Summary
Tbx3, a member of the T-box family, plays important roles in development, stem cells, nuclear reprogramming, and cancer. Loss of Tbx3 induces differentiation in mouse embryonic stem cells (mESCs). However, we show that mESCs exist in an alternate stable pluripotent state in the absence of Tbx3. In-depth transcriptome analysis of this mESC state reveals Dppa3 as a direct downstream target of Tbx3. Also, Tbx3 facilitates the cell fate transition from pluripotent cells to mesoderm progenitors by directly repressing Wnt pathway members required for differentiation. Wnt signaling regulates differentiation of mESCs into mesoderm progenitors and helps to maintain a naive pluripotent state. We show that Tbx3, a downstream target of Wnt signaling, fine tunes these divergent roles of Wnt signaling in mESCs. In conclusion, we identify a signaling-TF axis that controls the exit of mESCs from a self-renewing pluripotent state toward mesoderm differentiation.
Graphical Abstract
Highlights
-
•
An alternate and stable pluripotent state of Tbx3 knockout mESCs exists
-
•
Tbx3 maintains steady-state levels of Dppa3 in mESCs
-
•
Tbx3 directly represses mesoderm specification genes like T
-
•
Tbx3 represses Wnt pathway genes required for mesoderm differentiation
In this article, Lemischka and colleagues show that Tbx3 controls the exit of mESCs from pluripotency toward Wnt-mediated mesoderm differentiation. They describe the generation of Tbx3 knockout mESCs and identify Dppa3 as a direct downstream target of Tbx3 in mESCs.
Introduction
Mouse embryonic stem cells (mESCs) are cells derived from inner cell mass (ICM) that self-renew indefinitely in culture and can give rise to all somatic cell types both in vitro and in vivo (Evans and Kaufman, 1981; Martin, 1981; Murry and Keller, 2008). The balance between self-renewal and differentiation is maintained by a milieu of transcription factors (TFs) (Ivanova et al., 2006; Thomson et al., 2011), epigenetic modifiers (Ang et al., 2011; Liang and Zhang, 2013; Loh et al., 2007), and signaling cascades (Lee et al., 2012a; Niwa et al., 2009). Many ESC-specific TFs such as Nanog (Chambers et al., 2007), Rex1 (Toyooka et al., 2008), Klf4 (Niwa et al., 2009; Toyooka et al., 2008), and Tbx3 (Niwa et al., 2009) are heterogeneously expressed in a population of mESCs (Faddah et al., 2013; MacArthur et al., 2012). These factors display low and high protein expression levels (Chambers et al., 2007; Toyooka et al., 2008). In the absence of some of these TFs (like Nanog), mESCs can maintain self-renewal and pluripotency (Chambers et al., 2007).
Expression of TFs like Nanog, Sox2, Esrrb, Klf4, and Tbx3 is controlled by external signaling cues (Niwa et al., 2009). Wnt signaling pathway has been widely studied in development (Nusse and Varmus, 2012), plays important roles in maintaining pluripotency (ten Berge et al., 2011; Ying et al., 2008), and is important for acquisition of pluripotency during induced pluripotent stem cell (iPSC) reprogramming (Ho et al., 2013; Marson et al., 2008a). Downstream of Wnt ligand/receptor binding, the inhibition of GSK3β is necessary for stabilization of β-CATENIN (Nusse and Varmus, 2012). Inhibition of GSK3β and Mapk signaling with small molecules maintains a naive pluripotent state in mESCs (Sato et al., 2004; Ying et al., 2008). Wnt signaling stabilizes the mESC state in limiting amounts of LIF (Ogawa et al., 2006). Wnt3a prevents mESC differentiation from a naive to an epiblast-like or primed state (ten Berge et al., 2011). Apart from its role in maintenance of pluripotency, Wnt signaling is also required for differentiation of mESCs into early mesoderm derivatives (Gadue et al., 2006). Specifically, Wnt3 and Wnt8a are necessary for mesoderm differentiation (Kemp et al., 2005; Lindsley et al., 2006). During in vivo development, Wnt signaling is required for formation of primitive streak and mesoderm (Arnold et al., 2000; Martin and Kimelman, 2010; Yamaguchi et al., 1999). In summary, Wnt signaling is required both for stabilization of an undifferentiated pluripotent state and for promoting early mesoderm differentiation.
Expression of Tbx3 is regulated by Wnt signaling in mESCs (Kelly et al., 2011; Price et al., 2013) and cancer (Renard et al., 2007). Tbx3, the only member of its subfamily expressed in the ICM, is required for maintenance of ESC state (Chapman et al., 1996; Han et al., 2010; Ivanova et al., 2006). Tbx3 maintains self-renewal of mESCs in absence of LIF, a property shared by Nanog and Klf4 (Han et al., 2010). Tbx3 overexpressed with Oct4, Sox2, and Klf4 during iPSC-reprogramming improves germline competence of fully reprogrammed iPSCs (Han et al., 2010). Tbx3 overexpression also improves the efficiency of cell fusion-based reprogramming (Han et al., 2010). However, in vivo, Tbx3 null embryos survive until E10.5 (Davenport et al., 2003), beyond the blastocyst stage.
Many ESC-specific TFs like Oct4, Nanog, Sox2 (Mendjan et al., 2014; Thomson et al., 2011), and Sall4 (Lim et al., 2008) play important roles during early differentiation in addition to their roles in repressing differentiation of ESCs (Loh and Lim, 2011). Tbx3 promotes the formation of the mesendoderm lineage and is expressed both in ESCs and in vivo during primitive streak formation (Kartikasari et al., 2013; Weidgang et al., 2013). Furthermore, Tbx3 is required for generation of extraembryonic endoderm cells (Lu et al. 2011; Rugg-Gunn et al., 2010) and for opening up of Eomes promoter during differentiation to definitive endoderm (Kartikasari et al., 2013). However, the mechanisms by which Tbx3 regulates transition from a stable pluripotent state into differentiated progenitors remain unclear. Here, we explore how Tbx3 modulates the response of extracellular signaling and maintains balance between mESC self-renewal and differentiation. We isolate an alternate and stable pluripotent state in the absence of Tbx3 and identify Dppa3 (Stella/PGC7) as a direct downstream target of Tbx3. We present a model in which the Wnt/Tbx3/Dppa3 signaling-TF axis controls specification of mESCs into mesoderm lineage.
