Abstract
This study used ecological momentary assessment (EMA) data from adult daily smokers attempting to quit smoking to assess relations between exposure to contextual risk factors and cessation failure, latency to a first smoking lapse or progression from lapse to relapse (smoking seven days in a row). Participants were adult, daily smokers enrolled in a randomized controlled clinical trial of bupropion SR and individual counseling who were followed to one year post-quit. Participants reported exposure to high-risk contexts and behaviors, including being where cigarettes were available or smoking was permitted, being around others smoking in prospective, real-time assessment for two weeks pre- and four-weeks post-quit. Results showed that greater exposure to contextual risk factors during the pre-quit did not predict cessation failure. However, Cox regression survival analyses revealed that spending a greater proportion of time where cigarettes were easily available following at least one day of abstinence predicted shorter latency to a first lapse, even after controlling for baseline risk factors such as gender, nicotine dependence, depressive symptoms and living with a smoker. Greater cigarette availability following a lapse was not associated with progression from lapse to relapse with or without baseline risk factors in the model. This suggests that post-quit environmental risk factors such as cigarette availability increases lapse risk while stable risk factors such as living with smokers and higher baseline carbon monoxide level or depressive symptoms remain potent predictors of progression to relapse. Real-time contextual risk assessments post-quit predict lapse above and beyond stable, baseline risk factors.
Despite the well documented costs of smoking (World Health Report, 2008; Fiore et al., 2004), more than 18% of American adults still smoke (CDC, 2014). Although many want and try to quit smoking (CDC, 2011), failing to quit and relapse are the most common outcomes of quit attempts (Fiore et al., 2008). Returns to smoking tend to happen in some contexts more than others. Relapse episodes, for example, tend to occur in the context of alcohol consumption, eating, and being around other smokers (e.g., Shiffman, 1982). Living with a smoker or working where smoking is permitted also raises relapse risk (Hymowitz et al., 1991; Japuntich et al., 2011). This research has identified high-risk situations that counselors often advise quitters to avoid (e.g., bars), modify (e.g., impose smoking bans at home), or actively cope with (e.g., bring oral substitutes to work) during a quit attempt (Fiore et al., 2008; Brown et al., 2003).
To date, relapse contexts have been studied primarily using retrospective designs (i.e., asking people where they were when they relapsed). Retrospective accounts may be biased by one's internal state at the time of recall or the salience of events (Conway & Holmes, 2004; Pillemer, Rhinehart, & White,1986; Shiffman et al., 1997). Ecological momentary assessments (EMA) allow research participants to report smoking behavior, environment, events, and actions in real-time and in naturalistic settings (Stone et al., 2002; Stone &Shiffman, 2003). Shiffman et al. (1997) found that the correspondence between retrospective recall and real-time reports of smoking triggers preceding lapses and temptations was low, suggesting that participants have a hard time accurately recalling events that precede smoking. EMA gathers more accurate representations of contexts and their relations with smoking than do retrospective assessments.
Several prospective studies using EMA examined differences in the contexts of first lapse episodes, temptation episodes (i.e., situations in which individuals came to the brink of smoking but successfully refrained from smoking; Shiffman et al., 1996 a&b), and other random occasions during a quit attempt (Shiffman et al., 1996a;Shiffman et al., 1996b). For example, Shiffman et al. (1996b) showed that first lapses were more likely to occur, compared to temptation episodes, when smoking was permitted, when cigarettes were easily available, and in the presence of other smokers (Shiffman et al., 1996b). Further analyses (Shiffman et al., 1996a) showed that regardless of smoking status (lapsers or maintainers), temptations, compared to other occasions, were more likely to occur in the context of exposure of smoking cues, and eating and drinking. These studies focused on relations between contexts and immediate smoking outcome (i.e., lapse, temptation) within subjects. Potential accumulated effects of exposure to such contexts during a quit attempt on cessation success (ability to initiate cessation and maintain abstinence) have not been tested. Such a between-subjects approach would allow us to identify smokers at elevated risk for smoking post-quit based on their pre-event environments.
