to a quaternary ammonium/ phenolic compound in the beginning of November, suggesting that the second confirmed case received a different topical disinfectant. Next, most patients opted to apply ice contained in exam gloves or Ziplock-type bags to the intended injection site for further anesthetic effect. Finally, up to 50 injections were administered per session using 27- or 30-gauge needles; needles were changed every 3 to 4 injections, when they became dull. Although most needles were supplied with the dermal filler product, the clinic also stocked needles. After the injections, ice was typically again applied to the skin, followed by topical hydrocortisone and vitamin K cream. Both the topical anesthetics and hydrocortisone were purchased in bulk and repackaged in smaller containers. In response to the first case, the hydrocortisone cream and vitamin K stocks were discarded. The practitioner wore nonsterile gloves during dermal filler procedures.
Laboratory Studies
Environmental specimens obtained from the clinic were sent to the Oregon State Public Health Laboratory for mycobacterial culture. The water samples were filtered through a 0.2-μm filter and the residue was resuspended in a smaller volume. The resuspended sediment was then plated on Middlebrook 7H10 agar. Mycobacterium species grew from the tap water obtained from the faucet in the exam room. All of the other environmental specimens, including the swabs from the ice machine, were negative for acid-fast bacilli. Of note, the ice machine had been replaced 1 week prior to swabbing.
The specimens from the confirmed cases and the environmental isolates were forwarded from the original laboratory to the Centers for Disease Control and Prevention (CDC) for identification by high-performance liquid chromatography (HPLC), full-length 16S rRNA and rpoB gene sequencing, and comparison by pulsed-field gel electrophoresis. Mycolic acid analysis of the isolates with HPLC yielded profiles representative of the M chelonae–M abscessus complex. All of the isolates exhibited 100% 16S rRNA and rpoB gene sequence similarity to the GenBank M chelonae strain ATCC 19237. In addition, the 3 environmental isolates and the 2 clinical isolates matched when compared by pulsed-field gel electrophoresis, suggesting that the tap water was the source of contamination (Figure 1).
Figure 1.
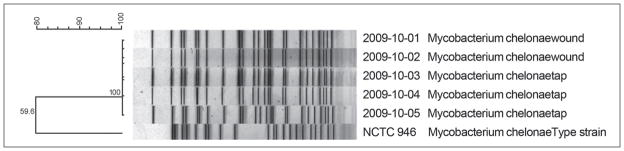
Pulsed-field gel electrophoresis patterns of clinical and environmental Mycobacterium chelonae isolates.
Our site investigation and procedural review suggested a potential mechanism for the introduction of contamination: ice made from tap water on site was applied after disinfection of the skin but before injection of the filler. Although the ice was sealed in Ziplock bags, leakage may have occurred, contaminating the skin surface and allowing for intradermal inoculation during the procedure. Other opportunities for tap water exposure of the injection site include rinsing of nonsterile equipment used in the procedure or the practitioner’s contamination of his non-sterile gloves.
The multiuse containers used to store topical agents could also have served as a source for M chelonae colonization. Although cultures for the topical disinfectant and anesthetic were negative, our samples were not taken from containers present on the days that the confirmed patients received their injections. Interestingly, a growing body of literature describes resistance and selection of more pathogenic strains of nontuberculous mycobacteria, including M chelonae in commonly used antiseptic agents such as quaternary ammonium disinfectants of the type used in the practice.20
Although the infections described in this report resolved with prolonged antibiotic therapy, their occurrence highlights the necessity for sterile injection practices with such products. The Juvederm Ultra Plus guidelines identify antisepsis as the step immediately prior to injection of the product, and application of ice is suggested for pain control after, not prior to, injection.21 Unlike most medicinal injections that are absorbed into tissues in short order, filler materials are intended to remain where injected indefinitely. For injections that rapidly diffuse in the subcutaneous tissues, a simple swab of the skin suffices as a disinfection step. On the other hand, longevity of these filler substances creates a volume of material that is less accessible to the immune system.22 Stricter adherence to antisepsis may need to be required for intradermal filler injections. Our findings suggest precautionary standards that (1) proscribe skin exposure to tap water immediately before and after an injection procedure, (2) standardize a consistent skin disinfection step immediately prior to injection, and (3) require documentation of disinfection steps and other topical treatments in patient medical records.
