Abstract
Myelodysplastic syndromes (MDS) are characterized by cytopenias resulting from ineffective hematopoiesis with a predisposition to transform to acute myeloid leukemia (AML). Recent evidence suggests that the hematopoietic stem cell microenvironment contributes to the pathogenesis of MDS. Inflammation and hypoxia within the bone marrow are key regulators of hematopoietic stem and progenitor cells that can lead to several bone marrow failure syndromes, including MDS. In this brief review, we provide an overview of the clinical and molecular features of MDS, the bone marrow microenvironment, and specific pathways that lead to abnormal blood cell development in MDS. Characterization of key steps in the pathogenesis of MDS will lead to new approaches to treat patients with this disease.
Keywords: myelodysplastic syndromes, bone marrow microenvironment, signaling pathways, hypoxia, ribosomal deficiency, inflammation
Introduction
Myelodysplastic syndromes (MDS) represent a heterogeneous group of clonal disorders characterized by ineffective hematopoiesis in the bone marrow leading to cytopenias in the blood and a predisposition to acute myeloid leukemia (AML) (1–4). The categorization of subclasses of MDS is based on the percentage of leukemia blasts in the peripheral blood and the bone marrow, the number and type of dysplastic cell lineages, the presence of ringed sideroblasts, and cytogenetic abnormalities. Low, intermediate or high-risk MDS is classified using the revised International Prognostic Scoring System (5, 6). The majority of MDS patients are diagnosed at greater than 70 years of age. A number of factors including environmental, genetic and prior exposure to chemotherapy or radiation therapies are associated with the development of MDS (7). In addition, there are a number of inherited bone marrow failure syndromes including Fanconi anemia (FA), Shwachman-Diamond syndrome (SDS), and dyskeratosis congenital (DC) that often develop during childhood and predispose patients to the development of MDS at an early age (7, 8).
A variety of morphological, genetic, and clinical features have been identified that distinguish pediatric MDS from adult MDS and have been previously discussed in detail (9). Although relatively uncommon in children, de novo and secondary MDS is often the first presentation of an inherited bone marrow failure syndrome. Unlike in adults, pediatric MDS is more often associated with monosomy 7 and a hypocellular bone marrow. Refractory cytopenia is more common than refractory anemia, which is seen in the elderly (9). Thus, there are biological and clinical aspects of pediatric MDS that are different from adult MDS.
Significant advances have been made to understand the pathogenesis of MDS to explain the spectrum of this disease. In addition to cytogenetic abnormalities including del(5q), −7 or del(7q), and +8, defects have been identified in RNA splicing machinery, epigenetic regulation of gene expression, and specific signaling pathways, including p38 Mitogen Activated Protein Kinase (MAPK) and Tissue Necrosis Factor alpha (TNFalpha) (3). Somatic mutations have been identified in hematopoietic stem cells from MDS patients and most likely contribute to the pathogenesis of the disease. Approximately 80% of MDS patients have a somatic mutation in their hematopoietic stem cells. Mutations in p53, EZH2, ETV6, RUNX1, and ASXL1, in MDS patients have been associated with a poor prognosis (4). In particular, p53 mutations predict patients who will progress to AML.
Treatment of MDS depends on the severity of the disease. For low-risk MDS, supportive care has been the primary mode of treatment, including growth factors, transfusions, and antibiotic therapy (4). For high risk disease, hypomethylating agents (decitabine and 5-azacytidine), immunomodulatory drugs (lenalidomide), and chemotherapy (daunomycin, cytarabine) are often used. High dose chemotherapy and stem cell transplantation can produce long-term remission in high-risk MDS patients.
