Abstract
Human exposure to polycyclic aromatic hydrocarbons (PAHs) can be assessed by biomonitoring of their urinary mono-hydroxylated metabolites (OH-PAHs). Limited information exists on the human pharmacokinetics of OH-PAHs. This study aimed to investigate the excretion half-life of 1-hydroxypyrene (1-PYR), the most used biomarker for PAH exposure, and 9 other OH-PAHs following a dietary exposure in 9 non-smoking volunteers with no occupational exposure to PAHs. Each person avoided food with known high PAH-content during the study period, except for a high PAH-containing lunch (barbecued chicken) on the first day. Individual urine samples (n = 217) were collected from 15 hours before to 60 hours following the dietary exposure. Levels of all OH-PAHs in all subjects increased rapidly by 9–141 fold after the exposure, followed by a decrease consistent with first order kinetics, and returned to background levels 24–48 hours after the exposure. The average time to reach maximal concentration ranged from 3.1 h (1-naphthol) to 5.5 h (1-PYR). Creatinine-adjusted urine concentrations for each metabolite were analyzed using a non-linear mixed effects model including a term to estimate background exposure. The background-adjusted half-life estimate was 3.9 h for 1-PYR and ranged 2.5–6.1 h for the other 9 OH-PAHs, which in general, were shorter than those previously reported. The maximum concentrations after the barbecued chicken consumption were comparable to the levels found in reported occupational settings with known high PAH exposures. It is essential to consider the relatively short half-life, the timing of samples relative to exposures, and the effect of diet when conducting PAH exposure biomonitoring studies.
Keywords: half-life, 1-hydroxypyrene, polycyclic aromatic hydrocarbon, PAH, biomarkers, metabolites, pharmacokinetics
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are incomplete combustion products present ubiquitously in the ambient air, soil, food, and in many occupational environments. Benzo(a)pyrene has been classified by the International Agency for Research on Cancer (IARC) as a human carcinogen, and many other PAHs, such as naphthalene, benz(a)anthracene and chrysene, have been classified as probable human carcinogens.1,2 PAHs have also been associated with a variety of health effects, as compiled previously.3 Notably, a recent study found a dose-response relationship between PAHs in placenta and the risk of neural tube defects.4
Exposure to PAHs in the general population occurs mostly through inhalation of polluted air and cigarette smoke, and ingestion of food containing PAHs, with ingestion reportedly as more dominant.5 In certain occupational settings, such as coke oven plants, dermal and inhalation exposure are the major routes. Urinary mono-hydroxylated PAHs (OH-PAHs) are metabolites of PAHs and have been used as biomarkers of PAH exposure. 1-Hydroxypyrene (1-PYR) is the most commonly used PAH biomarker.6 A number of studies have reported levels of OH-PAHs in occupational groups such as coke oven workers, aluminum plant workers and road pavers.7,8 PAH biomarkers have also been monitored in the general population from various countries.9–11
In order to properly conduct biomonitoring studies , it is essential to understand the excretion profile and pharmacokinetics of the biomarkers used, so that proper biological sampling strategies can be developed to capture the exposure, and the human biomarker data can be interpreted appropriately to derive information on exposure level which would aid in risk assessment. This is especially necessary for environmental chemicals with relatively short biological half-lives, such as PAHs. For example, the time window required to capture the excretion of urinary PAH biomarkers after an acute exposure event, defined by their half-lives, is very limited, and a prolonged lag between the exposure and the sampling would lead to inaccurate inference on exposed level and might lead to erroneous risk assessment conclusions. Several studies, predominantly focused on 1-PYR, have been conducted to estimate half-life (t1/2) from various exposure routes. The t1/2 after inhalation exposure has been determined to be 6.0–29 h.12–14 For ingestion exposure, the 1-PYR t1/2 was reported to be 4.4–12 h.15–17 For dermal absorption, the 1-PYR t1/2 was estimated at 11.5–15 h.17,18 In several occupational studies on workers exposed through both inhalation and dermal absorption, the average t1/2 ranged 10.4–18 h.19–21
Increasingly, PAH biomonitoring studies are being carried out using multiple biomarkers in addition to 1-PYR. Some biomarkers or metabolic patterns have been proposed to be associated with specific sources or risk factors due to the change of enzyme polymorphism. For example, 2-naphthol has been proposed as the inhalation biomarker,22 and the ratio of 1- and 2-hydroxyphenanthrenes (1-PHE, 2-PHE) to 3- and 4-hydroxyphenanthrenes (3-PHE, 4-PHE) has been linked to smoking status due to the induction of cytochrome P450 1A2 enzyme in smokers which led to higher formation of 3-PHE and 4-PHE.23 However, most of kinetics studies targeted only a single biomarker (1-PYR), while scarce or no information was available on other PAH biomarkers. Further, many investigations relied on only pre- and post-work shift samples collected over consecutive days. Due to the rapid elimination rate, an accurate estimate of the half-live cannot be obtained with so few data points. Moreover, many studies did not have an exposure-free or controlled exposure period following the exposure, and smokers were often included in the studies which could also bias the results.20,21 Furthermore, a majority of the published studies modeled individual excretion data to give one half-life result per subject, then took an arithmetic mean as the overall half-life estimate. Fitting a mixed-effects model to all subjects simultaneously would produce more reliable half-life estimates for individuals and allow formal inference regarding the extent of inter-individual variation in half-lives.24 Among the few studies modeling multiple subjects, no consideration was given on background levels in the models, which would bias modeling outcome because of the universal presence of these compounds.25
Taking into consideration of all factors addressed above, the present study was the first known conducted to investigate the excretion kinetics and estimate half-lives of 1-PYR and 9 additional urinary metabolites of naphthalene (NAP), fluorene (FLU) and phenanthrene (PHE) after a well-controlled dietary exposure in volunteers with no known occupational exposure and no self-reported exposure to tobacco smoke. The 9 volunteers avoided food with known high PAH-content (e.g., grilled or smoked food) during the 3-day study period, with the exception of a high PAH-containing lunch (barbecued chicken) on Day 1. We collected all individual urine excretions from the participants from 15 hours before to 60 hours after the dietary intake of the high PAH-containing meal. We studied the excretion profiles of these 10 PAH metabolites in the volunteers, and calculated their half-lives using a novel non-linear mixed effect models with a term accounting for background exposure.
EXPERIMENTAL PROCEDURES
Study design
The 9 volunteers in this study were healthy adults between 23 and 61 years of age (mean 37±12 years) at the time of the study in September–October, 2008. The participants (5 males and 4 females) lived in metropolitan Atlanta, USA. All volunteers commuted daily by car, worked in an office building at the Centers for Disease Control & Prevention (CDC), and were not occupationally exposed to PAHs. They were self-reported non-smokers with no exposure to second-hand smoke during the study.
During the three day study period, participants followed specific instructions, and consumed one high PAH-containing meal–shredded barbecued chicken–for lunch on Day 1, while eating only food with a low PAH content before and after. The barbecued chicken was purchased from a local restaurant, and each subject consumed a discretionary quantity of the barbecued chicken, sauce and side dishes (mashed potato and coleslaw, weight not recorded). Participants were instructed to avoid food with known high PAH-content, such as grilled or smoked food, two days prior to the study and were provided with a list of food items that were allowed during the study period (Supplemental Information Table S-1). In addition, participants were also encouraged, but not required, to drink the recommended daily intake of fluids―8 to 12 glasses of water (8 ounces per glass)―throughout the study period. Each person was informed of the importance of a continuous consistent intake of water for allowing an accurate estimation of the half-life of the PAHs consumed.
