Summary
Systemic and local signals must be integrated by mammary stem and progenitor cells to regulate their cyclic growth and turnover in the adult gland. Here, we show RANK-positive luminal progenitors exhibiting WNT pathway activation are selectively expanded in the human breast during the progesterone-high menstrual phase. To investigate underlying mechanisms, we examined mouse models and found that loss of RANK prevents the proliferation of hormone receptor-negative luminal mammary progenitors and basal cells, an accompanying loss of WNT activation, and, hence, a suppression of lobuloalveologenesis. We also show that R-spondin1 is depleted in RANK-null progenitors, and that its exogenous administration rescues key aspects of RANK deficiency by reinstating a WNT response and mammary cell expansion. Our findings point to a novel role of RANK in dictating WNT responsiveness to mediate hormone-induced changes in the growth dynamics of adult mammary cells.
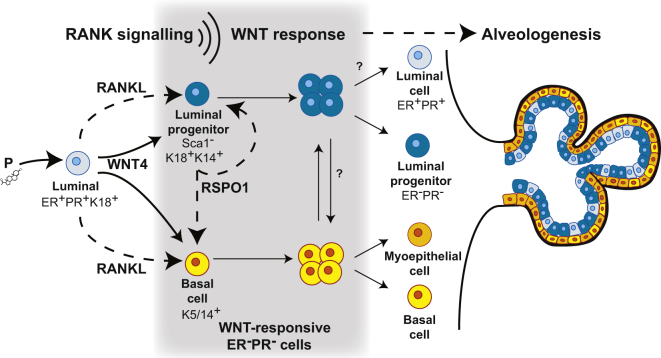
Graphical Abstract
Highlights
-
•
Luminal progenitors are targets of progesterone in the adult human breast
-
•
Progesterone-induced expansion of mammary epithelial subsets requires RANK
-
•
RANK signaling targets WNT-responsive ER–PR– luminal progenitors and basal cells
-
•
RANK controls RSPO1, which rescues defective progenitor expansion in Rank-null state
Cellular signals governing the WNT response are largely unknown despite the widely recognized function of this pathway in the control of stem and progenitor cells in diverse tissues. Khoka and colleagues describe RANK signaling as a requisite step for the expansion of ER–PR– adult mammary epithelial progenitors and acquisition of WNT responsiveness through its regulation of the WNT agonist R-SPONDIN1.
Introduction
The terminal ductal lobular units in the adult human mammary gland and their lobuloalveoli counterparts in the mouse are key hormone-sensitive structures (Cardiff and Wellings, 1999). They are also foci of milk-secreting cells following pregnancy and represent major sites of breast cancer development in both species. Increased progesterone levels that occur both during the reproductive cycle and pregnancy trigger a dynamic growth response in these structures, resulting in a documented marked expansion in the number of stem and progenitor cells in the mammary glands of mice (Asselin-Labat et al., 2010; Joshi et al., 2010). These primitive cells lack estrogen and progesterone receptors (ER–PR–) and therefore must respond to these hormones through indirect mechanisms via receipt of critical signals from other types of cells within the mammary stem cell niche that are ER+PR+ (Joshi et al., 2012). WNT signaling is thought to contribute to the regulation of stem cell self-renewal and differentiation responses in many tissues (Nusse et al., 2008), including the mouse mammary gland (van Amerongen et al., 2012; Zeng and Nusse, 2010). However, the specific mechanisms that control the ability of mammary stem and progenitor cells to respond to WNT ligands have remained largely undefined.
In this study, we show that the Receptor Activator of Nuclear factor Kappa B (RANK) ligand and WNT paracrine signals are conserved in adult mouse and human mammary tissue and fluctuate similarly during the cyclic progenitor expansion seen in both species. By exploiting a combination of genetic and pharmacological approaches, we also reveal an interaction between the RANK and WNT pathways that provides the molecular circuitry essential for the WNT response and the expansion of ER–PR– mammary progenitors in the adult mouse mammary gland.
Results
Luminal Progenitors Are Targeted by Progesterone in the Adult Human Breast
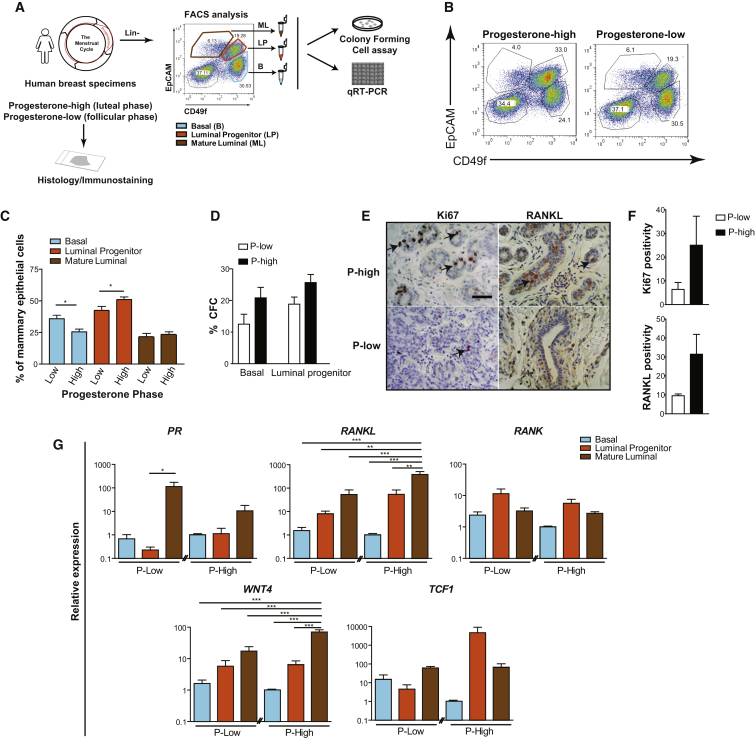
We have previously reported that the progesterone-dominant phase of the mouse estrous cycle is marked by an increase in stem and progenitor cells, likely arising from paracrine stimulation of ER–PR– primitive cells by RANKL and WNT4 produced from adjacent ER+PR+ cells (Joshi et al., 2010). To determine whether a similar mechanism is operative in the human mammary gland, we analyzed analogous subsets of mammary epithelial cells isolated from reduction mammoplasty samples obtained from 63 normal premenopausal women at different stages of the menstrual cycle (Figure 1A). Parallel sections of tissue from each of these samples were classified as luteal-phase (progesterone-high) or follicular-phase (progesterone-low) according to previously published histologic criteria (Ramakrishnan et al., 2002). Phenotypic analyses, using established marker sets (Eirew et al., 2008), showed that the proportion of CD49f+EpCAM+ luminal progenitor-enriched cells was significantly higher in the luteal as compared to the follicular phase, whereas the proportion of CD49f+EpCAMlo/– basal cells was lower, and the proportion of mature luminal cells remained unaffected by the menstrual phase of the donor (Figures 1B and 1C). In vitro colony-forming cell (CFC) assays performed on these subsets from progesterone-high and -low samples showed that even the frequencies of CFCs within both the basal and luminal progenitor fractions were slightly higher in the luteal phase samples (Figure 1D). Immunostaining of tissue sections prepared from these groups showed abundant RANKL+ cells and increased numbers of proliferating (Ki67+) cells in the luteal phase (Figures 1E and 1F). Taken together, these data suggest that transient elevated progesterone levels induce a parallel expansion of the luminal progenitor compartment in the mammary glands of normal premenopausal women.
Figure 1.
Increased Luminal Progenitors in the Progesterone-High Human Breast
(A) Schematic of the experimental procedure. Breast cells depleted of endothelial and hematopoietic cells are referred to as Lineage– (Lin–).