Bone marrow microenvironment and MDS
The bone marrow is comprised of hematopoietic stem cells (HSC) existing within a complex and dynamic microenvironment with multiple cellular and molecular factors that regulate hematopoiesis under physiologic and pathophysiologic conditions. The delicate interplay between the hematopoietic stem and progenitor cells, stromal cells, and cytokines or chemokines secreted within the microenvironment is needed to maintain hematopoiesis. Multiple cellular components of the bone marrow microenvironment including osteoblasts/osteoprogenitor cells, vascular endothelial cells, mesenchymal stem cells, monocytes, and macrophages support the hematopoietic stem cell niche (for a recent review (10)). It is likely that aberrant interactions between hematopoietic stem cells and the microenvironment also contribute to the pathogenesis of MDS. Indeed genetic studies in mice have shown that manipulation of the osteoblastic niche is sufficient to promote MDS and AML phenotypes. Genetic disruption of DICER, an RNAase III endonuclease that is essential for miRNA biogenesis and RNA processing resulted in the development of myelodysplasia and AML progression (11). Interestingly, microarray analysis of dysregulated gene expression in DICER deficient osteoblasts revealed significant down regulation of the Shwachman-Diamond-Bodian Syndrome gene (Sbds). Inactivating mutations in Sbds are associated with both skeletal abnormalities as well as bone marrow failure and a predisposition to develop MDS and AML (12). These findings indicate that dysregulation of Sbds in cells of the osteoblastic lineage may contribute to the pathogenesis of SDS. Further evidence to support a role for cells in the osteoblastic lineage in the pathogenesis of MDS was observed in mice with a single activating mutation of B-catenin in osteoblasts. These mice accumulated common chromosomal aberrations in myeloid cells as well as MDS features and the rapid development of AML (13). In this model, constitutive activation of beta-catenin in osteoblasts lead to increased expression of the Notch ligand, Jagged-1 that activated Notch signaling in HSCs. Importantly, nuclear accumulation and increased beta-catenin signaling in osteoblasts was also identified in 38% of patients with MDS/AML suggesting that this model may recapitulate cellular and molecular features within a subset of MDS/AML patients (13). In addition to murine models of MDS/AML, patient-derived bone marrow stromal cells have also been shown to promote the malignant behavior of human MDS cells in vivo. While human MDS cells injected into mice intrabone results in very little engraftment of the stem cells (14), co-injection of MDS cells with MDS MSCs significantly enhanced the engraftment rate of MDS cells within the bone marrow of immunocompromised mice (15). Previous studies have demonstrated that expression of CD146 on stromal cells is associated with enhanced engraftment of MDS cells. Additionally, overproduction of niche factors including N-Cadherin, IGFBP2, VEGFA, and LIF were also associated with the enhanced engraftment mediated by patient derived MDS MSC cells (14, 15). Despite the advances in this field, very little is known regarding the molecular basis of interaction between MDS cells and specific stromal cells in humans that could lead to development of dysplasia in the bone marrow.
Hypoxia and MDS
In addition to the cellular components of the HSC/MDS niche mentioned above, hypoxia, or low oxygen availability, is a prominent molecular feature of the bone marrow microenvironment that contributes to both normal and malignant hematopoiesis. Relative to most tissues, the bone marrow resides in a particularly hypoxic microenvironment. Oxygen tensions within the bone marrow cavity range from 0.6% to 4.2% O2, whereas oxygen tensions in most other adult tissues range from 2–9% O2 (16, 17). Hypoxia develops as a result of an imbalance between oxygen delivery and oxygen consumption. The bone marrow is thought to be particularly hypoxic tissue due to the low blood flow rate within bone marrow sinusoids and the high oxygen consumption rate of hematopoietic cells. It is estimated that the blood flow rate within bone marrow sinusoids is 1/10 to 1/20 of that found within bone marrow arterioles (18). In addition, it has been estimated using mathematical modeling that a layer of three myeloid progenitors is sufficient to utilize all oxygen delivered by a neighboring sinusoid cell (19).
The hypoxia inducible transcription factors HIF-1 and HIF-2 are the key molecular mediators of the cellular response to hypoxia. In response to oxygen tensions below 5% O2, the transcription factors HIF-1 and HIF-2 are stabilized and activate gene expression programs including angiogenesis, glycolytic metabolism, erythropoiesis, differentiation and apoptosis that help cells adapt to low oxygen (20). While HIF-1 and HIF-2 bind similar target DNA sequences, they have both overlapping and distinct functions (21, 22).