Results
A Subpopulation of mESCs Maintains a Tbx3 Null Pluripotent State
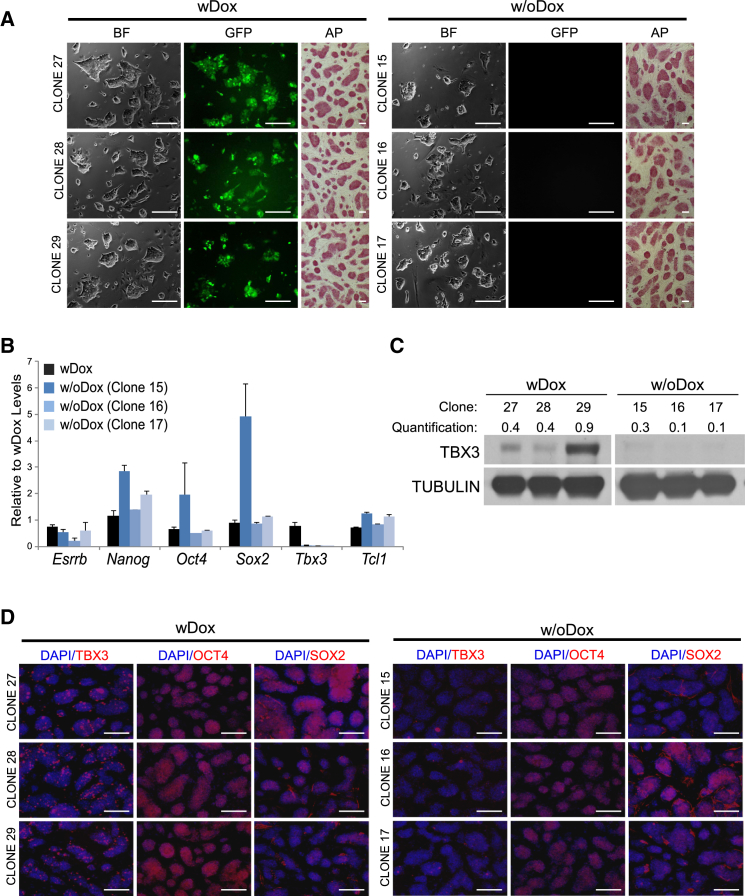
Tbx3 plays an important role in the maintenance of the mESC self-renewal state (Han et al., 2010; Ivanova et al., 2006). Upon RNAi-mediated Tbx3 depletion, mESCs differentiate even in the presence of LIF (Ivanova et al., 2006). We used a genetic complementation “rescue” system to control the expression of Tbx3 in ESCs (Figure S1A). In this system, called Tbx3R, endogenous Tbx3 is depleted by a constitutively expressed short-hairpin (sh) RNA and replaced with an exogenous shRNA-immune Tbx3 cDNA under the control of Doxycycline (Dox) (Ivanova et al., 2006). Five days after knockdown of Tbx3 (−Dox), mESCs lose their characteristic ESC-like morphology and alkaline phosphatase (AP) staining (Figure S1B). During the 5-day time course, mESCs also downregulate expression of pluripotency genes and upregulate differentiation markers (Figure S1C). Interestingly, upon prolonged culture of Tbx3-depleted cells, rare colonies with an ESC-like morphology emerge in a population of differentiating cells (Figure S1D). We picked and expanded three of these colonies (clones 15, 16, and 17) for weeks in the absence of Dox (Figure 1A, right). We also picked and expanded some colonies grown in the presence of Dox (clones 27, 28, and 29) as controls (Figure 1A, left). All of the six clones maintained ESC-like morphology and AP activity (Figure 1A). Tbx3 mRNA level was reduced in colonies without Dox, but they continued to express ESC markers such as Oct4, Nanog, Sox2, Esrrb, and Tcl1 (Figure 1B). The level of TBX3 protein was reduced in colonies without Dox (Figures 1C and 1D), but they continued to express OCT4 and SOX2 proteins (Figure 1D). Tbx3 mRNA and protein is heterogeneously expressed in populations of mESCs (Kumar et al., 2014; MacArthur et al., 2012; Niwa et al., 2009). We confirmed these results by analyzing the expression of Tbx3 mRNA in single mESCs discussed later. We therefore postulated that Tbx3 function might be dispensable in a subpopulation of mESCs upon long-term culture.
Figure 1.
Tbx3 Is Dispensable in a Subpopulation of mESCs
(A) Picked long-term cultured Tbx3R mESC clones grown in presence (wDox) or absence (w/oDox) of Dox in ESC media containing LIF. BF, GFP channel, and AP staining colony pictures are shown. Scale bars represent 200 μM.
(B) The mRNA expression signature of three independent Tbx3R-Dox mESC clones cultured in ESC media containing LIF. Data shown are the average of three independent clones for Tbx3R+Dox mESC.
(C) TBX3 protein expression in Tbx3R mESC clones cultured in ESC media containing LIF. Quantification of protein levels is shown.
(D) OCT4, SOX2, and TBX3 protein expression was checked by immunofluorescence (IF). Scale bars represent 200 μM.
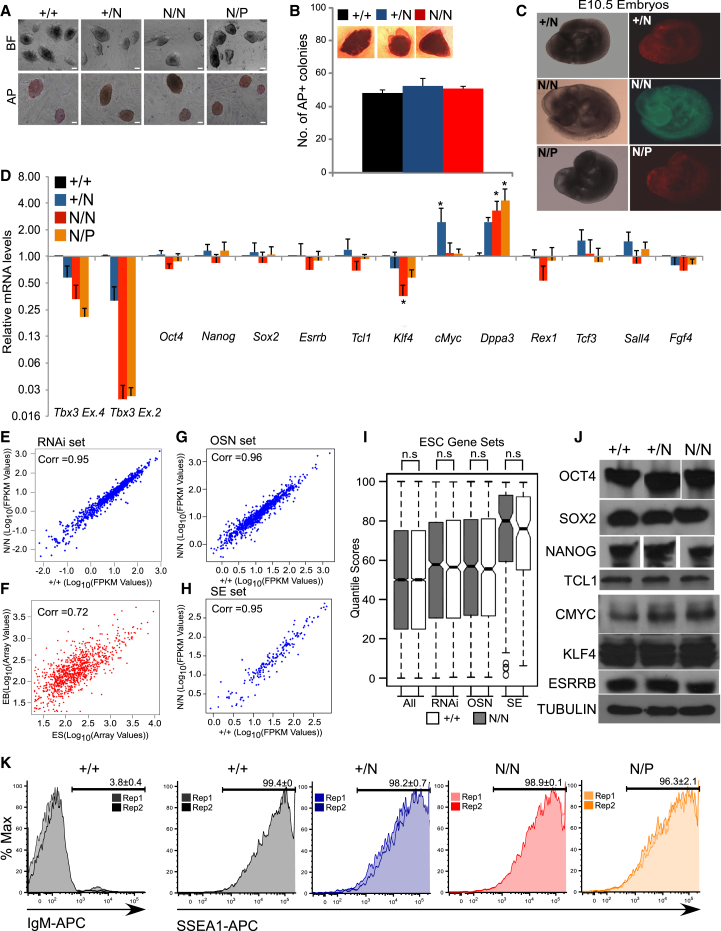
To explore this, we generated Tbx3 null mESCs from Tbx3 heterozygous (Tbx3+/N) mESCs (Davenport et al., 2003) using two approaches. First, Tbx3N/N cells were generated via homologous recombination by culturing Tbx3+/N cells in high concentrations of G418 (Figure S2A). Second, Tbx3N/P cells were generated by targeting the WT allele in Tbx3+/N cells (Figure S2A). We confirmed the targeted alleles by genomic PCR and Southern blotting (Figures S2B and 2C). Loss of TBX3 protein expression was confirmed (Figure S2D). Tbx3N/N cells also maintained a normal karyotype in culture (Figure S2E). Both Tbx3N/P and Tbx3N/N cells maintain ESC-like morphology and AP activity (Figure 2A). Single Tbx3N/N cells retain the ability to form AP-positive colonies with efficiencies equal to WT and Tbx3+/N cells (Figure 2B). We tested the developmental potential of Tbx3N/N and Tbx3N/P mESCs using the blastocyst injection assay. Tbx3+/N cells were used as control in the experiment. To mark ESCs and their descendants in chimeras, Tbx3+/N and Tbx3N/P cells were infected with lentiviral tdTomato vector, and Tbx3N/N cells were infected with lentiviral eGFP vector. Both Tbx3N/N and Tbx3N/P cells extensively contributed to various tissues of E10.5 embryos indicated by expression of GFP or tdTomato throughout the chimeric embryos (Figure 2C; Table S6). It has also been reported that Tbx3N/N embryos, derived by mating Tbx3+/N mice, give rise to live embryos that survive without any apparent defects until E10.5, after which they exhibit a variety of defects in various tissues, including the yolk sac, limbs, and emergent mammary glands (Davenport et al., 2003). The mRNA expression levels of a selected panel of ESC genes also did not change significantly (>2-fold) between Tbx3+/+, Tbx3+/N, Tbx3N/N, and Tbx3N/P mESCs with the exception of Dppa3, which is discussed in detail later (Figure 2D). To further characterize Tbx3 null mESCs, we used a heterokaryon-based reprogramming assay (Pereira and Fisher, 2009). We tested the ability of Tbx3N/N mESCs to confer an ESC-like state on human B cells following cell fusion (Figure S2F). The starting B cells do not express human TBX3 mRNA as shown using human-specific primers (Figure S2G). Tbx3N/N mESCs robustly induce a human ESC gene expression signature (Figure S2H) onto human B cells. Tbx3 null mESCs therefore retain the ability to reprogram differentiated cells into an ESC-like state, a property of self-renewing mESCs.
Figure 2.
Tbx3 Null mESCs Express an ESC-like Gene Expression Signature
(A) Tbx3 null mESCs maintain ESC-like morphology (top) and AP activity (bottom). Scale bars represent 200 μM.
