Abstract
Objective
Subependymomas are rare, indolent neoplasms that have been described in the brain and the spinal cord. The purpose of this study is to report the clinical and radiolographic features, and surgical outcomes of this entity.
Methods
Twenty-six patients with pathologically-verified subependymomas were treated from 1990 through 2009, with a mean follow-up of 39 months. The clinical and radiological records were reviewed and outcomes analyzed.
Results
There were 15 fourth ventricle tumors, 6 lateral ventricle tumors, and 5 spinal tumors. For the intracranial tumors, headaches, changes in vision, and difficulties with balance were the most common symptoms. Most tumors were heterogeneously enhancing and hypointense or isointense to gray matter on T1-imaging and hyperintense on T2-imaging.
All patients with tumors in the fourth ventricle underwent a suboccipital craniotomy and seven patients received an additional C1 laminectomy. Patients with lateral ventricular tumors underwent craniotomy with primarily a transcallosal resection. Patients with spinal tumors underwent laminectomy with intramedullary tumor resection. All tumors were resected employing microsurgical techniques. Overall, six patients had a sub-total resection. No recurrence of tumor or symptoms was noted at last follow-up for any patient, suggesting that maximal safe resection is often sufficient to provide symptomatic relief. Three patients had long-term complications from surgery. Tumor location was not associated with age at presentation, resection achieved, or development of complications.
Conclusions
Subependymomas are indolent tumors that when symptomatic can present with cerebrospinal fluid (CSF) obstructive symptoms in the brain and myelopathy in the spinal cord. There is no one symptom diagnostic for subependymomas. Surgical treatment can provide long term tumor control.
Keywords: Subependymoma, Intracranial ventricle neoplasm, Spinal neoplasm, Central nervous system
Introduction
Subependymomas are indolent, benign tumors of the central nervous system typically arising in the ventricular spaces. They were first described as a distinct pathological entity by Scheinker in 1945.1 Since then, only a handful of case series have been reported.2-5 Their true incidence has been difficult to determine as they often remains asymptomatic and are often found incidentally at autopsy.6 Some studies have estimated subependymomas to make up between 0.2 and 0.7% of all intracranial tumors.4-9
Subependymomas most often arise in the fourth (50–60%) and the lateral ventricles (30–40%).10 In some patients, however, they have a predilection for the spine, and present in the cervical and cervicothoracic regions.11 Histologically, they are categorized as World Health Organization Grade I tumors, appearing as clusters of cells embedded in a hypocellular fibrillary matrix.10 While a recent study focusing on genomic analysis has suggested a role for chromosomal copy number abnormalities in their pathogenesis, a precise understanding is still lacking and a progenitor cell has not yet been established.12
Patients with symptomatic masses have been reported to present with cerebrospinal fluid (CSF) obstruction, or less frequently, focal neurological deficits and seizures secondary to mass effect.4 Surgical treatment is employed in the vast majority of symptomatic cases.
The aim of the present study is to identify the clinical presentation, radiological features, surgical management, and outcomes of patients with pathologically-established subependymomas in a tertiary care academic medical center. We report these for our institution over a 20-year period.
Materials and Methods
Hospital records were identified for patients who underwent surgical intervention for brain tumors from 1990 through 2009 at our institution. Medical records including operative notes and clinical records were collected for patients who had a pathologically-confirmed subependymoma or those who had a mixed subependymoma–ependymoma tumor type on pathology. Patients whose pathology results indicated an alternate tumor type were excluded.
Radiology reports, including those for magnetic resonance imaging (MRI) and computed tomography (CT), were reviewed for each of the patients. Radiographs were used when the original images were available.
Basic patient demographic data, clinical data including presenting symptoms, tumor size and location, radiological findings, surgical approach and extent of resection, use of additional treatment modalities, presence of recurrent disease, and outcome at last follow-up were recorded. The study received approval from the Johns Hopkins Hospital Institutional Review Board.