The current study investigated relations between select contextual risk factors and initial cessation (successfully abstaining for one calendar day within the first two weeks of a quit attempt), latency to a first lapse (any smoking after achieving initial cessation), and latency to relapse (smoking seven days in a row) following a lapse (Shiffman et al., 2006). The use of cessation milestones allows us to look at relations between context and cessation outcomes at distinct phases of the quitting process. The aim of this between-subjects analysis was to determine whether EMA measures of trigger exposure told us anything above and beyond baseline measures to identify smokers at risk of rapid returns to smoking. We hypothesized that endorsing being where cigarettes were available, smoking was permitted, or others were smoking would increase the likelihood of cessation failure, and decrease the latency to a first lapse and subsequent relapse above and beyond baseline risk factors.
Method
Participants
The current study used data collected from a double-blind, randomized, placebo-controlled clinical trial of bupropion SR and individual counseling for smoking cessation described in detail elsewhere (McCarthy et al., 2008). Inclusion and exclusion criteria are presented in Table 1. The total enrollment included 463 participants who met screening criteria, and attended the first study visit. For the current analyses, we restricted the sample to those who completed at least three EMA reports per day, on average, during key assessment periods. We imposed this restriction to ensure that the data would provide adequate coverage of participants’ daily activities and environmental trigger exposure. Baseline characteristics of the individuals included in the full sample and restricted samples are shown in Table 2.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 18 years of age or greater | Living with someone enrolled in the study |
| Able to read and write English | Participation in a study in the past 30 days |
| Smoking at least 10 cigarettes per day | Current use of stop-smoking treatments |
| Baseline CO level of at least 10 parts per | Current illegal drug use |
| At least fairly motivated to quit smoking | Current heavy drinking |
| Willing to fulfill study requirements | Use of other tobacco products (e.g., cigar, chewing tobacco) in last 7 days |
| Current depression (CES-D score over 16) | |
| History of bipolar disorder or psychosis diagnosis or treatment | |
| Uncontrolled hypertension | |
| History of seizure | |
| Past negative reactions to bupropion | |
| Pregnancy or breast feeding |
Table 2.
Demographic characteristics of final samples used in analyses predicting cessation failure, lapse latency, and relapse latency following a lapse.
| Variable | Value | All Sample n (%) (n = 463) | Cessation Failure n (%) (n = 316) | Lapse n (%) (n = 284) | Relapse n (%) (n = 156) |
|---|---|---|---|---|---|
| Sex | Female | 233 (50.3%) | 162 (51.3%) | 135 (47.5%) | 81 (51.9%) |
| Race/Ethnicity | White | 412 (89.0%) | 282 (89.2%) | 253 (89.1%) | 134 (85.9%) |
| African-American | 26 (5.6%) | 17 (5.4%) | 17 (6.0%) | 10 (6.4%) | |
| Hispanic | 5 (1.1%) | 3 (0.9%) | 4 (1.4%) | 1 (0.6%) | |
| Other | 22 (4.8%) | 14 (5.0%) | 12 (4.2%) | 9 (5.8%) | |
| Marital Status | Married | 198 (42.8%) | 141 (44.6%) | 136 (47.9%) | 74 (47.4%) |
| Divorced | 85 (18.4%) | 60 (19.0%) | 55 (19.4%) | 31 (19.9%) | |
| Never married | 117 (25.3%) | 69 (21.8%) | 58 (20.4%) | 34 (21.8%) | |