(B) Single Tbx3 null mESCs sorted into 96-well plates and assayed for their ability to form AP-positive colonies. Data represent the average of two independent plates.
(C) Chimeric E10.5 embryos showing in vivo contribution of mESCs after blastocyst injection.
(D) mRNA levels of indicated ESC markers in mESCs cultured in the presence of LIF. Data are the average of three independent experiments. ∗p < 0.05 (t test).
(E–H) Correlation of mESC-specific gene set between WT and Tbx3N/N cells. Genes that have effects on mESC self-renewal from published genome-wide RNAi screens (E). Correlation of the same gene set between ESCs and EBs (G). Genes whose promoters are bounds by OCT4, SOX2, and NANOG (OSN) in mESCs from published datasets (F). Genes whose upstream or downstream DNA elements were described as ESC-specific super enhancers (H). “Corr” is the Pearson’s correlation coefficient.
(I) mRNA expression profile from normalized mRNA-Seq dataset from Tbx3+/+ and Tbx3N/N mESCs for the indicated gene sets. The expression data are represented as quantile scores, described in Supplemental Information. Significance calculated using Wilcoxon rank sum test with continuity correction.
(J) Protein levels of indicated ESC markers in mESCs cultured in presence of LIF.
(K) SSEA1-APC staining on mESCs grown in serum + LIF. IgM-APC stained cells used as negative control.
To rule out the possibility of cell culture side effects, we derived Tbx3−/− mESCs from Tbx3−/− blastocysts by mating Tbx3+/N mice (Figure S3A; Table S5). We refer to these in vivo derived cells as T3Blast mESCs. T3Blast+/+, T3Blast+/N, and T3BlastN/N cells maintain ESC-like morphology and stain positive for AP activity (Figure S3B). The mRNA expression levels of a selected panel of mESC genes did not change significantly (>2-fold) between T3Blast+/+, T3Blast+/N, and T3BlastN/N cells with the exception of Dppa3 (Figure S3C). To further characterize the function of Tbx3 in vivo, we assessed Tbx3+/+, Tbx3+/−, and Tbx3−/− blastocysts at stages E3.75 and E4.5. They had no apparent abnormality in size (Figures S3D and S3E) and cell number (Figure S3E; Table S5). The number of cells expressing GATA6 and NANOG proteins, markers for primitive endoderm (PrE) and epiblast, respectively, were also assessed in the blastocysts. No significant difference was found between Tbx3+/+, Tbx3+/−, and Tbx3−/− blastocysts at stages E3.75 and E4.5 (Figure S3F). In summary, our data suggest that Tbx3 null mESCs can be derived, and they maintain a stable self-renewing state and contribute to live chimeric embryos upon blastocyst injection.
Tbx3 Null mESCs Maintain an ESC-like Gene Expression Signature
To understand how ESC-like properties are maintained in the absence of Tbx3, we analyzed genome-wide mRNA expression profiles of Tbx3+/+, Tbx3+/N, and Tbx3N/N cells using mRNA-Seq (Table S1). Global gene expression profiles of Tbx3+/+, Tbx3+/N, and Tbx3N/N cells are strongly correlated with each other (0.96–0.98). We asked whether genes important for maintenance of the ESC state changed between Tbx3+/+, Tbx3+/N, and Tbx3N/N cells. To test this, we annotated a list of three ESC-specific gene sets. First, we looked at genes from published genome-wide RNAi screens in human and mouse ESCs that were found to be important for pluripotency, hereby-called “RNAi” set (Chia et al., 2010; Ding et al., 2009; Fazzio et al., 2008; Hu et al., 2009; Kagey et al., 2010). Second, we analyzed genes whose upstream DNA elements are bound by Oct4, Sox2, and Nanog (OSN), hereby-called “OSN” set. Third, we looked at genes with transcriptional start sites (TSSs) closest to mESC-specific super enhancers, hereby called “SE” set (Chen et al., 2008; Marson et al., 2008b; Whyte et al., 2013). The genes from “RNAi “set are expressed at similar levels (correlation coefficient = 0.95) in Tbx3+/+ and Tbx3N/N cells (Figure 2E). The mRNA expression pattern of the “RNAi” set genes between mESCs and mouse EBs (correlation coefficient = 0.72) is shown for comparison (Figure 2F) (Hailesellasse Sene et al., 2007). Also for genes from “OSN” and “SE” sets, mRNA expression levels correlated tightly (correlation coefficient = 0.95–0.96) between Tbx3+/+ and Tbx3N/N cells (Figures 2G and 2H). We therefore, saw no significant changes in mRNA expression levels between Tbx3+/+ and Tbx3N/N cells in any of the datasets tested (Figure 2I). In addition, we found comparable levels of key ESC proteins such as OCT4, SOX2, NANOG, ESRRB, CMYC, KLF4, and TCL1 in Tbx3+/+, Tbx3+/N, and Tbx3N/N cells (Figure 2J). The levels of SSEA1, a key cell surface marker of mESCs, also did not change between Tbx3+/+, Tbx3+/N, Tbx3N/N, and Tbx3N/P cells (Figure 2K). In conclusion, Tbx3 null mESCs maintain a functionally unchanged global transcriptional landscape in long-term cultures.
Tbx3 Transcriptionally Represses Dppa3 in mESCs
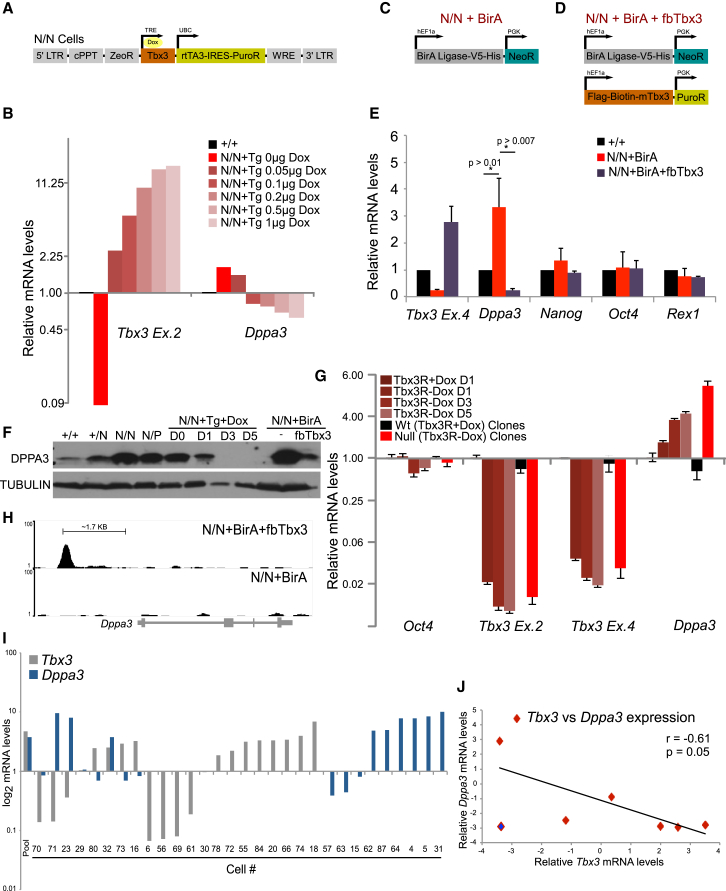
Our mRNA-Seq analysis revealed no global change in gene expression between Tbx3+/+, Tbx3+/N, and Tbx3N/N cells. However, few genes were upregulated (>2-fold) in Tbx3N/N cells. Dppa3 is one of those genes (Figure 2D). It is also upregulated in T3BlastN/N cells (Figure S3C). To investigate the molecular mechanism of Dppa3 induction, we expressed a Dox-inducible Tbx3 transgene (Figure 3A) into Tbx3N/N cells, hereby called Tbx3N/N+Tg cells. Dox-induced Tbx3 expression was verified at the mRNA and protein level (Figures S4A and S4B). Tbx3 controls Dppa3 mRNA expression in a concentration-dependent manner (Figure 3B). To confirm these results, we expressed Flag-Biotin-tagged Tbx3 (fbTbx3) transgene in Tbx3N/N cells expressing the biotin ligase BirA transgene (Tbx3N/N+ BirA cells) (Figures 3C and 3D). The expression of the biotin ligase BirA allows for in vivo biotinylation of the tagged TBX3 protein (de Boer et al., 2003; Kim et al., 2009; Wang et al., 2006). Expression of fbTBX3 protein in Tbx3N/N+ BirA cells downregulated Dppa3 mRNA expression (Figure 3E), while expression of other ESC TFs (Nanog, Oct4, Rex1) was unchanged. The effect of Tbx3 expression on DPPA3 levels was also validated at the protein level using the cell lines discussed above (Figure 3F). Dppa3 was also upregulated early upon shRNA-mediated depletion of Tbx3 (Tbx3R-Dox) in a population of differentiating mESCs and in stable Tbx3 knockdown clones (clones 15, 16, 17; discussed in Figure 1) (Figure 3G). We next asked whether Tbx3 directly regulates the expression of Dppa3 using ChIP-Seq in mESCs. TBX3 protein binds ∼1.7 kb upstream of the Dppa3 TSS (Figure 3H; Table S2). We also tested the expression of Tbx3 and Dppa3 mRNAs in 45 single mESCs. We confirmed that both Tbx3 and Dppa3 are heterogeneously expressed in a population of self-renewing (SSEA1+) mESCs (Figure 3I; Table S3), and their expression was anti-correlated (r = −0.61, p = 0.05) in cells that expressed Tbx3 and Dppa3 (Figure 3J). This anti-correlation of Tbx3 and Dppa3 expression in mESCs was also observed in other studies (r = −0.13; p = 0.03 in 185 cells) (Kumar et al., 2014). Tbx3 therefore transcriptionally represses Dppa3 in mESCs and may contribute to the heterogeneity of Dppa3 expression.