Student’s t-test, one-way analysis of variance (ANOVA) test, and Fisher’s exact test were used to analyze differences between the study groups. Statistical significance was set at P<0.05.
Results
Tumor location and patient characteristics
From 1990 through 2009, 26 patients were noted to have a subependymoma (24 patients with pathologically-confirmed subependymomas; two patients with mixed subependymoma–ependymoma tumors). The mean and median follow-up were 39 and 27 months, respectively. Patients were classified by tumor location into three different groups (Table 1): lateral ventricle tumors (LVTs), fourth ventricle tumors (FVTs), and spinal tumors (STs). Patient age, length of hospitalization, and length of follow-up are listed in Table 2. There was no statistical difference in age at presentation between the three groups (ANOVA test; P=0.2251).
Table 1. Subependymoma lesions were found in the brain and the spinal cord.
| Location | No. of patients |
|---|---|
| Lateral ventricle tumors (LVT) | 6 (23%) |
| Fourth ventricle tumors (FVT) | 15 (57%) |
| Spinal tumors (ST) | 5 (20%) |
| Cervical spine involvement only | 3 (12%) |
| Thoracic spine involvement only | 1 (4%) |
| Cervical and thoracic involvement | 1 (4%) |
Table 2. Patient characteristics by tumor location.
| LVT group | FVT group | ST group | All patients | |
|---|---|---|---|---|
| Sex | 4 F, 2 M | 5 F, 10 M | 3 F, 2 M | 12 F, 14 M |
| Mean age (range) | 54 yrs (28–73) | 46 yrs (19–59) | 41 yrs (36–45) | 47 yrs (19–73) |
| Mean hospital stay (range) | 10 d (3–16) | 10 d (2–61) | 8 d (4–16) | 10 d (2–61) |
| Mean follow-up (range) | 53 mo (8–126) | 39 mo (1–166) | 21 mo (1–57) | 39 mo (1–166) |
Note: F: female; M: male; yrs: years; d: days; mo: months.
Presentation
The clinical presentation varied between the three groups (Table 3). In the LVT group, all patients presented symptomatically, and headache was the most common presenting symptom (found in 67%).
Table 3. Clinical presentation of patients with subependymoma.
| Presenting symptom | LVT group | FVT group | ST group | All patients |
|---|---|---|---|---|
| Hydrocephalus-related | ||||
| Headache | 4 | 5 | … | 9 (35%) |
| Nausea and/or vomiting | 1 | 3 | … | 4 (15%) |
| Difficulty with balance and gait | 2 | 4 | … | 6 (23%) |
| Changes in mental status | 2 | 1 | … | 3 (12%) |
| Changes in memory | 2 | … | … | 2 (8%) |
| Focal neurological deficits | ||||
| Changes in vision | 1 | 6 | … | 7 (27%) |
| Changes in hearing | … | 3 | … | 3 (12%) |
| Vertigo | … | 3 | … | 3 (12%) |
| Motor weakness | 1 | 2 | 5 | 8 (31%) |
| Dysesthesia | 0 | 3 | 5 | 8 (31%) |
| Incidental findings | … | 5 | … | 5 (19%) |
In the FVT group, change in vision was the most common presenting symptom (found in 40%). Five patients in the FVT group were asymptomatic, and the tumor was an incidental finding on imaging obtained for workups relating to stroke, trauma, tinnitus, anisocoria, and post-herpetic pain, respectively.
In the ST group, all patients presented with extremity paresis and dysesthesias. In addition, three patients experienced urinary frequency and episodes of incontinence.
Radiographic features and tumor size
Generally, the subependymoma lesions were isointense or hypointense to gray matter on T1-imaging and hyperintense on T2-imaging, though this pattern was not universally seen.