Recent studies have defined an important functional role for hypoxia and HIF signaling in the regulation of HSC metabolism and maintenance. In particular, HIF-1 is highly expressed in HSCs where it regulates glycolytic metabolism (23). Genetic inactivation of HIF-1 in HSCs resulted in loss of cell cycle quiescence and decreased HSC numbers during stress conditions of bone marrow transplantation, myelosuppression, and aging (24). In contrast, loss of HIF-2 in HSCs had no significant effect on HSC maintenance or post-transplantation renewal indicating a predominant role for HIF-1 in the maintenance of HSC function (25).
Hypoxia and activation of HIF signaling is also associated with the development and pathogenesis of a variety of hematologic diseases (26–30). In MDS patients, HIF-1 expression correlates with poor patient survival and disease progression (31). Functionally, the role of HIF signaling in MDS remains to be elucidated. However, studies indicate that there may be both direct and indirect roles for hypoxia and HIF signaling in the pathogenesis of MDS. In vitro assays have demonstrated that culturing MDS cells in hypoxic conditions enhances the colony-forming unit (CFU) yield from MDS mononuclear cells (32). Additionally, gene expression profiling of supportive MDS MSCs in comparison to healthy MSCs revealed a strong hypoxic signature indicating that hypoxia and HIF signaling may also influence the malignant behavior of MDS MSCs (15). Future studies are needed to carefully dissect the role of HIF signaling within both MDS and key supportive cells of the MDS niche.
Inflammation and Immune Suppression in MDS
The role of inflammation and immune suppression is becoming increasingly recognized as an important factor in the pathogenesis of bone marrow failure syndromes, including MDS, DBA, and FA (33, 34). Dysregulation of cytokine expression in MDS bone marrow contributes to suppression of both ineffective hematopoiesis and malignant clone immune escape. The expression of TNFalpha, TGFbeta, IFNgamma, IL-4, IL-6, IL-7, and IL-10 are abnormally regulated in some MDS patients (1, 35)., TNFalpha has been implicated as a factor contributing to the increased apoptosis of stem cells in MDS, DBA and FA patients (33, 34). Additionally, TNFalpha, TGFbeta, and IFNgamma exhibit myelosuppressive activities within MDS marrow (36). IL-10 is an immunosuppressive cytokine is elevated in CD3+ peripheral blood cells in high risk compared to low risk MDS patients (37, 38). Polymorphisms within the IL10 promoter are also present associated with poor patient prognosis in MDS patients, further indicating a role for IL10 in the pathogenesis of MDS (39).
Recent studies have began to identify key cellular components of the MDS bone marrow microenvironment that contribute to altered cytokine production, the development of ineffective hematopoiesis, and immune escape in MDS patients. Chen and colleagues discovered that myeloid-derived suppressor cells (MDSCs) are expanded in MDS patients and can actively suppress hematopoiesis through the production of IL-10 and TGFbeta (40). In addition to myeloid suppressor cells, regulatory T cells (Tregs) are also expanded in MDS patients. The activation of Treg cells in MDS is thought to promote immune tolerance and allow for the expansion of blasts harboring genetic mutations (41, 42). Along with the increase in suppressive immune populations, patients with high risk MDS also exhibit impaired natural killer (NK) cells function and expansion of autoreactive CD8+ T cells (43).
There is now significant evidence that activation of the innate immune system, e.g. macrophages and neutrophils, contributes to HSC senescence and MDS pathogenesis (1). Signaling pathways of the toll-like receptors (TLRs) are among the important mediators of the inflammatory response. TLR4 is overexpressed in MDS HSCs, which leads to apoptosis and cytopenias (44). Increased TLR1, 2, and 6 and downstream immune modulating kinase IRAK1 have also been reported in MDS HSCs. There are now inhibitors of IRAK1 that have been demonstrated to induce cell cycle arrest and apoptosis of the MDS HSCs in vitro (1, 45). Several other immune signaling molecules downstream of TLRs such as TRAF6 and NFκB have also been shown to mediate the proinflammatory response and have increased activation in low- and high-risk MDS stem cells (1, 46).