Figure 3.
Tbx3 Represses the Expression of Dppa3 in mESCs
(A) Construct used for inducible expression of Tbx3 transgene in Tbx3 null mESCs.
(B) Tbx3 and Dppa3 mRNA levels in Tbx3+/+ and Tbx3N/N+Tg cells treated with indicated concentrations of Dox/ml.
(C and D) Constructs used in making Tbx3N/N + BirA (C) and Tbx3N/N + BirA + fbTbx3 (D) lines.
(E) mRNA levels of indicated genes in Tbx3+/+, Tbx3N/N+BirA, and Tbx3N/N+BirA+fbTbx3 cells. Data are the average of three independent replicates. The p values were generated using t test.
(F) DPPA3 protein expression in Tbx3+/+, Tbx3+/N, Tbx3N/N, Tbx3N/P, Tbx3N/N+Tg, Tbx3N/N+BirA, and Tbx3N/N+BirA+fbTbx3 cells.
(G) mRNA expression profile from qRT-PCR experiment for the indicated genes. Tbx3R data are cells grown with or without Dox (Tbx3) over 5 days. Wild-type (Tbx3R+Dox) data are an average of three independent clones 27, 28, and 29 from Figure 1. Null (Tbx3R-Dox) data are an average of three independent clones 15, 16, and 17 from Figure 1.
(H) TBX3 binds 1.7 kb upstream of the Dppa3 TSS in mESCs.
(I) Tbx3 and Dppa3 mRNA levels in SSEA1+ single mESCs.
(J) Correlation of mRNA expression values in cells expressing Dppa3 and Tbx3.
Dppa3 is a marker of naive mESCs downregulated in epiblast stem cells (EpiSCs) (Hayashi et al., 2008; Tesar et al., 2007) and during EB differentiation (Figure S4C). We therefore asked whether Dppa3 overexpression compensates for absence of Tbx3 and helps to maintain the mESC state. We utilized our genetic complementation strategy (Lee et al., 2012b) and established Tbx3N/N cells stably expressing an shRNA targeting Dppa3 as well as a Dox-inducible shRNA immune Dppa3 Tg, named Tbx3N/N-Dp3R (Figure S4D). These rescue clones were generated in the presence of Dox (Figure S4E). Upon removal of Dox, Tbx3N/N-Dp3R cells displayed a partially differentiated morphology (Figure S4F) and decreased levels of the ESC marker SSEA1 (Figure S4G). These changes were accompanied by increased expression of the mesoderm marker Brachyury (T) and decreased expression of the ESC markers Tcl1 and Esrrb (Figure S4H). Given this partial differentiation phenotype, it is likely that other factors besides Dppa3 also aid in the maintenance of the mESC state in the absence of Tbx3. Taken together, our results indicate that Dppa3 overexpression in Tbx3 null mESCs, at least in part, compensates for the function of Tbx3 in the maintenance of ESC state.
Tbx3 Controls Initiation of Wnt Signaling-Mediated Mesoderm Differentiation
We wanted to better understand the function of Tbx3 during the exit of mESCs from pluripotency toward differentiation. During exit from pluripotency, Tbx3 expression is downregulated (Figure S4I). Tbx3 expression is also downregulated in mouse EpiSCs (Tesar et al., 2007) and mouse epiblast-like stem cells (EpiLCs) (Buecker et al., 2014). We used Wnt mediated serum-free derivation of mesoderm from mESCs to further assess levels of Tbx3 (Figure S4J) (Kanke et al., 2014). During 2 days of differentiation, mesoderm genes (T, Mixl1, Wnt8a) were upregulated (Figure S4K), and other pluripotency-associated genes (Nanog, Tcl1) as well as Tbx3 were downregulated (Figure S4L).
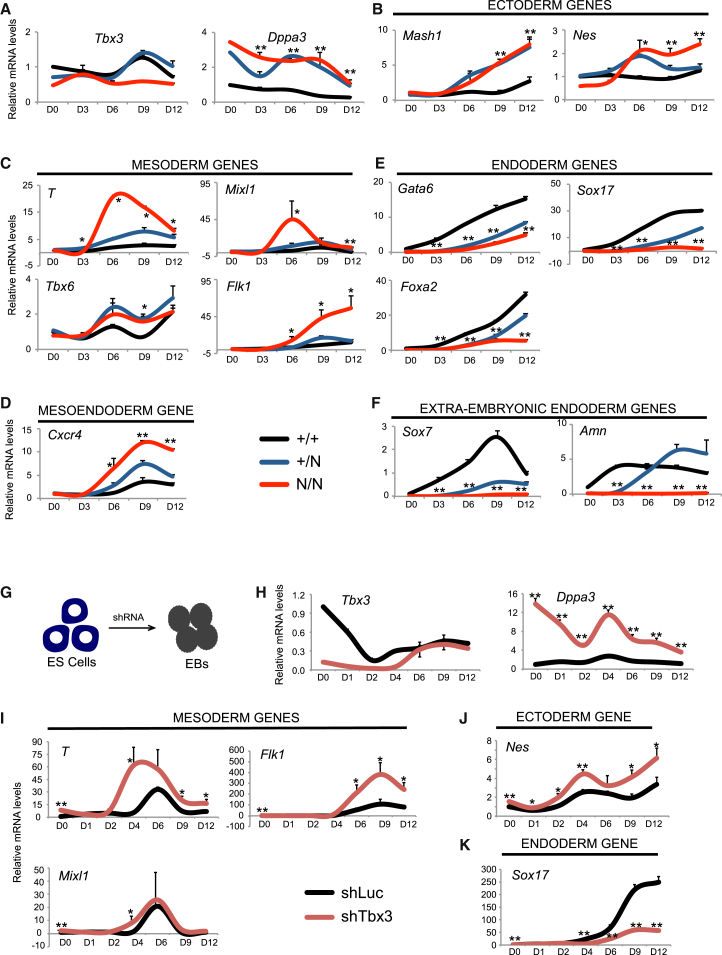
We tested the ability of Tbx3 null mESCs to differentiate into the three germ layers using the EB differentiation assay. Absence of Tbx3 expression in Tbx3N/N cells is accompanied by overexpression of Dppa3 (Figure 4A), a number of markers of ectoderm (Mash1/Ascl1, Nes) (Figure 4B), mesoderm (T, Mixl1, Flk1, Tbx6) (Figure 4C), and mesendoderm (Cxcr4) markers (Figure 4D). However, endoderm (Gata6, Sox17, Foxa2) and extraembryonic endoderm (Sox7, Amn) genes were significantly downregulated in EBs of Tbx3N/N cells (Figures 4E and 4F), consistent with the reported role of Tbx3 in regulating extraembryonic endoderm differentiation (Kartikasari et al., 2013; Lu et al., 2011). We confirmed these results by knocking down Tbx3 in mESCs using shRNA and testing its effect on EB differentiation (Figure 4G). Tbx3 expression was downregulated, and Dppa3 expression was significantly upregulated (Figure 4H). We saw significant upregulation of mesoderm (T, Flk1, Mixl1) and ectoderm (Nes) genes (Figures 4I and 4J), while endoderm gene (Sox17) was downregulated (Figure 4K). The same effect was also seen during differentiation of Tbx3 N/P cells. In absence of Tbx3 expression (Figure S5A), Dppa3 was upregulated (Figure S5B). The upregulation of mesoderm genes (T, Tbx6, Wnt8a) (Figure S5C) and downregulation of endoderm genes (Sox17, FoxA2, Gata6, Amn, Sox7) (Figure S5D) were confirmed. To confirm the effect at the protein level, we used a serum-free differentiation (SFD) protocol to direct mESCs to mesoderm progenitors (Figure S5E). Mesoderm progenitors are ECADHERIN− FLK1+ population (Nostro et al., 2008; Yamashita et al., 2000; Yasunaga et al., 2005). A population of ECADHERIN− mesoderm cells also expresses CXCR4 protein unlike endoderm cells that are ECADHERIN+CXCR4+ (Yasunaga et al., 2005). Therefore, to define mesoderm progenitors, we measured either ECADHERIN− FLK1+ or ECADHERIN− FLK1+ CXCR4+after 5–6 days of differentiation of Tbx3+/+, Tbx3+/N, and Tbx3N/P cells (Figure S5F). We see a significant upregulation of mesoderm differentiation in Tbx3N/P cells compared with Tbx3+/+ cells (Figure S5G). Tbx3 therefore represses differentiation into mesoderm lineage.