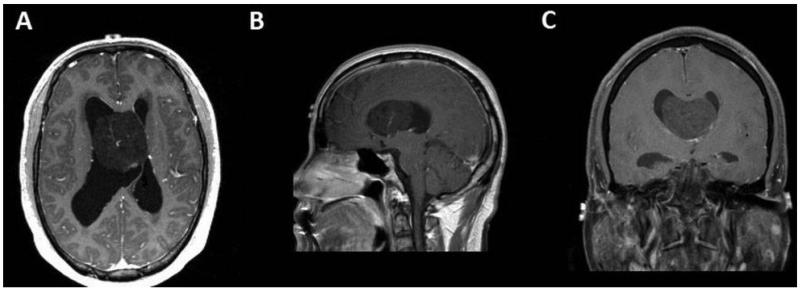
In the LVT group, there was heterogeneous enhancement with gadolinium contrast on T1-weighted images in four (67%) patients; two patients had tumors that did not enhance. The lesions in the LVT group had a mean tumor diameter of 27 mm in transverse plane, 30 mm in anterioposterior plane, and 30 mm in craniocaudal plane (Fig. 1).
Figure 1. A 45-year-old male presenting with headache and fatigue with a 4.6 cm transverse × 4.8 cm anteroposterior × 3.8 cm craniocaudal, heterogeneously-enhancing mass in the anterior body of the right lateral ventricle. Patient had prominent lateral ventricles and received an intra-operative ventricular catheter. (A) Minimally enhancing lesion on an axial T1-weighted post-contrast image. (B) Minimally enhancing lesion on a coronal T1-weighted post-contrast image. (C) Minimally enhancing lesion on a sagittal T1-weighted post-contrast image.
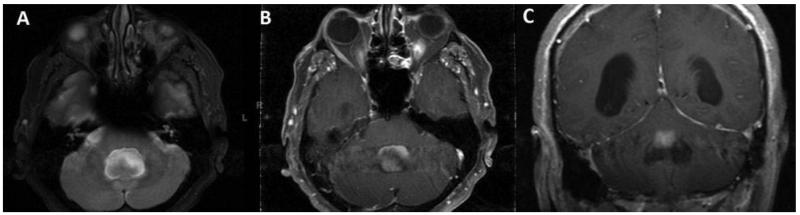
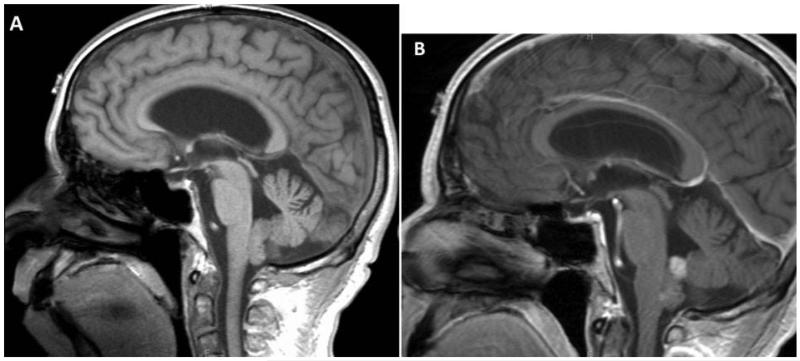
In the FVT group, there was heterogeneous enhancement with gadolinium contrast on T1-weighted images in 12 (80%) patients (Fig. 2). Two patients had tumors that did not enhance and one patient had homogeneous enhancement on the superior aspect of the lesion (Fig. 3). The lesions in the FVT group had a mean tumor diameter of 17 mm in transverse plane, 17 mm in anterioposterior plane, and 22 mm in craniocaudal plane.
Figure 2. A 59-year-old male presented with alternating levels of consciousness, difficulties with balance, vision and hearing found to have a 2.2 cm transverse × 2.1 cm anteroposterior × 4.2 cm craniocaudal, heterogeneously-enhancing mass in the fourth ventricle. The mass extends out of the foramen of Megendie and foramen of Luscka extending inferiorly to the level of C2 and causing fourth ventricular outflow obstruction. This resulted in enlargement of the entire ventricular system. Patient was treated with suboccipital craniotomy with C1 laminectomy. (A) Lesion is hyperintense to gray matter on the axial T2-weighted image. (B) Lesion enhances heterogeneously on the axial T1-weighted image after contrast administration. (C) Lesion enhances on the coronal T1-weighted post-contrast image.