(B) FACS profiles of human breast cell populations derived from morphologically categorized progesterone-high and -low breast tissue samples: basal (CD49f+EpCAM−/lo), luminal progenitor (CD49f+EpCAM+), mature luminal (CD49f−EpCAM+), and stromal (CD49f−EpCAM−) cell subsets.
(C) Quantification of basal, luminal progenitors, and mature cells in progesterone-high and -low human breast samples; data represent mean ± SEM (n = 34 for progesterone-high, n = 29 for progesterone-low).
(D) Colony forming cell (CFC) frequency in FACS-purified basal and luminal progenitor subsets from P-high and P-low phases; data represent mean ± SEM (n = 6 breast samples/phase).
(E) Ki67 and RANKL immunostaining in progesterone-high (P-high) and -low (P-low) tissue sections; scale bar, 50 μm.
(F) Quantification of Ki67 and RANKL positivity in P-high and -low tissues from E, measured as a ratio of Ki67 or RANKL positive pixel to total pixel count within glandular regions; data represent mean ± SEM (n = 3 breast specimens/group).
(G) qRT-PCR for the indicated genes on FACS-purified subsets from reduction mammoplasty samples shown in (B); GAPDH normalized transcript levels plotted relative to the P-high group; data represent mean ± SEM (n = 6 and n = 7 breast samples for P-low and P-high, respectively, except for PR where n = 4 per group).
Statistical significance is indicated as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.0001.
We also found levels of PR, RANK, RANKL, WNT4 transcripts and the WNT downstream target TCF1 to vary in expression between luteal and follicular phase samples of purified human breast epithelial subpopulations (Figure 1G). PR expression was found to be the highest in the mature luminal cell compartment and relatively lower in the other fractions. RANKL, WNT4, and TCF1 were expressed in all three epithelial subsets, but RANKL and WNT4 transcript levels were highest in the mature luminal cells and further elevated in the luteal phase samples. In contrast, TCF1 was elevated in the expanded luminal progenitor subset in the luteal phase. RANK expression was also detected in all epithelial subsets but at relatively higher levels in the luminal progenitor-enriched fraction. This pattern of gene expression is consistent with a mechanism of progesterone effects on human mammary tissue being mediated by a stimulation of mature PR-expressing cells to produce RANKL, which then potentiates WNT responsive luminal progenitors to induce their proliferation, akin to the hormone-triggered mitogenic response previously identified in the mouse mammary gland.
RANK Signaling Expands Mammary Epithelial Subsets and Alveolar Progenitors
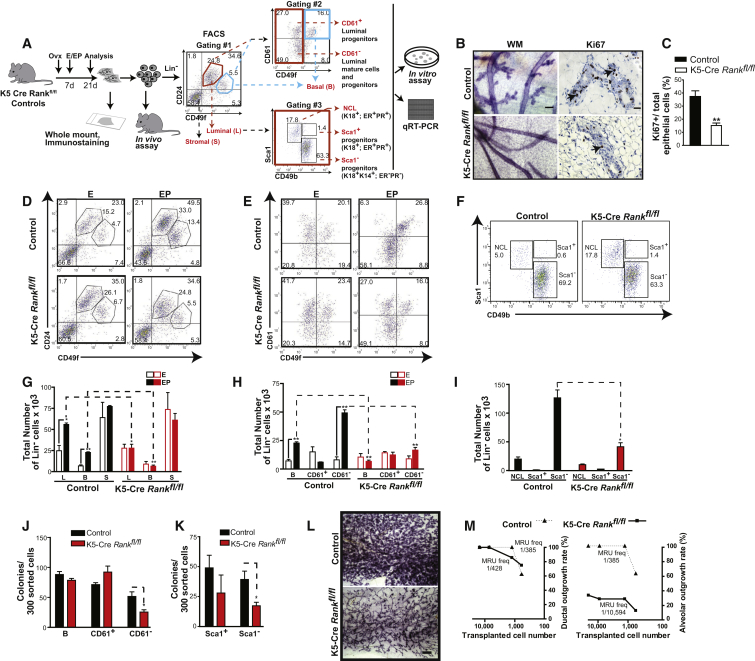
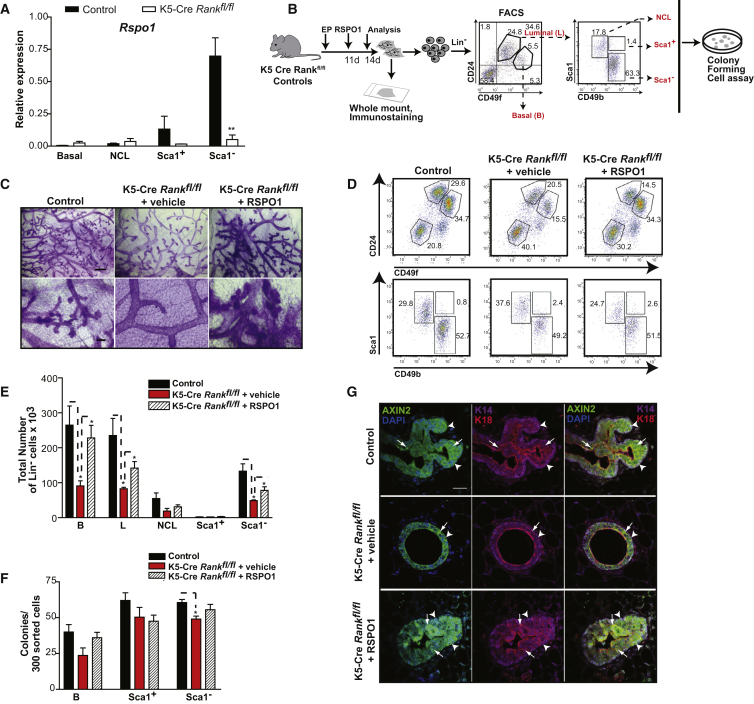
RANKL is a known, and sufficient, mediator of mammary lobuloalveologenesis downstream of progesterone (Beleut et al., 2010; Fata et al., 2000), but little is known of its effect on different adult mammary epithelial subsets and progenitors. In contrast, WNT4 is known to be essential during early phases of alveoli development in the mammary gland, but is not sufficient for this process (Brisken et al., 2000; Kim et al., 2009), although WNTs are accepted as important stem cell growth factors (Nusse et al., 2008; Zeng and Nusse, 2010). We therefore designed a series of experiments in mice to investigate the role of RANK signaling in mediating progesterone-driven changes in distinct mammary epithelial subsets, and whether this involved activation of a WNT response. Accordingly, we compared the mitogenic response of mammary cells in adult keratin 5 (K5)-Cre Rankfl/fl (RANK null) to control Rankfl/fl ovariectomized mice treated with 17β-estradiol alone (E) or with progesterone (EP) (Figure 2A). Mammary cell preparations were depleted of hematopoietic and endothelial cells during flow cytometry, and the resulting lineage– (Lin–) population was analyzed using CD49f and CD24 cell-surface markers (Stingl et al., 2006) to identify the luminal (CD24+CD49flo), basal (CD24+CD49fhi) epithelial, and stromal (CD24–CD49f–) subsets (gating #1 in Figure 2A). The luminal CD24+CD49flo subset was further segregated (gating #2) into CD24+CD49floCD61+ luminal progenitors (hereafter CD61+), and CD24+CD49floCD61– luminal cells (hereafter CD61–), which includes mature cells and progenitors (Asselin-Labat et al., 2007). To discern ER+PR+ and ER–PR– luminal epithelial subsets, the luminal subset was also refined based on Sca1 and CD49b markers (gating #3) into “ER+PR+” CD24+CD49floSca1+CD49b– non-clonogenic luminal cells (hereafter NCL), “ER+PR+” CD24+CD49floSca1+CD49b+ progenitors (hereafter Sca1+) and “ER–PR–“ CD24+CD49floSca1–CD49b+ progenitors (hereafter Sca1–) (Shehata et al., 2012). The latter, ER–PR– luminal cells have a high clonogenic capacity and are considered enriched for alveolar progenitors due to their significant levels of milk protein genes (Shehata et al., 2012; Sleeman et al., 2007). These gating strategies were used to comprehend the phenotypic and functional defects arising from Rank deficiency across the spectrum of cell compartments and progenitors reported to date. Co-staining for CD61 together with Sca1 and CD49b markers showed that the CD61+/– and Sca1+/– subpopulations within the luminal compartment do not entirely overlap such that Sca1– cells are found in both the CD61+ and CD61– luminal cell fractions, whereas