Figure 4.
Effect of Tbx3’s Loss on Differentiation of mESCs
(A–F) The mRNA expression pattern of indicated genes during a 12-day EB differentiation time course. Data are average of three independent replicates. ∗p < 0.05 and ∗∗p < 0.005 (t test).
(G) Schematic of EB differentiation of mESCs after infection with the control shRNA or Tbx3 shRNA.
(H–K) The mRNA expression pattern of indicated genes during a 12-day EB differentiation time course after infection with the control shRNA or Tbx3 shRNA. The data are an average of three independent replicates. ∗p < 0.05 and ∗∗p < 0.005 (t test).
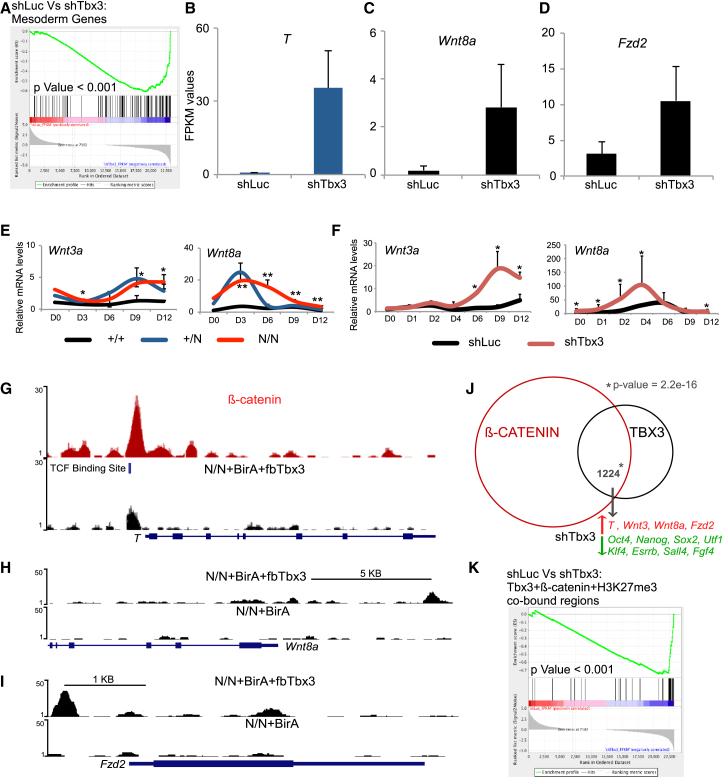
Furthermore, in ESC growth conditions, knockdown of Tbx3 in mESCs (shTbx3) causes a significant upregulation of mesoderm genes (Figure 5A). T, a primitive streak marker, is overexpressed in shTbx3 cells (∼60-fold) (Figure 5B). Wnt signaling regulates and is regulated by T expression during primitive streak emergence and early mesoderm formation (Martin and Kimelman, 2010; Narayanan et al., 2011; Yamaguchi et al., 1999). We saw that shTbx3 cells have significantly increased expression of Wnt pathway genes (Figures S6A). Specifically, Wnt8a and Fzd2 mRNAs are upregulated in shTbx3 cells (Figures 5C and 5D). Wnt8a is expressed during early stages of mESC differentiation (Lindsley et al., 2006) and in mesoderm progenitors of a developing embryo (Narayanan et al., 2011). During a time course of EB differentiation, Wnt8a and Fzd2 are significantly upregulated in EBs derived from Tbx3 null cells (Figure 5E) and in EBs derived upon knockdown of Tbx3 (Figure 5F). TBX3 protein also binds upstream of the T, Wnt8a, and Fzd2 gene promoters in mESCs (Figures 5G–5I; Table S2) directly repressing their expression.
Figure 5.
Tbx3 Represses Wnt Signaling-Mediated Mesoderm Differentiation
(A) Gene set enrichment analysis (GSEA) to detect statistical significance of mesoderm genes in shTbx3 versus shLuc. Cells grown in ESC media containing LIF were infected with lentiviral shRNA constructs against Luciferase (shLuc) or Tbx3 (shTbx3).
(B–D) mRNA-Seq FPKM values of T, Wnt8a, and Fzd2 in cells infected the indicated shRNAs.
(E) mRNA expression profile of indicated genes during EB differentiation time course of 12 days for indicated cell lines. Data are the average of three independent replicates. ∗p < 0.05 and ∗∗p < 0.005 (t test).
(F) mRNA expression profile of the indicated genes during EB differentiation time course of 12 days in WT mESCs infected with indicated shRNAs. Data are the average of three independent replicates. ∗p < 0.05 and ∗∗p < 0.005 (t test).
(G–I) TBX3 ChIP-seq binding data from N/N-BirA (control) and N/N-BirA+fbTbx3 cells. β-CATENIN binding from published data.
(J) Venn diagram representing overlap between β-CATENIN and TBX3 genome-wide binding sites. The p value was generated using the fisher exact test.
(K) GSEA to detect statistical significance of TBX3, β-CATENIN, and H3K27me3 co-bound gene set in shTbx3 versus shLuc.
Apart from its role in differentiation, Wnt signaling is known to promote pluripotency in ESC culture conditions. The addition of WNT3A into mESC culture media increased levels of Tbx3 mRNA (Figures S5B and S5C). A significant number of genes bound by TBX3 are also bound by β-CATENIN, a downstream effector of Wnt signaling (Figure 5J). We found that the TBX3 binding site upstream of the T promoter (Figure 5G) overlaps with a TCF binding motif previously shown to activate T expression during mesoderm differentiation (Yamaguchi et al., 1999). This binding site is also co-bound by β- CATENIN upon Wnt stimulation (Figure 5G) in mESCs (Zhang et al., 2013) and mediates the differentiation of human ESCs and iPSCs into mesoderm lineage (Mendjan et al., 2014). This region is also marked by repressive modification H3K27me3 in mESCs (encode data). A significant number of TBX3 and β-CATENIN co-bound genes are highly expressed in mESCs, including Oct4, Nanog, Sox2, and Esrrb (Figure S5D). However, TBX3, β-CATENIN, and H3K27me3 co-bound genes are significantly upregulated upon knockdown of Tbx3, which included mesoderm genes such as T, Wnt8a, Hes7 (Figure 5K). Therefore, Tbx3 along with β-catenin represses mesoderm and Wnt pathway genes required for initiation of mesoderm differentiation.