Proinflammatory signaling pathways have recently been shown to be critical for normal hematopoietic stem cell fate (47, 48). In zebrafish, TNFalpha signaling through TNFR2 expressed on HSCs resulting in activation of the Notch and NFkappaB signaling pathways is required for definitive but not primitive hematopoiesis (47). Interestingly, TNFalpha is produced by neutrophils in the microenvironment, not macrophages or monocytes suggesting the innate immune systems plays a crucial role in HSC production. Another study demonstrated that interferon-gamma (IFNgamma) and its receptor Crfb17 regulates HSC development in zebrafish (49). IFNgamma does not regulate HSC proliferation or survival, but rather transition from endothelial cells to HSCs in the hemogenic endothelium or aorta-gonad-mesonephros region. IFNgamma appears to activate Stat3 signaling pathways downstream of Notch and blood flow to regulate HSC fate (49). Therefore, inflammatory signals are not only critical for pathogenesis of MDS, but also for normal HSC development (48).
Ribosomal deficiency, MDS, and inflammatory response
One of the most common chromosomal abnormalities in adult MDS is (del)5q. Somatic chromosomal deletions in 5q leads to the development of MDS that is characterized by a defect in erythroid differentiation. Ebert et al. employed an siRNA approach to identify downregulated genes that would phenocopy the hematopoietic defects associated with 5q deletion. These studies revealed that partial loss of the ribosomal protein subunit 14 (RPS14) was sufficient to phenocopy the disease in hematopoietic progenitor cells. Moreover, RSP14 expression was sufficient to rescue the erythroid defect in patient derived progenitors (50). The mechanism by which RSP14 regulated erythroid differentiation was linked to a defect in the processing of pre-RNA (50). Interestingly, this defect was analogous to the ribosomal biogenesis defects found in Diamond-Blackfan Anemia (DBA) providing a functional link between MDS in somatic 5q del with a congenital bone marrow failure syndrome (50). Indeed, inherited mutations in several other proteins involved in ribosomal synthesis are associated with the development of pediatric bone marrow failure syndromes including Diamond-Blackfan Anemia (DBA), SDS, dyskeratosis congenital, and cartilage hair hypoplasia (51, 52).
DBA is characterized by a selective erythroid defect leading to anemia at an early age in childhood (53). In 1999, mutations in the ribosomal protein subunit, RPS19, was first described in patients with DBA (54). Approximately 25% of patients with DBA have mutations in RPS19. We recently described that knockdown of RPS19 in cord blood CD34+ cells results in decreased GATA1 protein levels and increased TNFalpha production by CD71+ (nonerythroid) cells including stromal cells, such as macrophages (33). The induction of TNFalpha was mediated by p53 activation and phosphorylation of p38 Mitogen Activated Protein (MAP) kinase. The erythroid defect was corrected in vitro by treatment with TNFalpha (33). This was the first report linking ribosomal deficiency to inflammatory responses in human hematopoietic stem and progenitor cells. In addition, these data suggest that perhaps other bone marrow failure syndromes, including RPS14 deficient del(5q) MDS, could lead to stress hematopoiesis that activates p53 and p38MAPkinase, and decreased levels of GATA1. The precise mechanism by which ribosomal insufficiency in hematopoietic stem and progenitor cells produces inflammation is not known and is a focus of investigation in the future.
Targeting the microenvironment for treatment of MDS
Several approaches to decrease inflammation in the bone marrow of MDS patients have been taken. Unfortunately, immune modulators have variable responses in MDS patients. Antithymocyte globulin and cyclosporine alone or in combination have responses between 0 and 30% (1). One study showed that lenalidomide is effective in 83% of patients with del(5q) MDS, while hypomethylating agents seem to be more effective in MDS patients with greater numbers of blasts in the bone marrow (55). An obvious approach to treating MDS would be to target TNFalpha or its receptor. In one pilot study with 12 MDS patients treated with Etanercept, four patients had improvement in their blood counts or transfusion requirements while others had decreased cell counts. Although baseline TNFalpha levels did not correlate with response, the HSCs from these patients showed an increase in myeloid progenitor cells in vitro (56). Clearly, more studies are required to determine the optimal administration or MDS patient populations who might respond to Etanercept (2). The TNFalpha antibody, infliximab, treatment in low-risk MDS patients yielded a low response rate (3/22 patients compared to 0/21) (57). Thus, TNFalpha blockade alone may not be optimal therapy for MDS patients. Other components of the microenvironment that are potential targets for therapy include Dicer, where targeted deletion of Dicer in the osterix-expressing osteoprogenitor cells affected proliferation, survival, and differentiation of HSCs of MDS patients (1, 58). Greater understanding of the relationship between inflammation in the bone marrow microenvironment of MDS patients and their HSCs will provide novel approaches to treat this disease.