Participants collected all individual urine specimens approximately from 15 hours prior to the PAH-containing lunch (i.e. 9PM the night before), to 60 hours after the ingestion of the barbecued chicken. During each restroom visit, participants collected all urine excretions in a graduated beaker, recorded the total volume and time of each excretion, and transferred a portion (~100 mL) to a pre-labeled sterile urine cup, which was then stored in an ice cooler. The urine samples were retrieved from each participant daily, and frozen at −70°C until analysis. Participants kept detailed records of each urine excretion, dietary intake, driving, and other activities. The study protocol was approved by the CDC Human Research Protection Office. Written informed consent was obtained from all participants prior to the study.
Analytical methods
A sample of the shredded barbecued chicken (10 g) was analyzed for 16 PAHs by a licensed commercial laboratory. The chicken consumed by participants was shredded and pre-mixed with sauce during preparation and therefore, had a relatively consistent texture. The extraction method was EPA Method 3541 and the analysis was carried out using a gas chromatography/mass spectrometry (GC/MS) method based on EPA Method 8270C. No internal standards were used in the food analysis. Fluorene-d10 and fluoranthene-d10 were used as surrogates. A method blank and two laboratory control samples were prepared along with the barbecued chicken sample.
The method26 used to measure the urinary OH-PAH metabolites in clinical specimens has been certified by the U.S. Centers for Medicare & Medicaid Services according to the guidelines set forth in the Clinical Laboratory Improvement Amendments Act (CLIA). Briefly, urine samples were spiked with a mixture of ten 13C-labeled internal standards and sodium acetate buffer containing β-glucuronidase and sulfatase, hydrolyzed overnight at 37 °C, and then extracted by use of pentene through semi-automated liquid-liquid extraction. The extracts were evaporated, derivatized, and analyzed on a 6890 GC (Agilent Technology, Palo Alto, CA) coupled with a MAT 95XL high resolution MS (Thermo Fisher Scientific Inc., Waltham, MA). Ten OH-PAHs, including 1-, 2-naphthols (1-, 2-NAP), 2-, 3-, 9-hydroxyfluorenes (2-, 3-, 9-FLU), 1-, 2-, 3-, 4-hydroxyphenanthrenes (1-, 2-, 3-, 4-PHE) and 1-PYR, were quantified. Each analyte had its own 13C-labeled internal standard. All analyses were subjected to a series of quality control and quality assurance checks as described elsewhere.26 The limits of detection (LOD) for the 10 OH-PAHs ranged 2.6–18 pg/mL, and the detection rate ranged 91.3% for 4-PHE to 100% for 5 of the 10 OH-PAHs. Urinary creatinine was measured on a Roche Hitachi 912 Chemistry Analyzer (Hitachi Inc., Pleasanton, CA) by use of the Creatinine Plus Assay, as described in Roche’s Creatinine Plus Product Application # 03631761003.
Data and statistical analysis
Participants’ tabulated diaries on urine excretion, dietary intake, driving, and other activities were entered into an electronic document. The diaries were inspected by the investigators to check the compliance to the study protocol and make certain that no obvious alternative PAH exposure occurred during the study period.
The weight of barbecued chicken consumed by each participant was recorded during the organized lunch on Day 1. The ingested PAH amount for each participant was calculated by multiplying the mass of consumed barbecued chicken with the PAH concentration determined in a sample of the barbecued chicken.
All concentrations were blank-subtracted. Concentrations below LOD were replaced with the LOD value. We used creatinine-adjusted concentrations for analyses to account for urine dilution. All statistical analyses were performed through SAS 9.2 (SAS Institute, Cary, NC) or R software (R Development Core Team, 2010). A total of 217 samples were collected from 9 participants (14–36 samples/person) during the study period. Concentrations of 1-naphthol (1-NAP) in 3 persons reached maximum before the exposure and decreased throughout the study period indicating a significant episodic contribution from sources other than the dietary ingestion of the barbecued chicken. Therefore, 1-NAP data from these 3 persons were excluded in all analyses.
We defined the time of the dietary exposure as 0 h, the pre-exposure period as −15 h to 0 h, and the post-exposure baseline period as 48–60 h. The pre-exposure level in each person was calculated as the average concentrations in all urine specimens taken during −15–0 h, and the post-exposure level was the average concentration in all urine collected during 48–60 h. Excretion rate (ng/h) was calculated as the total amount excreted for each metabolite over a 12-h or 15-h time segment. A total of 6 time segments were available for each person, i.e. −15 to 0 h, and five 12-hour segments thereafter (0–60 h).
Linear regression analysis was used to study the correlation among the different OH-PAHs in all samples. Non-parametric Spearman’s rank order correlation was conducted between the ingested barbecued chicken mass and the excreted urinary OH-PAH mass over 24-h after the dietary exposure due to the small sample size (N=6 for 1-NAP and N=9 for the remaining OH-PAHs). Correlation coefficients (R) were considered statistically significant when p-value was equal or less than 0.05, and marginally significant when p was between 0.05 and 0.10.
Pharmacokinetic modeling
In a first analysis, we extracted, for each person and metabolite, data from the peak concentration until 20–30 h later. Regression of these data generally showed highly statistically significant first-order rate constants. The data for each metabolite were then combined from the different people, and analyzed using a non-linear mixed effects model to calculate the mean background level (µC), mean uptake level (µB), mean decay rate parameter (µk) and median half-life. In order to estimate the terminal half-life we omitted the data during uptake and selected data for analysis in the following way: i) include all data prior to the controlled dietary exposure; ii) exclude data between the time of controlled exposure (0 h) and the observed time of peak urinary concentration (tmax); iii) include all data after the peak. We modeled these data using a non-linear mixed effects model that takes into account both the first order decline of metabolites following exposure and the background exposure, as well as between-subject variation in pharmacokinetics.25 The post-peak model (t ⩾ tmax) is:
Cij = C0i + Bi exp[kitij] + εij
and the pre-exposure model (t < 0 h) is:
Cij = C0i + εij
where tij (h) is time since peak (t – tmax), i is an index for person, j is an index for sampling time point, Cij is the creatinine adjusted urine concentration (µg/g creatinine) at time t in person i, C0i is the background creatinine-adjusted metabolite concentration for each person, B is the initial concentration increase above background after the exposure for each person, k is the first-order elimination rate constant (1/h), and εij is the error term. The observed time at peak concentration (tmax) was determined separately for each person and each metabolite. The terms C0i, Bi, and k are further assumed to be normally distributed among participants (i.e., random effects), with mean parameters µC, µB, and µk, respectively. This model assume that creatinine adjusted concentration in levels are proportional to central compartment levels and does not examine the uptake phase (0 < t < tmax). The model was fit separately to each metabolite. The population-median half-life for each metabolite is
t1/2 = ln(2)/µk
RESULTS
The shredded barbecued chicken contained 200, 77, 220 and 99 µg/kg of NAP, FLU, PHE and PYR, respectively. The concentration of benzo(a)pyrene was 8.6 µg/kg and concentration of all 16 PAHs measured in the barbecued chicken is given in Supplemental Information, Table S-2. The 9 participants ingested 144–233 g of the barbecued chicken (172±33 g) at the lunch, which resulted in 25–44 µg NAP, 10–17 µg FLU, 28–49 µg PHE and 12–22 µg PYR ingested by each participants during the exposure (Supplemental Information, Table S-3). There was no significant difference on consumed barbecued chicken quantity and ingested PAH amount with regard to gender, age and weight (data not shown).