| Cohabitating | 44 (9.5%) | 30 (9.5%) | 24 (8.5%) | 13 (8.3%) | |
| Separated | 10 (2.2%) | 7 (2.2%) | 5 (1.8%) | 1 (0.6%) | |
| Widowed | 7 (1.5%) | 7 (2.2%) | 5 (1.8%) | 2 (1.3%) | |
| Education | < High school graduate | 20 (4.3%) | 13 (4.1%) | 9 (3.2%) | 7 (4.4%) |
| High school graduate | 104 (22.5%) | 69 (21.8%) | 55 (19.4%) | 29 (18.6%) | |
| Some college | 224 (48.4%) | 160 (50.6%) | 139 (48.9%) | 72 (46.2%) | |
| College degree or more | 113 (24.4%) | 72 (22.8%) | 79 (27.8%) | 46 (29.5%) | |
| Employment Status | Employed | 376 (81.2%) | 260 (82.3%) | 230 (81.0%) | 121 (77.6%) |
| Unemployed | 25 (5.4%) | 16 (5.1%) | 28 (9.9%) | 9 (5.8%) | |
| Homemaker | 18 (3.9%) | 15 (4.7%) | 15 (5.3%) | 9 (5.8%) | |
| Student | 12 (2.6%) | 6 (1.9%) | 7 (2.5%) | 4 (2.6%) | |
| Retired | 14 (3.0%) | 9 (2.8%) | 9 (3.2%) | 6 (3.8%) | |
| Disabled | 10 (2.2%) | 6 (1.9%) | 5 (1.8%) | 3 (1.9%) | |
| Household Income | < $25,000 | 141 (30.5%) | 86 (27.2%) | 70 (24.6%) | 47 (30.1%) |
| $25,000-$34,999 | 70 (15.1%) | 48 (15.2%) | 48 (16.9%) | 17 (10.9%) | |
| $35,000-$49,999 | 88 (19.0%) | 69 (21.8%) | 59 (20.8%) | 30 (19.2%) | |
| >$50,000 | 154 (33.3%) | 105 (33.2%) | 99 (34.9%) | 58 (37.2%) | |
| All Sample M (SD) | Cessation Failure M (SD) | Lapse M (SD) | Relapse M (SD) | |
|---|---|---|---|---|
| Age | 38.76 (12.16) | 39.09 (11.58) | 39.19 (11.52) | 40.04 (12.13) |
| Age at first cigarette | 13.48 (3.82) | 13.39 (3.95) | 13.56 (4.00) | 13.49 (4.04) |
| Age at daily smoking | 16.40 (3.52) | 16.35 (3.59) | 16.60 (3.65) | 16.56 (3.63) |
| Cigarettes smoked per day | 21.93 (10.44) | 21.92 (10.56) | 21.83 (10.83) | 21.83 (11.22) |
| Years of smoking | 25.28 (12.36) | 25.70 (11.88) | 25.63 (11.82) | 26.45 (12.28) |
| Previous quit attempts | 5.47 (10.23) | 5.23 (10.51) | 5.89 (11.16) | 6.59 (14.04) |
| Baseline CO level | 24.37 (11.55) | 24.40 (11.44) | 24.32 (11.68) | 23.78 (11.73) |
| Baseline FTND Score | 5.13 (2.36) | 5.10 (2.30) | 4.94 (2.33) | 4.86 (2.31) |
| Baseline CES-D Score | 6.10 (5.24) | 5.95 (5.22) | 6.00 (5.24) | 6.20 (5.32) |
Note: CO = carbon monoxide; FTND = Fagerström Test for Nicotine Dependence; CES-D = Center for Epidemiological Studies-Depression scale.
Procedure
Participants were assigned to one of four groups in a two (active bupropion SR vs. placebo) by two (counseling vs. no counseling) factorial design. Random EMA reports were administered using an electronic diary (on a palmtop computer) from two weeks pre-quit to four weeks after a target quit date. Participants were followed to one year after a quit date to assess smoking status with biochemical verification of abstinence at 6 and 12 months post-quit.
Measures
Baseline Assessment
Participants provided demographic information and answered self-report measures of nicotine dependence, smoking history, affect, and depression symptoms. Nicotine dependence was assessed with the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Ecological Momentary Assessment
Participants were prompted to complete two-minute EMA reports four to seven times per day based on the length of their waking day, separated by at least 30-minute intervals. Participants had two minutes to respond to random prompts and reports not completed within two minutes were considered missed. Randomly prompted EMA reports assessed thoughts, emotions, tobacco withdrawal symptoms, temptations to smoke, stressful events, coping, and smoking. Participants also completed evening reports that assessed the number of cigarettes smoked over the past 24 hours and initiated a “slip” report following lapses.