Based on mechanistic studies, several signaling pathways to target the microenvironment to treat MDS patients have been identified. Recently the RPS19 and RPS14 deficient HSCs were demonstrated to be responsive to the amino acid and translational enhancer, L-Leucine, by activating the mTOR pathway (59). P38 MAP kinase is constitutively activated in MDS HSCs and an inhibitor of the p38MAPK alpha isoform increases proliferation of MDS stem cells in vitro. A Phase I/II clinical trial for low- and intermediate-risk MDS with the small molecule, SCIO-469, demonstrated a 30% response in cytopenia (2). Another p38MAPK inhibitor known as ARRY-614 also targets the Tie-2 ligands are overexpressed in MDS patients and are associated with a worse prognosis (36). A phase I study of ARRY-614 demonstrated decreased platelet transfusion requirements in a subset of patients with low- and intermediate-risk MDS (60). Correlative studies demonstrated decrease in p38MAPK activation and apoptosis in the bone marrow cells from MDS patients who responded. Additional targeted therapies have been developed for TGFbeta ligand Activin (2). A ligand trap developed for TGFbeta receptor, ACE-536, stimulates erythropoiesis in preclinical studies and is presently in phase I/II clinical trials for MDS (61). Sotatercept is a fusion protein of Activin receptor type IIa and IgG, inhibits SMAD2/3 signaling (62, 63). Clinical trials with other multi- kinase inhibitors and mTOR inhibitors, e.g. tensirolimus, for MDS patients are currently in progress. MEK inhibitors, farnesyl transferase inhibitors, EGF inhibitors, glutathione-S-Transferase-1 inhibitors are all under investigation (2).
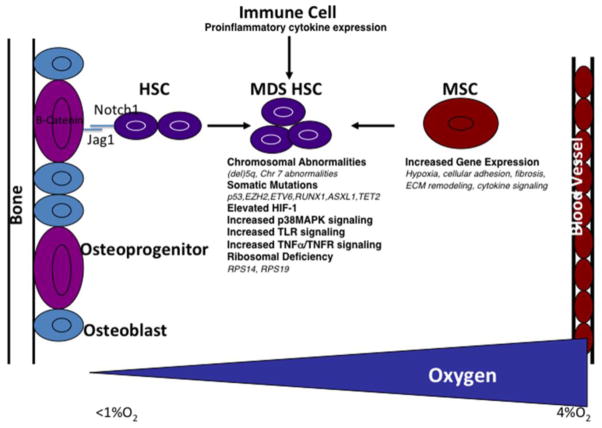
In conclusion, the bone marrow microenvironment plays a critical role in the fate of HSCs and contributes to the pathogenesis of MDS. Recent work has demonstrated that key cellular factors within the bone marrow microenvironment including cells of the osteoblast lineage, immune, and mesenchymal stromal cells produce signaling molecules that promote the initiation and progression of MDS (Figure 1). Hypoxia in the bone marrow also controls normal hematopoiesis and when altered likely contributes to the pathogenesis of MDS (Figure 1). Future studies will be necessary to better define the interactions between stromal cells, cytokines/chemokines, and their specific effects on HSC proliferation, survival, and differentiation. This information will be crucial for development of novel therapies to treat MDS.
Figure 1. Cellular and molecular mechanisms of MDS.
Immune cells, osteoblasts, and mesenchymal stromal cells (MSCs) express signaling molecules that influence MDS HSC signaling and function. Hypoxia also has the capacity to directly and indirectly influence the behavior of MDS HSCs.
Research Highlights.