Figure 3. A 59-year old male presented with positional nausea found to have a 0.7 cm transverse × 0.9 cm anteroposterior × 1.3 cm craniocaudal, homogeneously-enhancing mass in the floor of the fourth ventricle. Patient was treated with suboccipital craniotomy and C1 laminectomy. (A) Lesion is isointense to gray matter on a sagittal T1-weighted image before gadolinium contrast administration. (B) Superior portion of lesion enhances homogeneously on a sagittal T1-weighted image post-contrast.
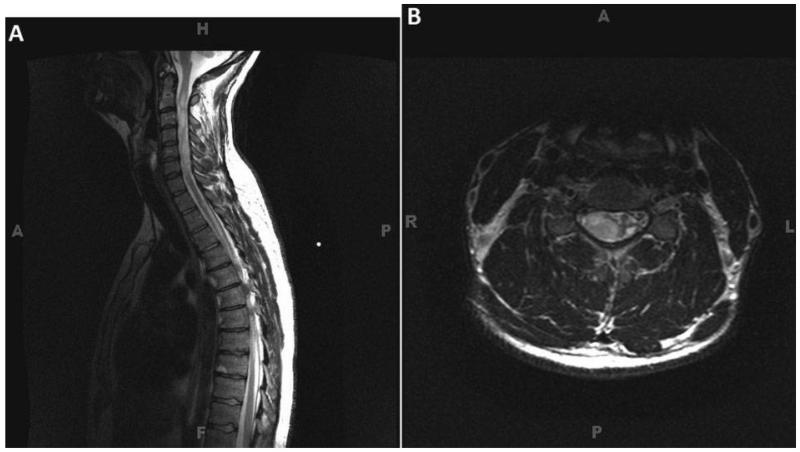
In the ST group, the tumors spanned on average of five vertebral levels, and appeared as lobular, infiltrative intramedullary masses involving the cervical and upper thoracic spinal cord, with heterogeneous features on T2-weighted images (Fig. 4). Marked cord expansion and distortion of the cord substances was seen throughout the affected levels. Two patients had cervical spine only tumors (C1 to C7 and C3 to C6); two patients had tumors that spanned the cervicothoracic junction (C2 to T3 and C4 to T1); and one patient had a tumor spanning the upper thoracic vertebral levels (T1 to T5).
Figure 4. A 36-year-old male presenting with right arm dysesthesia with a large, lobular, infiltrative intradmedullary mass involving the entire cervical spine from C1 to C7. (A) T2-weighted sagittal image, the mass is manifested by heterogeneous T2 signal hyperintensity. Marked cord expansion and distortion of the cord substance is seen throughout the affected levels. (B) T2-weighted axial MRI demonstrating marked distortion of the spinal cord with large predominantly right sided, hyperintense subependymoma.
Management of hydrocephalus
In the LVT group, there was evidence of hydrocephalus on imaging of five (83%) patients. None of the patients received pre-operative CSF drainage in the form of a ventricular drain or shunt. However, three patients received intraoperative ventricular catheters that were all removed in the immediate post-operative period without complication.
In the FVT group, there was evidence of hydrocephalus in three patients. One of these patients demonstrated dilation of the entire ventricular system (Fig. 2). None of these patients received pre-operative or intraoperative CSF drainage.
None of the patients in the ST group had radiographic evidence of hydrocephalus or syrinx formation.
Surgical treatment
In the LVT group, complete resection was achieved in five (83%) cases. Five of the cases employed a frontal craniotomy with a transcallosal approach; one case required a temporoparietal craniotomy with an approach between the inferior temporal gyrus and the most inferior portion of the parietal lobe.
In the FVT group, all patients were treated surgically with a suboccipital craniotomy and microsurgical resection. In seven cases, an additional C1 laminectomy was performed. Complete resection was achieved in 10 (67%) cases and partial resection in five (33%) cases.