After the dietary exposure, levels of the urinary OH-PAH biomarkers increased by 9–141 folds in the participants (Table 1). This rapid increase was then followed by a decrease consistent with first order kinetics (i.e., a relatively linear decrease of log metabolites vs. time), and a flattening out after about 24–48 h to a baseline concentration consistent with background exposure. The time course of creatinine adjusted concentrations of the 10 metabolites from participant S3 is given in Figure 1 and other participants are represented in Supplementary Material (Figures S-1~8). The median, minimum and maximum metabolite concentrations in each participant are given in Supplemental Information, Table S-4. Figure 2 shows the levels of 1-PYR during the study period for all 9 participants. Most metabolites were highly correlated (r>0.8) for most people (data not shown). There were, however, some exceptions. The most notable exception is that 1-NAP had low or even negative correlations with other metabolites for three individuals, suggesting the presence of other sources for this compound. This is also supported by the time course graphs (Figure 3), showing that for three participants, the 1-NAP concentrations peaked before the exposure, and decreased throughout the monitoring period (Figure 3C). In addition, the maximum concentration in these three persons was 10–60 fold higher than that in the remaining 6 participants, suggesting a substantial source of 1-NAP other than the barbecued chicken in these three persons. For example, 1-NAP is also a main metabolite of the wide-spectrum carbamate insecticide carbaryl,27 hence, 1-NAP data from these three persons were excluded from further data analysis.
Table 1.
Pre (−15~0 h), post (48~60 h) and maximum urinary OH-PAH concentrations (creatinine adjusted, µg/g creatinine) from the 9 volunteers consumed barbecued chicken
| Analyte | Abbr. | Median (range) concentrations (µg/g creatinine) |
Median (range) folds of increase (max/pre) |
US populationb |
|||
|---|---|---|---|---|---|---|---|
| Pre (−15–0 h) | Maximum | Post (48–60 h) | Median | 95th | |||
| 1-naphthola | 1-NAP | 2.12 (0.62–4) | 70.1 (41.1–94.3) | 2.03 (0.78–3.23) | 35 (11–79) | 1.56 | 17.8 |
| 2-naphthol | 2-NAP | 1.88 (0.49–9) | 61.2 (49.8–116) | 3.72 (2.18–20.3) | 33 (10–113) | 1.94 | 16.7 |
| 9-hydroxyfluorene | 9-FLU | 0.41 (0.06–0.77) | 12.2 (4.28–18.4) | 0.28 (0.14–0.47) | 27 (21–99) | 0.24 | 1.89 |
| 3-hydroxyfluorene | 3-FLU | 0.06 (0.02–0.24) | 2.24 (1.69–3.1) | 0.13 (0.05–0.29) | 36 (10–141) | 0.094 | 1.06 |
| 2-hydroxyfluorene | 2-FLU | 0.16 (0.09–0.32) | 5.57 (3.85–7.26) | 0.31 (0.2–0.49) | 28 (19–66) | 0.206 | 0.852 |
| 4-hydroxyphenanthrene | 4-PHE | 0.02 (0.01–0.22) | 0.71 (0.45–2.02) | 0.02 (0.02–0.2) | 22 (9–90) | 0.125 | 0.464 |
| 3-hydroxyphenanthrene | 3-PHE | 0.11 (0.04–0.24) | 3.04 (2.06–3.45) | 0.09 (0.04–0.24) | 27 (13–81) | 0.053 | 0.231 |
| 1-hydroxyphenanthrene | 1-PHE | 0.17 (0.04–0.45) | 2.94 (1.71–4.02) | 0.16 (0.08–0.37) | 16 (9–75) | 0.087 | 0.428 |
| 2-hydroxyphenanthrene | 2-PHE | 0.06 (0.03–0.24) | 2.19 (1.63–3.21) | 0.07 (0.04–0.24) | 32 (10–67) | 0.057 | 0.347 |
| 1-hydroxypyrene | 1-PYR | 0.06 (0.02–0.21) | 1.86 (1.57–2.95) | 0.09 (0.03–0.17) | 29 (10–87) | 0.044 | 0.243 |
Data of 1-naphthol from 3 participants were excluded.
U.S. population levels are from reference #11.
Figure 1.
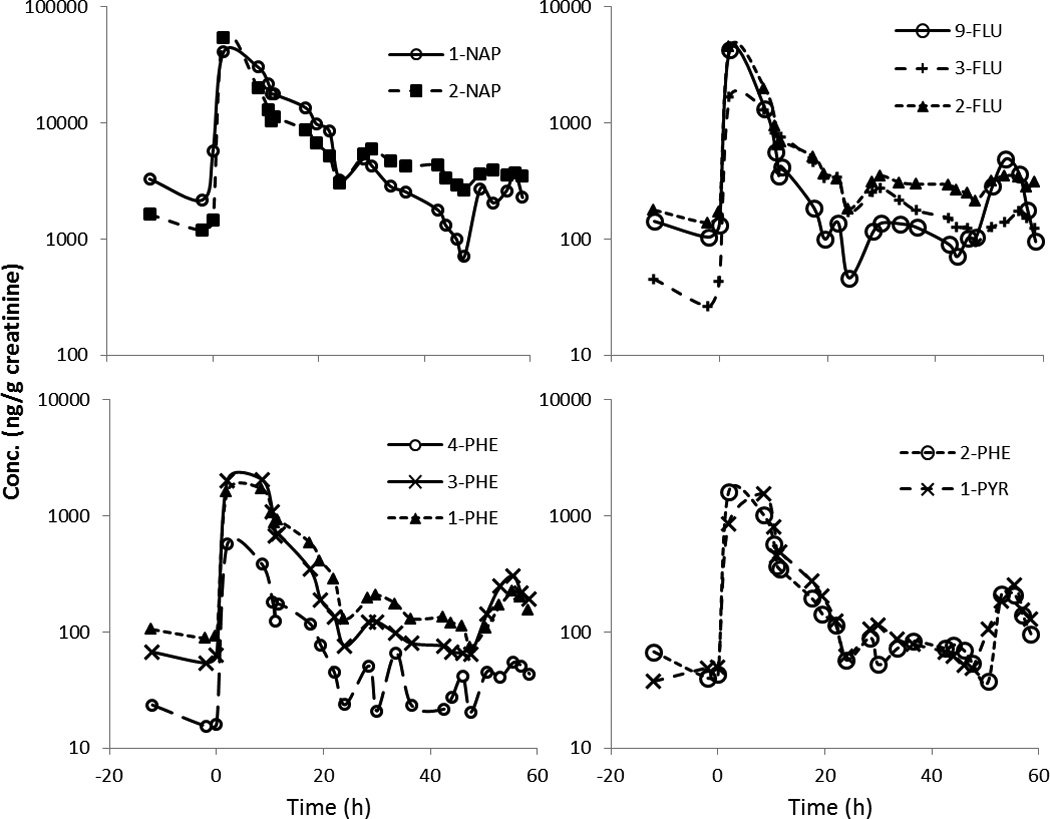
Creatinine adjusted concentration (ng/g creatinine) of 10 OH-PAH metabolites in urine excretions from participant S3 over 3 days. Barbecued chicken consumption took place at time 0h.
Figure 2.