Only Mycobacterium simiae has been previously reported in association with a similar cosmetic intradermal microinjection procedure associated with an unlicensed compound.23 To our knowledge, this is the first report of a rapidly growing mycobacterial infection associated with a dermal filler injection.
This retrospective investigation is subject to several limitations. First, the environmental investigation was incomplete. Because of the long incubation period of NTM infections, we did not become aware of this cluster until many weeks after the clinic visits. By the time the investigation began, we could not test the bulk anesthetics, disinfectants, and other topical products in use at the time of the dermal filler injections since they had been discarded. In addition, the ice machine in use at the time of the injections had also been replaced. Second, the patient medical records lacked sufficient detail for a case control study; we were unable to explore procedure-related risk factors, such as anesthetic choice or injection details, or patient-related risk factors, such as concurrent immunological or dermatological conditions among dermal filler recipients. Finally, although we did identify M chelonae in the clinic water supply, this organism is common and its identification could be coincidental, although coincidence is less likely since the environmental and clinical isolates were identical species by mycolic acid analysis and gene sequencing.24–26 Most important, the isolates matched by pulsed-field gel electrophoresis, an established standard for distinguishing M chelonae.27
CONCLUSIONS
These 3 cases of M chelonae contamination illustrate a need to optimize antisepsis and minimize water exposure during simple cosmetic procedures such as intradermal injections. Providers should recognize that tap water, ice made from tap water, and open multiuse containers of aqueous solutions can be contaminated by rapidly growing mycobacteria.
Acknowledgments
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Footnotes
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Level of Evidence: 5
References
- 1.Vaerewijck MJM, Huys G, Palomino JC, Swings J, Portaels F. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol Rev. 2005;29(5):911–934. doi: 10.1016/j.femsre.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Fox C, Smith B, Brogan O, Rayner A, Harris G, Watt B. Non-tuberculous mycobacteria in a hospital’s piped water supply. J Hosp Infect. 1992;21(2):152–154. doi: 10.1016/0195-6701(92)90036-l. [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky E, Rynearson TK. Mycobacteria in soil and their relation to disease-associated strains. Am Rev Respir Dis. 1968;97(6):1032–1037. doi: 10.1164/arrd.1968.97.6P1.1032. [DOI] [PubMed] [Google Scholar]
- 4.Goslee S, Wolinsky E. Water as a source of potentially pathogenic mycobacteria. Am Rev Respir Dis. 1976;113(3):287–292. doi: 10.1164/arrd.1976.113.3.287. [DOI] [PubMed] [Google Scholar]
- 5.Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis. 2001;33(8):1363–1374. doi: 10.1086/323126. [DOI] [PubMed] [Google Scholar]
- 6.Wallace RJ, Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166(2):405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- 7.Kullavanijaya P. Atypical mycobacterial cutaneous infection. Clin Dermatol. 1999;17(2):153–158. doi: 10.1016/s0738-081x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 8.Gruft H, Falkinham JO, III, Parker BC. Recent experience in the epidemiology of disease caused by atypical mycobacteria. Rev Infect Dis. 1981;3(5):990–996. doi: 10.1093/clinids/3.5.990. [DOI] [PubMed] [Google Scholar]
- 9.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 10.McFarland EJ, Kuritzkes DR. Clinical features and treatment of infection due to mycobacterium fortuitum/chelonae complex. Curr Clin Top Infect Dis. 1993;13:188–202. [PubMed] [Google Scholar]