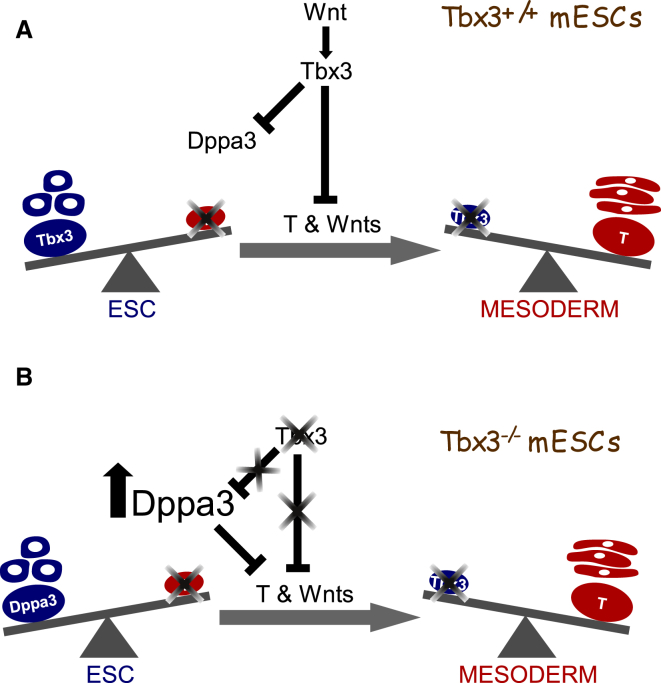
Tbx3 is rapidly downregulated upon differentiation of mESCs and is subsequently upregulated during later stages of differentiation. To address the role of Tbx3 during the exit from pluripotency, we overexpressed Tbx3 in WT mESCs prior to and during differentiation (Figure S6E). Overexpression of Tbx3 for 2 days prior to differentiation repressed expression of mesoderm genes (T, Mixl1, Tbx6) and Wnt genes required for mesoderm differentiation (Wnt8a, Wnt3a) (Figure S6F). Collectively, these data indicate that Tbx3 plays two separate roles, one during exit from pluripotency where it represses mesoderm genes and another during gastrulation, as reported, where it promotes the development of a mesendoderm population (Weidgang et al., 2013). In pluripotent Tbx3 null cells, but not in WT cells (data not shown), Dppa3 is overexpressed and represses T and Wnt8a (Figure S6G). Dppa3 therefore compensates for Tbx3 function in repressing mesoderm differentiation and maintains pluripotency in the absence of Tbx3. Taken together, these findings suggest Tbx3 orchestrates the exit of mESCs from pluripotency by directly regulating members of the Wnt signaling pathway and repressing expression of mesoderm genes (Figures 6A and 6B).
Figure 6.
Tbx3-Mediated Repression of Dppa3, Mesoderm Genes, and Wnt Signaling Components Maintains the Balance between Self-renewal and Differentiation of mESCs
(A) In WT mESCs, Tbx3 controls expression of Dppa3, mesoderm genes, and Wnt signaling components to maintain mESC pluripotency.
(B) In Tbx3 null mESCs, Dppa3 is overexpressed and partially takes over function of Tbx3 to repress mesoderm genes and Wnt signaling components to maintain mESC pluripotency.
Discussion
Many key pluripotency TFs bind within close proximity to specific genomic locations and therefore cooperatively control the expression of key genes (Chen et al., 2008). Overlapping functional TF binding sites (Whyte et al., 2013) ensure the stability of the overall transcriptional network. At the same time, TF expression heterogeneity (Chambers et al., 2007; Kumar et al., 2014; MacArthur and Lemischka, 2013; MacArthur et al., 2012; Niwa et al., 2009; Toyooka et al., 2008) allows the network to respond quickly and specifically to external differentiation cues. We expect there exists Tbx3 high and low self-renewing states in an ESC population. Such states maybe dynamic and possess different developmental potentials. In the absence of Tbx3, mESCs can self-renew in LIF containing media and maintain an alternate and stable pluripotent state. Like other ESC TFs such as Nanog and Sox2 (Thomson et al., 2011), Tbx3 maintains the pluripotent state and promotes differentiation into specific lineages like embryonic and extra-embryonic endoderm (Kartikasari et al., 2013; Lu et al., 2011).
Using a loss-of-function approach, we identify direct versus indirect targets of Tbx3 in mESCs. Tbx3 maintains normal steady-state levels of Dppa3. In the absence of Tbx3, upregulation of Dppa3 in part contributes to the maintenance of a stable pluripotent state. Varying levels of Dppa3 in mESCs marks distinct developmental states (Hayashi et al., 2008). The mechanisms by which expression heterogeneity of Dppa3 is maintained in mESCs have not been defined. Tbx3 prevents the overexpression of Dppa3 in mESCs and may contribute to its heterogeneous expression.
Downstream components of key signaling pathways such as Bmp/Smad, Lif/Stat (Niwa et al., 1998; van Oosten et al., 2012), Wnt/β-catenin (Habib et al., 2013; Merrill, 2012), and PI3K/Akt (Niwa et al., 2009) balance self-renewal and differentiation. External signaling cues integrate into a response generated by activation or repression of key lineage-specific TFs (Chen et al., 2008; Lee et al., 2012a; Mullen et al., 2011; Niwa et al., 2009). Tbx3 expression is regulated by key signaling cascades such as Bmp/Smad (Chen et al., 2008; Yang et al., 2006), Jak/Stat (Chen et al., 2008), PI3K/Akt (Niwa et al., 2009), Grb2/Mapk (Niwa et al., 2009), and Wnt/β-catenin (Price et al., 2013; Renard et al., 2007). Wnt signaling and Mapk inhibition in particular play important roles in pluripotency (ten Berge et al., 2011) and mesoderm differentiation (Gadue et al., 2006). Activation of Wnt signaling by repression of GSK3β alone stimulates mesoderm differentiation from mESCs grown in ground state condition (Kanke et al., 2014; Ying et al., 2008). In vivo loss-of-function studies demonstrate the requirement of Wnt signaling in the generation of primitive streak during gastrulation (Tortelote et al., 2013; Yamaguchi et al., 1999). Wnt signaling is transduced by regulation of TFs such as Tcf1 and Tcf3 (Yi et al., 2011; Zhang et al., 2013). We show that downstream of Wnt signaling Tbx3 is required to repress premature differentiation of mESCs into mesoderm cells. We propose that the seemingly paradoxical functions of the Wnt signaling pathway in pluripotency versus differentiation are mediated in part through its control of Tbx3 expression. In the absence of Tbx3 expression, overexpression of T and Wnt pathway genes such as Wnt8a promotes mesoderm differentiation. Tbx3 therefore fine tunes the response of Wnt signaling to maintain the balance between pluripotency and mesoderm differentiation.
Recent studies indicated that overexpression of Tbx3 in differentiating cell populations promotes differentiation toward a mesendoderm fate (Kartikasari et al., 2013; Weidgang et al., 2013). We show that overexpression of Tbx3 in mESCs prior to differentiation blocks emergence of mesoderm lineage (Ivanova et al., 2006; Lu et al., 2011). Tbx3 expression is downregulated during the early stages of differentiation and upregulated at later stages (Hailesellasse Sene et al., 2007; Hayashi et al., 2008). Tbx3 expression is also highly downregulated during in vivo development of epiblast from the ICM and in the in vitro established EpiSCs and EpiLCs (Brons et al., 2007; Buecker et al., 2014; Tesar et al., 2007). Tbx3 therefore has a dual role in blocking the premature exit from pluripotency by directly repressing mesoderm and Wnt pathway genes and at a later stage by promoting mesoderm differentiation during gastrulation.