CD61− cells are dispersed between the Sca1+ and Sca1− fractions (Figures S1A and S1B). PCR on genomic DNA extracted from fluorescence-activated cell sorting (FACS)-purified CD24+CD49fhi stem cell-enriched basal cells and CD24+CD49flo luminal cell populations of K5-Cre Rankfl/fl mammary glands showed that Rank is efficiently deleted from both of these mammary cell compartments (Figure S1C). K5 is a basal cell marker in the adult mammary gland, but an absence of Rank in the luminal cells of the adult mammary gland is not surprising since these arise during early development from multipotent K5+ basal cells (Van Keymeulen et al., 2011). We observed that administration of progesterone to K5-Cre Rankfl/fl mice failed to stimulate development of mammary alveoli and the glands of these mice contained markedly fewer proliferating cells than Rankfl/fl controls (Figures 2B and 2C), although they retained normal basal- and luminal-restricted patterns of cytokeratin and PR expression (Figure S2A). Importantly, K5-Cre Rankfl/fl mice did not exhibit the progesterone-stimulated expansion of basal and luminal epithelial subsets, characteristic of control mice (Figures 2D–2I). The stromal cell population did not appear altered in the absence of RANK. In the luminal subsets, CD61− cells were significantly reduced and the number of Sca1− progenitors in EP-treated K5-Cre Rankfl/fl mice was 3-fold lower than in controls (Figures 2E, 2F, 2H, and 2I). In contrast, the number of Sca1+ cells in the same glands remained similar to controls.
Figure 2.
RANK Is Required for Progesterone-Induced Expansion of Mouse Mammary Epithelial Subsets and Alveolar Progenitor Activity
(A) Schema of the experimental procedure; 17β-estradiol (E), 17β-estradiol + progesterone (EP), Lineage− (Lin−) i.e., cells depleted of endothelial and hematopoietic cells, non-clonogenic luminal (NCL) cells, estrogen receptor (ER), and progesterone receptor (PR).
(B) Whole-mount (WM; scale bar, 100 μm) and Ki67 immunostaining (scale bar, 20 μm) of control and K5-Cre Rankfl/fl mammary glands following EP treatment.
(C) Quantification of Ki67+ proliferating cells in Rank-null and control glands; data represent mean ± SEM (n = 3 mice/group).
(D) FACS profiles of luminal (CD24+CD49flo), basal (CD24+CD49fhi), and stromal (CD24−CD49f−) mammary cell subpopulations in ovariectomized control and K5-Cre Rankfl/fl mice following treatment with E or EP (gating #1).
(E) Representative FACS plots of basal, CD61+ luminal, and CD61− luminal cell populations within CD24+ mammary epithelial cells (gating #2).
(F) Proportions of NCL cells (hormone receptor-positive), Sca1+ (hormone receptor-positive) progenitors, and Sca1− (hormone receptor-negative) alveolar progenitors in the luminal population of EP-treated control and K5-Cre Rankfl/fl mice (gating #3).
(G–K) Quantification of (G) luminal (L), basal (B), and stromal (S) cell subsets from (D), (H) indicated cell populations in (E), and (I) luminal cell populations in (F); data represent mean ± SEM of n = 3 mice/group for (G) and (H) and n = 4 mice/group for (I). Colony forming cell capacity of FACS-sorted (J) basal and CD61+, CD61− luminal progenitors, (K) Sca1+ and Sca1− progenitors, on fibroblast feeders in vitro from EP-stimulated control and K5-Cre Rankfl/fl mice; data represent mean ± SEM of n = 3 mice/group for (J) and (K).
(L) Representative images of ductal and alveolar-containing outgrowths from 5,000 mammary cells of EP-stimulated K5-Cre Rankfl/fl and control donor mice injected into fat pads of subsequently impregnated recipients (scale bar, 500 μm).
(M) Gland repopulation rates in limiting dilution assays in vivo scored for either ductal or alveolar outgrowths; mammary repopulating unit frequency (MRU freq). Data are pooled from at least two independent experiments for each group and are consistent with a single-hit process; control cells: p = 0.81 (ductal and alveolar), K5-Cre Rankfl/fl cells: p = 0.96 (ductal) and p = 0.13 (alveolar).
We then performed functional assays to examine whether Rank loss affects progenitor activity. A previous study reported an increase in the relative numbers of basal and luminal cells and a decline in CD61+ luminal cells in Rank overexpressing mice (Pellegrini et al., 2013). However, we found that the loss of Rank did not significantly alter the total numbers of CD61+ luminal cells or their CFC content (Figures 2H and 2J). In contrast, the CFC frequencies in FACS-purified CD61− cells (Figure 2J) and ER−PR−, Sca1− (Figure 2K) luminal cells were significantly lower than in controls, whereas the CFC frequency in basal or ER+PR+ Sca1+ cells was not significantly altered. This suggests that RANK signaling acts primarily by instructing ER−PR− alveolar progenitors contained within the Sca1− and CD61− luminal subsets. In vivo limiting dilution transplantation experiments on mammary cells from ovariectomized EP-treated K5-Cre Rankfl/fl mice further showed that the frequency of mammary stem cells (able to produce a macroscopic ductal outgrowth in a pre-cleared mammary fat pad) was comparable to control cells (Figures 2L and 2M). In contrast, the capacity of Rank-deficient cells to generate alveoli was severely compromised (Figures 2L and 2M; Table S1). Thus, RANK is critical for ensuring the activity and repopulation capacity of adult mouse mammary epithelial progenitors that normally generate alveoli.