The bone marrow microenvironment plays a critical role in the pathogenesis of MDS.
Characterization of molecular pathways that contribute to MDS could lead to identification of novel approaches to treat MDS.
Inflammatory signals in the bone marrow determine hematopoietic stem cell fate and are aberrantly regulated in MDS hematopoietic stem cells.
Hypoxia is important for normal hematopoietic stem cell development and potentially plays a role in MDS.
Dysregulation of inflammatory signaling, including upregulation of TNFalpha, is associated with the development of acquired and inherited bone marrow failure syndromes. Dysregulation of inflammatory signaling contributes to the pathogenesis of MDS through both direct mechanisms on HSCs and by altering the bone marrow microenvironment.
Several targeted therapies have been developed, which inhibit molecules that are abnormally regulated in MDS patients.
Acknowledgments
K.M.S. is supported by the NIH (R01 HL75826, R01 GM087305, DOD BMFRP Idea Award BM110060), Leukemia and Lymphoma Society of America Screen to Lead Program, Hyundai Hope on Wheels, Stanford SPARK/Child Health Research Institute, A.N. (ASH Scholar Award, K08 DK090145-01A1, CHRI Pilot Early Career Award), J.K.P. (T32DK098132, Paul and Yuanbi Ramsay Endowed Postdoctoral Fellowship, Child Health Research Institute and the Stanford CTSA UL1 TR001085)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang L, Qian Y, Eksioglu E, Epling-Burnette PK, Wei S. The inflammatory microenvironment in MDS. Cell Mol Life Sci. 2015;72(10):1959–66. doi: 10.1007/s00018-015-1846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachegowda L, Gligich O, Mantzaris I, Schinke C, Wyville D, Carrillo T, Braunschweig I, Steidl U, Verma A. Signal transduction inhibitors in treatment of myelodysplastic syndromes. J Hematol Oncol. 2013;6(50) doi: 10.1186/1756-8722-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jhanwar SC. Genetic and epigenetic pathways in myelodysplastic syndromes: A brief overview. Adv Biol Regul. 2015;58:28–37. doi: 10.1016/j.jbior.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Visconte V, Tiu RV, Rogers HJ. Pathogenesis of myelodysplastic syndromes: an overview of molecular and non-molecular aspects of the disease. Blood Res. 2014;49(4):216–27. doi: 10.5045/br.2014.49.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg PL, Stone RM, Bejar R, Bennett JM, Bloomfield CD, Borate U, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, et al. Myelodysplastic syndromes, version 2.2015. J Natl Compr Canc Netw. 2015;13(3):261–72. doi: 10.6004/jnccn.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzi A, Sekeres MA. Myelodysplastic syndromes: a practical approach to diagnosis and treatment. Cleveland Clinic journal of medicine. 2010;77(1):37–44. doi: 10.3949/ccjm.77a.09069. [DOI] [PubMed] [Google Scholar]
- 8.Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, Carr AG, Greene MH, Rosenberg PS. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glaubach T, Robinson LJ, Corey SJ. Pediatric myelodysplastic syndromes: they do exist! Journal of pediatric hematology/oncology. 2014;36(1):1–7. doi: 10.1097/MPH.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 10.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dror Y, Freedman MH. Shwachman-Diamond syndrome: An inherited preleukemic bone marrow failure disorder with aberrant hematopoietic progenitors and faulty marrow microenvironment. Blood. 1999;94(9):3048–54. [PubMed] [Google Scholar]
- 13.Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–4. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Deeg HJ. Murine xenogeneic models of myelodysplastic syndrome: an essential role for stroma cells. Exp Hematol. 2014;42(1):4–10. doi: 10.1016/j.exphem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell stem cell. 2014;14(6):824–37. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell. 2010;7(2):150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Rankin EB, Giaccia AJ, Schipani E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Current osteoporosis reports. 2011;9(2):46–52. doi: 10.1007/s11914-011-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophysical journal. 2001;81(2):685–96. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Molecular and cellular biology. 2006;26(9):3514–26. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. The Journal of biological chemistry. 2009;284(25):16767–75. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell stem cell. 2010;7(3):380–90. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, Gezer D, Vukovic M, Dunn K, Pollard P, Holyoake TL, et al. Hif-2alpha is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122(10):1741–5. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 26.Rouault-Pierre K, Lopez-Onieva L, Foster K, Anjos-Afonso F, Lamrissi-Garcia I, Serrano-Sanchez M, Mitter R, Ivanovic Z, de Verneuil H, Gribben J, et al. HIF-2alpha protects human hematopoietic stem/progenitors and acute myeloid leukemic cells from apoptosis induced by endoplasmic reticulum stress. Cell stem cell. 2013;13(5):549–63. doi: 10.1016/j.stem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell stem cell. 2011;8(4):399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100(10):3767–75. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh AK, Shanafelt TD, Cimmino A, Taccioli C, Volinia S, Liu CG, Calin GA, Croce CM, Chan DA, Giaccia AJ, et al. Aberrant regulation of pVHL levels by microRNA promotes the HIF/VEGF axis in CLL B cells. Blood. 2009;113(22):5568–74. doi: 10.1182/blood-2008-10-185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellmann S, Guschmann M, Griethe W, Eckert C, von Stackelberg A, Lottaz C, Moderegger E, Einsiedel HG, Eckardt KU, Henze G, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18(5):926–33. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- 31.Tong H, Hu C, Zhuang Z, Wang L, Jin J. Hypoxia-inducible factor-1alpha expression indicates poor prognosis in myelodysplastic syndromes. Leukemia & lymphoma. 2012;53(12):2412–8. doi: 10.3109/10428194.2012.696637. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JE, Conlon JP, Yang X, Sanchez PV, Carroll M. Enhanced growth of myelodysplastic colonies in hypoxic conditions. Experimental hematology. 2007;35(1):21–31. doi: 10.1016/j.exphem.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibikova E, Youn MY, Danilova N, Ono-Uruga Y, Konto-Ghiorghi Y, Ochoa R, Narla A, Glader B, Lin S, Sakamoto KM. TNF-mediated inflammation represses GATA1 and activates p38 MAP kinase in RPS19 deficient hematopoietic progenitors. Blood. 2014 doi: 10.1182/blood-2014-06-584656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du W, Erden O, Pang Q. TNF-alpha signaling in Fanconi anemia. Blood Cells Mol Dis. 2014;52(1):2–11. doi: 10.1016/j.bcmd.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allampallam K, Shetty V, Mundle S, Dutt D, Kravitz H, Reddy PL, Alvi S, Galili N, Saberwal GS, Anthwal S, et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int J Hematol. 2002;75(3):289–97. doi: 10.1007/BF02982044. [DOI] [PubMed] [Google Scholar]
- 36.Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, Parmar S, Haghnazari E, Zhou L, Collins R, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108(13):4170–7. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopes MR, Traina F, de Campos PM, Pereira JK, Machado-Neto JA, da Machado HC, Gilli SC, Saad ST, Favaro P. IL10 inversely correlates with the percentage of CD8(+) cells in MDS patients. Leuk Res. 2013;37(5):541–6. doi: 10.1016/j.leukres.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124(11):4678–80. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasamatsu T, Saitoh T, Minato Y, Shimizu H, Yokohama A, Tsukamoto N, Handa H, Sakura T, Murakami H. Polymorphisms of IL-10 affect the severity and prognosis of myelodysplastic syndrome. Eur J Haematol. 2015 doi: 10.1111/ejh.12577. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, Epling-Burnette P, Van Bijnen S, Dolstra H, Cannon J, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123(11):4595–611. doi: 10.1172/JCI67580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, Lombardi G, Wlodarski MW, Maciejewski JP, Farzaneh F, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110(3):847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 42.Mailloux AW, Sugimori C, Komrokji RS, Yang L, Maciejewski JP, Sekeres MA, Paquette R, Loughran TP, Jr, List AF, Epling-Burnette PK. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol. 2012;189(6):3198–208. doi: 10.4049/jimmunol.1200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816–24. doi: 10.1182/blood-2006-07-035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13(4):1154–60. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 45.Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, Mathews L, Ferrer M, Southall N, Guha R, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013;24(1):90–104. doi: 10.1016/j.ccr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fang J, Rhyasen G, Bolanos L, Rasch C, Varney M, Wunderlich M, Goyama S, Jansen G, Cloos J, Rigolino C, et al. Cytotoxic effects of bortezomib in myelodysplastic syndrome/acute myeloid leukemia depend on autophagy-mediated lysosomal degradation of TRAF6 and repression of PSMA1. Blood. 2012;120(4):858–67. doi: 10.1182/blood-2012-02-407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, Traver D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159(5):1070–85. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veldman MB, Lin S. Stem cells on fire: inflammatory signaling in HSC emergence. Dev Cell. 2014;31(5):517–8. doi: 10.1016/j.devcel.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 49.Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev Cell. 2014;31(5):640–53. doi: 10.1016/j.devcel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, Raza A, Root DE, Attar E, Ellis SR, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451(7176):335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs O. Important genes in the pathogenesis of 5q- syndrome and their connection with ribosomal stress and the innate immune system pathway. Leukemia research and treatment. 2012;2012:179402. doi: 10.1155/2012/179402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu JM, Ellis SR. Ribosomes and marrow failure: coincidental association or molecular paradigm? Blood. 2006;107(12):4583–8. doi: 10.1182/blood-2005-12-4831. [DOI] [PubMed] [Google Scholar]
- 53.Sakamoto KM, Shimamura A, Davies SM. Congenital disorders of ribosome biogenesis and bone marrow failure. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S12–7. doi: 10.1016/j.bbmt.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet. 1999;21(2):169–75. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 55.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 56.Deeg HJ, Gotlib J, Beckham C, Dugan K, Holmberg L, Schubert M, Appelbaum F, Greenberg P. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia. 2002;16(2):162–4. doi: 10.1038/sj.leu.2402356. [DOI] [PubMed] [Google Scholar]
- 57.Raza A, Candoni A, Khan U, Lisak L, Tahir S, Silvestri F, Billmeier J, Alvi MI, Mumtaz M, Gezer S, et al. Remicade as TNF suppressor in patients with myelodysplastic syndromes. Leuk Lymphoma. 2004;45(10):2099–104. doi: 10.1080/10428190410001723322. [DOI] [PubMed] [Google Scholar]
- 58.Raaijmakers MH. Disease progression in myelodysplastic syndromes: do mesenchymal cells pave the way? Cell stem cell. 2014;14(6):695–7. doi: 10.1016/j.stem.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Narla A, Payne EM, Abayasekara N, Hurst SN, Raiser DM, Look AT, Berliner N, Ebert BL, Khanna-Gupta A. L-Leucine improves the anaemia in models of Diamond Blackfan anaemia and the 5q- syndrome in a TP53-independent way. Br J Haematol. 2014;167(4):524–8. doi: 10.1111/bjh.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Manero G, Khoury HJ, Jabbour E, Lancet J, Winski SL, Cable L, Rush S, Maloney L, Hogeland G, Ptaszynski M, et al. A phase I study of oral ARRY-614, a p38 MAPK/Tie2 dual inhibitor, in patients with low or intermediate-1 risk myelodysplastic syndromes. Clin Cancer Res. 2015;21(5):985–94. doi: 10.1158/1078-0432.CCR-14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suragani RN, Cadena SM, Cawley SM, Sako D, Mitchell D, Li R, Davies MV, Alexander MJ, Devine M, Loveday KS, et al. Transforming growth factor-beta superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408–14. doi: 10.1038/nm.3512. [DOI] [PubMed] [Google Scholar]
- 62.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8(12):970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 63.Lotinun S, Pearsall RS, Davies MV, Marvell TH, Monnell TE, Ucran J, Fajardo RJ, Kumar R, Underwood KW, Seehra J, et al. A soluble activin receptor Type IIA fusion protein (ACE-011) increases bone mass via a dual anabolic-antiresorptive effect in Cynomolgus monkeys. Bone. 2010;46(4):1082–8. doi: 10.1016/j.bone.2010.01.370. [DOI] [PubMed] [Google Scholar]