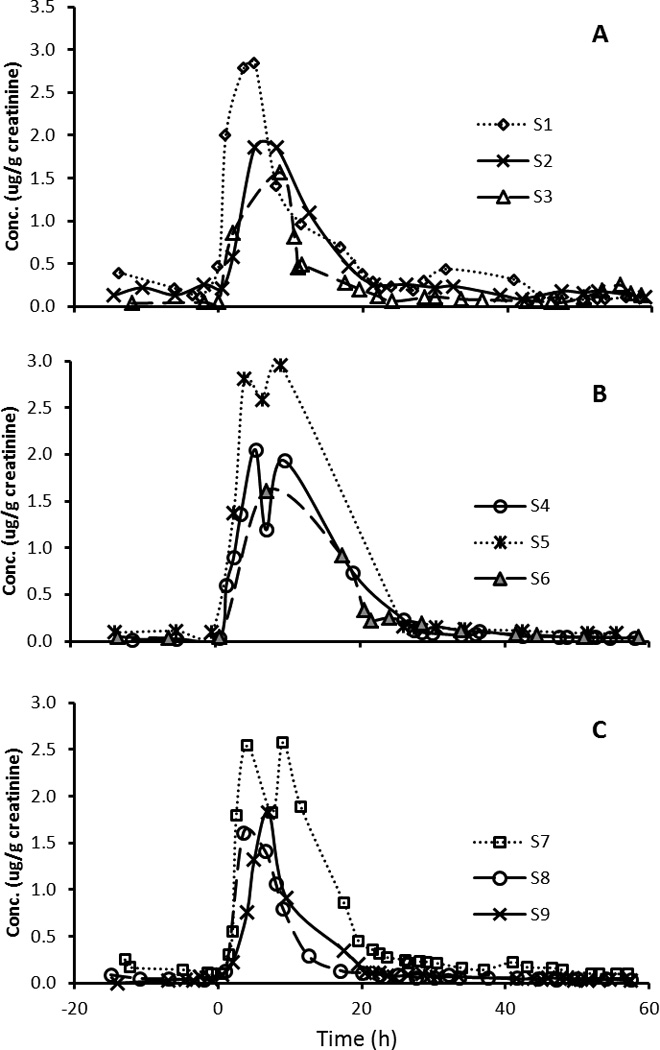
Creatinine-adjusted concentration (µg/g creatinine) of 1-hydroxypyrene in urine excretions from 9 participants over 3 days. Barbecued chicken consumption took place at time 0 h.
Figure 3.
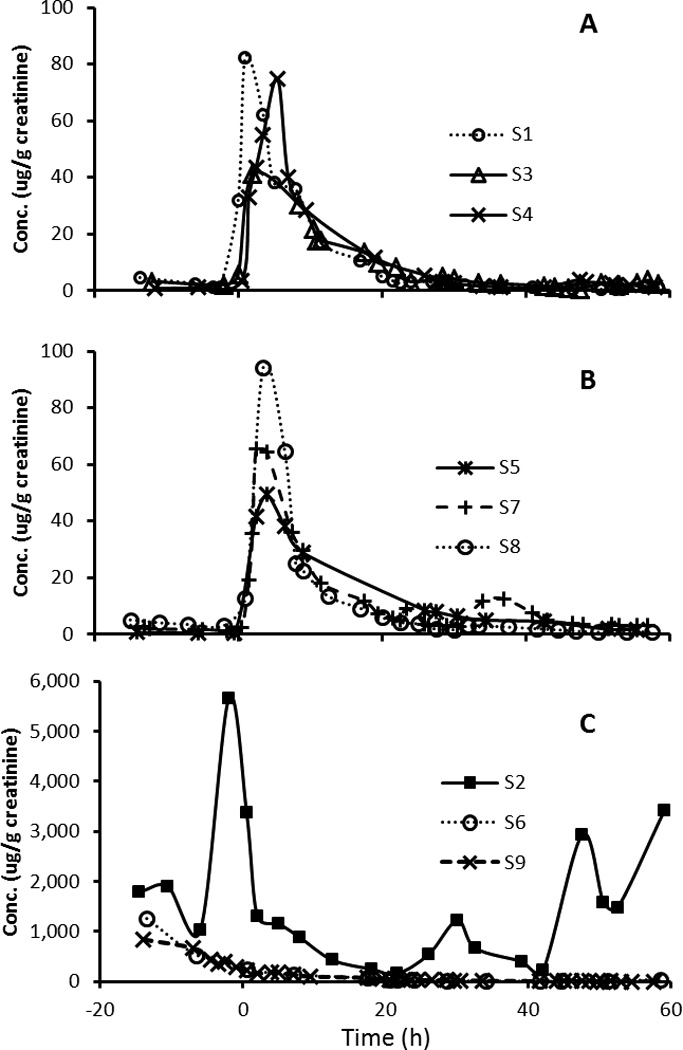
Creatinine-adjusted concentration (µg/g creatinine) of 1-naphthol in urine excretions from 9 participants over 3 days. Barbecued chicken consumption took place at time 0 h.
On average, 58–79% of urinary OH-PAHs were excreted within the first 12 h after the exposure. For 1-PYR, 51–90% (mean 67%) was eliminated within the first 12 h. At 3.5–8.5 h (mean 5.5 h) after the exposure, the urinary 1-PYR concentration increased by 10–85 fold among the participants. The excretion rate in 12-h time segments (or 15-h for pre-exposure) among all participants are given for 2-NAP and 1-PYR in Figure 4. The excretion rates were highest within the first 12-h post exposure. After 36–48 h, the excretion rate approached the pre-exposure level. However, there were outliers with elevated post-exposure excretion, most of which came from participant S1. The highest post-exposure excretion (24 h and later) was found for 2-NAP in S1, which was approaching the median of maximum excretion rate in all participants (Figure 4B).
Figure 4.

Box-and-Whisker Plots of the excretion rate (ng/h) for 1-hydroxypyrene (A) and 2-naphthol (B) in all participants. Barbecued chicken consumption took place at time 0 h.
Mean percentage of PYR excreted as 1-PYR in urine over 24 h was 6.8% (range 4.5–14.6%). For NAP, FLU and PHE, the mean percentages of their excreted hydroxylated metabolites (sum of the metabolites from the same PAH) were 182% (99–248%), 60% (30–73%) and 11% (7.5–16%), respectively. The ingested barbecued chicken mass was correlated or marginally correlated with the excreted 2-FLU, 3-FLU, 2-PHE, 3-PHE, 4-PHE and 1-PYR over 24-h post the exposure, with Spearman R’s ranged 0.60–0.68 (Table 2).
Table 2.
Excretion parameters for the 10 OH-PAH metabolites and Spearman rank order correlation coefficients between the ingested barbecued chicken mass and the excreted OH-PAH mass over 24-h following the dietary exposure among the 9 participants.
| Observed time to peak exposure, tmax, mean and range (h) |
Modeled pharmacokinetic parameters |
Correlation to BBQ |
|||||
|---|---|---|---|---|---|---|---|
| Mean background level, µC (µg/g crea) |
Mean uptake level, µB (µg/g crea) |
Mean decay rate parameter, µk (1/h) |
Median half-life, t1/2, with 95% CI (h) |
Spearman R, 24-h excreted OH-PAH vs. BBQ |
p | ||
| 1-NAPa | 3.1 (1.01–5.5) | 2.3 | 66.3 | 0.16 | 4.3 [3.3–6.2] | 0.43 | 0.397 |
| 2-NAP | 3.8 (1.01–7) | 5.8 | 64.4 | 0.27 | 2.5 [2.0–3.4] | 0.35 | 0.356 |
| 9-FLU | 3.8 (1.01–7) | 0.31 | 11.4 | 0.23 | 3.1 [2.6–3.8] | 0.37 | 0.332 |
| 3-FLU | 3.9 (1.01–7) | 0.12 | 2.2 | 0.11 | 6.1 [4.9–8.1] | 0.63b | 0.067 |
| 2-FLU | 3.9 (1.01–7) | 0.29 | 5.2 | 0.24 | 2.9 [2.3–4.0] | 0.62 | 0.077 |
| 4-PHE | 3.8 (1.01–7) | 0.04 | 0.77 | 0.2 | 3.5 [2.7–4.8] | 0.68c | 0.042 |
| 3-PHE | 5.1 (3.5–8.5) | 0.09 | 2.8 | 0.17 | 4.1 [3.3–5.6] | 0.68 | 0.042 |
| 1-PHE | 5.3 (3.5–8.5) | 0.18 | 2.7 | 0.14 | 5.1 [4.3–6.1] | 0.52 | 0.154 |
| 2-PHE | 4.1 (1.01–7) | 0.08 | 2.2 | 0.18 | 3.9 [3.4–4.6] | 0.62 | 0.077 |
| 1-PYR | 5.5 (3.5–8.5) | 0.08 | 2.0 | 0.18 | 3.9 [3.0–5.7] | 0.60 | 0.088 |
Data on 1-naphthol from 3 participants were excluded.