In the ST group, all patients had their intradural intramedullary tumors treated with laminectomy and microsurgical resection. An average of 5.8 vertebral levels (range: 4–8) were operated upon.
There was no significant relationship between tumor location and extent of resection (Fisher’s exact test; P=0.623). None of the patients in our study received pre- or post-operative radiation or chemotherapy.
Perioperative complications
In the LVT group, one of the six patients suffered a major complication. Immediately post-operation, the patient developed ventilatory insufficiency, pneumocephalus, hydrocephalus, aphasia, and lethargy. The patient was treated conservatively but required a 16-day hospital stay and was discharged to an inpatient rehabilitation facility for continuation of care.
In the FVT group, six of the 15 patients developed post-operative complications. Five of these patients had transient symptoms that resolved by the time of discharge from the hospital. The sixth patient suffered from a left middle cerebral artery stroke requiring a decompressive craniotomy in the immediate post-operative period. He was discharged to an inpatient rehabilitation facility for continuation of care.
In the ST group, one of the five patients suffered from major complications. The patient developed a pulmonary embolism post-operatively and was started on an intravenous heparin drip. While on anticoagulation, the patient developed acute neurological deficits and MRI of the spine demonstrated significant cord edema. He was taken emergently to the operating room for intradural exploration, which revealed extensive cord infarction. He subsequently underwent decompression of the spinal cord and received instrumentation and fusion that extended from C2 to T3. He was discharged to a long-term inpatient rehabilitation facility with lower extremity paralysis.
There was no significant relationship between tumor location and likelihood of developing major complications (Fisher’s exact test; P=0.381).
Long-term outcomes
In the LVT group, mean length of follow-up was 53 months (range: 8–126 months). At most recent follow-up, five of the six patients reported resolution of their original symptoms and denied development of any new symptoms. The one patient who had suffered perioperative complications was noted to have developed severe memory loss, incontinence, complete inability to ambulate and a Parkinsonian syndrome at 2-year follow-up. It was unclear whether her worsened memory was due to the surgery or independent Parkinsonian-type dementia.
In the FVT group, mean length of follow-up was 39 months (range: 1–166 months). At most recent follow-up, 14 (93%) of the patients reported resolution of their original symptoms and denied development of any new symptoms. The one patient who had developed a left middle cerebral artery stroke continued to suffer from right hemiparesis and aphasia.
In the ST group, mean length of follow-up was 21 months (range: 1–57 months). At last follow-up, four of the five patients reported resolution of their original symptoms and denied development of any new symptoms. The one patient who suffered a spinal cord infarct continued to report symptoms consistent with C7 quadriplegia at the 56-month follow-up.
Radiographic outcomes
One patient was lost to follow-up, but the remaining 25 patients underwent follow-up MRI. The mean MRI follow–up length was 37 months (range: 1–166 months). There was no radiographic evidence of tumor recurrence in any patient receiving gross total resection and no evidence of tumor progression in any patient who underwent subtotal resection.