The timing of a first lapse (any puff from a cigarette) after the target quit day set by experimenters and the timing of the first relapse (first day smoking seven days in a row) were determined from the composite of EMA random, bedtime, and slip reports and the timeline follow-back smoking calendar completed at visits. Data were coded such that any smoking reported in any report or modality was considered a sign that smoking occurred on that day.
Exposure to high-risk contexts was assessed at every random report. Participants were asked to report “just before prompt” the following whether: smoking was permitted, cigarettes were available, they were with anyone, and if so, anyone was smoking. Drinking alcohol just before the prompt was also assessed, but occurred at very low rates (in 1.6% of reports and in only 46% of participants), so these data will not be analyzed. All context variables were coded as binary (1=exposed to high-risk context, 0=low/no exposure) at each report. Items that represented exposure to high-risk contexts included: smoking was allowed (vs. forbidden or discouraged=0), being where cigarettes were easily available (vs. not available or available with difficulty=0), and being with someone who was smoking (vs. being alone or being with someone who was not smoking=0). On average, in the 403 participants retained through the quit day, participants completed 3.81 reports per day (Range=0-6.50, SD=1.08).
Final Sample
Of the 463 enrollees, 60 dropped out prior to the target quit day and were excluded from these analyses. The final sample for cessation failure was 316 (78.4%) because 87 (21.6%) of 403 participants retained through the quit date responded to fewer than three random reports on average pre-quit. The final sample for the first lapse analysis was 282 of 349 (80.8%) who achieved initial cessation because 67 (19.2%) responded to fewer than an average of three reports per day. A total of 156 of 295 (52.9%) participants who lapsed after achieving initial cessation were included in the relapse latency model because 66 (22.4%) did not lapse during the EMA period and 73 (24.7%) who lapsed during the EMA period did not respond to at least three random report prompts post-lapse and pre-relapse.
There were no significant differences in baseline measures (Table 2) between the samples included versus excluded for low responses rates in any model (all ps >.05). We conducted sensitivity analyses (i.e., repeated analyses with the different subsamples of participants with at least an average of one or at least two reports per day for inclusion). The pattern of results described below remained unchanged, except where noted for the relapse model.
Data Analysis
The mean proportion of reports in which participants endorsed exposure to each smoking trigger during each period (i.e., pre-quit, pre-lapse, and between lapse and relapse) were used as between-subject variables capturing contextual risk factor exposure. Logistic regression analyses were conducted to examine the associations between pre-quit rates of exposure to smoking triggers and cessation failure. Cox regression survival analyses were conducted to model separately latency to a first lapse after achieving initial cessation and latency to relapse following a first lapse. Participants who were lost to follow-up or who never lapsed or relapsed were treated as censored in analyses. Analyses were conducted both with and without controlling for baseline predictors of cessation failure, lapse, and relapse, including treatment condition (bupropion SR = 1, placebo =0), gender, age, education level, baseline depression score, baseline nicotine dependence level, baseline CO level, and living with smokers. Smoking cessation counseling condition was not included in the set of baseline covariates given that counseling was not associated with smoking outcomes (McCarthy et al., 2008) and inclusion of counseling in the models did not change the pattern of results. Non-significant context predictors were pruned from models to enhance model parsimony.
Results
Cessation Failure
Multiple logistic regression analyses were conducted to determine whether the proportion of reports in which risk factors were endorsed pre-quit predicted cessation failure, or being unable to quit for one calendar day within two weeks of a quit attempt. A total of 316 participants provided 17,596 reports during the pre-quit period that were used to compute individual participants’ rate of risk exposure.
None of the hypothesized contextual risk factors significantly predicted cessation failure (ps> .05). Exposure to environments in which others were smoking (M=12.5%, SD=14.0%), smoking was permitted (M=64.6%, SD=27.5%), or cigarettes were easily available (M=85.3%, SD=17.3%) prior to quitting had no detectable relation with cessation failure. Inclusion of baseline covariates in the model did not change the results. Women were significantly less likely to achieve initial cessation compared to men (Odds Ratio [OR]= 2.57, 95% Confidence Interval [CI] = 1.18-5.59, p = .017).