Correlation coefficients in italic are marginally significant with 0.05<p<0.1
Correlation coefficients in bold are significant with p<0.05
The median pre-exposure levels ranged 0.02 µg/g creatinine for 4-PHE to 2.12 µg/g creatinine for 2-NAP (Table 1). After the dietary exposure, the OH-PAH biomarker concentrations increased by 9–141 fold in the urine from the participants. The maximum concentration ranged 0.71 (4-PHE) to 70.1 µg/g creatinine (1-NAP).
Among the 9 participants, the biomarker levels reached their maximum concentration at 1.01–8.5 hour after the exposure (tmax). The tmax for the naphthalene and fluorene metabolites averaged 3.1–3.9 h. The mean tmax for the phenanthrene metabolites were 3.8–5.3 h, while for 1-PYR, the mean tmax was 5.5±1.7 h among the 9 participants. As shown in Table 2, median half-life estimates from the non-linear mixed effects model ranged 2.5 h (95% CI: 2.0 h, 3.4 h) in 2-NAP to 6.1 h (4.9 h, 8.1 h) in 3-hydroxyfluorene (3-FLU). For 1-PYR, the half-life was 3.9 h (3.0 h, 5.7 h) in this study.
DISCUSSION
Urinary OH-PAHs, particularly 1-PYR, have been used as biomarkers for PAH exposure. Most published pharmacokinetics studies have been focused on 1-PYR only, while there are hundreds of PAHs and each is biotransformed into multiple metabolites. Our analytical method could measure up to 24 OH-PAHs in urine,26 however, many of the metabolites from PAHs larger than pyrene had zero or low detection rate,11 most likely due to their major excretion route through feces as reported previously.3 Therefore, we had stopped measuring those metabolites and only have been focusing on 10 detectable urinary biomarkers, i.e. metabolites of NAP, FLU, PHE and PYR, in all recent research projects,28 including this pharmacokinetic investigation in 9 non-smoking and non-occupationally exposed participants after dietary exposure.
Excretion kinetics and half-life estimates
PAHs were metabolized and excreted rapidly. The observed OH-PAH levels reached maximum in less than 8.5 h among the participants, and the majority of the urinary OH-PAHs were excreted within 12 h after the exposure. This is consistent with existing information on elimination of urinary 1-PYR following dietary exposure. Chien and Yeh reported mean tmax of 4.0 h (2.2–9.7 h) among 9 non-smoking subjects after consuming barbecued meat.15 In another study, the time to reach the maximal elimination rate was 6.3 h (2.2–9.7 h) after grilled beef consumption.16
It is common to calculate half-life based on a two-step approach: first estimating the elimination rate or half-life individually for each person, then averaging those estimates to obtain a group mean. However, that method is problematic because it tends to overestimate the variability in half-lives, a phenomenon known as "Stein's paradox" (i.e., separately calculated means are not as accurate as simultaneous estimates).24 Fitting the model to all subjects simultaneously (accounting for within-subject correlations) produces more reliable estimates.24,29,30 In addition, PAHs are ubiquitously present in the environment and humans are exposed to background levels of PAHs continuously, as indicated in studies of various general populations.9,11 Although background exposures are often believed to be negligible, ignoring their contribution in half-life estimation can cause severe upward bias.25,30,31 In our modeling, absent of the background term would result in an increase of 18%-147% on the half-live estimates for the 10 OH-PAHs, compared to the final model accounting for the background. Therefore, it is advantageous to include both within-subject correlations and background exposure levels in the pharmacokinetic model. We included both elements using a non-linear mixed effects model with a background term as described in the methods section. We decided to use creatinine-adjusted concentration in the modeling, with the assumption that creatinine-adjusted metabolite concentrations are proportional to metabolite concentrations in the central compartment at that time. This is a reasonable and common approach for such models but not the only choice—alternatives include direct modeling of the bladder compartment and its urine accumulation and void times.
The model-estimated background levels (µC, Table 2) were similar to measured pre-exposure levels (Table 1), and the modeled uptake levels (µB, Table 2) were similar to the calculated uptake of the differences between the pre-exposure and post-exposure maximum concentrations for all metabolites (Table 1), demonstrating the effectiveness of the model that we developed. It should be noted that our statistical model has extensive data requirements and may not be feasible in settings with more sparse data collection. However, our model also has significantly fewer data needs than physiologically-based pharmacokinetic models that attempt to model every detail of uptake and excretion, for which parameters are typically estimated using a variety of external sources in addition to data collected from study participants.
Our estimated t1/2 for urinary 1-PYR was 3.9 h, which is in general lower than reported half-lives for 1-PYR (Table 3). Buckley and Lioy conducted a 6-day study collecting 8-hour composite urine samples from 5 subjects after consuming grilled ground beef, and determined the mean 1-PYR t1/2 of 4.4 h (3.1–5.9 h).16 Chien and Yeh collected all urine voids over 7 days in 9 subjects who ate barbecued meat; the average t1/2 was 5.7 h (3.0–9.9 h).15 Viau et al. determined t1/2 of 12 h in two volunteers who ingested 500 µg pyrene dissolved in olive oil.17 The same author also determined a mean t1/2 of 12.8 h in three volunteers who were dermally exposed.18 It should be noted that these earlier studies did not adjust for background exposures, which could have biased their half-life estimates upwards as discussed earlier.
Table 3.