Subependymoma–ependymoma mixed tumors
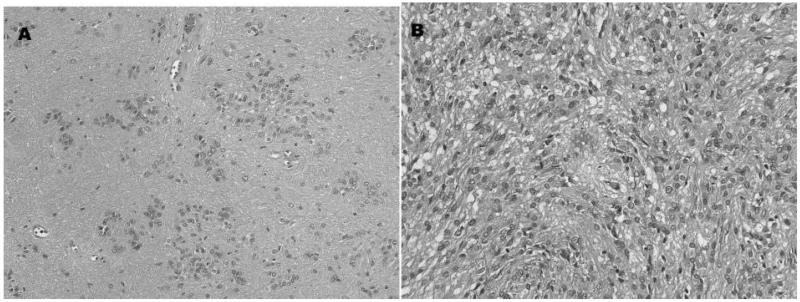
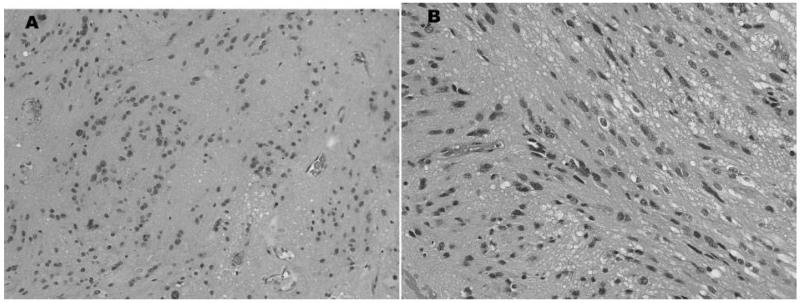
Two patients had histology consistent with a mixed subependymoma–ependymoma tumor. One patient had a FVT and pathological analysis (Fig. 5) revealed a lesion where 90% of the specimen was consistent with subependymoma, and the remainder was consistent with a classic grade II ependymoma. The other patient had a ST and pathological analysis (Fig. 6) revealed features of both tanycytic ependymoma and subependymoma. Both patients had complete resolution of symptoms post-operatively and no evidence of disease progression at last follow-up.
Figure 5. Pathological sections of a mixed subependymoma–ependymoma section from a fourth ventricular tumor. (A) Areas of the tumor have classic clusters of nuclei in a finely-fibrillar tumor background. (B) Other regions of the same tumor have class ependymoma features, i.e. high-cellularity and peri-vascular pseudoreosettes. The latter were GFAP-positive.
Figure 6. Pathological sections of a mixed subependymoma–ependymoma section from a cervical spine tumor. (A) Tumor has cell clustering and fibrillar background typical of subependymoma. (B) Elsewhere, the tumor was composed of elongated, spindle-shaped cells in a tanycytic pattern.
Discussion
Subependymomas are indolent tumors that comprised 26 cases resected at our institution during the 20-year period from 1990 through 2009. The rarity of the tumor is consistent with the dearth of large case reports on the topic.2-5 In this study, we report our experience with subependymomas found in the lateral ventricles, fourth ventricles, and the cervical and thoracic spinal cord. We found that tumor location is not associated with age at presentation, extent of resection, or development of major complications.
Mean age of presentation for our patients was 47 years, which is consistent with the mean reported age of 51 years in other studies.4 While our subependymoma patients contained about an equal number of males and females, the literature5,9-10 presents this primarily as a disease of older males.
The majority of the patients in our study were symptomatic and eight patients had clear evidence of obstructive hydrocephalus with ventricular enlargement on imaging. We postulate that in patients with subependymomas, CSF obstruction is much more likely to be the cause of symptoms than direct compression of neural structures. This is consistent with our finding that the most common presenting symptoms were headaches, changes in vision, and difficulty with balance and gait — problems that are a known result of altered CSF dynamics. Similarly, others have reported that symptoms in up to 94% patients can be attributed to some form of CSF obstruction.2
In our study, only three of the eight patients with hydrocephalus received an intra-operative ventricular catheter for CSF drainage. All three catheters were removed in the immediate post-operative period. Although these patients had evidence of prominent ventricles on imaging before surgery, none of them received hydrocephalus treatment pre-operatively or required permanent CSF diversion after the ventricular catheter was removed. The catheters were mainly used to provide brain relaxation during surgery and help drain blood and proteinaceous material released during the tumor resection. Thus, we suggest that the majority of patients with subependymomas do not require permanent CSF diversion to treat hydrocephalus and that tumor resection is often sufficient to regain normal CSF flow.
Anatomically, the fourth ventricular floor is a common location for lesions that obstruct CSF flow. Interestingly, such lesions constituted approximately 75% of the intracranial subependymomas in our study. Ragel et al. found that subependymomas arising in the fourth ventricle often led to symptoms associated with severe neurological deficits because of their ability to obstruct CSF flow.6 As such, at our institution, fourth ventricular subependymomas are routinely resected, even if the patient is initially asymptomatic at presentation. In most cases, the advantage of removing the lesion from this location and obtaining a pathological diagnosis out-weighs the potential harms related to surgical intervention.