Latency to a First Lapse
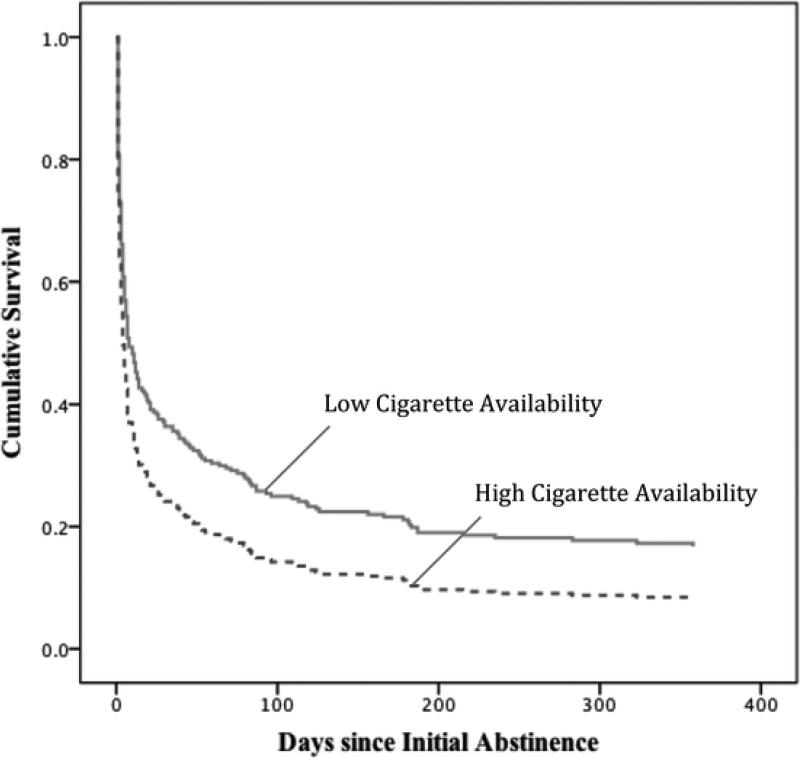
Cox regression survival analyses were conducted to assess the effects of contextual risk factors on the latency to a first lapse. A total of 14,259 reports from 284 participants were aggregated to get estimates of the percentage of reports in which each trigger was endorsed in the pre-quit and post-initial cessation, pre-lapse periods separately. Spending a higher proportion of time where cigarettes were easily available (M=20.8%, SD=30.0%) during the post-initial cessation, pre-lapse period, but not pre-quit period, was associated with a significantly shorter latency to a first lapse (Figure 1). Real-time reports of being where smoking was permitted (M=40.9%, SD=34.3%) and exposure to others smoking (M=7.8%, SD=13.5%) were not predictive of lapse latency. The pattern of results remained unchanged when baseline covariates were included in the survival model. Pruning non-significant predictors of latency to lapse from the final lapse model (Table 3) also did not change the results. Bupropion SR treatment increased the latency to lapse following initial cessation.
Figure 1.
Latency to Lapse by Post-quit (pre-lapse) Cigarette Availability (mean split).
Table 3.
Cox regression estimates of pre-lapse (post-initial cessation) trigger exposure rates on latency to a first lapse.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Female | 1.106 | (0.852, 1.435) | 0.451 |
| Racial/Ethnic Minority | 1.184 | (0.786, 1.783) | 0.420 |
| College Education | 1.000 | (0.720, 1.388) | 0.999 |
| Bupropion SR | 0.748 | (0.573, 0.975) | 0.032 |
| Age | 1.004 | (0.991, 1.016) | 0.581 |
| Baseline CO | 0.997 | (0.986, 1.009) | 0.655 |
| FTND | 0.977 | (0.918, 1.040) | 0.470 |
| CES-D | 1.024 | (0.998, 1.051) | 0.075 |
| Lives w/ Smoker | 1.189 | (0.892, 1.585) | 0.237 |
| Pre-lapse Cigarette Availabilitya | 1.007 | (1.002, 1,011) | 0.007* |
The hazard ratio in the table represents the increase in the hazard of lapsing associated with a 1% increase in exposure to contexts in which cigarettes were available because cigarette availability was coded as a percentage ranging from 0 to 100%.