The Adult Mammary Epithelial WNT Response Is Dependent on RANK Signaling
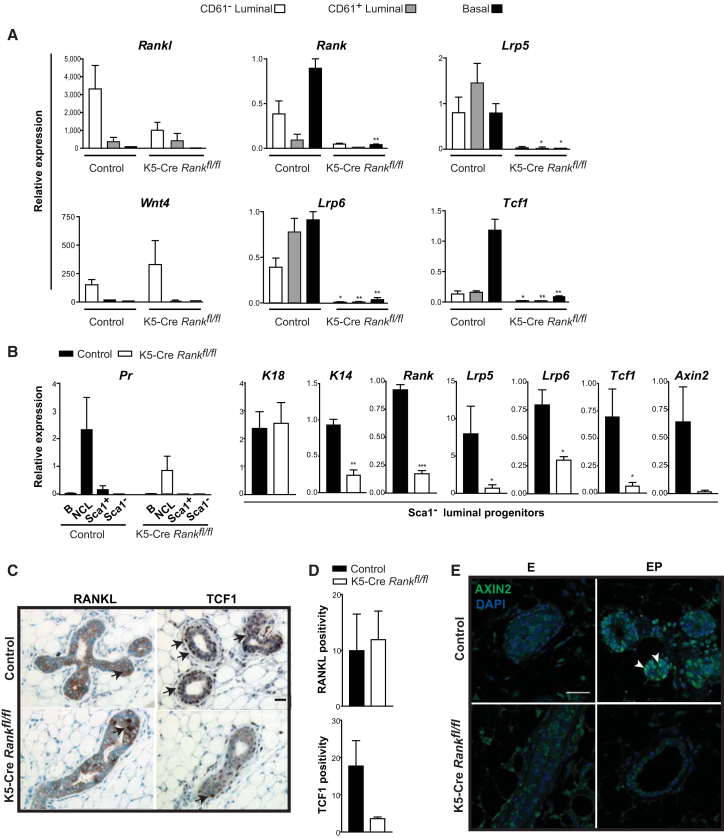
Next, we determined whether and how an absence of RANK affects the adult mammary WNT response (Figures 3A–3E). qRT-PCR measurements on FACS-purified cells showed that the progesterone-mediated induction of Wnt4 ligand in the luminal compartment still occurred in K5-Cre Rankfl/fl mice, where Wnt4 was specifically detected in the CD61− luminal cells that also express high levels of Rankl (Figure 3A). Rank expression was absent, as expected, in K5-Cre Rankfl/fl mice and expression of Rankl appeared reduced in CD61− cells from these mice (Figure 3A). However, immunostaining revealed a comparable number of RANKL-positive cells to be detectable (Figures 3C and 3D). Transcript levels for the cell-cycle activators Cyclin D1 and Cyclin D2 were significantly decreased in CD61− luminal cells and basal cells, respectively, of RANK-deficient glands (Figure S2B) consistent with their lack of a progesterone-stimulated proliferative response. Rank-null CD61− luminal cells also had significantly reduced expression of the milk protein, β-casein, in comparison to controls (Figure S2B), consistent with the compromised development of functional lobuloalveoli in Rank-deficient glands. Recently, CXCR4, the receptor for CXCL12, was shown to be important for mammary progenitor cell fate (Shiah et al., 2015) and we found its expression to be also decreased in the Rank-deficient Sca1− luminal progenitor population, although this effect did not reach statistical significance (Figure S2C). ELF5 is a transcription factor that dictates alveolar development, and its expression has been reported to be decreased in CD61− and CD61+ luminal cells following the loss of Rank (Lee et al., 2013). Measurement of Elf5 mRNA levels within the Sca1− luminal progenitor fraction showed no overt changes in the absence of Rank (Figure S2C). STAT5A is another transcription factor that regulates alveologenesis (Liu et al., 1997), and we found its expression in Sca1− progenitors of Rank-deficient mice to be reduced (Figure S2C).
Figure 3.
Progesterone-Triggered WNT Responsiveness Is Dependent on RANK Signaling
(A) Expression of RANKL and WNT pathway components measured by qRT-PCR relative to β-actin in FACS-purified CD61−, CD61+ luminal, and CD49fhi basal epithelial subpopulations from control and K5-Cre Rankfl/fl mice stimulated with 17β-estradiol and progesterone (EP). Expression was normalized to β-actin levels and plotted relative to that of CD49fhi basal cells from controls, set at 1. Data represent mean ± SEM; (n = 3 mice for each group).
(B) Pr expression status of FACS-sorted basal (B), non-clonogenic luminal (NCL), Sca1+, Sca1− luminal cells, and Keratin, Rank, Wnt signaling gene expression in the latter hormone receptor-negative alveolar progenitor population, from control and Rank-null mice in response to EP; β-actin-normalized transcript levels are shown plotted relative to control NCL cells for Pr, and control Sca1− cells for the other genes. Data represent mean ± SEM of n = 3 mice/cell fraction/group.
(C) Immunostaining of RANKL and WNT target, TCF1, in paraffin-embedded K5-Cre Rankfl/fl and control mammary tissue sections; scale bar, 20 μm.
(D) Quantification of RANKL and TCF1 protein levels in mammary tissues shown in (C), measured as a ratio of RANKL or TCF1 positive pixel to total pixel count, averaged from ten glandular regions per mouse mammary gland specimen; data are mean ± SEM (n = 3 mice/group).
(E) Immunofluorescent detection of AXIN2-expressing cells (green; white arrowheads) in control and Rank-deficient mammary glands in response to 17β-estradiol (E) and EP; scale bar, 30 μm.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S2.
Since progesterone has been shown previously to upregulate the expression of the WNT co-receptor, Lrp5, and the WNT target, Tcf1, in both luminal and basal mammary cells (Joshi et al., 2010), we also examined the expression of these elements in progesterone-stimulated K5-Cre Rankfl/fl purified mammary cells. Remarkably, there was no induction in the expression of Lrp5, Lrp6, or Tcf1 in either the luminal or basal mammary subpopulations of EP-stimulated K5-Cre Rankfl/fl mice (Figure 3A), and all three genes, as well as the Axin2 target gene, were also markedly reduced in the ER−PR−, Sca1− luminal progenitors isolated by FACS from K5-Cre Rankfl/fl glands by comparison to controls (Figure 3B). Moreover, these Sca1− cells in the luminal compartment that co-express luminal and basal keratins are considered bipotent (Shehata et al., 2012) and showed a lower level of K14 expression (a basal marker) without an alteration in K18 (a luminal marker) in Rank-null mice (Figure 3B). This suggests that RANK may help to promote the retention of basal characteristics in luminal cells. Immunostaining of mammary tissue from progesterone-treated K5-Cre Rankfl/fl mice showed that TCF1+ cells remained scarce in contrast to the prominent numbers of TCF1+ cells in similarly treated control mammary tissue (Figures 3C and 3D). Immunostaining for AXIN2, a key WNT target gene, also demonstrated a lack of AXIN2-expressing cells in the RANK-deficient mammary gland compared to their increased abundance following progesterone stimulation in controls (Figure 3E). This complete lack of expression of key WNT signaling components points to a role for RANK in controlling the molecular machinery that generates WNT-responsive adult mammary cells when progesterone levels increase. It has been reported that Wnt4-deficient mammary glands lack early lobuloalveolar development during pregnancy (Brisken et al., 2000), and that transgenic Wnt4 expression is not sufficient to generate this morphogenetic program (Kim et al., 2009), whereas RANKL signaling is (Fernandez-Valdivia et al., 2009). Our findings here explain these previous observations since we demonstrate that, despite Wnt4 ligand expression, RANKL-RANK signaling is essential to establish the WNT response in progenitor cell-enriched epithelial compartments.