Reported 1-hydroxypyrene half-lives (hours) in published studies on human populations
| Study design | No. person, smoking status |
Exposure source | Average t1/2 (range) |
Reference |
|---|---|---|---|---|
| Ingestion exposure | ||||
| 3-day sampling from office workers | 9, NS | Barbecued chicken | 3.9 [3.0–5.7]a | This study |
| 7-day sampling from college students | 9, NS | Barbecued meat | 5.7 (3.0–9.9) | 15 |
| 3-day sampling from male adults | 2, NS | 500 µg pyrene in olive oil | 12 | 17 |
| 6-day sampling (8-hour composite urine) from male adults | 5, NS | Grilled beef | 4.4 (3.1–5.9) | 16 |
| Inhalation exposure | ||||
| 3-day sampling from subjects exposed at an aluminum plant | 5, n/a | 6-h aluminum plant air | 9.8 [7.9–11.7]a | 12 |
| 4-day samples from shooting target factory workers | 7, n/a | Petroleum pitch | 6.1 (1.9–12.5) | 13 |
| 4-day pre and post samples from locomotive plant workers | 17, NS,S | Diesel exhaust | 29 (6.4–128) | 32 |
| 10-day sampling from smokers | 8,S | Cigarette smoke | 6.0 (3.7–9.9) | 14 |
| Dermal exposure | ||||
| 3-day sampling from 1 psoriasis patient and 2 volunteers | 3, NS | Creosote or 500 µg pyrene | 12.8 (11.5–15) | 17,18 |
| Inhalation and dermal occupational exposure | ||||
| 3-day pre/post/bedtime samples from asphalt pavers | 20,NS,S | Asphalt | 13.3 [7.8–46]a | 20 |
| 3-day of 5 composite urine/day from creosote workers | 2, S | Coal tar creosote | 5–6 h; 22–24 hb | 36 |
| 5-day pre and post samples from needle coke plant workers | 16, NS,S | Workplace | 10.4 (3.9–26.7) | 21 |
| 4-day pre and post samples from coke oven and graphite electrode workers |
15, NS,S | Workplace | 18 (13.4–26.3) | 19 |
| 3-day pre and post samples from coke oven workers | 18, NS,S | Workplace | n/a (6–35) | 33 |
Abbreviations: NS-non-smoker; S-smoker; n/a-not available
calculated t1/2 with 95% confidence interval;
half-lives in two-phase excretion
Urinary 1-PYR elimination kinetics after inhalation and/or dermal exposure has also been reported. In a study during which 5 subjects breathed workplace air at an aluminum plant for 6 h and collected urine samples for 71 h following the exposure, the 1-PYR excretion process was described by a one-compartment model with t1/2 of 9.8 h (95% CI 7.9−12 h).12 In another inhalation study on 7 workers at an artificial shooting target factory using petroleum pitch as the basic binder, the mean t1/2 was 6.1 h (1.9–12.5 h).13 Excretion kinetics after inhalation exposure to PAHs in cigarette smoke was studied in 8 smokers, and the half-life averaged 6.0 h (3.7–9.9 h).14 Huang et al. studied diesel exhaust exposure in 17 locomotive engine workers, and found the mean t1/2 was 29 h (6.4–128 h) based on pre and post-shift urine samples over 4 consecutive workdays.32 In four studies involving 15–20 workers with both inhalation and dermal exposure who provided pre and post-shift samples over 3–5 consecutive days (Table 3), the reported t1/2 ranged from 5 to 35 hours.19–21,33
In contrast to the consistent half-life estimations from this and existing studies on ingestion exposure, the published results after inhalation and dermal absorption were generally higher and more variable. Such difference could be due to the fact that most investigations on inhalation and dermal exposure occurred in occupational settings and often involved only pre- and post-shift samples over consecutive workdays, rather than frequent and continuous sampling in feeding experiments. A potential residual amount would be carried over to the next workday, which would affect the pre-shift levels and consequently affect the kinetics modeling. As shown in this study and others,12,15,17 an exposure-free period of 24–48 h is often required for PAH biomarkers to reach pre-exposure baseline. In addition, there could be substantial within-person and within-day variability for these PAH metabolites,34 therefore, estimation based on individual’s pre and post shift samples over several days could be largely influenced by such variability, which could lead to biased and/or erroneous results.
In this study, the half-lives ranged 2.5–6.1 h among the 9 other OH-PAHs (Table 2). 2-FLU had the longest t1/2 of 6.1 h (95%CI: 4.9h, 8.1h). 2-NAP has the shortest t1/2 of 2.5 h (2.0h, 3.4h), potentially because of its relatively high background (Figure 4B). A small number of studies have reported the elimination kinetics of urinary PAH biomarkers other than 1-PYR. The t1/2 for 1-NAP was 4 h in workers conducting naphthalene oil distillation,35 and 1.2–1.9 h and14–46 h (two-phase excretion) in 2 smoking workers.36 Sobus et al. collected pre-, post-shift, bedtime and morning samples from 20 asphalt pavers; the calculated half-lives were 26 h (95% CI: 14−116 h) for naphthols (summation of 1-NAP and 2-NAP) and 14 h (9.0–28 h) for phenanthrols (summation of 1-, 2-, 3-, 4- and 9-PHE).20 St. Helen et al. followed 8 smokers and investigated excretion kinetics of urinary PAHs after cigarette smoking; the average t1/2 were 9.4 h (4.9–12.2 h) for 2-NAP and 4.1–8.2 h for the fluorene metabolites. 14 A recent study recruited 12 smokers to smoke a cigarette fortified with D10-phenanthrene and found that the average t1/2 for plasma phenanthrene diol epoxide was 7.3 h (4.8–11 h),37 while half-lives of the 4 urinary phenanthrene metabolites were 3.5–5.1 h in our study.
Excreted metabolite amounts in comparison to ingested PAHs
Within 24 h after the dietary exposure, 6.8% of ingested PYR was excreted as urinary 1-PYR, which was at similar scale, though slightly higher than previously reported 4.4% 15 and 2.9–4.5%.38 We calculated the ingested dose based only on the barbecued chicken, and did not measure PAH contents in the sauce and side dishes, which resulted in an underestimate of the actual ingested amount.
The excreted amount of urinary naphthols (sum of 1- and 2-NAP) was higher than calculated ingested naphthalene, which can be explained by several factors. As discussed above, the ingested amount was underestimated. The urinary biomarkers reflect total exposure from all sources, and inhalation can be the dominant exposure route for NAP in general population.39,40 In addition, naphthalene analysis can be challenging and is often omitted in food analyses.41 In this study, the food was analyzed by a commercial lab without using isotopically labeled internal standards and we did not have control over the data quality.
Generally, the percentage of ingested PAHs excreted as their perspective urinary metabolite was inversely related to the molecule size. NAP metabolites (2-ring) also had the shortest tmax, while the largest biomarker in the assay, 1-PYR (4-ring), had the longest tmax (Table 2). Smaller PAHs have better efficiency to diffuse across lipid/lipoprotein cell membranes, and therefore, contribute to more rapid and efficient biological absorption, transport, distribution and excretion processes.1 Also, smaller compounds are generally excreted preferentially in urine as hydroxy metabolites, while bigger PAHs are more lipophilic and could have a different metabolic pattern, either by forming alternative metabolites/adducts, or by excreting in feces.3 Furthermore, inhalation is the dominant exposure route for small PAHs such as NAP in general population.39,40
For most FLU, PHE and PYR metabolites, the amount of OH-PAHs excreted within 24 h after the dietary exposure was correlated or marginally correlated with the amount of ingested barbecued chicken, and thus, correlated with the amount of ingested PAHs. This is encouraging, especially considering the small sample size (9 participants or N=9). The two naphthalene metabolites were not correlated with the ingested chicken and ingested NAP in the chicken, further confirming that even such high PAH-containing diet was not a major source for the urinary naphthols, and that alternative source through inhalation is more dominant for NAP exposure.