Note: CO = carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; CES-D = Center for Epidemiological Studies-Depression scale.
p<.05
Lapse-Relapse Latency
Cox regression survival analysis tested relations between contextual risk factor exposure between a first lapse and relapse and the latency in days between lapse and relapse, both with and without baseline covariates. Estimates of risk exposure during this interval were based on 10,305 random reports from 156 participants. Contextual risk factors during the pre-quit period were also entered in the model.
Contrary to the lapse model, spending more time in places where cigarettes were easily available after lapsing (M=29.4%, SD=30.0%), being where smoking was permitted (M=42.3%, SD=34.0%), and exposure to others smoking (M=9.6%, SD=14.0%) were not significantly associated with a progression from a first lapse to a relapse, controlling for pre-quit contextual risk factors. Sensitivity analyses with baseline covariates showed that estimated hazard ratios were very similar across the subsamples tested, with a hazard ratio or 1.006 in the sample with three or more reports per day (95% CI=.998-1.008, n=156), and 1.007 in the samples with at least one (95% CI=1.001-1.013, n=214) or two reports per day (95% CI=1.000-1.015, n=193). None of the pre-quit contextual risk factors predicted relapse latency and including the baseline covariates in the model did not change the pattern of results. Those with higher baseline depressive symptoms and those who had higher baseline CO levels had significantly shorter latencies to relapse after a first lapse. Living with smokers also significantly predicted shorter latency to relapse following a first lapse. Excluding non-significant pre-quit predictors of lapse-relapse latency from the final lapse-relapse model did not change the pattern of results (Table 4).
Table 4.
Cox regression estimates of post-lapse, pre-relapse trigger exposure rates on latency to relapse.
| Hazard Ratio | 95% CI | p-value | |
|---|---|---|---|
| Female | 1.189 | (0.809, 1.747) | 0.378 |
| Racial/Ethnic Minority | 1.221 | (0.687, 2.171) | 0.496 |
| College Education | 1.054 | (0.658, 1.688) | 0.828 |
| Bupropion SR | 0.585 | (0.391, 0.875) | 0.009* |
| Age | 0.990 | (0.972, 1.008) | 0.261 |
| Baseline CO | 1.023 | (1.003, 1.044) | 0.027* |
| FTND | 1.081 | (0.984, 1.186) | 0.104 |
| CES-D | 1.043 | (1.006, 1.080) | 0.022* |
| Lives w/ Smoker | 1.748 | (1.148, 2.663) | 0.009* |
| Pre-relapse Cigarette Availabilitya | 1.006 | (0.998, 1.014) | 0.138 |
| Pre-relapse Smoking Permitteda | 1.002 | (0.996, 1.008) | 0.558 |
| Pre-relapse Others Smokinga | 0.995 | (0.980, 1.011) | 0.559 |
The odds ratio in the table represents the increase in the hazard of lapsing associated with a 1% increase in exposure to contexts in which cigarettes were available because cigarette availability was coded as a percentage ranging from 0 to 100%.
Note: CO = carbon monoxide; FTND = Fagerstrom Test for Nicotine Dependence; CES-D = Center for Epidemiological Studies-Depression scale.
p<.05
Discussion
The purpose of the study was to test hypotheses regarding frequency of exposure to contextual risk factors before and after a quit attempt and key milestones in the relapse process. Results of this between-subjects analysis indicated that pre-quit exposure to high-risk contexts was not associated with likelihood of achieving initial cessation. In addition, contextual variables during the pre-quit period were also unrelated to lapse or relapse risk. On the other hand, spending more time in situations where cigarettes were easily available after achieving initial cessation predicted shorter latency to lapse. This result is consistent with a finding from an early EMA study (Shiffman et al., 1996b) showing that cigarettes were perceived as more available on lapse occasions than comparison temptation or randomly prompted control occasions. Shiffman et al (1996b) demonstrated that exposure to contextual risk factors (vs. non-exposure) predicted increased immediate lapse risk. Results of this study add to these findings, suggesting that there are potential accumulated effects of access to cigarettes on lapse risk. Taken together, these results suggest that failing to reduce access to cigarettes upon cessation increases lapse risk both between and within subjects and supports the importance of promoting avoidance of cigarette access in recent quitters. This effect of cigarette availability on lapse latency remained unchanged, even after controlling for baseline risk factors for smoking and pre-quit cigarette availability. Assessing environmental cigarette availability post-quit therefore adds value when attempting to identify smokers at high-risk for lapse.