In our study, the five patients with spinal subependymomas had lesions located in the cervical spine. This is consistent with the previous work of Jallo et al., where they showed that the spinal subependymomas have a predilection for cervical and cervicothoracic regions.11 We do not include any of the patients from Jallo et al.’s prior series in this study. While rare, one should keep subependymomas in mind when encountered with a patient with an upper spinal cord intramedullary lesion.
Radiographic analysis of the lesions in our patients revealed that subependymomas are hypointense or isointense to gray matter on T1 images, hyperintense on T2 images, and heterogeneously enhancing on post-gadolinium contrast images. This is largely consistent with what has been reported in literature.6,13,14 For instance, in 12 cases studied with MR imaging, Rushing et al. also found the tumors to be isointense or hypointense to gray matter on T1 and hyperintense on T2, with 80% enhancing after gadolinium-based contrast administration.4 Further, Chiechi and colleagues reported that fourth ventricular tumors are more likely to have calcifications on unenhanced CT images.2 Incidentally, there were only two cases in our series where a pre-operative CT image was obtained for fourth ventricular tumors; a calcified mass was seen in both.
All patients in the current study were treated surgically. The fourth ventricular tumors were resected primarily via a suboccipital craniotomy and the tumors within the lateral ventricles were resected primarily via transcallosal approaches. All patients survived the procedure and most had no new neurological deficits post-operatively. This is in contrast to the high mortality rates reported previously,4,5 including a staggering 33% operative mortality reported by Scheithauer in 1978 and a 23.5% mortality rate reported by Rushing et al. for patients operated on between 1970 and 1999. The difference can likely be attributed to the improvements in intraoperative microscopy and intra-operative neurophysiological monitoring and recent advancements in microsurgical techniques that allows for safe tumor resection and CSF flow restoration. However, it should be noted that surgery for these tumors is certainly not free of complications.
There were two patients in our series with a mixed subependymoma–ependymoma histology and both presented in similar fashion to those with pure subependymomas. Additionally, the radiographic characteristics of the mixed tumors were very similar to that of pure subependymomas. The outcomes were also comparable between the two groups. However, with only two patients, it is hard to draw any conclusions on whether outcomes of pure and mixed subependymomas are truly similar.
None of the patients in our series received radiation treatment. Lombardi et al. recommended that post-operative radiation may be appropriate for patients with symptomatic residual tumors.3 Similarly, Maiuri et al. treated two patients who had partial tumor resection with post-operative radiation.13 Other authors including Scheithauer and Fujisawa have recommended against use of radiation post sub-total resection.5,15 This is perhaps because residual tumors grow slowly, do not cause significant CSF flow obstruction and as such are often asymptomatic. Rushing et al. found that there was not a significant survival difference between patients who received a gross versus sub-total surgical tumor resection.4 Our data are consistent with this finding, as imaging and examination of the six patients with partial resection showed all to be well at their last follow-up. Further, there was no evidence of residual tumor in four cases and only asymptomatic small nodules in the other two cases. This is likely because such a small fraction was left behind that it was not visible on follow-up imaging. No recurrence or tumor growth was observed in any of the patients during follow-up; however, it must be noted that our mean clinical follow-up of 39 months and mean MRI follow-up of 37 months may be too short for this benign, slow growing lesion. Nonetheless, all the tumors in our series behaved indolently, supporting a strategy of observation for asymptomatic incidentally discovered subependymomas.
Conclusions
In summary, subependymomas are primarily ventricular tumors that often become symptomatic secondary to CSF obstruction. They most often present as headaches, changes in vision and balance, and have characteristic findings on MRI. Surgery remains the mainstay in treating these lesions and with the use of microsurgical techniques, complete tumor resection is possible and curative. If minimal residual disease is left behind, it may be appropriate it follow patients conservatively and monitor for tumor progression.