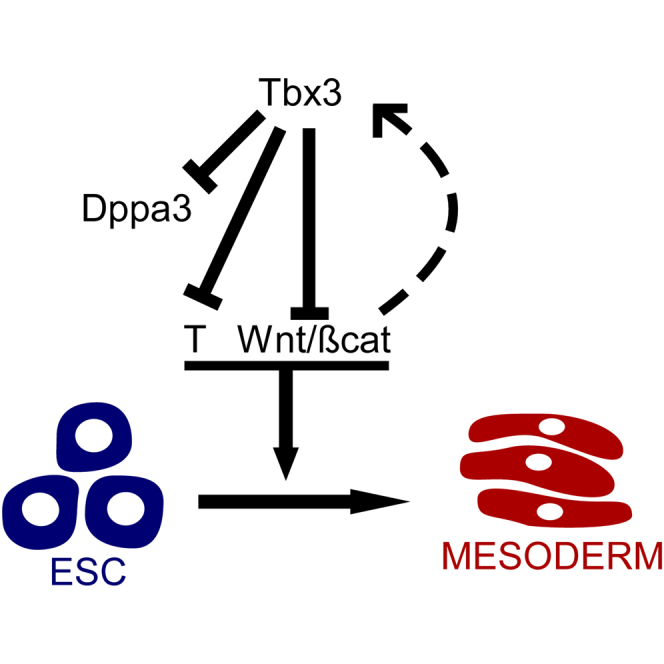
Collectively, we suggest a model in which interplay of Tbx3 and Dppa3 regulates early differentiation decisions into mesoderm lineage by controlling components of Wnt pathway and mesoderm genes in response to external cues. The regulation of Dppa3 expression by Tbx3 in mESCs may act as a “buffer” to allow responses to varying concentrations of these two key TFs, thus maintaining a dynamic and responsive transcriptional landscape. Our model reconciles the dual role of Wnt signaling in maintaining the balance between mESC self-renewal versus differentiation. In conclusion, since Tbx3 is downstream of key signaling cascades and overlaps with many TF circuits understanding the direct versus indirect targets of Tbx3 and mechanisms by which it maintains pluripotency is vital to understanding the process of differentiation from self-renewing mESCs.
Experimental Procedures
Cell Culture
The mESCs in the study were generated from R1, J1, AINV15, KH2, and CCE mESC lines. Tbx3 null mESCs were generated from Tbx3+/N cells (Davenport et al., 2003). The procedures followed to make the lines are mentioned in greater detail in Supplemental Information.
Differentiation Assays in Serum and Serum-free Conditions
EB differentiation assay was performed in serum containing media without LIF by plating 0.5- to 2-million cells per well of a low attachment six-well plate over a time course of days. Serum-free mesoderm differentiation assay were performed as described (Gouon-Evans et al., 2006). Briefly, mESCs were plated in low attachment plates for 48 hr in SFD media and then replated in the same plates with VEGF (5 ng/μl), ACTIVIN A (25 ng/μl), and BMP4 (2.5 ng/μl). The cells were stained with respective antibodies (Table S4) and analyzed by FACS described in Supplemental Information.
ChIP-Seq and mRNA-Seq
ChIP-Seq experiment was done as described (Ang et al., 2011). mRNA-Seq experiment was done using the polyA pull down method as described in Supplemental Information. Sequencing analysis for ChIP-seq and mRNA-seq data were performed in house as described in supplemental methods (Tables S1 and S2). Raw data were deposited in Gene Expression Omnibus (GEO), accession number GSE60066.
Blastocyst Injection Assay
Indicated mESCs were injected into isolated C7Bl6 blastocysts and placed into pseudo-pregnant mice at the mouse genetics core at Mount Sinai. The embryos were harvested at E10.5, and pictures were taken using a florescence microscope. Mouse work was subject to approval by and carried out in accordance with guidelines from Mount Sinai’s Institutional Animal Care and Use Committee.
Acknowledgments
We thank Dr. E. Bernstein, Dr. M. Rendl, Dr. J. Wang and Dr. P. Soriano, Dr. V. Gouon-Evans, and their laboratories for their critical guidance and assistance. We thank members of the Lemischka/Moore laboratory for useful discussions. This research was funded by grants from the NIH (5R01GM078465) to I.R.L. B.C. is a trainee in NIDCR-Interdisciplinary Training in Systems and Developmental Biology and Birth Defects (T32HD075735). A.G.F. was supported by Fundação para a Ciência e a Tecnologia-FCT (SFRH/BD/64715/2009).
Published: June 18, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, six figures, and six tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.05.009.
Supplemental Information
References
- Ang Y.S., Tsai S.Y., Lee D.F., Monk J., Su J., Ratnakumar K., Ding J., Ge Y., Darr H., Chang B. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S.J., Stappert J., Bauer A., Kispert A., Herrmann B.G., Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech. Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Buecker C., Srinivasan R., Wu Z., Calo E., Acampora D., Faial T., Simeone A., Tan M., Swigut T., Wysocka J. Reorganization of enhancer patterns in transition from naive to primed pluripotency. Cell Stem Cell. 2014;14:838–853. doi: 10.1016/j.stem.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Chapman D.L., Garvey N., Hancock S., Alexiou M., Agulnik S.I., Gibson-Brown J.J., Cebra-Thomas J., Bollag R.J., Silver L.M., Papaioannou V.E. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev. Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V.B., Wong E., Orlov Y.L., Zhang W., Jiang J. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chia N.Y., Chan Y.S., Feng B., Lu X., Orlov Y.L., Moreau D., Kumar P., Yang L., Jiang J., Lau M.S. A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature. 2010;468:316–320. doi: 10.1038/nature09531. [DOI] [PubMed] [Google Scholar]
- Davenport T.G., Jerome-Majewska L.A., Papaioannou V.E. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- de Boer E., Rodriguez P., Bonte E., Krijgsveld J., Katsantoni E., Heck A., Grosveld F., Strouboulis J. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA. 2003;100:7480–7485. doi: 10.1073/pnas.1332608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Paszkowski-Rogacz M., Nitzsche A., Slabicki M.M., Heninger A.-K., de Vries I., Kittler R., Junqueira M., Shevchenko A., Schulz H. A genome-scale RNAi screen for Oct4 modulators defines a role of the Paf1 complex for embryonic stem cell identity. Cell Stem Cell. 2009;4:403–415. doi: 10.1016/j.stem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Faddah D.A., Wang H., Cheng A.W., Katz Y., Buganim Y., Jaenisch R. Single-cell analysis reveals that expression of nanog is biallelic and equally variable as that of other pluripotency factors in mouse ESCs. Cell Stem Cell. 2013;13:23–29. doi: 10.1016/j.stem.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzio T.G., Huff J.T., Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T.L., Paddison P.J., Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V., Boussemart L., Gadue P., Nierhoff D., Koehler C.I., Kubo A., Shafritz D.A., Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Habib S.J., Chen B.C., Tsai F.C., Anastassiadis K., Meyer T., Betzig E., Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailesellasse Sene K., Porter C.J., Palidwor G., Perez-Iratxeta C., Muro E.M., Campbell P.A., Rudnicki M.A., Andrade-Navarro M.A. Gene function in early mouse embryonic stem cell differentiation. BMC Genomics. 2007;8:85. doi: 10.1186/1471-2164-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Yuan P., Yang H., Zhang J., Soh B.S., Li P., Lim S.L., Cao S., Tay J., Orlov Y.L. Tbx3 improves the germ-line competency of induced pluripotent stem cells. Nature. 2010;463:1096–1100. doi: 10.1038/nature08735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Lopes S.M., Tang F., Surani M.A. Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell. 2008;3:391–401. doi: 10.1016/j.stem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R., Papp B., Hoffman J.A., Merrill B.J., Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Kim J., Xu Q., Leng Y., Orkin S.H., Elledge S.J. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 2009;23:837–848. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N., Dobrin R., Lu R., Kotenko I., Levorse J., DeCoste C., Schafer X., Lun Y., Lemischka I.R. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Kagey M.H., Newman J.J., Bilodeau S., Zhan Y., Orlando D.A., van Berkum N.L., Ebmeier C.C., Goossens J., Rahl P.B., Levine S.S. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanke K., Masaki H., Saito T., Komiyama Y., Hojo H., Nakauchi H., Lichtler A.C., Takato T., Chung U.I., Ohba S. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Rev. 2014;2:751–760. doi: 10.1016/j.stemcr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartikasari A.E., Zhou J.X., Kanji M.S., Chan D.N., Sinha A., Grapin-Botton A., Magnuson M.A., Lowry W.E., Bhushan A. The histone demethylase Jmjd3 sequentially associates with the transcription factors Tbx3 and Eomes to drive endoderm differentiation. EMBO J. 2013;32:1393–1408. doi: 10.1038/emboj.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K.F., Ng D.Y., Jayakumaran G., Wood G.A., Koide H., Doble B.W. β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C., Willems E., Abdo S., Lambiv L., Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev. Dyn. 2005;233:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Kim J., Cantor A.B., Orkin S.H., Wang J. Use of in vivo biotinylation to study protein-protein and protein-DNA interactions in mouse embryonic stem cells. Nat. Protoc. 2009;4:506–517. doi: 10.1038/nprot.2009.23. [DOI] [PubMed] [Google Scholar]