Progression from lapse to relapse was not significantly related to pre-relapse cigarette availability in the relatively small sample of participants (n=156) with high response rates. In broader samples with lower response rates, this relation achieved significance with similar point estimates of the hazard ratio, however. This suggests that lack of significant relations between cigarette access and progression to relapse in this study may be due to inadequate statistical power to detect small effects. However, it is also possible that cigarette availability adds less to relapse prediction than to lapse prediction. Relapse progression may be more related to individual difference variables or to lapse reactions than to contextual factors. In even the most restrictive samples, baseline variables, including living with a smoker, higher baseline CO levels, and elevated depressive symptoms were predictive of relapse latency, when cigarette access was not.
Contrary to our prediction, being around other smokers and being in places in which smoking was permitted were not significantly related to lapse or relapse latencies. In an earlier study (Shiffman et al., 1996), being where smoking was permitted and seeing others smoking also differentiated lapses from both temptations and random control occasions within subjects. Our analyses suggest that, in a between-subjects analysis, spending more time where cigarettes seem readily available is more closely related with both latencies to lapse and relapse than are perceptions of smoking bans or awareness of others smoking. The perception of cigarette availability may have more personal relevance than smoking permission and others’ smoking behavior. Research has shown that perceiving a proximal opportunity to smoke can enhance attention to smoking cues (Wertz & Sayette, 2001) and that exposure to smoking cues, including contexts, can induce strong urges to smoke (Conklin et al., 2009). Other studies have also demonstrated that “availability” of cigarettes can induce strong craving to smoke as well as withdrawal-like symptoms (Carter & Tiffany, 2001; Dols, Willems, van den Hout, & Bittoun, 2000; Juliano & Brandon, 1998; Thewissen, van den Hout, Havermans, & Jansen, 2005).
Limitations
Although we preserved the temporal ordering of the predictor and outcome in this analysis, we cannot rule out the possibility that a third variable might influence pre-event choices about contexts and also accelerate lapses and relapses. Second, the present study selected a few contexts as risk factors for smoking based on previous research. Contextual triggers to smoke may be idiosyncratic based on specific learning histories and smoker-specific triggers were not assessed in this study. Third, while reports were randomly prompted to minimize sampling biases, missing reports may be associated with certain contexts or situations. Fourth, the selected sample used for analyses may not represent the general population of smokers trying to quit, especially given the relative homogeneity of the sample and use of high motivation to quit as an inclusion criterion. Finally, more data were available for participants who did not lapse compared to those who did. We corrected this imbalance by computing risk exposure as a percentage of reports in which risk was endorsed rather than using counts of exposure.
Conclusion
The present study investigated the between-subjects relations between aggregated real-time assessments of exposure to contextual risk factors and key milestones of smoking cessation. Results suggested that those with greater access to cigarettes after achieving initial cessation lapsed sooner than did those with less access to cigarettes. The goal of the current research was not to establish exposure to high-risk contexts as a cause of lapse or relapse, but rather to determine whether assessing smoker's exposure to contextual risk factors during each of the critical milestone periods could serve as a useful marker of lapse or relapse risk. The results suggest that monitoring smokers’ environments for cigarette availability can tell us something about their lapse risk above and beyond baseline measures while achieving initial abstinence and relapse risk are more strongly predicted by stable, baseline risk factors. Future research that explores relations between more refined or individualized contexts and cessation milestones may further contribute to our understanding of ways in which environments predict smoking cessation success.