RANK Signaling Activates WNT-Responsive ER−PR− Subsets of Mammary Epithelial Cells
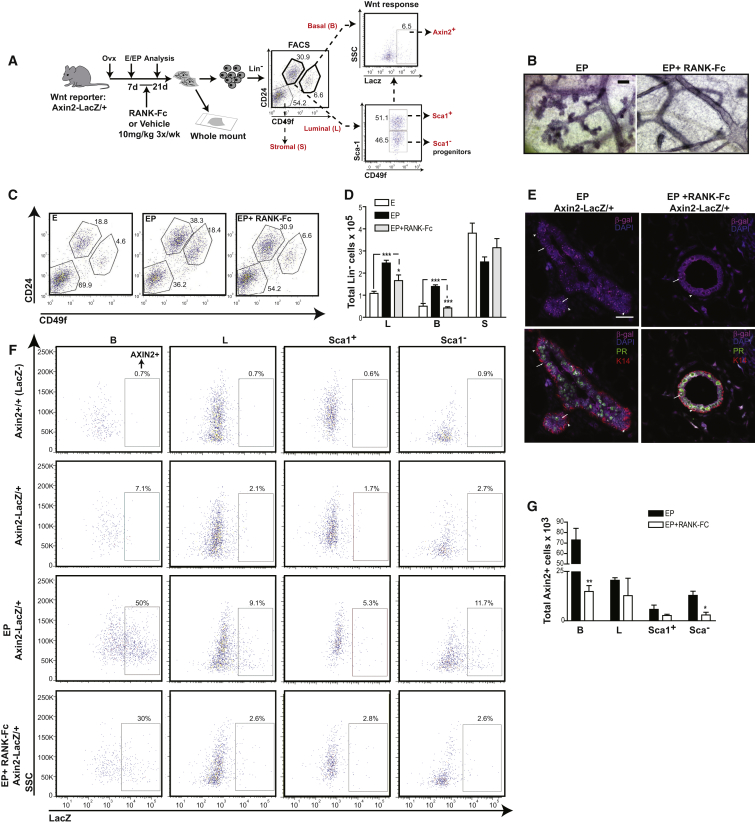
We next utilized Axin2-LacZ WNT reporter mice to investigate whether and how RANK signaling might affect the dynamics of WNT-responsive cell populations. These reporter mice express lacZ through the endogenous promoter of Axin2, a WNT/β-catenin target gene (Zeng and Nusse, 2010). Ovariectomized EP-treated Axin2-LacZ mice were co-administered the RANKL inhibitor, RANK-Fc (Figure 4A). As seen using our genetic approach to delete Rank, mice treated with RANK-Fc exhibited a blunted progesterone-induced expansion of both the luminal (CD24+CD49flo) and basal (CD24+CD49fhi) populations, and also suppressed lobuloalveologenesis (Figures 4B–4D). Previous studies using WNT reporter mice have documented the presence of WNT-responsive cells (∼5%) within basal, but not luminal cells, of adult virgin mice (van Amerongen et al., 2012; Zeng and Nusse, 2010). During pregnancy, however, lineage tracing experiments have identified WNT-responsive luminal cells adjacent to basal cells within alveolar structures, suggesting their origin from a common bipotent progenitor (van Amerongen et al., 2012). In mammary tissue from EP-stimulated WNT reporter mice, we detected Axin2-LacZ+ cells by immunostaining in the basal layer and in luminal cells, including those that are PR− (Figure 4E). Abundance of these cells was decreased following RANK-Fc treatment (Figure 4E). Using flow cytometry, we observed a small AXIN2+ WNT-responsive population in the basal fraction and negligible numbers in luminal subsets of the adult virgin mammary gland compared to LacZ– controls (Figure 4F), consistent with previous reports. AXIN2+ cell numbers were found to be greatly elevated in ovariectomized EP-treated mice, predominantly existing within ER–PR–, basal (∼50%) and Sca1– luminal subsets (∼12%) (Figure 4F). RANK-Fc treatment of these mice resulted in a marked depletion of WNT-responsive AXIN2+ cells in both the basal and Sca1– luminal fractions (Figures 4F and 4G). Together, these results reveal that RANK signaling selectively amplifies hormone receptor-negative, WNT-responsive basal cell and luminal progenitor numbers in response to progesterone.
Figure 4.
RANK Signaling Controls WNT-Responsive Mammary Epithelial Subsets
(A) Schema of the experimental procedure involving RANKL blockade in WNT reporter mice; 17β-estradiol (E), 17β-estradiol + progesterone (EP).
(B) Mammary gland whole-mounts following EP treatment with/without RANK-Fc (scale bar, 100 μm).
(C) FACS analysis of luminal (CD24+CD49flo), basal (CD24+CD49fhi), and stromal (CD24−CD49f−) mammary subpopulations from mice treated with E, EP, or EP + RANK-Fc.
(D) Histogram of total Lin− cells in the luminal (L), basal (B), and stromal (S) cell fractions. Data represent mean ± SEM of n = 4 (E), n = 5 (EP), n = 6 (EP+RANK-Fc) mice.
(E) Immunofluorescent images of mammary tissues from mice stimulated with EP ± RANK-Fc stained for Axin2-LacZ (β-gal); magenta, DAPI+ nuclei; blue (top) and co-stained for progesterone receptor (PR); green, keratin14 (K14); red (bottom). White arrows indicate Axin2-LacZ+ cells within the PR− luminal compartment and arrowheads point to Axin2-LacZ+ cells within the K14+PR− basal compartment; scale bar, 25 μm.
(F) FACS analysis of Axin2-Lacz+ WNT-responsive cells in B, L and segregated Sca1+ and Sca1− luminal cell populations of Axin2+/+ (LacZ–; row 1), and Axin2-LacZ/+ WNT reporter adult virgin mice untreated (row 2) and treated with EP ± RANK-Fc (rows 3 and 4).
(G) Quantification of the total numbers of AXIN2+ cells in B, L, Sca1+, and Sca1− epithelial subsets in EP ± RANK-Fc treated mice; data represent mean ± SEM of n = 3 mice/cell fraction/group.
∗p < 0.05, ∗∗p < 0.05, ∗∗∗p < 0.0001.
R-SPONDIN1 Is a Key Mediator of the RANK-Induced Activation of WNT Responsiveness
Given the role of R-SPONDIN1 (RSPO1) as a potent WNT signal enhancer and stem cell renewal factor (de Lau et al., 2014), we next interrogated whether RSPO1 is altered when RANK signaling is prevented. In purified cell fractions from EP-stimulated mice, we observed Rspo1 to be highly expressed in control Sca1− luminal progenitor cells similar to a previous report (Cai et al., 2014). However, its expression was severely impaired in Sca1− cells derived from K5-Cre Rankfl/fl mice (Figure 5A). To determine whether RSPO1 is a key downstream mediator of RANK effects on mammary progenitors, fat pads of EP-treated K5-Cre Rankfl/fl mice were injected once with recombinant RSPO1 or vehicle, 3 days prior to their analysis (Figure 5B). RSPO1 injection resulted in the formation of structures reminiscent of alveoli in Rank-null mammary glands, albeit not as uniformly as in controls, suggesting that other factors downstream of RANK likely cooperate with RSPO1 to maximize alveolar differentiation (Figure 5C). At the cellular level, RSPO1 led to a robust rescue in the expansion of basal, total luminal cells, and Sca1− progenitor cell numbers that were typically defective in the Rank-null mammary gland (Figures 5D and 5E). The functional clonogenic capacity of Rank-null basal cells and Sca1− cells also modestly increased following in vivo RSPO1 treatment (Figure 5F). Of note, the WNT response, as determined by AXIN2 immunostaining, was restored in luminal and basal cells of Rank-deficient mammary tissue following RSPO1 rescue (Figure 5G). These data collectively uncover RSPO1 as a central executor of RANK signaling effects on epithelial progenitors and the WNT response in the adult mammary gland.
Figure 5.
RSPO1 Rescues the Expansion of Mammary Epithelial Subsets, Progenitors, and the WNT Response in the Absence of RANK Signaling
(A) R-spondin1 (Rspo1) expression in the basal and luminal (NCL, Sca1+, Sca1−) mammary epithelial subsets of EP-treated control and K5-Cre Rankfl/fl mice. β-actin-normalized transcript levels are plotted relative to control Sca1− cells; data represent mean ± SEM of n = 4 mice/group.
(B) Schema of the RSPO1 rescue experimental procedure.
(C) Whole mounts of mammary glands from EP-stimulated control and K5-Cre Rankfl/fl mice treated with vehicle or RSPO1; scale bar, 500 μm (top), 100 μm (bottom, higher magnification).