- 11.Camargo D, Saad C, Ruiz F, et al. Iatrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117(1):113–119. doi: 10.1017/s0950268800001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias M, Antony B, Scaria B, Pinto H. Cutaneous infection caused by M. chelonae following thorn prick. J Clin Diagn Res. 2009;3(3):1577–1579. [Google Scholar]
- 13.Safranek TJ, Jarvis WR, Carson LA, et al. Mycobacterium chelonae wound infections after plastic surgery employing contaminated gentian violet skin-marking solution. N Engl J Med. 1987;317(4):197–201. doi: 10.1056/NEJM198707233170403. [DOI] [PubMed] [Google Scholar]
- 14.Satyanarayana S, Mathur AD. Atypical mycobcterial injection abscess. J Indian Med Assoc. 2003;101(1):36, 38, 40. [PubMed] [Google Scholar]
- 15.Zhibang Y, BiXia Z, Qishan L, Lihao C, Xiangquan L, Huaping L. Large-scale outbreak of infection with Mycobacterium chelonae subsp. abscessus after penicillin injection. J Clin Microbiol. 2002;40(7):2626–2628. doi: 10.1128/JCM.40.7.2626-2628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace RJ, Jr, Musser JM, Hull SI, et al. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989;159(4):708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- 17.Meyers H, Brown-Elliott BA, Moore D, et al. An outbreak of Mycobacterium chelonae infection following liposuction. Clin Infect Dis. 2002;34(11):1500–1507. doi: 10.1086/340399. [DOI] [PubMed] [Google Scholar]
- 18.Yuan J, Liu Y, Yang Z, et al. Mycobacterium abscessus post-injection abscesses from extrinsic contamination of multiple-dose bottles of normal saline in a rural clinic. Int J Infect Dis. 2009;13(5):537–542. doi: 10.1016/j.ijid.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Wenger JD, Spika JS, Smithwick RW, et al. Outbreak of Mycobacterium chelonae infection associated with use of jet injectors. JAMA. 1990;264(3):373–376. [PubMed] [Google Scholar]
- 20.Cortesia C, Lopez GJ, de Waard JH, Takiff HE. The use of quaternary ammonium disinfectants selects for persisters at high frequency from some species of non-tuberculous mycobacteria and may be associated with outbreaks of soft tissue infections. J Antimicrob Chemother. 2010;65(12):2574–2581. doi: 10.1093/jac/dkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allergan, Inc. Juvederm Ultra. http://www.juvederm.com/content/pdf/juvederm_dfu.pdf.
- 22.Johl SS, Burgett RA. Dermal filler agents: a practical review. Curr Opin Ophthalmol. 2006;17(5):471–479. doi: 10.1097/01.icu.0000243021.20499.4b. [DOI] [PubMed] [Google Scholar]
- 23.Piquero J, Casals VP, Higuera EL, Yakrus M, Sikes D, de Waard JH. Iatrogenic Mycobacterium simiae skin infection in an immunocompetent patient. Emerg Infect Dis. 2004;10(5):969–970. doi: 10.3201/eid1005.030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler WR, Ahearn DG, Kilburn JO. High-performance liquid chromatography of mycolic acids as a tool in the identification of Corynebacterium, Nocardia, Rhodococcus, and Mycobacterium species. J Clin Microbiol. 1986;23(1):182–185. doi: 10.1128/jcm.23.1.182-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler WR, Guthertz LS. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin Microbiol Rev. 2001;14(4):704–726. doi: 10.1128/CMR.14.4.704-726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogall T, Flohr T, Böttger EC. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J Gen Microbiol. 1990;136(9):1915–1920. doi: 10.1099/00221287-136-9-1915. [DOI] [PubMed] [Google Scholar]
- 27.Wallace RJ, Jr, Zhang Y, Brown BA, Fraser V, Mazurek GH, Maloney S. DNA large restriction fragment patterns of sporadic and epidemic nosocomial strains of Mycobacterium chelonae and Mycobacterium abscessus. J Clin Microbiol. 1993;31(10):2697–2701. doi: 10.1128/jcm.31.10.2697-2701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