Urinary 1-NAP: pesticide or PAH biomarker
While 9 out of the 10 OH-PAHs correlated well with each other, 1-NAP was not correlated other OH-PAHs measured, including 2-NAP, another metabolite formed from the same parent compound (naphthalene) as 1-NAP. The absence of a significant correlation was driven by high 1-NAP levels in three participants (S2, S6 and S9) that peaked before the dietary exposure with concentrations at 10–60 folds higher than the maximal post-BBQ exposure levels in the other 6 participants (Figure 3). 1-NAP is also the main metabolite of the broad spectrum carbaryl insecticide, accounting for more than 85% of urinary carbaryl metabolites.27 Carbaryl (1-naphthyl-N-methylcarbamate), the major active ingredient in commonly used pesticides, is an agricultural and garden insecticide widely applied to commercial and residential lawns and gardens, including fruits and vegetables. The utility of urinary 1-NAP as biomarker to carbaryl and naphthalene exposure has been discussed by Meeker et al., who proposed the use of correlations between 1-NAP and 2-NAP as well as the ratio of 1-NAP/2-NAP to distinguish the two different sources for urinary 1-NAP.42 Indeed, 1-NAP/2-NAP ratio averaged 442, 91 and 180 in persons S2, S6 and S9, respectively, in contrast to 0.73 (SD 0.52) in the remaining 6 participants. After excluding the 1-NAP data from these 3 participants, the correlation coefficients between 1-NAP and the other 9 OH-PAHs were 0.82–0.97 among all 9 participants. Therefore, it appears that the elevated 1-NAP in participants S2, S6 and S9 were most likely a result of carbaryl pesticide exposure. Similar 1-NAP-specific spikes have also been observed in several previous studies on non-occupationally exposed reference populations.34,40 Considering the wide usage of the carbaryl pesticides and its dominant contribution to urinary 1-NAP when exposed, we recommend not using 1-NAP, and relying on 2-NAP instead as the biomarker to naphthalene exposure in future biomonitoring studies.
Comparison of the biomarker levels to other studies
The maximum 1-PYR concentrations after the dietary exposure were 8.6 times higher than the 95th percentile in the general US population,11 over 8-fold higher than heavy smokers smoking over 20 cigarettes a day,43 and at similar magnitude to that of coke oven workers44 and graphite electrode plant workers.45 This is not to suggest that the risk of eating barbecued chicken were similar to those of smoking heavily or working at highly exposed workplace, since we were comparing the maximum concentration after a single dose to those in consistently long-term high exposure scenario. Rather, this demonstrated the need to control for diet in PAH exposure biomonitoring studies, even in occupational studies with high exposure.
In conclusion, this is the first reported pharmacokinetic study for a panel of 10 OH-PAH biomarkers. The excretion half-life after a dietary exposure was estimated at 2.5–6.1 h using a novel non-linear mixed effect model with a term accounting for background exposure levels, which was shorter, and in many cases, substantially shorter than previously reported half-lives. The majority of metabolites were excreted within 12 h after the exposure. The maximum concentration after the dietary exposure was comparable in magnitude to occupations with known high PAH exposure. Diet is an important and often unavoidable source and should be controlled for even in occupational studies with high exposure. The information obtained from this study, such as the shorter half-lives, clearance time and potential large impact from dietary intake, are crucial for study design, data analysis and result interpretation in future PAH biomonitoring studies.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the study participants for their time and devotion. We also would like to thank Pam Olive for supplying the creatinine measurements and Donald Hilton for commenting on the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the Centers for Disease Control and Prevention.
ABBREVIATIONS
- PAH
polycyclic aromatic hydrocarbon
- OH-PAH
mono-hydroxy polycyclic aromatic hydrocarbon
- IARC
International Agency for Research on Cancer
- CLIA
Clinical Laboratory Improvement Amendments
- CDC
Centers for Disease Control & Prevention
- NAP
naphthalene
- FLU
fluorene
- PHE
phenanthrene
- PYR
pyrene
- 1-PYR
1-hydroxypyrene
- 1-NAP
1-naphthol
- 2-NAP
2-naphthol
- 2-FLU
2-hydroxyfluorene
- 3-FLU
3-hydroxyfluorene
- 9-FLU
9-hydroxyfluorene
- 1-PHE
1-hydroxyphenanthrene
- 2-PHE
2-hydroxyphenanthrene
- 3-PHE
3-hydroxyphenanthrene
- 4-PHE
4-hydroxyphenanthrene
Footnotes
DISCLAIMER
The co-authors of this manuscript do not have any financial conflict of interest.
SUPPORTING INFORMATION AVAILABLE
The following contents are given in the Supporting Information: dietary intake instruction (table S-1), concentration of 16 PAHs in the shredded barbecued chicken (table S-2), calculated ingested PAHs from barbecued chicken and excreted OH-PAH metabolites in 24 h (table S-3), and concentration of 10 urinary OH-PAHs in all participants (figures S-1~8).
REFERENCES
- 1.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 92. Lyon, France: International Agency for Research on Cancer; 2010. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. http://monographs.iarc.fr/ENG/Monographs/vol92/ [PMC free article] [PubMed] [Google Scholar]
- 2.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 82. Lyon, France: International Agency for Research on Cancer; 2002. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. http://monographs.iarc.fr/ENG/Monographs/vol82/ [PMC free article] [PubMed] [Google Scholar]
- 3.ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta: Agency for Toxic Substances and Disease Registry; 1995. http://www.atsdr.cdc.gov/toxprofiles/tp69.html. [PubMed] [Google Scholar]
- 4.Ren A, Qiu X, Jin L, Ma J, Li Z, Zhang L, Zhu H, Finnell RH, Zhu T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12770–12775. doi: 10.1073/pnas.1105209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies-A review. International Journal of Hygiene and Environmental Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Levin JO, Rhen M, Sikstrom E. Occupational PAH exposure: urinary 1-hydroxypyrene levels of coke oven workers, aluminium smelter pot-room workers, road pavers, and occupationally non-exposed persons in Sweden. Sci. Total Environ. 1995;163:169–177. doi: 10.1016/0048-9697(95)04488-m. [DOI] [PubMed] [Google Scholar]
- 8.Grimmer G, Dettbarn G, Jacob J. Biomonitoring of polycyclic aromatic hydrocarbons in highly exposed coke plant workers by measurement of urinary phenanthrene and pyrene metabolites (phenols and dihydrodiols) Int. Arch. Occup. Environ Health. 1993;65:189–199. doi: 10.1007/BF00381155. [DOI] [PubMed] [Google Scholar]
- 9.Mucha AP, Hryhorczuk D, Serdyuk A, Nakonechny J, Zvinchuk A, Erdal S, Caudill M, Scheff P, Lukyanova E, Shkiryak-Nyzhnyk Z, Chislovska N. Urinary 1-hydroxypyrene as a biomarker of PAH exposure in 3-year-old Ukrainian children. Environ. Health Perspect. 2006;114:603–609. doi: 10.1289/ehp.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AM, Raaschou-Nielsen O, Knudsen LE. Urinary 1-hydroxypyrene in children living in city and rural residences in Denmark. Sci. Total Environ. 2005;347:98–105. doi: 10.1016/j.scitotenv.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG., Jr Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environmental Research. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Brzeznicki S, Jakubowski M, Czerski B. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Int. Arch. Occup. Environ. Health. 1997;70:257–260. doi: 10.1007/s004200050216. [DOI] [PubMed] [Google Scholar]
- 13.Lafontaine M, Payan JP, Delsaut P, Morele Y. Polycyclic aromatic hydrocarbon exposure in an artificial shooting target factory: assessment of 1-hydroxypyrene urinary excretion as a biological indicator of exposure. Ann. Occup. Hyg. 2000;44:89–100. [PubMed] [Google Scholar]