(D) FACS profiles of luminal, basal, and stromal mammary cell subpopulations (top) and further segregated luminal NCL, Sca1+, and Sca1− subsets (bottom) from EP-stimulated control and K5-Cre Rankfl/fl mice treated with vehicle or RSPO1.
(E) Quantification of basal (B), total luminal (L) as well as NCL, Sca1+, and Sca1− luminal subsets from (D).
(F) Colony forming cell capacity of FACS-sorted basal (B), Sca1+, and Sca1− mammary progenitors from EP-stimulated control and Rank-deficient mice treated with vehicle or RSPO1. Data represent mean ± SEM of n = 4 (control), n = 3 (K5-Cre Rankfl/fl+vehicle), and n = 4 (K5-Cre Rankfl/fl+RSPO1) mice for (E) and (F).
(G) Immunofluorescent staining of mammary tissues from control and K5-Cre Rankfl/fl mice treated with vehicle or RSPO1 for AXIN2 (green), DAPI (blue), Keratin 14 (K14; magenta), and Keratin 18 (K18; red); white arrows indicate AXIN2+ WNT-responsive cells in the luminal compartment, and white arrowheads point to WNT-responsive cells found in the basal layer; scale bar, 25 μm.∗p < 0.05, ∗∗p < 0.01.
Discussion
In this study, we provide evidence that human breast luminal progenitors undergo a similar type of progesterone-mediated expansion of their numbers during the luteal phase of the menstrual cycle as previously documented in the mouse (Asselin-Labat et al., 2010; Joshi et al., 2010). This cyclical rise in luminal progenitor numbers would be anticipated to pose repeated opportunities for mutagenic events to accumulate, and thus offer an explanation for an increasing risk of breast cancer with more menstrual cycles (Kelsey et al., 1993). Luminal progenitors have been implicated as the cell of origin in BRCA1-related breast cancers (Lim et al., 2009; Molyneux et al., 2010), and in more recent work we have shown that these cells possess short dysfunctional telomeres and higher levels of reactive oxygen species, consistent with a higher propensity for transformation (Kannan et al., 2013, 2014). Previous studies investigating the effects of progesterone on human mammary cells have relied on progestin treatment of cultured human breast cells or organoids (Graham et al., 2009; Tanos et al., 2013). In a recent study, ex vivo progestin treatment of human mammary epithelial organoids or microstructures did not shift the distribution of epithelial subpopulations, although increased RANKL was observed (Tanos et al., 2013). The significant differences that we observed may reflect the fact that the cells we analyzed were obtained directly from tissues removed from women in progesterone-high and progesterone-low menstrual phases, thus pointing to the potential importance of avoiding in vitro treatments that may not faithfully recapitulate conditions operative in vivo. Our data also provide a snapshot of RANKL and WNT activity during the luteal phase in the human breast, thus indicating their involvement in mediating the physiological changes in circulating progesterone levels on the human luminal progenitor compartment.
WNT signaling is well recognized for its role in regulating stem and progenitor cells in diverse systems (Nusse et al., 2008). Elevated levels of β-catenin, indicative of aberrant WNT signaling, are reported in more than 50% of human breast carcinomas, correlate with poor prognosis, and are preferentially found in the aggressive triple-negative (ER−PR−HER2−) basal-like subtype (Geyer et al., 2011; Lin et al., 2000; Ryo et al., 2001). RANK signaling is also emerging as a significant variable in breast cancer (Schramek et al., 2010) with microarray data showing the highest levels of RANK in poor prognosis basal-like human breast cancers in comparison to other molecularly defined subtypes (Santini et al., 2011). Progesterone is likewise becoming increasingly recognized as a critical contributor to human breast cancer development, largely through lessons learned from increased breast cancer incidence in hormone replacement therapy trials with progestin combinations (Beral, 2003; Chlebowski et al., 2015; Joshi et al., 2015; Rossouw et al., 2002). A recent study also reported significantly higher levels of progesterone in BRCA1/2 mutation carriers who are at increased risk of ovarian and breast cancer, suggesting a progesterone influence on premenopausal breast cancer risk (Widschwendter et al., 2013).
RANKL and WNT signaling are known to be downstream effectors of progesterone (Beleut et al., 2010; Brisken et al., 2000; Fata et al., 2000; Joshi et al., 2010; Rajaram et al., 2015), but their underlying mechanism of action on distinct cellular targets in the normal and neoplastic mammary gland remains poorly understood. We now demonstrate that RANKL from ER+PR+ luminal epithelial cells acts on the mouse ER–PR– luminal alveolar progenitor compartment to activate RSPO1 and enable WNT responsiveness, resulting in the expansion of ER−PR− luminal alveolar progenitors and basal cells that jointly culminate in the generation of lobuloalveoli (Figure 6). Interestingly, RSPO1 has been shown to be required for alveolar formation in the mouse mammary gland, where its expression pattern during gestation paralleled that of Wnt4 (Chadi et al., 2009). RSPO1 enhances WNT signaling through its stabilizing effects on the WNT receptor complex and promotes the expansion of intestinal LGR5+ stem cells (de Lau et al., 2014). Recent work also shows its cooperation with WNT4 for mammary stem cell renewal (Cai et al., 2014). Here, we discover a crucial role for RANKL in inducing RSPO1 and engaging the WNT response in primitive mammary cells, thus enabling them to respond to progesterone. Given the widely documented role of WNT signals in controlling stem and progenitor cells in other tissues, our findings may prove to have broader relevance.
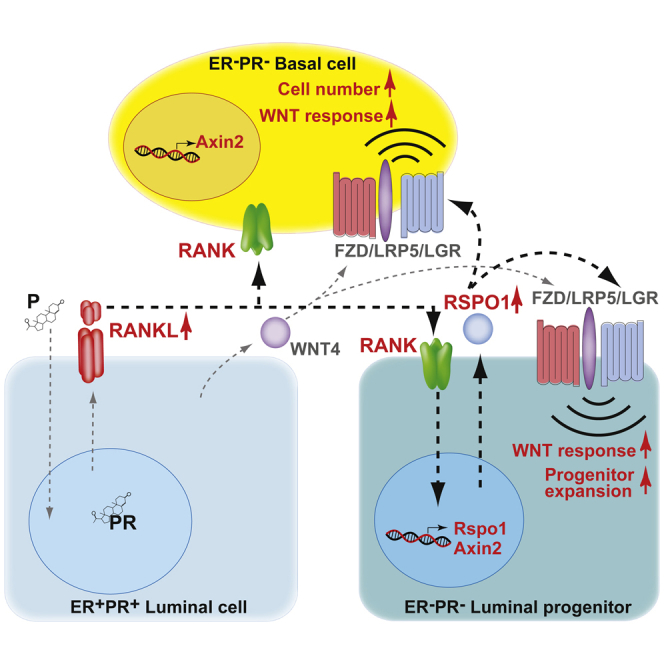
Figure 6.
Model Illustrating the Impact of RANK Signaling on WNT-Responsive Mammary Progenitors
Progesterone (P)-triggered RANKL-RANK paracrine signaling within the mammary stem cell niche stimulates the expression of R-SPONDIN1 (RSPO1) from the hormone-receptor negative (ER−PR−) luminal alveolar progenitor compartment (Sca1−), potentiating the WNT response and amplifying the numbers of ER−PR− luminal progenitors and basal cells. ER−PR− luminal alveolar progenitors express basal markers such as K14 and are considered bipotent, likely contributing to the basal lineage within alveolar structures, while basal WNT-responsive cells may continue to replenish the luminal progenitor pool. Together, these cellular events converge to generate lobuloalveolar units in the adult progesterone-exposed gland.