References
- 1.Scheinker IM. Subependymoma: a newly recognized tumor of subependymal derivation. J Neurosurg. 1945;2:232–40. [Google Scholar]
- 2.Chiechi MV, Smirniotopoulos JG, Jones RV. Intracranial subependymomas: CT and MR imaging features in 24 cases. AJR Am J Roentgenol. 1995;165:1245–50. doi: 10.2214/ajr.165.5.7572512. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi D, Scheithauer BW, Meyer FB, Forbes GS, Shaw EG, Gibney DJ, et al. Symptomatic subependymoma: a clinicopathological and flow cytometric study. J Neurosurg. 1991;75:583–8. doi: 10.3171/jns.1991.75.4.0583. [DOI] [PubMed] [Google Scholar]
- 4.Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, Smirniotopoulos JG, et al. Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol. 2007;85:297–305. doi: 10.1007/s11060-007-9411-6. [DOI] [PubMed] [Google Scholar]
- 5.Scheithauer BW. Symptomatic subependymoma: report of 21 cases with review of the literature. J Neurosurg. 1978;49:689–96. doi: 10.3171/jns.1978.49.5.0689. [DOI] [PubMed] [Google Scholar]
- 6.Ragel BT, Osborn AG, Whang K, Townsend JJ, Jensen RL, Couldwell WT. Subependymomas: an analysis of clinical and imaging features. Neurosurgery. 2006;58:881–90. doi: 10.1227/01.NEU.0000209928.04532.09. [DOI] [PubMed] [Google Scholar]
- 7.Brown DF, Rushing EJ. Subependymomas: clinicopathologic study of 14 tumors. Arch Pathol Lab Med. 1999;123:873. doi: 10.5858/1999-123-0873-SCSOT. [DOI] [PubMed] [Google Scholar]
- 8.Lobato RD, Sarabia M, Castro S, Esparza J, Cordobes F, Portillo JM, et al. Symptomatic subependymoma: report of four new cases studied with computed tomography and review of the literature. Neurosurgery. 1986;19:594–8. doi: 10.1227/00006123-198610000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Prayson RA, Suh JH. Subependymomas: clinicopathologic study of 14 tumors, including comparative MIB-1 immunohistochemical analysis with other ependymal neoplasms. Arch Pathol Lab Med. 1999;123:306–9. doi: 10.5858/1999-123-0306-S. [DOI] [PubMed] [Google Scholar]
- 10.Wiestler OD, Schiffer D. Subependymoma: tumors of the nervous system. In: Kleihues P, Cavenee WK, editors. Pathology and genetics. IARC Press; Lyon: 2000. pp. 80–81. [Google Scholar]
- 11.Jallo GI, Zagzag D, Epstein F. Intramedullary subependymoma of the spinal cord. Neurosurgery. 1996;38:251–7. doi: 10.1097/00006123-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kurian KM, Jones DT, Marsden F, Openshaw SW, Pearson DM, Ichimura K, et al. Genome-wide analysis of subependymomas shows underlying chromosomal copy number changes involving chromosomes 6, 7, 8 and 14 in a proportion of cases. Brain Pathol. 2008;18:469–73. doi: 10.1111/j.1750-3639.2008.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiuri F, Gangemi M, Iaconetta G, Signorelli F, del Basso de Caro M. Symptomatic subependymomas of the lateral ventricles. report of eight cases. Clin Neurol Neurosurg. 1997;99:17–22. doi: 10.1016/s0303-8467(96)00554-9. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein JE, Lenchik L, Stanciu MG, Shimkin PM. MRI of intracranial subependymomas. J Comput Assist Tomogr. 1995;19:264–7. doi: 10.1097/00004728-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa H, Hasegawa M, Ueno M. Clinical features and management of five patients with supratentorial subependymoma. J Clin Neurosci. 2010;17:201–4. doi: 10.1016/j.jocn.2009.05.028. [DOI] [PubMed] [Google Scholar]