- Kumar R.M., Cahan P., Shalek A.K., Satija R., DaleyKeyser A.J., Li H., Zhang J., Pardee K., Gennert D., Trombetta J.J. Deconstructing transcriptional heterogeneity in pluripotent stem cells. Nature. 2014;516:56–61. doi: 10.1038/nature13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.F., Su J., Ang Y.S., Carvajal-Vergara X., Mulero-Navarro S., Pereira C.F., Gingold J., Wang H.L., Zhao R., Sevilla A. Regulation of embryonic and induced pluripotency by aurora kinase-p53 signaling. Cell Stem Cell. 2012;11:179–194. doi: 10.1016/j.stem.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.F., Su J., Sevilla A., Gingold J., Schaniel C., Lemischka I.R. Combining competition assays with genetic complementation strategies to dissect mouse embryonic stem cell self-renewal and pluripotency. Nat. Protoc. 2012;7:729–748. doi: 10.1038/nprot.2012.018. [DOI] [PubMed] [Google Scholar]
- Liang G., Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49–69. doi: 10.1038/cr.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.Y., Tam W.L., Zhang J., Ang H.S., Jia H., Lipovich L., Ng H.H., Wei C.L., Sung W.K., Robson P. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3:543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Lindsley R.C., Gill J.G., Kyba M., Murphy T.L., Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Loh K.M., Lim B. A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell. 2011;8:363–369. doi: 10.1016/j.stem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Loh Y.H., Zhang W., Chen X., George J., Ng H.H. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Yang A., Jin Y. Dual functions of T-box 3 (Tbx3) in the control of self-renewal and extraembryonic endoderm differentiation in mouse embryonic stem cells. J. Biol. Chem. 2011;286:8425–8436. doi: 10.1074/jbc.M110.202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur B.D., Lemischka I.R. Statistical mechanics of pluripotency. Cell. 2013;154:484–489. doi: 10.1016/j.cell.2013.07.024. [DOI] [PubMed] [Google Scholar]
- MacArthur B.D., Sevilla A., Lenz M., Müller F.J., Schuldt B.M., Schuppert A.A., Ridden S.J., Stumpf P.S., Fidalgo M., Ma’ayan A. Nanog-dependent feedback loops regulate murine embryonic stem cell heterogeneity. Nat. Cell Biol. 2012;14:1139–1147. doi: 10.1038/ncb2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A., Foreman R., Chevalier B., Bilodeau S., Kahn M., Young R.A., Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A., Levine S.S., Cole M.F., Frampton G.M., Brambrink T., Johnstone S., Guenther M.G., Johnston W.K., Wernig M., Newman J. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.L., Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24:2778–2783. doi: 10.1101/gad.1962910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendjan S., Mascetti V.L., Ortmann D., Ortiz M., Karjosukarso D.W., Ng Y., Moreau T., Pedersen R.A. NANOG and CDX2 pattern distinct subtypes of human mesoderm during exit from pluripotency. Cell Stem Cell. 2014;15:310–325. doi: 10.1016/j.stem.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Merrill B.J. Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb. Perspect. Biol. 2012;4:a007971. doi: 10.1101/cshperspect.a007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen A.C., Orlando D.A., Newman J.J., Lovén J., Kumar R.M., Bilodeau S., Reddy J., Guenther M.G., DeKoter R.P., Young R.A. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. doi: 10.1016/j.cell.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C.E., Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Narayanan A., Thompson S.A., Lee J.J., Lekven A.C. A transgenic wnt8a:PAC reporter reveals biphasic regulation of vertebrate mesoderm development. Dev. Dyn. 2011;240:898–907. doi: 10.1002/dvdy.22599. [DOI] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I., Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Ogawa K., Shimosato D., Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Cheng X., Keller G.M., Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Nishinakamura R., Iwamatsu Y., Shimosato D., Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem. Biophys. Res. Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Pereira C.F., Fisher A.G. Heterokaryon-based reprogramming for pluripotency. Curr. Protoc. Stem Cell Biol. 2009;Chapter 4:4B.1. doi: 10.1002/9780470151808.sc04b01s9. [DOI] [PubMed] [Google Scholar]
- Price F.D., Yin H., Jones A., van Ijcken W., Grosveld F., Rudnicki M.A. Canonical Wnt signaling induces a primitive endoderm metastable state in mouse embryonic stem cells. Stem Cells. 2013;31:752–764. doi: 10.1002/stem.1321. [DOI] [PubMed] [Google Scholar]
- Renard C.A., Labalette C., Armengol C., Cougot D., Wei Y., Cairo S., Pineau P., Neuveut C., de Reyniès A., Dejean A. Tbx3 is a downstream target of the Wnt/beta-catenin pathway and a critical mediator of beta-catenin survival functions in liver cancer. Cancer Res. 2007;67:901–910. doi: 10.1158/0008-5472.CAN-06-2344. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn P.J., Cox B.J., Ralston A., Rossant J. Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc. Natl. Acad. Sci. USA. 2010;107:10783–10790. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R.K., Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson M., Liu S.J., Zou L.N., Smith Z., Meissner A., Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortelote G.G., Hernández-Hernández J.M., Quaresma A.J., Nickerson J.A., Imbalzano A.N., Rivera-Pérez J.A. Wnt3 function in the epiblast is required for the maintenance but not the initiation of gastrulation in mice. Dev. Biol. 2013;374:164–173. doi: 10.1016/j.ydbio.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- van Oosten A.L., Costa Y., Smith A., Silva J.C. JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nat Commun. 2012;3:817. doi: 10.1038/ncomms1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Rao S., Chu J., Shen X., Levasseur D.N., Theunissen T.W., Orkin S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Weidgang C.E., Russell R., Tata P.R., Kühl S.J., Illing A., Müller M., Lin Q., Brunner C., Boeckers T.M., Bauer K. TBX3 Directs Cell-Fate Decision toward Mesendoderm. Stem Cell Rev. 2013;1:248–265. doi: 10.1016/j.stemcr.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte W.A., Orlando D.A., Hnisz D., Abraham B.J., Lin C.Y., Kagey M.H., Rahl P.B., Lee T.I., Young R.A. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T.P., Takada S., Yoshikawa Y., Wu N., McMahon A.P. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Itoh H., Hirashima M., Ogawa M., Nishikawa S., Yurugi T., Naito M., Nakao K., Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yang L., Cai C.L., Lin L., Qyang Y., Chung C., Monteiro R.M., Mummery C.L., Fishman G.I., Cogen A., Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga M., Tada S., Torikai-Nishikawa S., Nakano Y., Okada M., Jakt L.M., Nishikawa S., Chiba T., Era T., Nishikawa S. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat. Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- Yi F., Pereira L., Hoffman J.A., Shy B.R., Yuen C.M., Liu D.R., Merrill B.J. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat. Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Peterson K.A., Liu X.S., McMahon A.P., Ohba S. Gene regulatory networks mediating canonical Wnt signal-directed control of pluripotency and differentiation in embryo stem cells. Stem Cells. 2013;31:2667–2679. doi: 10.1002/stem.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.