The RANKL-RANK driven, WNT-responsive ER−PR− luminal progenitors in the mouse and RANK-expressing luminal progenitors that expand in the human breast during the progesterone-high menstrual phase appear to represent analogous, highly proliferative progenitors within lobuloalveolar units. Since lobuloalveoli are the most common site of mammary cancer development in both mouse and human, it is tempting to nominate this progenitor as a strong candidate for breast carcinogenesis. Targeting RANK signaling in clinical trials utilizing Denosumab, a fully human monoclonal antibody to RANKL, has proved effective in reducing skeletal-related events and bone metastasis in breast cancer patients (Stopeck et al., 2010). Thus, it may be important to test the therapeutic capacity of Denosumab against primary breast cancer for treatment or prevention. However, in light of breast cancer heterogeneity and emerging RANK expression data, variable effects on different breast cancer subtypes and stages would also be anticipated to affect therapeutic outcomes. Since the WNT pathway is considered therapeutically elusive, RANK signaling blockade may also serve as a means to curtail WNT activity during breast pathogenesis.
Experimental Procedures
Mice
Rankflox mice and K5-Cre Rankfl/fl knockout mice have been described previously (Schramek et al., 2010) and were maintained on a pure C57BL/6 background. Cre-mediated deletion is described in the Supplemental Experimental Procedures. Wild-type mice and Axin2-LacZ/+ (Stock #009120) mice were purchased from the Jackson Laboratory. All mice were cared for according to the Canadian Council for Animal Care guidelines under protocols approved by the Animal Care Committee of the Ontario Cancer Institute, Toronto, Ontario.
Ovariectomy and Treatments
K5-Cre Rankfl/fl, Rankfl/fl controls and wild-type mice (10–13 weeks old) were bilaterally ovariectomized and allowed to recover for 1 week. Mice were subcutaneously implanted with 14-day slow release pellets (Innovative Research of America) of 17β-estradiol (E, 0.14 mg) or 17β-estradiol + progesterone (EP, 0.14 mg E + 14 mg P). For RANKL inhibition, RANK-Fc (kindly provided by William Dougall, Amgen) was injected subcutaneously (10 mg/kg RANK-Fc or PBS; three times/week) over the 14-day period. For Rank knockout rescue experiments, R-SPONDIN1 (R&D Systems) or PBS + 0.1%BSA (vehicle) alone was injected into the mammary fat pad (5 μg/gland) following 11 days of EP treatment.
Whole-Mount, Immunostaining, RNA Isolation, and Real-Time PCR Analysis
Please see Supplemental Experimental Procedures for detail description.
Mammary Cell Preparation and Flow Cytometry
Single mammary cell suspensions were generated from each pair of fourth inguinal mammary glands from individual mice by enzymatic digestion and flow cytometry performed as reported (Joshi et al., 2010) and described in Supplemental Experimental Procedures. To analyze β-galactosidase activity and WNT-responsive cells in Axin2-LacZ/+ mice, a fluorogenic β-galactosidase substrate (FDG) with the FluoReporter lacZ Flow Cytometry kit (Molecular Probes) was used according to the manufacturer’s protocol. FACS analysis was performed using FACSCalibur or FACSCanto II (BD Biosciences) and FlowJo software (Tree Star). Gating was defined based on single color, isotype, and fluorescence minus one negative controls. Cell sorting was performed on a FACSAria (BD Biosciences). The purity of sorted populations was routinely >95%. Histologically confirmed normal, anonymized discard breast tissue samples from 63 non-lactating, premenopausal women (17–50 years) undergoing reduction mammoplasties for indications other than neoplasia were obtained with informed consent according to procedures approved by the University of British Columbia Research Ethics Board. Their menstrual phase-specific morphology was identified as defined previously (Ramakrishnan et al., 2002). Viable single cell suspensions were prepared from previously isolated and cryopreserved organoids as previously described (Eirew et al., 2008). The cells were then labeled with fluorochrome-conjugated antibodies CD31 (BioLegend, cat. #303118), CD45 (BioLegend; cat. #304016) for endothelial and hematopoietic cell exclusion, respectively; CD49f (BD Biosciences; cat. #555735) and EpCAM (BioLegend; cat. #324206) for mammary cells; DAPI for cell viability in subpopulation distribution analysis by flow cytometry or to enable highly purified subsets to be isolated by FACS (>95%) using either an Influx-II or FACSAria (Becton Dickinson) (Eirew et al., 2008).
CFC Assays and In Vivo Limiting Dilution Assays
In vitro 2D CFC assays were performed as reported (Kannan et al., 2013; Makarem et al., 2013; Stingl et al., 2006). Limiting dilution transplantation assays of total mammary cells were performed to determine ductal and alveolar outgrowth rates in vivo. Details can be found in Supplemental Experimental Procedures.
Statistical Analysis
Values are reported as mean ± SEM comparison of data between multiple groups was performed using one-way ANOVA followed by Tukey’s post hoc multiple comparison test, and analysis between two specific groups was made using Student’s t test. Statistical significance is recognized at p < 0.05.
Author Contributions
P.A.J. designed and performed majority of the experiments and data analyses. P.D.W. performed limiting dilution assays and facilitated R-SPONDIN1 rescue experiments. N.K. generated and analyzed human breast samples from specific reproductive phases, S.N. assisted with RT-PCR, H.F. maintained mouse strains, M.A.D.G. contributed to in vitro experiments and RANK-Fc administration, H.W.J. assisted with cell preparations, J.M.P. provided Rank-deficient mice, and C.E. facilitated human breast work and advised on study presentation. R.K. directed the study. R.K. and P.A.J. wrote the manuscript with C.E. and N.K.
Acknowledgments
This work was supported by grants from the Canadian Cancer Society Research Institute (CCSRI) to R.K. and C.E. P.A.J. holds a postdoctoral fellowship from the Canadian Breast Cancer Foundation (CBCF) and H.W.J. was supported by a CBCF Studentship. N.K. holds a MITACS Elevate Fellowship. Mammoplasty tissue was obtained with the assistance of D. Wilkinson and surgeons J. Sproul, P. Lennox, N. Van Laeken, and R. Warren. The expert technical assistance of D. Wilkinson in performing menstrual staging analyses on human breast samples is gratefully acknowledged.
Published: June 18, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, two figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.05.012.