Acknowledgments
Funding: The project described was supported by Transdisciplinary Tobacco Use Research Center grant P50CA084724 from the National Cancer Institute and P50DA19706 from the National Institute of Drug Abuse awarded to Michael C. Fiore, M.D., M.P.H. and Timothy B. Baker, Ph.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Abrams DB, Niaura RS. Social learning theory. In: Blanc HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. Guilford Press; New York: 1987. pp. 131–178. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bower GH, Forgas JP. Mood and social memory. In: Forgas J, editor. Handbook of affect and social cognition. Erlbaum; Mahwah, NJ: 2001. pp. 95–120. [Google Scholar]
- Bower GH, Monteiro KP, Gilligan SG. Emotional mood as a context for learning and recall. Journal of Verbal Learning and Verbal Behavior. 1978;17:573–585. [Google Scholar]
- Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health. 2000;90:1122–1127. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA. Intensive behavioral treatment. In: Abrams DB, Niaura R, Brown R, Emmons KM, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook: A guide to best practices. Guilford Press; New York, NY: 2003. pp. 118–177. [Google Scholar]
- Carter BL, Tiffany ST. Meta analysis of cue reactivity in addiction research. Addiction. 1999;92:15–26. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9(2):183–90. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Current Cigarette Smoking Among Adults—United States, 2005–2012. Morbidity and Mortality Weekly Report. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Quitting Smoking Among Adults—United States, 2001–2010.Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Christakis NA, Fowler JH. The Collective Dynamics of Smoking in a Large Social Network. New England Journal of Medicine. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: Implication for human extinction-based research and treatment. Experimental and Clinical Psychopharmacology. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: The effects of environments on smokers’ cue reactivity. Experimental and Clinical Psychopharmacology. 2008;16:207–214. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Cook JW, Fucito LM, Piasecki TM, Piper ME, Schlam TR, Berg KM, Baker TB. Relations of alcohol consumption with smoking cessation milestones and tobacco dependence. Journal of Consulting and Clinical Psychology. 2012;80:1075–1085. doi: 10.1037/a0029931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Holmes A. Psychological stages and availability of autobiographical memories. Journal of Personality. 2004;72:461–480. doi: 10.1111/j.0022-3506.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addictive Behaviors. 2000;25(1):103–8. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O'Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20(5):657–73. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose, and Throat Journal. 1992;69:763–767. [PubMed] [Google Scholar]
- Fiore MC, Croyle RT, Curry SJ, Baker TB. Preventing 3 million deaths and helping 5 million smokers quit: a national action plan for tobacco cessation. American Journal of Public Health. 2004;94(2):205–210. doi: 10.2105/ajph.94.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, et al. Clinical practice guideline. U.S.: Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: systematic review. British Medical Journal. 2002;325(7357):188–125. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundey J, Thirugnanasambandam N, Kaminsky K, Drees A, Skwirba AC, Lang N, Paulus W, Nitsche MA. Neuroplasticity in cigarette smokers is altered under withdrawal and partially restituted by nicotine exposition. Journal of Neuroscience. 2012;32(12):4156–62. doi: 10.1523/JNEUROSCI.3660-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology. 2005;114:649–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert WS, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoking per day. Addiction. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Sexton M, Ockene J, Grandits G. Baseline factors associated with smoking cessation and relapse. Preventive Medicine. 1991;20:590–601. doi: 10.1016/0091-7435(91)90057-b. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB. Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine. 2011;40:286–294. doi: 10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6(1):45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research. 2008;10(4):717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? Journal of Psychopharmacology. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104(10):1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piasecki T, McCarthy DE, Fiore M, Baker TB. Alcohol consumption, smoking urge, and reinforcing effects of cigarettes: An ecological study. Psychology of Addictive Behaviors. 2008;22(2):230–239. doi: 10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73:1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Fischer LA, Paty JA, Gnys M, Kassel JD, Hickcox M, Perz W. Drinking and smoking: a field study of their association. Annals of Behavioral Medicine. 1994;16:203–209. [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychology. 1996a;15:455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gyns M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996b;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100(3):387–96. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence Phenotype Workgroup Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Report WHO report on the global tobacco epidemic. 2008 Available at: http://whqlibdoc.who.int/publications/2008/9789241596282_eng.pdf (accessed September 8, 2013)