- 14.St. Helen G, Goniewicz ML, Dempsey D, Wilson M, Jacob P, III, Benowitz NL. Exposure and Kinetics of Polycyclic Aromatic Hydrocarbons (PAHs) in Cigarette Smokers. Chem. Res. Toxicol. 2012;25:952–964. doi: 10.1021/tx300043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien YC, Yeh CT. Amounts and proportion of administered pyrene dose excreted as urinary 1-hydroxypyrene after dietary exposure to polycyclic aromatic hydrocarbons. Archives of Toxicology. 2010;84:767–776. doi: 10.1007/s00204-010-0570-4. [DOI] [PubMed] [Google Scholar]
- 16.Buckley TJ, Lioy PJ. An examination of the time course from human dietary exposure to polycyclic aromatic hydrocarbons to urinary elimination of 1-hydroxypyrene. Br. J. Ind. Med. 1992;49:113–124. doi: 10.1136/oem.49.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viau C, Carrier G, Vyskocil A, Dodd C. Urinary excretion kinetics of 1-hydroxypyrene in volunteers exposed to pyrene by the oral and dermal route. Sci. Total Environ. 1995;163:179–186. doi: 10.1016/0048-9697(95)04494-l. [DOI] [PubMed] [Google Scholar]
- 18.Viau C, Vyskocil A. Patterns of 1-hydroxypyrene excretion in volunteers exposed to pyrene by the dermal route. Sci. Total Environ. 1995;163:187–190. doi: 10.1016/0048-9697(95)04495-m. [DOI] [PubMed] [Google Scholar]
- 19.Buchet JP, Gennart JP, Mercadocalderon F, Delavignette JP, Cupers L, Lauwerys R. Evaluation of Exposure to Polycyclic Aromatic-Hydrocarbons in A Coke Production and A Graphite Electrode Manufacturing Plant - Assessment of Urinary-Excretion of 1-Hydroxypyrene As A Biological Indicator of Exposure. British Journal of Industrial Medicine. 1992;49:761–768. doi: 10.1136/oem.49.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobus JR, Mcclean MD, Herrick RF, Waidyanatha S, Onyemauwa F, Kupper LL, Rappaport SM. Investigation of PAH biomarkers in the urine of workers exposed to hot asphalt. Ann. Occup. Hyg. 2009;53:551–560. doi: 10.1093/annhyg/mep041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boogaard PJ, van Sittert NJ. Exposure to polycyclic aromatic hydrocarbons in petrochemical industries by measurement of urinary 1-hydroxypyrene. Occup. Environ. Med. 1994;51:250–258. doi: 10.1136/oem.51.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Cho SH, Kang JW, Kim YD, Nan HM, Lee CH, Lee H, Kawamoto T. Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int. Arch. Occup. Environ. Health. 2001;74:59–62. doi: 10.1007/s004200000193. [DOI] [PubMed] [Google Scholar]
- 23.Heudorf U, Angerer J. Urinary monohydroxylated phenanthrenes and hydroxypyrene - the effects of smoking habits and changes induced by smoking on monooxygenase-mediated metabolism. International Archives of Occupational and Environmental Health. 2001;74:177–183. doi: 10.1007/s004200000215. [DOI] [PubMed] [Google Scholar]
- 24.Efron B, Morris C. Stein’s Paradox in Statistics. Scientific American. 1977;236:119–127. [Google Scholar]
- 25.Bartell SM. Bias in half-life estimates using log concentration regression in the presence of background exposures, and potential solutions. J. Expo. Sci. Environ. Epidemiol. 2012;22:299–303. doi: 10.1038/jes.2012.2. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, Patterson DG, Jr, Sjodin A. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal. Chem. 2006;78:5744–5751. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- 27.Maroni M, Colosio C, Ferioli A, Fait A. Biological monitoring of pesticide exposure: a review. Toxicology. 2000;143:5–118. doi: 10.1016/s0300-483x(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Sjodin A, Romanoff LC, Horton K, Fitzgerald CL, Eppler A, Aguilar-Villalobos M, Naeher LP. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ. Int. 2011;37:1157–1163. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 2006;114:176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirai JH, Kissel JC. Uncertainty in estimated half-lives of PCBS in humans: impact on exposure assessment. Sci. Total Environ. 1996;187:199–210. doi: 10.1016/0048-9697(96)05142-x. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Smith TJ, Ngo L, Wang T, Chen H, Wu F, Herrick RF, Christiani DC, Ding H. Characterizing and Biological Monitoring of Polycyclic Aromatic Hydrocarbons in Exposures to Diesel Exhaust. Environ Sci. Technol. 2007;41:2711–2716. doi: 10.1021/es062863j. [DOI] [PubMed] [Google Scholar]
- 33.Jongeneelen FJ, Van Leeuwen FE, Oosterink S, Anzion RB, van der LF, Bos RP, van Veen HG. Ambient and biological monitoring of cokeoven workers: determinants of the internal dose of polycyclic aromatic hydrocarbons. Br. J. Ind. Med. 1990;47:454–461. doi: 10.1136/oem.47.7.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Romanoff LC, Lewin MD, Pittman EN, Trinidad D, Needham LL, Patterson DG, Jr, Sjodin A. Variability of Urinary Polycyclic Aromatic Hydrocarbon Metabolite Levels in Adults and Comparison of Spot, First-Morning, and 24-Hour Void Sampling. J. Expo. Sci. Environ. Epidemiol. 2010;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bieniek G. The presence of 1-naphthol in the urine of industrial workers exposed to naphthalene. Occup. Environ. Med. 1994;51:357–359. doi: 10.1136/oem.51.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heikkila P, Luotamo M, Pyy L, Riihimaki V. Urinary 1-naphthol and 1-pyrenol as indicators of exposure to coal tar products. Int. Arch. Occup. Environ. Health. 1995;67:211–217. doi: 10.1007/BF00626355. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y, Carmella SG, Upadhyaya P, Hochalter JB, Rauch D, Oliver A, Jensen J, Hatsukami D, Wang J, Zimmerman C, Hecht SS. Immediate Consequences of Cigarette Smoking: Rapid Formation of Polycyclic Aromatic Hydrocarbon Diol Epoxides. Chemical Research in Toxicology. 2011;24:246–252. doi: 10.1021/tx100345x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viau C, Diakite A, Ruzgyte A, Tuchweber B, Blais C, Bouchard M, Vyskocil A. Is 1-hydroxypyrene a reliable bioindicator of measured dietary polycyclic aromatic hydrocarbon under normal conditions? J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:165–177. doi: 10.1016/s0378-4347(01)00465-0. [DOI] [PubMed] [Google Scholar]
- 39.ATSDR. Toxicological profile for naphthalene, 1-methylnaphthalene and 2-methylnaphthalene. Atlanta: Agency for Toxic Substances and Disease Registry; 2005. http://www.atsdr.cdc.gov/toxprofiles/tp67.html. [Google Scholar]
- 40.Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD, Sjodin A. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. Journal of Environmental Monitoring. 2010;12:1110–1118. doi: 10.1039/c000689k. [DOI] [PubMed] [Google Scholar]
- 41.ECSCF. Polycyclic Aromatic Hydrocarbons - Occurrence in Foods, Dietary Exposure and Health Effects. Brussels: European Commission Scientific Committee on Food; 2002. [Google Scholar]
- 42.Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Expo. Sci. Environ. Epidemiol. 2007;17:314–320. doi: 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- 43.Hecht SS, Carmella SG, Le KA, Murphy SE, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on levels of 1-hydroxypyrene in urine. Cancer Epidemiology Biomarkers & Prevention. 2004;13:834–842. [PubMed] [Google Scholar]
- 44.Simioli P, Lupi S, Gregorio P, Siwinska E, Mielzynska D, Clonfero E, Pavanello S. Non-smoking coke oven workers show an occupational PAH exposure-related increase in urinary mutagens. Mutation Research. 2004;562:103–110. doi: 10.1016/j.mrgentox.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Angerer J, Mannschreck C, Gundel J. Occupational exposure to polycyclic aromatic hydrocarbons in a graphite-electrode producing plant: biological monitoring of 1-hydroxypyrene and monohydroxylated metabolites of phenanthrene. Int. Arch. Occup. Environ Health. 1997;69:323–331. doi: 10.1007/s004200050155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