Supplemental Information
References
- Asselin-Labat M.L., Sutherland K.D., Barker H., Thomas R., Shackleton M., Forrest N.C., Hartley L., Robb L., Grosveld F.G., van der Wees J. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Asselin-Labat M.L., Vaillant F., Sheridan J.M., Pal B., Wu D., Simpson E.R., Yasuda H., Smyth G.K., Martin T.J., Lindeman G.J., Visvader J.E. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- Beleut M., Rajaram R.D., Caikovski M., Ayyanan A., Germano D., Choi Y., Schneider P., Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl. Acad. Sci. USA. 2010;107:2989–2994. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V., Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Brisken C., Heineman A., Chavarria T., Elenbaas B., Tan J., Dey S.K., McMahon J.A., McMahon A.P., Weinberg R.A. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- Cai C., Yu Q.C., Jiang W., Liu W., Song W., Yu H., Zhang L., Yang Y., Zeng Y.A. R-spondin1 is a novel hormone mediator for mammary stem cell self-renewal. Genes Dev. 2014;28:2205–2218. doi: 10.1101/gad.245142.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R.D., Wellings S.R. The comparative pathology of human and mouse mammary glands. J. Mammary Gland Biol. Neoplasia. 1999;4:105–122. doi: 10.1023/a:1018712905244. [DOI] [PubMed] [Google Scholar]
- Chadi S., Buscara L., Pechoux C., Costa J., Laubier J., Chaboissier M.C., Pailhoux E., Vilotte J.L., Chanat E., Le Provost F. R-spondin1 is required for normal epithelial morphogenesis during mammary gland development. Biochem. Biophys. Res. Commun. 2009;390:1040–1043. doi: 10.1016/j.bbrc.2009.10.104. [DOI] [PubMed] [Google Scholar]
- Chlebowski R.T., Rohan T.E., Manson J.E., Aragaki A.K., Kaunitz A., Stefanick M.L., Simon M.S., Johnson K.C., Wactawski-Wende J., O’Sullivan M.J. Breast cancer after use of estrogen plus progestin and estrogen alone. Analyses of data from 2 women’s health initiative randomized clinical trials. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.0494. Published online April 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W., Peng W.C., Gros P., Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirew P., Stingl J., Raouf A., Turashvili G., Aparicio S., Emerman J.T., Eaves C.J. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat. Med. 2008;14:1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- Fata J.E., Kong Y.Y., Li J., Sasaki T., Irie-Sasaki J., Moorehead R.A., Elliott R., Scully S., Voura E.B., Lacey D.L. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R., Mukherjee A., Ying Y., Li J., Paquet M., DeMayo F.J., Lydon J.P. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev. Biol. 2009;328:127–139. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Geyer F.C., Lacroix-Triki M., Savage K., Arnedos M., Lambros M.B., MacKay A., Natrajan R., Reis-Filho J.S. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod. Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- Graham J.D., Mote P.A., Salagame U., van Dijk J.H., Balleine R.L., Huschtscha L.I., Reddel R.R., Clarke C.L. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–3326. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P.A., Jackson H.W., Beristain A.G., Di Grappa M.A., Mote P.A., Clarke C.L., Stingl J., Waterhouse P.D., Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- Joshi P.A., Di Grappa M.A., Khokha R. Active allies: hormones, stem cells and the niche in adult mammopoiesis. Trends Endocrinol. Metab. 2012;23:299–309. doi: 10.1016/j.tem.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Joshi P.A., Goodwin P.J., Khokha R. Progesterone exposure and breast cancer risk. Understanding the biological roots. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.0512. Published online April 16, 2015. [DOI] [PubMed] [Google Scholar]
- Kannan N., Huda N., Tu L., Droumeva R., Aubert G., Chavez E., Brinkman R.R., Lansdorp P., Emerman J., Abe S. The luminal progenitor compartment of the normal human mammary gland constitutes a unique site of telomere dysfunction. Stem Cell Rev. 2013;1:28–37. doi: 10.1016/j.stemcr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N., Nguyen L.V., Makarem M., Dong Y., Shih K., Eirew P., Raouf A., Emerman J.T., Eaves C.J. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc. Natl. Acad. Sci. USA. 2014;111:7789–7794. doi: 10.1073/pnas.1403813111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey J.L., Gammon M.D., John E.M. Reproductive factors and breast cancer. Epidemiol. Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Kim Y.C., Clark R.J., Pelegri F., Alexander C.M. Wnt4 is not sufficient to induce lobuloalveolar mammary development. BMC Dev. Biol. 2009;9:55. doi: 10.1186/1471-213X-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Gallego-Ortega D., Ledger A., Schramek D., Joshi P., Szwarc M.M., Cho C., Lydon J.P., Khokha R., Penninger J.M., Ormandy C.J. Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development. 2013;140:1397–1401. doi: 10.1242/dev.088948. [DOI] [PubMed] [Google Scholar]
- Lim E., Vaillant F., Wu D., Forrest N.C., Pal B., Hart A.H., Asselin-Labat M.L., Gyorki D.E., Ward T., Partanen A., kConFab Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Lin S.Y., Xia W., Wang J.C., Kwong K.Y., Spohn B., Wen Y., Pestell R.G., Hung M.C. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Robinson G.W., Wagner K.U., Garrett L., Wynshaw-Boris A., Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Makarem M., Kannan N., Nguyen L.V., Knapp D.J., Balani S., Prater M.D., Stingl J., Raouf A., Nemirovsky O., Eirew P., Eaves C.J. Developmental changes in the in vitro activated regenerative activity of primitive mammary epithelial cells. PLoS Biol. 2013;11:e1001630. doi: 10.1371/journal.pbio.1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G., Geyer F.C., Magnay F.A., McCarthy A., Kendrick H., Natrajan R., Mackay A., Grigoriadis A., Tutt A., Ashworth A. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., ten Berge D., Kalani Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008;73:59–66. doi: 10.1101/sqb.2008.73.035. [DOI] [PubMed] [Google Scholar]
- Pellegrini P., Cordero A., Gallego M.I., Dougall W.C., Purificación M., Pujana M.A., Gonzalez-Suarez E. Constitutive activation of RANK disrupts mammary cell fate leading to tumorigenesis. Stem Cells. 2013;31:1954–1965. doi: 10.1002/stem.1454. [DOI] [PubMed] [Google Scholar]
- Rajaram R.D., Buric D., Caikovski M., Ayyanan A., Rougemont J., Shan J., Vainio S.J., Yalcin-Ozuysal O., Brisken C. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34:641–652. doi: 10.15252/embj.201490434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R., Khan S.A., Badve S. Morphological changes in breast tissue with menstrual cycle. Mod. Pathol. 2002;15:1348–1356. doi: 10.1097/01.MP.0000039566.20817.46. [DOI] [PubMed] [Google Scholar]
- Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A., Howard B.V., Johnson K.C. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ryo A., Nakamura M., Wulf G., Liou Y.C., Lu K.P. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat. Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- Santini D., Schiavon G., Vincenzi B., Gaeta L., Pantano F., Russo A., Ortega C., Porta C., Galluzzo S., Armento G. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE. 2011;6:e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek D., Leibbrandt A., Sigl V., Kenner L., Pospisilik J.A., Lee H.J., Hanada R., Joshi P.A., Aliprantis A., Glimcher L. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M., Teschendorff A., Sharp G., Novcic N., Russell I.A., Avril S., Prater M., Eirew P., Caldas C., Watson C.J., Stingl J. Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14:R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiah Y.J., Tharmapalan P., Casey A.E., Joshi P.A., McKee T.D., Jackson H.W., Beristain A.G., Chan-Seng-Yue M.A., Bader G.D., Lydon J.P. A progesterone-CXCR4 axis controls mammary progenitor cell fate in the adult gland. Stem Cell Rev. 2015;4:313–322. doi: 10.1016/j.stemcr.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman K.E., Kendrick H., Robertson D., Isacke C.M., Ashworth A., Smalley M.J. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J. Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J., Eirew P., Ricketson I., Shackleton M., Vaillant F., Choi D., Li H.I., Eaves C.J. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Tanos T., Sflomos G., Echeverria P.C., Ayyanan A., Gutierrez M., Delaloye J.F., Raffoul W., Fiche M., Dougall W., Schneider P. Progesterone/RANKL is a major regulatory axis in the human breast. Science Trans. Med. 2013;5:182ra155. doi: 10.1126/scitranslmed.3005654. [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Bowman A.N., Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A., Rocha A.S., Ousset M., Beck B., Bouvencourt G., Rock J., Sharma N., Dekoninck S., Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Widschwendter M., Rosenthal A.N., Philpott S., Rizzuto I., Fraser L., Hayward J., Intermaggio M.P., Edlund C.K., Ramus S.J., Gayther S.A. The sex hormone system in carriers of BRCA1/2 mutations: a case-control study. Lancet Oncol. 2013;14:1226–1232. doi: 10.1016/S1470-2045(13)70448-0. [DOI] [PubMed] [Google Scholar]
- Zeng Y.A., Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.